Introduction
Ischemic preconditioning (IPC) is an important
endogenous adaptive phenomenon first described by Murry et
al (1) in 1986. IPC involves
single or multiple brief periods of sublethal ischemia, which
increase myocardial resistance to a greater subsequent insult. It
is now well established that IPC confers two separate phases of
cardioprotection; an early phase occurring instantly and continuing
for 3–4 h and a late (or delayed) phase occurring ~12 h after the
preconditioning stimulus that may persist to 72 h (2,3). The
late phase is clinically relevant due to its persistent and
effective cardioprotection against myocardial stunning and
infarction (4); therefore,
extensive investigations have been performed to elucidate its
underlying mechanisms.
Previous studies have revealed that the delayed
cardioprotective mechanisms underlying IPC are complex and involve
upregulation of endogenous cardioprotective proteins and oxidative
stress inhibition (5–7). Genetic and pharmacological studies
have identified aldose reductase, heme oxygenase-1 (HO-1), and
Mn-superoxide dismutase (MnSOD) as critical mediators of the
antioxidative stress effects of late preconditioning (8–10).
However, accumulating evidence indicates that IPC is a complex
polygenic adaptation (3);
therefore, as yet unidentified antioxidant proteins may
additionally be involved.
DJ-1, a novel oncogene product identified in 1997,
is a ubiquitously expressed and highly conserved intracellular
protein (11). Subsequent research
suggested that DJ-1 has various potential functions, including
transcriptional regulation, oxidative stress inhibition, acting as
a chaperone or protease and mitochondrial regulation (12). DJ-1 may act as an antioxidant and
be important for cellular defense in response to oxidative stress
(13–16). Our previous study revealed that
hypoxia preconditioning of H9c2 cardiomyocytes significantly
increased the de novo synthesis of DJ-1 and induced
cardioprotection against prolonged hypoxic injury 24 h later
(17). However, whether the
increase in DJ-1 expression mediates protection against
ischemia/reperfusion (I/R) injury in vivo during the late
phase of IPC remains to be determined.
The present study therefore used an in vivo
rat model of IPC and I/R to determine whether DJ-1 was upregulated
24 h after IPC, which has previously been demonstrated to induce
delayed cardioprotection against oxidant stress caused by I/R. It
was subsequently investigated whether in situ knockdown of
DJ-1 interfered with the delayed cardioprotective effects mediated
by IPC and blocked the inhibition of oxidative stress generated by
I/R. The results of the present study demonstrated that IPC
upregulates DJ-1 protein expression levels in the heart and that
DJ-1 is essential for the antioxidative stress effects of late
phase IPC in vivo, thereby identifying DJ-1 as an endogenous
cardioprotective protein.
Materials and methods
Chemicals and reagents
Anti-DJ-1 (N-20; catalog no. sc-27004) and
anti-β-actin (I-19; catalog no. sc-1616) goat polyclonal primary
antibodies, and the horseradish peroxidase-conjugated rabbit
anti-goat secondary antibody (catalog no. sc-2768) were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Dihydroethidium (DHE) was obtained from Molecular Probes; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Malondialdehyde (MDA),
glutathione peroxidase (GPx), catalase (CAT) and superoxide
dismutase (SOD) assay kits were purchased from Beijing Solarbio
Science & Technology Co., Ltd. (Beijing, China). All other
chemicals were purchased from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany) unless otherwise stated.
Animals
A total of 75 adult, healthy, male Sprague-Dawley
rats (weight, 210–240 g) were purchased from the Animal Center of
Nanchang University (Nanchang, China). Rats were housed at a
temperature of 23±1°C and a relative humidity of 55±10%, under a
12-h light/dark cycle and were allowed free access to water and a
standard diet. All procedures performed in the present study were
in accordance with the Guidelines on the Use of Laboratory Animals
(National Institutes of Health, Bethesda, MD, USA) and were
approved by the Ethics Committee for the Use of Experimental
Animals at Nanchang University.
In situ knockdown of DJ-1
A lentiviral vector containing DJ-1 short hairpin
(sh)RNA (lenti-shDJ-1; GeneChem Co., Ltd., Shanghai, China) was
used to selectively knockdown DJ-1 in situ according to the
method of Das et al (18).
In brief, rats were anesthetized with an intraperitoneal injection
of 50 mg/kg sodium pentobarbitone (Sigma-Aldrich; Merck Millipore)
and orotracheally intubated; a positive-pressure ventilator was
used to maintain breathing. A left thoracotomy was performed at the
fourth intercostal space. Following exposure of the heart by
stripping the pericardium, three volumes of 10 µl containing
0.15×106 infectious units lenti-shDJ-1 or control
lenti-shRNA (lenti-shC) were injected into the muscle surrounding
the left ventricle using 27 gauge needles. The rats were extubated
and received analgesia (0.02 mg/kg buprenex injected
subcutaneously; Sigma-Aldrich; Merck Millipore) and antibiotics
(0.7 mg/kg gentamicin injected intramuscularly for 3 days). A total
of three weeks later, myocardial IPC and I/R was performed. In
addition, a subset of hearts was harvested for analysis of protein
expression levels by western blotting. These rats were in the sham
group. The lenti-shC-injected, lenti-shDJ-1-injected and control
wild-type (WT) rats were harvested for western blotting without IPC
and I/R.
In vivo models of myocardial IPC and
I/R
The surgical procedures of IPC and I/R by left
coronary artery (LCA) occlusion in rats were performed as
previously described by Patel et al (19). Briefly, rats were anesthetized with
an intraperitoneal injection of 50 mg/kg sodium pentobarbitone and
ventilated using carefully selected parameters. A left thoracotomy
was performed at the fourth intercostal space, and the heart was
exposed by stripping the pericardium. A 7/0 silk suture was placed
around the LCA 3–4-mm distal to the LCA origin, and an occlusive
snare was placed around it. Artery occlusion was achieved by
tightening the snare and verified by epicardial cyanosis.
Successful reperfusion of the heart was achieved by releasing the
snare, and confirmed by visualizing a clear epicardial hyperemic
response.
For IPC, a sequence of three cycles of 5-min
coronary occlusion/5-min reperfusion was performed. I/R was induced
24 h following IPC, in the late phase of delayed preconditioning,
and achieved by 30 min of coronary occlusion followed by 120 min of
reperfusion.
Experimental groups
The present study consisted of two successive
phases. The objective of the first phase was to determine the
effect of IPC on the expression of DJ-1 protein in rat myocardium.
Male Sprague-Dawley rats were assigned to six groups (n=5/group).
