Introduction
Cervical cancer is one of the most common
gynecological malignancies, with 500,000 new cases diagnosed
annually worldwide, and approximately one-third of these cases
leading to mortality (1).
High-risk human papillomavirus (HPV) types, such as HPV16, 18 and
31 are associated with >90% of cervical cancer cases (2). The high-risk HPV oncoproteins, E6 and
E7, contribute to cervical carcinogenesis through inactivation of
the cellular tumor suppressor proteins p53 (3) and retinoblastoma (4), respectively. The current treatments
for cervical cancer are comprehensive and include surgery,
radiotherapy, chemotherapy and immunotherapy. Previous studies have
predicted that immunotherapy may be a promising strategy as it has
a record of feasibility and safety in the treatment of cancer
(5,6). However, cytotoxic T lymphocyte
dysfunction is the principal obstacle for cancer immunotherapy. The
negative co-stimulating signaling molecules in the programmed death
ligand 1/programmed death 1 (PD-L1/PD-1) signaling pathway have
been previously correlated with tumor-immune escape (7). The interaction between PD-L1 on
antigen-presenting cells (APCs) and its receptor PD-1 on T
lymphocytes, leads to inhibition of T lymphocyte activation and
induction of apoptosis or anergy of T lymphocytes (8,9).
Aberrant high expression of PD-L1 is common in various tumors and
has been correlated with tumor progression and patient survival
(10,11). The aim of the present study was to
investigate whether HPV oncoprotein-induced cervical epithelial
cells circumvent the host immune system via the PD-L1/PD-1
signaling pathway.
Materials and methods
Cell culture and transfection
The HPV16-associated cervical cancer cell line,
CaSki, and a HPV-negative epithelial cell line, PC3, were obtained
from the China Center for Type Culture Collection (Wuhan, China).
Cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% newborn
calf serum (NBCS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (North China Pharmaceutical Co., Ltd.,
Hebei, China) at 37°C in 5% CO2. Purified plasmids
MSCVPIG (Addgene, Cambridge, MA, USA) and MSCVPIG-sPD-1
(constructed in the lab) were stably transfected into PC3 cells
(1×105/ml) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After 2 weeks, positive clones were selected based on their
resistance to G418 (800 mg/ml; North China Pharmaceutical Co.,
Ltd.), which were analyzed by reverse transcription-polymerase
chain reaction (RT-PCR), flow cytometry and western blot
analysis.
Cell viability assay
Stable transfection clones were seeded into 96-well
plates (1×104 cells/well) and cultured in RPMI-1640 medium
supplemented with 10% NBCS and 1% penicillin/streptomycin at 37°C
and 5% CO2. MTT reagent (5 mg/ml; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was added to the cells at different
time-points (12, 24, 48, 72 and 96 h) and were incubated for 4 h.
After discarding the supernatant, 100 µl dimethyl sulfoxide was
added to each well and the plates were agitated at 37°C for 15 min.
The absorbance of samples was recorded at 490 nm using a Multiskan
Spectrum (Thermo Fisher Scientific, Inc.).
Flow cytometry
PC3 cells (0.5–1×106) were collected, washed with 1X
phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde at
4°C for 30 min, and then incubated with 0.1% Triton X-100
(Sigma-Aldrich; Merck Millipore), containing 2% bovine serum
albumin (BSA Sigma-Aldrich; Merck Millipore) at room temperature
for 15 min. After blocking with 2% BSA at 37°C for 30 min, the
cells were incubated with mouse anti-HPV16E7 (cat no. sc-6981) or
rabbit anti-human PD-L1 (cat no. sc-50298) primary antibodies
(dilution 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 30 min at 37°C, and then incubated with either fluorescein
isothiocyanate (FITC)-conjugated donkey anti-mouse (cat. no.
715–005-151) or goat anti-rabbit (cat. no. 111-095-003) secondary
antibodies (dilution 1:3,000; Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA) for 30 min at room temperature. Cells
were resuspended in 500 µl 1X PBS and analyzed by
fluorescence-activated cell sorting to determine the expression
levels of E7 and PD-L1. The same protocol was used, with the
exception of the use of an isotype control (cat. no. sc-2025 or
sc-2027; Santa Cruz Biotechnology, Inc.) in place of the primary
antibodies.
In order to perform cell cycle analysis, PC3 cells
were collected, washed with 1X PBS and fixed with 70% ethanol for 1
h at 4°C. The cells were then treated with 0.1% Triton X-100 and 50
µg/ml RNase (Sigma-Aldrich; Merck Millipore), and labeled with 20
µg/ml propidium iodide (Sigma-Aldrich; Merck Millipore) for 30 min
at room temperature. A total of 1×104 cells were
analyzed for each sample with flow cytometry using the Coulter
EPICS flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
RT-PCR
PC3 cells (1×106) were collected and the total RNA
was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and cDNA was synthesized using the SuperScript™
II reverse transcriptase (Invitrogen; Thermo Scientific, Inc.) at
65°C for 5 min, 42°C for 1 h and 70°C for 5 min, according to the
manufacturer's instructions. PCR amplification was performed using
specific oligonucleotide primers (Sangon Biotech Co., Ltd.,
Shanghai, China). β-actin was used as an endogenous control.
HPV16E7 forward, 5′-GCTCAGAGGAGGAGGATGAAATAGA-3′ and reverse,
5′-CACAACCGAAGCGTAGAGTCAC-3′; PD-L1 forward,
5′-GCTATGGTGGTGCCGACTACA-3′ and reverse,
5′-CTTGATGGTCACTGCTTGTCCA-3′; β-actin forward,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. PCR reactions contained 25 ng cDNA
template, 100 ng sense and antisense oligonucleotide primers, 2.5
µl Taq PCR buffer (Promega Corporation, Madison, WI, USA), 0.4 mM
dNTP mixture, and 1U Taq polymerase (Promega Corporation, Madison,
WI, USA) in a total reaction volume of 25 µl. Following an initial
3 min incubation step at 94°C, PCR was performed using 30 cycles of
denaturation at 94°C for 30 s, annealing at 55°C for 30 s and
elongation at 72°C for 30 s. PCR products were separated by
electrophoresis at 100 V for 40 min using a 2% agarose gel, and
were detected by ethidium bromide staining.
Western blotting
CaSki cell (5×106) were collected and lyzed in 1X
SDS sample buffer containing 5% β-mercaptoethanol (Sigma-Aldrich;
Merck Millipore) at 4°C. The protein samples were denatured by
heating at 95°C for 5 min, and 30 µg/well protein was separated in
a 12% SDS-PAGE Bis-Tris gel (Invitrogen; Thermo Fisher Scientific,
Inc.) in 1X MES SDS running buffer (Invitrogen; Thermo Fisher
Scientific, Inc.), and then transferred to a polyvinylidene
difluoride (PVDF) membrane (current, 250 mA; duration, 2 h). The
PVDF membrane was blocked with 5% non-fat milk in Tris-buffered
saline with 0.1% Tween-20 (TBST; Sigma-Aldrich; Merck Millipore) at
4°C overnight. After rinsing with TBST, the membrane was incubated
at room temperature for 2 h with a primary rabbit anti-human PD-1
antibody (cat no. sc-10295; Santa Cruz Biotechnology, Inc.) at
1:500 dilution in TBST and then washed three times with TBST.
Proteins were incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody at 1:3,000 dilution in TBST for 1 h
at room temperature, before washing three times with TBST. The
immunoreactive proteins were detected with a chemiluminescence
substrate (Pierce; Thermo Fisher Scientific, Inc.), and the signals
were captured on an X-ray film in a darkroom. The relative protein
level in each sample presented in a bar graph was calculated based
on the protein band density after normalizing to β-actin (cat. no.
sc-47778; Santa Cruz Biotechnology, Inc.) (1:1,000 dilution in
TBST) for sample loading.
Co-culture and lymphocyte
proliferation
Peripheral blood mononuclear cells (PBMCs) were
isolated from the blood of 20 healthy donors (Center Blood Bank of
Yichang City, Yichang, China) by density gradient centrifugation
(536 × g for 20 min) over Ficoll/Hypaque (Sigma-Aldrich; Merck
Millipore), according to the manufacturer's instructions. PBMCs
were resuspended to a final concentration of 1×106/ml in PBS with
0.1% BSA, before they were stained with 5 µmol/l
5-(−6)-carboxyfluorescein diacetate succinimidyl ester (CFSE;
Molecular Probes; Thermo Fisher Scientific, Inc.) for 10 min at
37°C. The stain was quenched by incubating cells for 5 min in PBS
containing 10% fetal bovine serum. Excess CFSE was removed by
adding PBS and centrifuging (134 × g for 5 min). This wash-step was
repeated twice. The CFSE-labeled PBMCs were co-cultured with stable
transfection clone sPD1-PC3 cells at a ratio of 1:100. Following 2
days of culture, the PBMCs were harvested and washed three times,
resuspended with 300 µl PBS, subjected to FACS analysis
(Ex/Em=488/518 nm), and analyzed using CellQuest Pro software
version 5.1 (BD Biosciences, San Diego, CA, USA) to determine the
cell proliferation.