Group I (control) did not undergo coronary occlusion. Groups II,
III, IV, V, and VI underwent IPC with no treatment and were
sacrificed by cervical dislocation 0 (group II), 12 (group III), 24
(group IV), 48 (group V) or 72 h (group VI) following the final
reperfusion. Myocardial samples were rapidly removed, frozen in
liquid nitrogen, and stored at −140°C until analysis of DJ-1
protein expression levels by western blotting.
The aim of the second phase was to determine whether
in situ knockdown of DJ-1 interferes with delayed
cardioprotection induced by IPC against oxidative stress caused by
I/R. Male Sprague-Dawley rats were randomly assigned to one of
three experimental groups (n=15/group). Rats in group VII (WT) were
untreated. Rats in group VIII (lenti-shC) received left
intramyocardial injection of control virus lenti-shC. Rats in group
IX (lenti-shDJ-1) received left intramyocardial injection of
lenti-shDJ-1. A total of three weeks later, rats in each of these
three experimental groups were randomly divided into three
subgroups (n=5/group): i) Sham, in which rats underwent the
surgical procedure without coronary occlusion; ii) I/R, in which
rats were subjected to 30 min coronary occlusion followed by 120
min reperfusion; and iii) lPC + I/R, in which rats were
preconditioned with a sequence of three cycles of 5-min coronary
occlusion/5-min reperfusion 24 h prior to coronary I/R. Lactate
dehydrogenase (LDH) and creatine kinase-MB (CK-MB) release, infarct
size, cardiac function, CAT, SOD and GPx activities, MDA, and
intracellular reactive oxygen species (ROS) were assessed following
I/R. Rats were sacrificed immediately following I/R.
Determination of cardiac function
I/R-induced cardiac dysfunction was evaluated by
invasive hemodynamic evaluation methods. A microcatheter was
inserted into the left ventricle via the right carotid artery to
measure the left ventricular pressure (LVP). LVP was tracked on a
RM-6200C polygraph. Computer algorithms measured left ventricular
end-diastolic pressure (LVEDP), left ventricular systolic pressure
(LVSP), and first derivative of LVP (±dP/dtmax) 120 min
following reperfusion.
LDH and CK-MB release evaluation
Myocardial cellular damage was evaluated by
measuring LDH and CK-MB activities. Blood samples were collected
from the carotid artery after 120 min reperfusion and placed in
heparinized tubes. The blood was centrifuged at 3,000 × g for 10
min at 4°C. The plasma was recovered and used for measuring the
activities of LDH and CK-MB using commercially available assay kits
(cat nos. BC0681 and 1855105; Beijing Solarbio Science &
Technology Co., Ltd.).
Determination of infarct size
Myocardial infarct size was evaluated by Evans
blue/2,3,5-triphenyl-2H-tetrazolium chloride (TTC) staining as
previously described (20).
Following 120 min coronary artery reperfusion, the snare around the
LCA was retightened and 1 ml 2% Evans blue was perfused into the
aorta and coronary arteries to delineate the area at risk (AAR).
AAR was defined as the area of myocardium that was not stained with
Evans blue. The heart was excised and cut transversely into 1-mm
thick slices from the apex to the base. The slices were incubated
with 1% TTC in 0.2 M Tris buffer (pH 8.0) at 37°C for 10 min.
Following TTC staining, the infarcted area appears white, whereas
the healthy tissue appears red. Each slice was imaged and analyzed
using Image-Pro Plus (version, 6.0; Media Cybernetics, Inc.,
Rockville, MD, USA). The myocardial infarct size was expressed as
the percentage of the infarct area/AAR.
Measurement of the activities of the
antioxidant enzymes CAT, SOD and GPx, and MDA content
The border zone was identified as ‘Evans blue
unstained’ and ‘TTC stained’. A 2 mm section of myocardium tissue
from the border zone was obtained for CAT, SOD, GPx and MDA assays.
Following 120 min reperfusion, ventricular tissues were removed and
stored in radioimmunoprecipitation assay buffer (Beijing Solarbio
Science & Technology Co., Ltd.) containing 1 mM ethylene
glycol-bis (β-aminoethyl ether)-N, N,N',N'-tetraacetic acid, 5 mM
sodium fluoride, 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 220 mM
mannitol, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium
orthovanadate, 70 mM sucrose and 1 mM Na2 β-glycerol
phosphate (Sigma-Aldrich; Merck Millipore), supplemented with 5
µl/ml protease inhibitor mixture, pH 7.4 at 4°C. The tissues were
cut into pieces and homogenized on ice with a Teflon Potter
homogenizer. Following centrifugation at 13,000 × g, 4°C for 20
min, the supernatants were used to determine the activities of
cellular CAT, SOD and GPx, and MDA content, using commercial assay
kits (cat nos. BC0200, BC0170, BC1190, BC0020). The activities of
CAT, SOD and GPx, and MDA content, were expressed relative to the
protein levels in the supernatant determined by the Lowry method
using the Detergent-Compatible Protein assay kit II, (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Measurement of in situ ROS
production
As before, the border zone was used for
determination of ROS level. Myocardial ROS generation was assessed
by observing DHE staining under a fluorescence microscope (Olympus
IX-81; Olympus Corporation, Tokyo, Japan). DHE staining was
performed on 10-µm thick frozen myocardial sections as previously
described (21–23). In brief, following 120 min
reperfusion, hearts were snap-frozen in Tissue-Tek embedding medium
(Sakura Finetek USA, Inc., Torrance, CA, USA), and 10-µm thick
sections were cut using a cryostat. Sections were air-dried,
hydrated with PBS and incubated with 10 µM DHE at 37°C for 30 min
in a dark humidified chamber. The sections were washed briefly and
the relative DHE fluorescence was quantified by calculating the
mean value of fluorescence intensity within three identical circles
using Image-Pro Plus software version 6.0. Three to five sections
from each rat were analyzed.
Western blot analysis
A total of three weeks following left
intramyocardial injection of lenti-shDJ-1 or control lenti-shC, or
at various time points following IPC, total proteins were extracted
with RIPA buffer (cat. no. R0010; Beijing Solarbio Science &
Technology Co., Ltd.) from cardiac left ventricular samples and
quantified by the Lowry method using the Detergent-Compatible
Protein assay kit II. Total proteins (50 µg) were subjected to 12%
SDS-PAGE and transferred onto a polyvinylidine fluoride membrane.
The membrane was blocked for 2 h at 4°C with 5% nonfat milk and
subsequently probed with primary antibodies directed against DJ-1
(1:1,000) at 4°C overnight, followed by incubation with a
horseradish peroxidase-conjugated secondary antibody (1:5,000) at
4°C for 2 h. The signals were visualized with an Enhanced
Chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc.).
To normalize lane loading, the same membranes were reprobed with
anti-β-actin (1:1,000). The levels of DJ-1 protein were
standardized to the loading control and were quantified using
Quantity One® software version 4.62 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error.
Statistical comparisons between groups were performed by one-way
analysis of variance followed by a least significant difference
post hoc test. Statistical analyses were performed in SPSS software
version 11.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
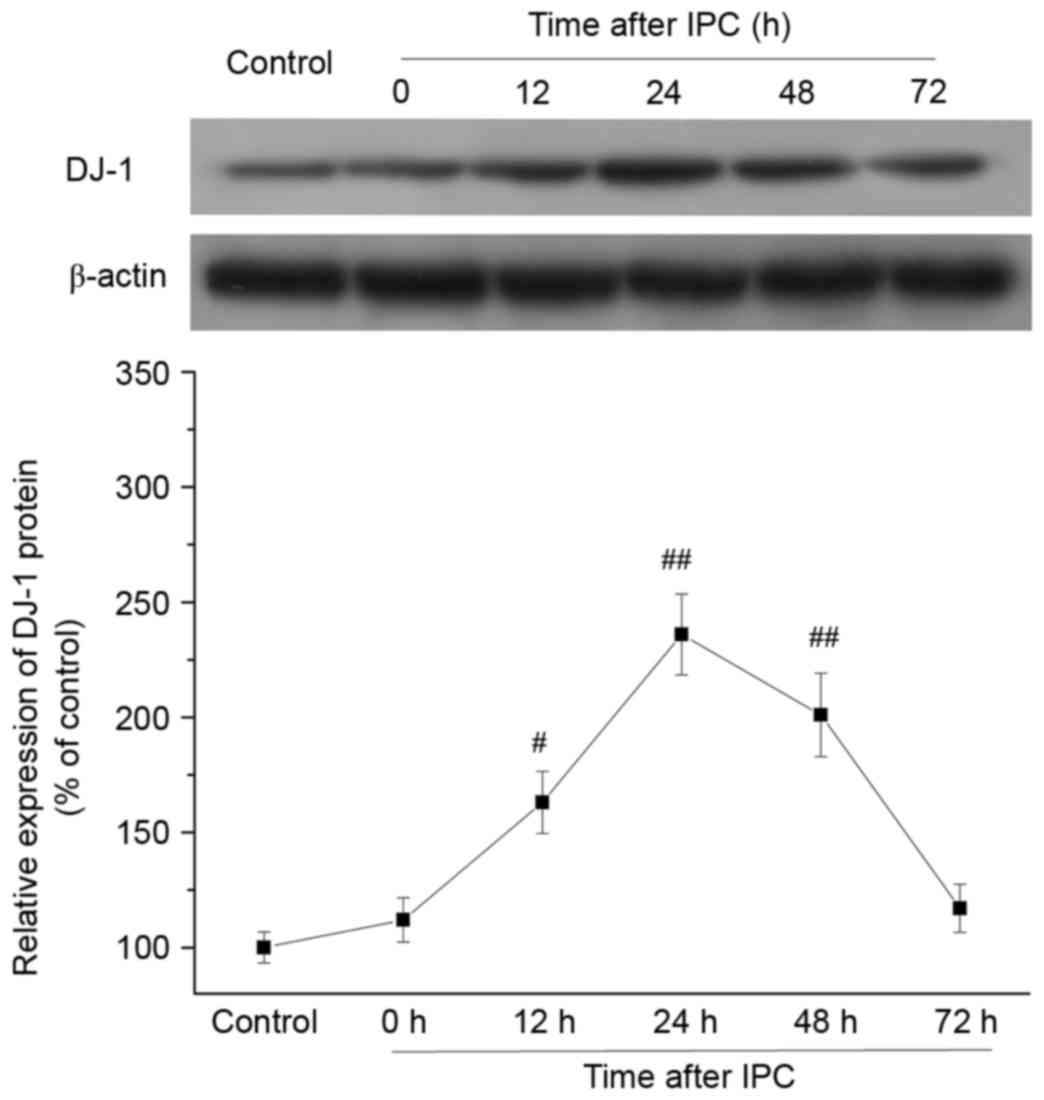
Effect of IPC on DJ-1 protein
expression levels in rat myocardium
To provide in vivo evidence that DJ-1 is
involved in the delayed protection of IPC, DJ-1 protein expression
levels were analyzed in rat myocardium at 0, 12, 24, 48 and 72 h
after IPC. DJ-1 protein expression levels were increased at 12 h
(P=0.0112), peaked at 24 h (P=0.0002), and persisted at 48 h
(P=0.0016) following IPC compared with control group (Fig. 1). The time points of DJ-1
upregulation were consistent with the established time window of
delayed cardioprotection induced by IPC in vivo (24,25).
These results demonstrated that IPC increased DJ-1 protein
expression levels in the late phase.