Lymphocyte cytotoxicity assay
Using above PBMCs stimulated with tumor cells
serving as effector cells, the target cells (1×104/well) were
incubated at different effector-to-target ratios (1:1, 50:1 and
100:1). The following three control groups were established: PBMCs
with 1% NP-40 (Sigma-Aldrich; Merck Millipore), denoted as the
‘maximum lytic group’; target cells, denoted as the ‘effector
spontaneous release group’; and the remaining group containing
phenolsulfonphthalein medium as the ‘blank control group’. All of
the cells were incubated at 37°C for 6 h. Centrifuged supernatant
(536 × g for 10 min; 50 µl) was collected to assess the quantity of
lactate dehydrogenase by quantitative measurements using the
Cytotox 96 Non-Radioactive Cytotoxicity assay kit (Promega
Corporation) according to the manufacturer's instructions. The
lytic activity of lymphocytes was assessed using the mean value
from triplicate repeats according to the following formula:
Percentage of specific lysis = (experimental release - spontaneous
release)/(maximum release - spontaneous release ×100.
Luciferase reporter gene assay
PC3 cells (1×105/ml) were transiently transfected
with the PD-L1-firefly luciferase reporter gene generated at the
Institute of Molecular Biology of China Three Gorges University
(Yichang, China). Following 48 h of transfection, luciferase
activity was measured using the Dual-Luciferase®
Reporter assay system (E1910, Promega Corporation) according to the
manufacturer's instructions. Luminescence was read from the 96-well
plate using an Infinite™ 200 luminometer (Tecan, Männedorf,
Switzerland).
Histopathological analysis and
immunohistochemical staining
Cervical carcinoma (40 patients) and normal cervical
tissue (8 individuals) samples were obtained from the Department of
Obstetrics and Gynecology, the Second Affiliated Hospital of China
Three Gorges University (Yichang, China) between March 2010 and May
2011. All patients did not accept any pretreatment for six months
prior to the study. The study met the criteria of the Ethics
Committee of The Second Affiliated Hospital of China Three Gorges
University (Yichang, China). All cervical tissue specimens were
fixed with 10% neutral formalin, embedded with paraffin, and
serially-sectioned at 5 µm. The sections were mounted onto the
histostick-coated slides. A total of 4–5 adjacent sections were
collected for histopathological analysis and immunohistochemical
staining. The clinical histopathological diagnosis of all cases was
performed according to the WHO (2003) standard (12).
The biotin-streptavidin complex kit (Boshide
Biological engineering Ltd., Wuhan, China) was used for the
immunostaining of PD-L1 and HPV16E7. All of the following agents
were part of the kit, except the primary antibodies. According to
the manufacturer's protocol the procedures were briefly as follows:
Following de-waxing, inactivation of endogenous peroxidase activity
and inhibition of cross-reactivity using normal non-immune goat
serum, the sections were incubated at 4°C overnight with a diluted
solution of the primary antibodies (as aforementioned).
Localization of the primary antibodies was achieved by subsequent
application of a biotin-conjugated IgG secondary antibody, and a
streptavidin-conjugated peroxidase. Signals were visualized with
diaminobenzidine, and cellular nuclei were counterstained with
instant hematoxylin. Negative controls were established by
replacing the primary antibody with normal isotype control (the kit
supplied). The quantity of positive staining was analysed as
follows: Opening the image analysis system, defining the negative
and positive standard, followed by scanning the samples. The
relative brightness values were obtained using the Image-Pro Plus
6.0 software (Media Cybernetics, Washington DC, USA).
Statistical analysis
Data were analyzed using SPSS software (version
13.0; SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean
± standard deviation. Differences between two groups were analyzed
with Student's t-test or chi-square test. P<0.05 and P<0.01
were considered to indicate statistically significant
differences.