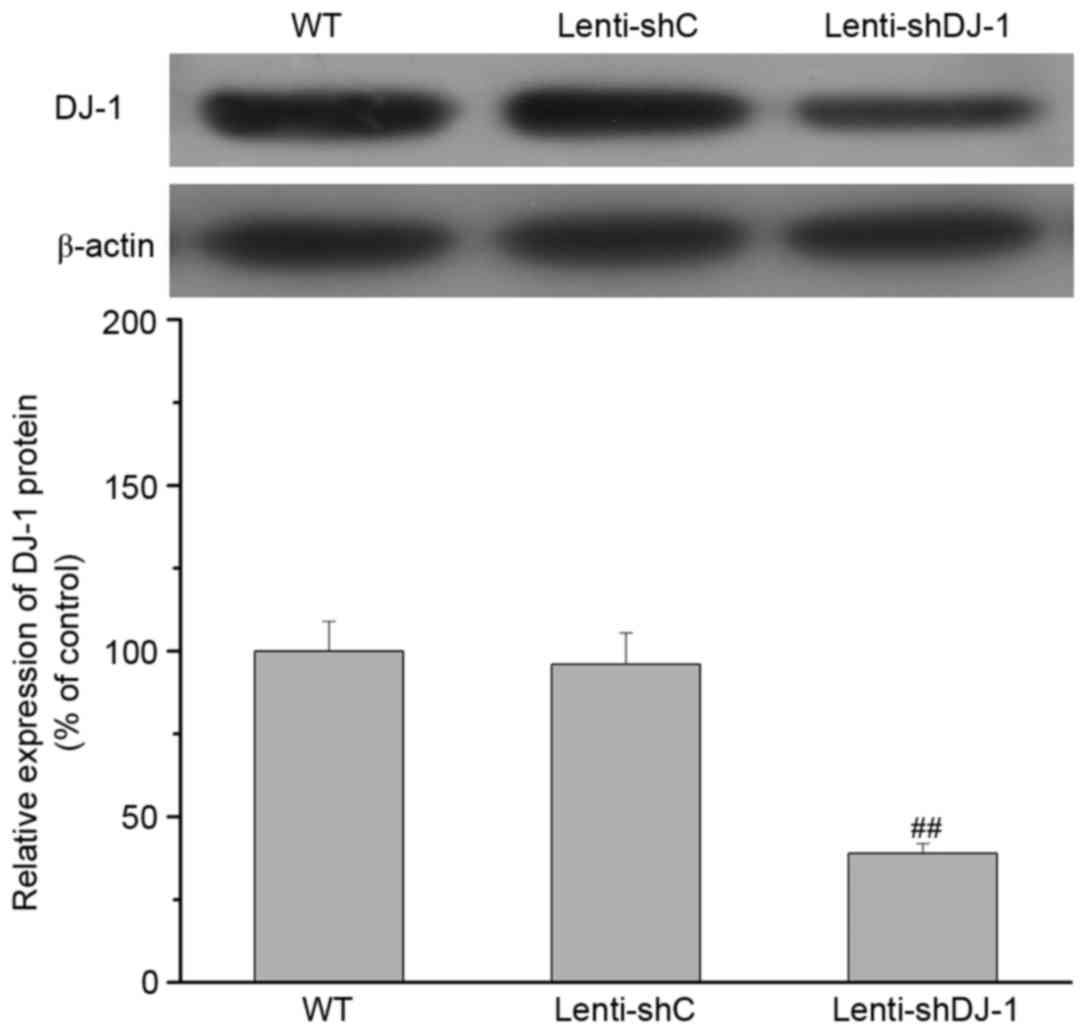
Effect of DJ-1 knockdown on the
IPC-induced improvement of cardiac function following I/R
To determine the in vivo contribution of DJ-1
to IPC-induced delayed cardioprotection, cardiac function was
measured in rats subjected to IPC 24 h prior to I/R. As presented
in Fig. 2, a 61% decrease in DJ-1
protein expression levels was observed in the heart of sham rats
three weeks after left intramyocardial injection of lenti-shDJ-1
compared with injection with control lenti-shC (P=0.0003). Notably,
in WT and lenti-shC-infected rats, IPC significantly attenuated the
reduction of LVSP (P=0.0056 and P=0.0082, respectively) and
±dP/dtmax (P=0.0003 and 0.0011 for +dP/dtmax;
P=0.0020 and 0.0005 for -dP/dtmax, respectively) induced
by I/R injury (Fig. 3).
Additionally, IPC significantly inhibited the I/R-induced increase
of LVEDP in WT (P=0.0025) and lenti-shC-infected rats (P=0.0039).
However, IPC did not attenuate these effects in
lenti-shDJ-1-infected rats. These results indicated that DJ-1
knockdown abrogates the recovery effect of IPC on rat cardiac
function following I/R.
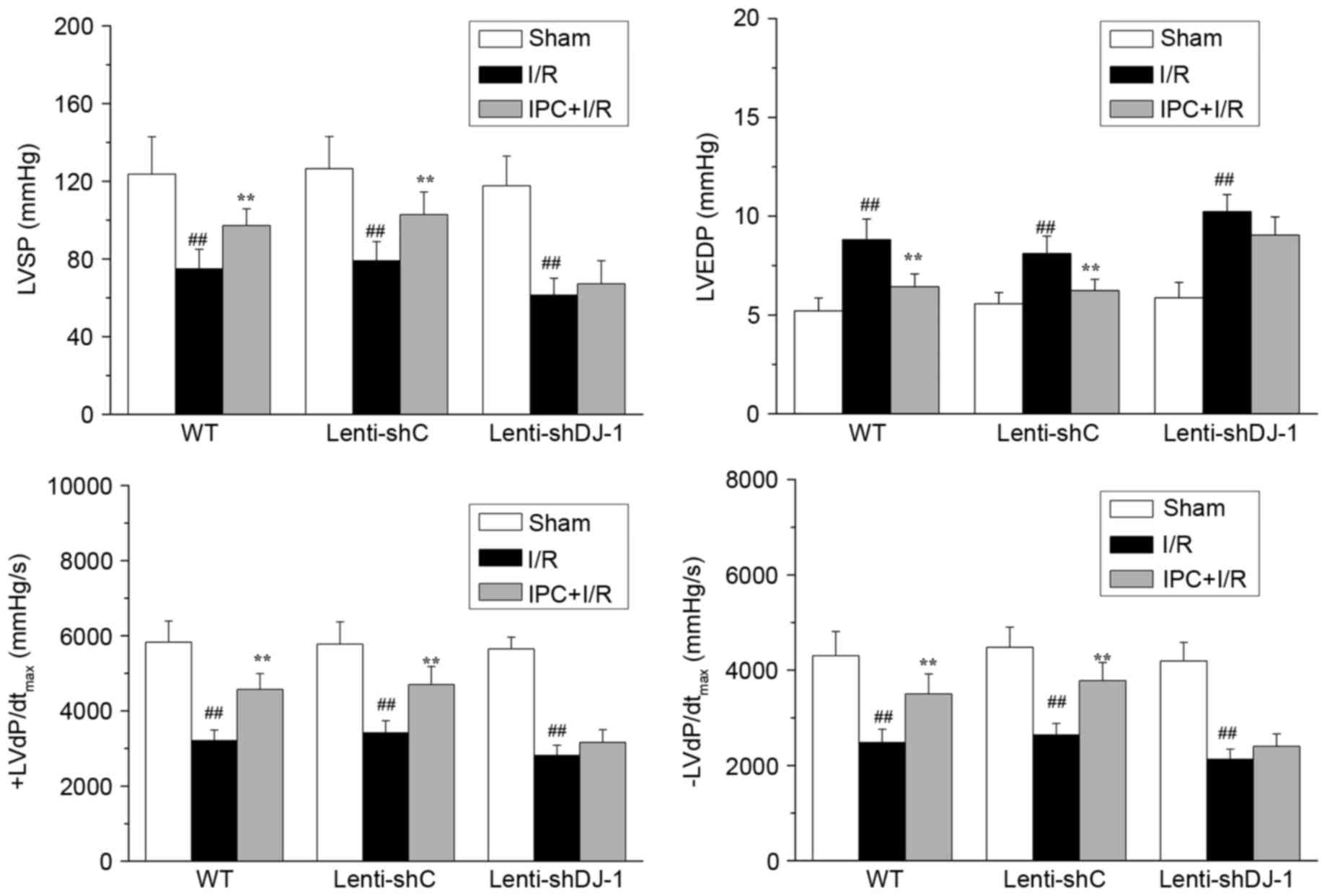 | Figure 3.Effects of DJ-1 knockdown on cardiac
function following IPC and I/R. A total of three weeks following
left intramyocardial injection of lenti-shDJ-1 or control
lenti-shC, rats were subjected to IPC 24 h prior to I/R.
Subsequently, LVSP, LVEDP and ± LVdP/dtmax were
measured. Data are presented as the mean ± standard error (n=5).
##P<0.01 vs. sham group; **P<0.01 vs. I/R group.
IPC, ischemic preconditioning; I/R, ischemia reperfusion; sh, short
hairpin; C, control; WT, wild-type; LVSP, left ventricular systolic
pressure; LVEDP, left ventricular end diastolic pressure; ±
LVdP/dtmax, first derivation of left ventricle
pressure. |
Effect of DJ-1 knockdown on the
delayed cytoprotection of IPC against rat myocardial I/R
injury
The effect of DJ-1 knockdown on the delayed
cytoprotection of IPC in vivo was determined by measuring
myocardial infarct size and plasma CK-MB and LDH levels post I/R.
As presented in Fig. 4, no
myocardial infarction (Fig. 4A)
was observed in sham-operated hearts. I/R resulted in significant
infarction in the I/R compared with sham group rats (P<0.0001).
However, IPC pretreatment significantly decreased I/R-induced
myocardial infarction in WT (P=0.0002) and lenti-shC-infected rats
(P=0.0002). Notably, the infarct-decreasing effect of IPC was
attenuated following knockdown of DJ-1 by lenti-shDJ-1
infection.
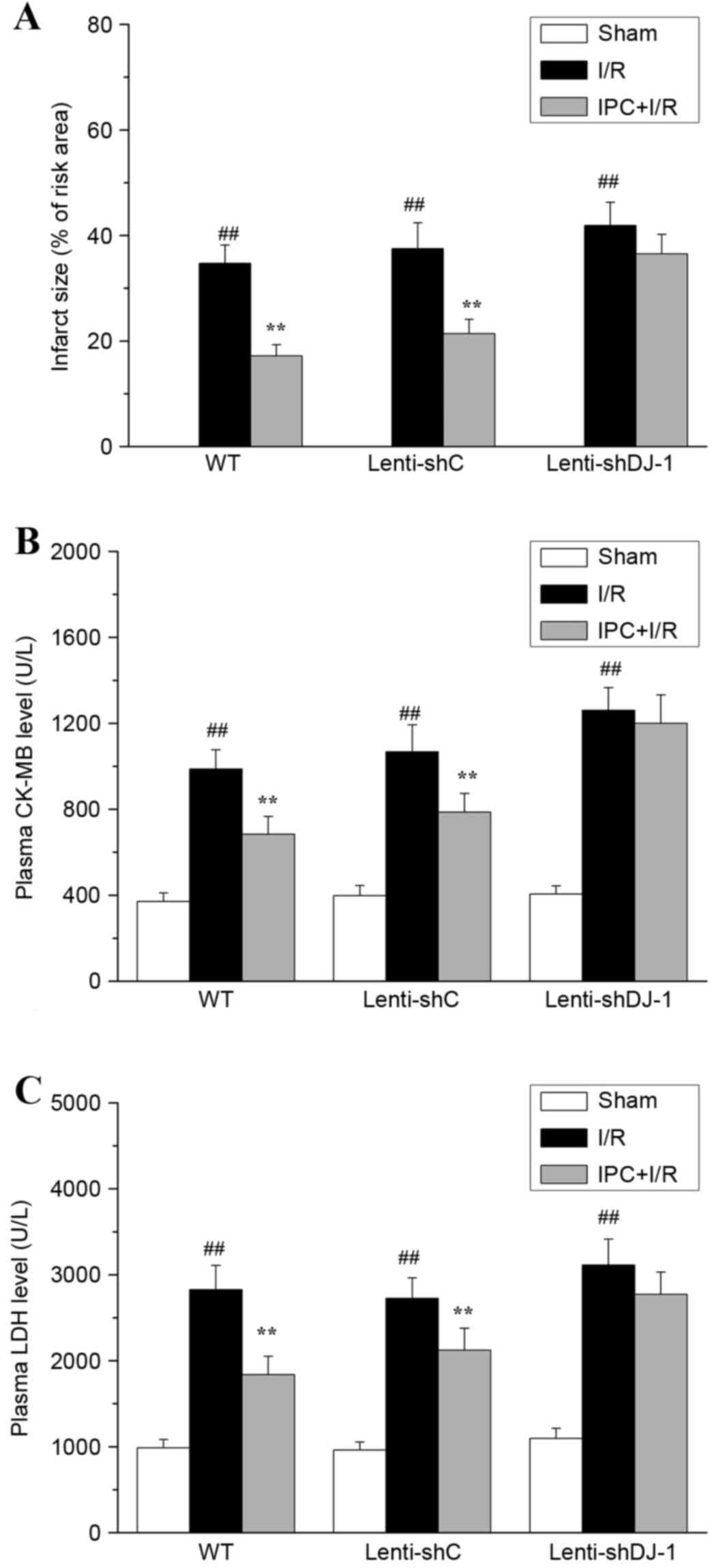 | Figure 4.Effects of DJ-1 knockdown on
myocardial infarct size, plasma CK-MB and LDH following IPC and
I/R. A total of three weeks following left intramyocardial
injection of lenti-shDJ-1 or control lenti-shC, rats were subjected
to IPC 24 h prior to I/R. Subsequently, (A) myocardial infarct
size, (B) CK-MB and (C) LDH levels were examined. Data are
presented as the mean ± standard error (n=5).
##P<0.01 vs. sham group; **P<0.01 vs. I/R group.
CK-MB, creatine kinase-MB; LDH, lactate dehydrogenase; IPC,
ischemic preconditioning; I/R, ischemia reperfusion; sh, short
hairpin; C, control; WT, wild-type. |
The serum levels of the necrotic cell
death markers, LDH and CK-MB, were evaluated
I/R significantly increased CK-MB (all P<0.0001;
Fig. 4B) and LDH (all P<0.0001;
Fig. 4C) release in all groups.
IPC significantly attenuated the increases in CK-MB (P=0.0005 and
P=0.0033, respectively) and LDH (P=0.0002 and P=0.0049,
respectively) levels caused by I/R injury in WT and
lenti-shC-infected rats (P<0.01); however, no significant effect
was observed following lenti-shDJ-1-infection. These results
provided direct evidence that DJ-1 knockdown abrogated the delayed
cardioprotective effect of IPC against rat myocardial I/R
injury.
Effect of DJ-1 knockdown on the
inhibitory action of IPC on oxidative stress caused by I/R
Oxidative stress is one of the primary causes of I/R
injury; IPC-induced delayed cardioprotection is associated with the
attenuation of oxidative stress. As DJ-1 serves an important role
in regulating cell survival and oxidative stress, the present study
determined whether DJ-1 knockdown abrogates the inhibitory effect
of IPC on I/R-induced oxidative stress. To evaluate oxidative
stress, MDA content, ROS levels and the activities of SOD, CAT and
GPx were measured. As presented in Fig. 5, in WT or lenti-shC-infected rats,
IPC attenuated the I/R-induced accumulation of ROS (all
P<0.0001; Fig. 5A) and MDA (all
P<0.0001; Fig. 5B) and
partially reversed I/R-induced effects on the activities of the
cellular antioxidant enzymes SOD (P=0.0096 and 0.0032,
respectively), CAT (P=0.0037 and P=0.0096, respectively) and GPx
(all P<0.0001; Fig. 5C), again
indicating that IPC attenuates I/R-induced oxidative stress in
vivo. However, when DJ-1 expression was specifically knocked
down in lenti-shDJ-1-infected rats, the inhibitory effect of IPC on
oxidative stress was abrogated. These data demonstrated that DJ-1
is required for the delayed protective effect of IPC against
oxidative stress induced by I/R.
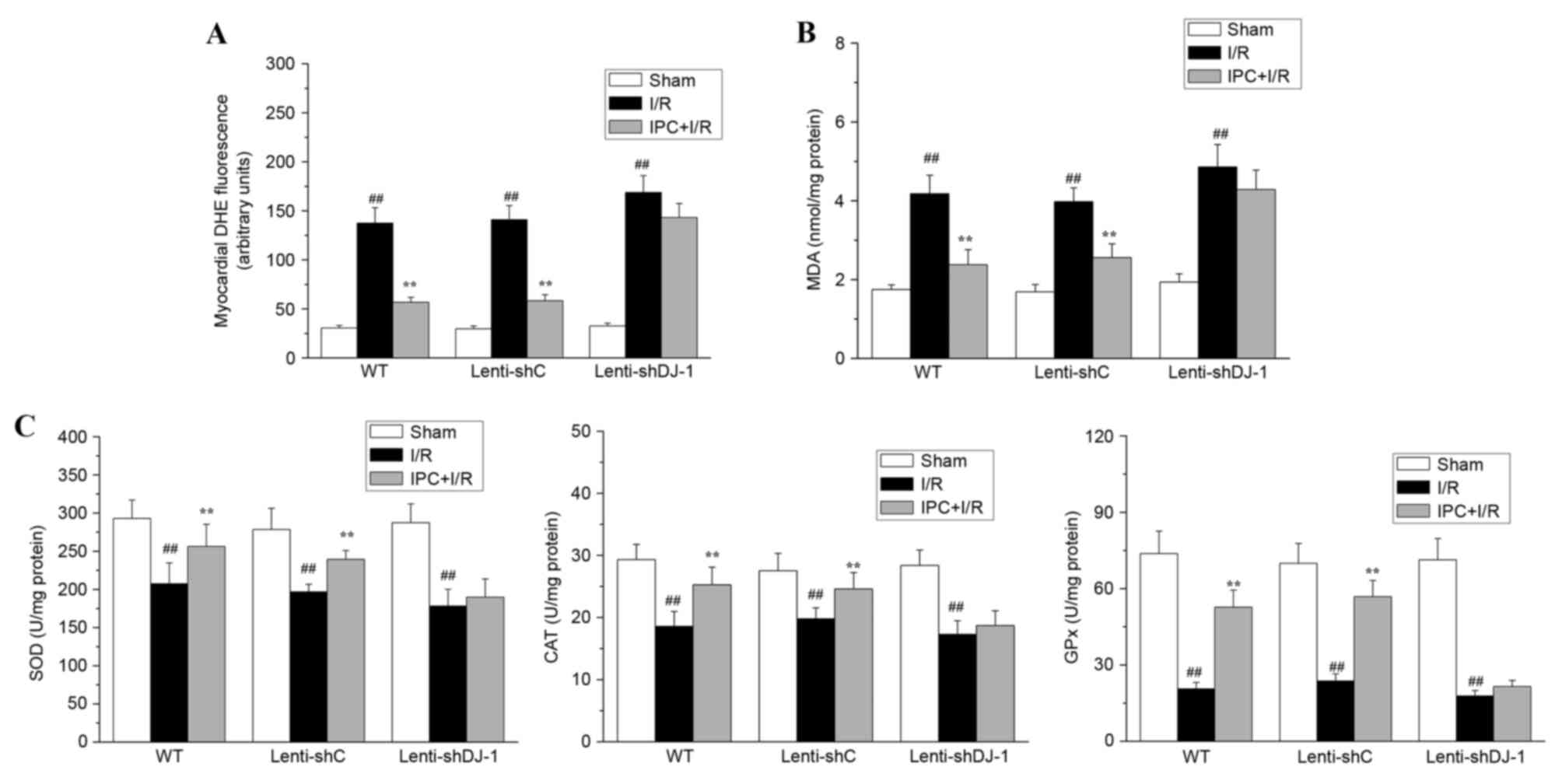 | Figure 5.Effects of DJ-1 knockdown on ROS
generation, MDA content, and SOD, CAT and GPx activities following
IPC and I/R. A total of three weeks following left intramyocardial
injection of lenti-shDJ-1 or control lenti-shC, rats were subjected
to IPC 24 h prior to I/R. Subsequently, (A) ROS generation, (B) MDA
content, and (C) the activities of the antioxidant enzymes SOD, CAT
and GPx were measured. The generation of ROS was expressed as the
mean fluorescence intensity of DHE. The MDA content and the
activities of SOD, CAT and GPx were normalized to the total protein
levels. Data are presented as the mean ± standard error (n=5).
##P<0.01 vs. sham; **P<0.01 vs. I/R group. ROS,
reactive oxygen species; MDA, malondialdehyde; SOD, superoxide
dismutase; CAT, catalase; GPx, glutathione peroxidase; IPC,
ischemic preconditioning; I/R, ischemia reperfusion; sh, short
hairpin; C, control; WT, wild-type; DHE, dihydroethidium. |
Discussion
The present study used a well-characterized rat
model of I/R and IPC to demonstrate that DJ-1 is involved in the
delayed cardioprotection of IPC in vivo. DJ-1 protein
expression levels were increased following IPC, peaking at 24 h and
being maintained for up to 72 h. This time course was consistent
with delayed preconditioning. Targeted in vivo knockdown of
DJ-1 using lenti-shRNA abrogated the antioxidative stress effects
of IPC, and significantly inhibited the delayed cardioprotection
provided by IPC. These findings improve understanding of delayed
preconditioning by identifying DJ-1 as an essential molecular
effector of this cardioprotective phenomenon in vivo.
It is widely accepted that oxidative stress is a
primary cause of I/R injury (26–28),
whereas delayed cardioprotection is associated with limited
intracellular oxidative stress following I/R (29,30).
In the present study, using a well-characterized in vivo
model of IPC and I/R, IPC efficiently reduced LDH and CK-MB
release, attenuated myocardial infarct size and improved cardiac
function when performed 24 h prior to I/R, suggesting that IPC may
exert delayed cardioprotection against I/R in vivo.
Furthermore, IPC significantly inhibited I/R-induced increases in
intracellular ROS production and MDA content, and decreases in the
activities of the antioxidant enzymes SOD, CAT and GPx. Therefore,
these results additionally demonstrated that the delayed
cardioprotection induced by IPC is associated with the attenuation
of oxidative stress caused by I/R.
Although the mechanism underlying IPC-induced
delayed cardioprotection remains to be fully elucidated, it is
clear that the delayed cardioprotection of IPC is dependent upon
de novo protein synthesis (5–7).
Recent genetic and pharmacological studies have identified aldose
reductase, MnSOD and HO-1 as essential antioxidative stress
mediators in late preconditioning (8–10).
However, as myocardial preconditioning is a complex polygenic
adaptation (3), other endogenous
antioxidant proteins may be involved.
DJ-1 is a highly conserved and ubiquitously
expressed intracellular protein with multiple functions. The
inhibition of oxidative stress is a primary function of DJ-1. To
exert this effect, DJ-1 eliminates ROS by self-oxidation (15) and modulates the expression of genes
including glutamate cysteine ligase, extracellular SOD (SOD3) and
MnSOD by activating Nrf2, a master transcription factor in the
redox system (16,31,32).
Therefore, DJ-1 may serve as a general survival factor by enhancing
cellular antioxidant capacity whilst suppressing ROS production.
Consistent with this, Yokota et al (33) reported that cell death induced by
hydrogen peroxide exposure was markedly inhibited by overexpression
of WT DJ-1, while Taira et al (15) reported that DJ-1 knockdown rendered
neuroblastoma cells more susceptible to hydrogen peroxide-induced
cell death. These reports further suggest that DJ-1 is a stress
responder and may serve a potentially cytoprotective role due to
its antioxidant activity. Our previous study demonstrated that
hypoxia preconditioning may induce delayed cardioprotection against
hypoxia/reoxygenation-induced oxidative stress in an H9c2 cellular
model. This was accompanied by enhanced expression of DJ-1, and
DJ-1 knockdown abrogated the delayed cardioprotection, indicating
that DJ-1 may be involved in the delayed cardioprotection induced
by hypoxia preconditioning against oxidative stress caused by
hypoxia/reoxygenation (17).
However, there was no in vivo evidence that the induction of
DJ-1 was responsible for the acquisition of tolerance to I/R in the
late phase of IPC. Therefore, the present study used an in
vivo model of IPC and I/R to investigate the role of DJ-1. DJ-1
protein expression levels were increased at 12 h, peaked at 24 h
and persisted for up to 72 h following IPC. This was in accordance
with the time course of the attenuation of cell injury induced by
subsequent prolonged ischemia. To further verify the in vivo
contribution of DJ-1 to IPC-induced delayed cardioprotection, the
effect of DJ-1 knockdown in situ was investigated. IPC
efficiently reduced LDH and CK-MB release, attenuated myocardial
infarct size and improved cardiac function following I/R injury in
WT rats, but not in lenti-shDJ-1-infected rats, suggesting that
DJ-1 is required for the delayed cardioprotective effect induced by
IPC.
DJ-1 protein serves a critical role in the
regulation of cell viability and oxidative stress. Oxidative stress
is a primary cause of I/R injury, and delayed cardioprotection of
IPC is associated with the attenuation of oxidative stress.
Therefore, it was hypothesized that DJ-1 may be essential for the
antioxidative stress effect of IPC. To test this hypothesis, the
effect of DJ-1 knockdown on oxidative stress during the late phase
of IPC was investigated. IPC attenuated the I/R-induced production
of ROS and MDA and maintained the activities of the cellular
antioxidant enzymes SOD, CAT and GPx. However, following DJ-1
knockdown by lenti-shRNA, the antioxidative stress effects of IPC
were abrogated. Taken together, these data provide, to the best of
our knowledge, the first in vivo evidence to suggest that
DJ-1 has a crucial role in the antioxidative stress effects of late
phase IPC, and further demonstrated that DJ-1 contributes to the
delayed protection induced by IPC via its antioxidant action.
In conclusion, the present study identified DJ-1 as
an essential mediator responsible for the beneficial effects of the
late phase of IPC in vivo. In addition, the results of the
present study suggested that IPC exerts delayed cardioprotective
effects by a hitherto unrecognized underlying mechanism; the
attenuation of I/R-induced oxidative stress via the upregulation of
DJ-1. These findings provide a basis for the further investigations
that are required to elucidate the detailed molecular mechanism
underlying the effect of DJ-1 in the delayed protection of
myocardial preconditioning in vivo.
Acknowledgements
The present study was supported by the Natural
Scientific Foundation of China (grant nos. 81060022 and 81460060)
and the Natural Scientific Foundation of Jiangxi Province (grant
no. 2010GZY0220).
References
|
1
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuzuya T, Hoshida S, Yamashita N, Fuji H,
Oe H, Hori M, Kamada T and Tada M: Delayed effects of sublethal
ischemia on the acquisition of tolerance to ischemia. Circ Res.
72:1293–1299. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bolli R: The late phase of
preconditioning. Circ Res. 87:972–983. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bolli R: The early and late phases of
preconditioning against myocardial stunning and the essential role
of oxyradicals in the late phase: An overview. Basic Res Cardiol.
91:57–63. 1996.PubMed/NCBI
|
|
5
|
Rizvi A, Tang XL, Qiu Y, Xuan YT, Takano
H, Jadoon AK and Bolli R: Increased protein synthesis is necessary
for the development of late preconditioning against myocardial
stunning. Am J Physiol. 277:H874–H884. 1999.PubMed/NCBI
|
|
6
|
Hoek T Vanden, Becker LB, Shao ZH, Li CQ
and Schumacker PT: Preconditioning in cardiomyocytes protects by
attenuating oxidant stress at reperfusion. Circ Res. 86:541–548.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morihira M, Hasebe N, Baljinnyam E,
Sumitomo K, Matsusaka T, Izawa K, Fujino T, Fukuzawa J and Kikuchi
K: Ischemic preconditioning enhances scavenging activity of
reactive oxygen species and diminishes transmural difference of
infarct size. Am J Physiol Heart Circ Physiol. 290:H577–H583. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoshida S, Yamashita N, Otsu K and Hori M:
The importance of manganese superoxide dismutase in delayed
preconditioning: Involvement of reactive oxygen species and
cytokines. Cardiovasc Res. 55:495–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shinmura K, Bolli R, Liu SQ, Tang XL,
Kodani E, Xuan YT, Srivastava S and Bhatnagar A: Aldose reductase
is an obligatory mediator of the late phase of ischemic
preconditioning. Circ Res. 91:240–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jancsó G, Cserepes B, Gasz B, Benkó L,
Borsiczky B, Ferenc A, Kürthy M, Rácz B, Lantos J, Gál J, et al:
Expression and protective role of heme oxygenase-1 in delayed
myocardial preconditioning. Ann N Y Acad Sci. 1095:251–261. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagakubo D, Taira T, Kitaura H, Ikeda M,
Tamai K, Iguchi-Ariga SM and Ariga H: DJ-1, a novel oncogene which
transforms mouse NIH3T3 cells in cooperation with ras. Biochem
Biophys Res Commun. 231:509–513. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cremer JN, Amunts K, Schleicher A,
Palomero-Gallagher N, Piel M, Rösch F and Zilles K: Changes in the
expression of neurotransmitter receptors in Parkin and DJ-1
knockout mice-A quantitative multireceptor study. Neuroscience.
311:539–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lev N, Ickowicz D, Barhum Y, Lev S,
Melamed E and Offen D: DJ-1 protects against dopamine toxicity. J
Neural Transm (Vienna). 116:151–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lev N, Ickowicz D, Melamed E and Offen D:
Oxidative insults induce DJ-1 upregulation and redistribution:
Implications for neuroprotection. Neurotoxicology. 29:397–405.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taira T, Saito Y, Niki T, Iguchi-Ariga SM,
Takahashi K and Ariga H: DJ-1 has a role in antioxidative stress to
prevent cell death. EMBO Rep. 5:213–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou W and Freed CR: DJ-1 up-regulates
glutathione synthesis during oxidative stress and inhibits A53T
alpha-synuclein toxicity. J Biol Chem. 280:43150–43158. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu HS, Chen HP, Wang S, Yu HH, Huang XS,
Huang QR and He M: Hypoxic preconditioning up-regulates DJ-1
protein expression in rat heart-derived H9c2 cells through the
activation of extracellular-regulated kinase 1/2 pathway. Mol Cell
Biochem. 370:231–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Das A, Salloum FN, Durrant D, Ockaili R
and Kukreja RC: Rapamycin protects against myocardial
ischemia-reperfusion injury through JAK2-STAT3 signaling pathway. J
Mol Cell Cardiol. 53:858–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel HH, Gross ER, Peart JN, Hsu AK and
Gross GJ: Sarcolemmal KATP channel triggers delayed ischemic
preconditioning in rats. Am J Physiol Heart Circ Physiol.
288:H445–H447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugano M, Hata T, Tsuchida K, Suematsu N,
Oyama J, Satoh S and Makino N: Local delivery of soluble TNF-alpha
receptor 1 gene reduces infarct size following ischemia/reperfusion
injury in rats. Mol Cell Biochem. 266:127–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera
JS, Halkos ME, Kerendi F, Guyton RA and Vinten-Johansen J:
Postconditioning attenuates myocardial ischemia-reperfusion injury
by inhibiting events in the early minutes of reperfusion.
Cardiovasc Res. 62:74–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adluri RS, Thirunavukkarasu M, Zhan L,
Dunna NR, Akita Y, Selvaraju V, Otani H, Sanchez JA, Ho YS and
Maulik N: Glutaredoxin-1 overexpression enhances neovascularization
and diminishes ventricular remodeling in chronic myocardial
infarction. PLoS One. 7:e347902012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thandavarayan RA, Giridharan VV, Arumugam
S, Suzuki K, Ko KM, Krishnamurthy P, Watanabe K and Konishi T:
Schisandrin B prevents doxorubicin induced cardiac dysfunction by
modulation of DNA damage, oxidative stress and inflammation through
inhibition of MAPK/p53 signaling. PLoS One. 10:e01192142015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das DK and Maulik N: Cardiac genomic
response following preconditioning stimulus. Cardiovasc Res.
70:254–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yellon DM and Downey JM: Preconditioning
the myocardium: From cellular physiology to clinical cardiology.
Physiol Rev. 83:1113–1151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saeed SA, Waqar MA, Zubairi AJ, Bhurgri H,
Khan A, Gowani SA, Waqar SN, Choudhary MI, Jalil S, Zaidi AH and
Ara I: Myocardial ischaemia and reperfusion injury: Reactive oxygen
species and the role of neutrophil. J Coll Physicians Surg Pak.
15:507–514. 2005.PubMed/NCBI
|
|
27
|
Zweier JL and Talukder MA: The role of
oxidants and free radicals in reperfusion injury. Cardiovasc Res.
70:181–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JL and Lucchesi BR: Mechanisms of
myocardial reperfusion injury. Ann Thorac Surg. 68:1905–1912. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dhalla NS, Elmoselhi AB, Hata T and Makino
N: Status of myocardial antioxidants in ischemia-reperfusion
injury. Cardiovasc Res. 47:446–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rui T, Cepinskas G, Feng Q and Kvietys PR:
Delayed preconditioning in cardiac myocytes with respect to
development of a proinflammatory phenotype: Role of SOD and NOS.
Cardiovasc Res. 59:901–911. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clements CM, McNally RS, Conti BJ, Mak TW
and Ting JP: DJ-1, a cancer- and Parkinson's disease-associated
protein, stabilizes the antioxidant transcriptional master
regulator Nrf2. Proc Natl Acad Sci USA. 103:15091–15096. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhong N and Xu J: Synergistic activation
of the human MnSOD promoter by DJ-1 and PGC-1alpha: Regulation by
SUMOylation and oxidation. Hum Mol Genet. 17:3357–3367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yokota T, Sugawara K, Ito K, Takahashi R,
Ariga H and Mizusawa H: Down regulation of DJ-1 enhances cell death
by oxidative stress, ER stress, and proteasome inhibition. Biochem
Biophys Res Commun. 312:1342–1348. 2003. View Article : Google Scholar : PubMed/NCBI
|