Results
Expression of HPV16E7 and PD-L1 in
cervical tissues was positively correlated
In order to explore the correlation between HPV16E7
and PD-L1 expression, the present study examined their mRNA and
protein expression levels in clinically diagnosed cervical cancer
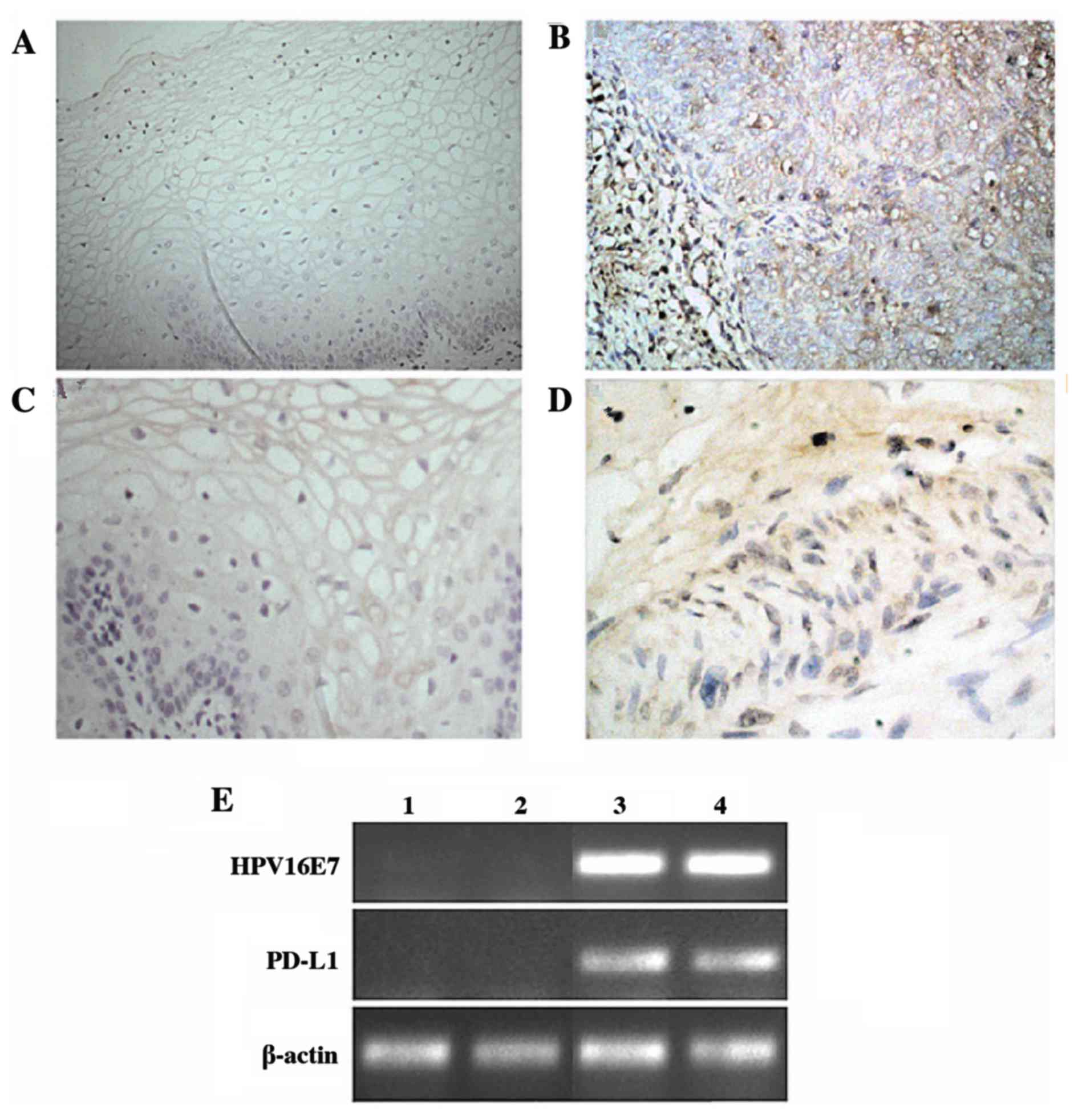
tissues. Immunohistochemical analysis demonstrated that the normal
cervical epithelium rarely expressed PD-L1, while the majority of
cervical cancer samples exhibited strong expression of PD-L1
(P=0.017; Table I). HPV16E7
expression was detected using immunohistochemistry and RT-PCR
analyses. The results demonstrated that HPV16E7 was expressed at
low levels in normal tissues and at high levels in cervical cancer
tissues, with HPV16E7 protein primarily localized to the nucleus
and cytoplasm of the cervical cancer cells (Fig. 1). Statistical analysis of these
data indicated that HPV16E7 and PD-L1 protein expression was
positively correlated (r=0.531) in cervical cancer tissues and
reached statistical significance (P=0.043).
 | Table I.PD-L1 and HPV16E7 expression in normal
cervical and cervical cancer tissues. |
Table I.
PD-L1 and HPV16E7 expression in normal
cervical and cervical cancer tissues.
|
| n | PD-L1 (%) | HPV16E7 (%) |
|---|
| Normal control | 8 | 0 (0) | 1 (12.5) |
| Cervical
carcinoma | 40 | 38 (95)a | 33
(82.5)a |
HPV16E7 upregulated PD-L1 expression
and inhibited lymphocyte activity
The expression of HPV16E7 and PD-L1 demonstrated a
positive correlation in cervical carcinoma tissues. In order to
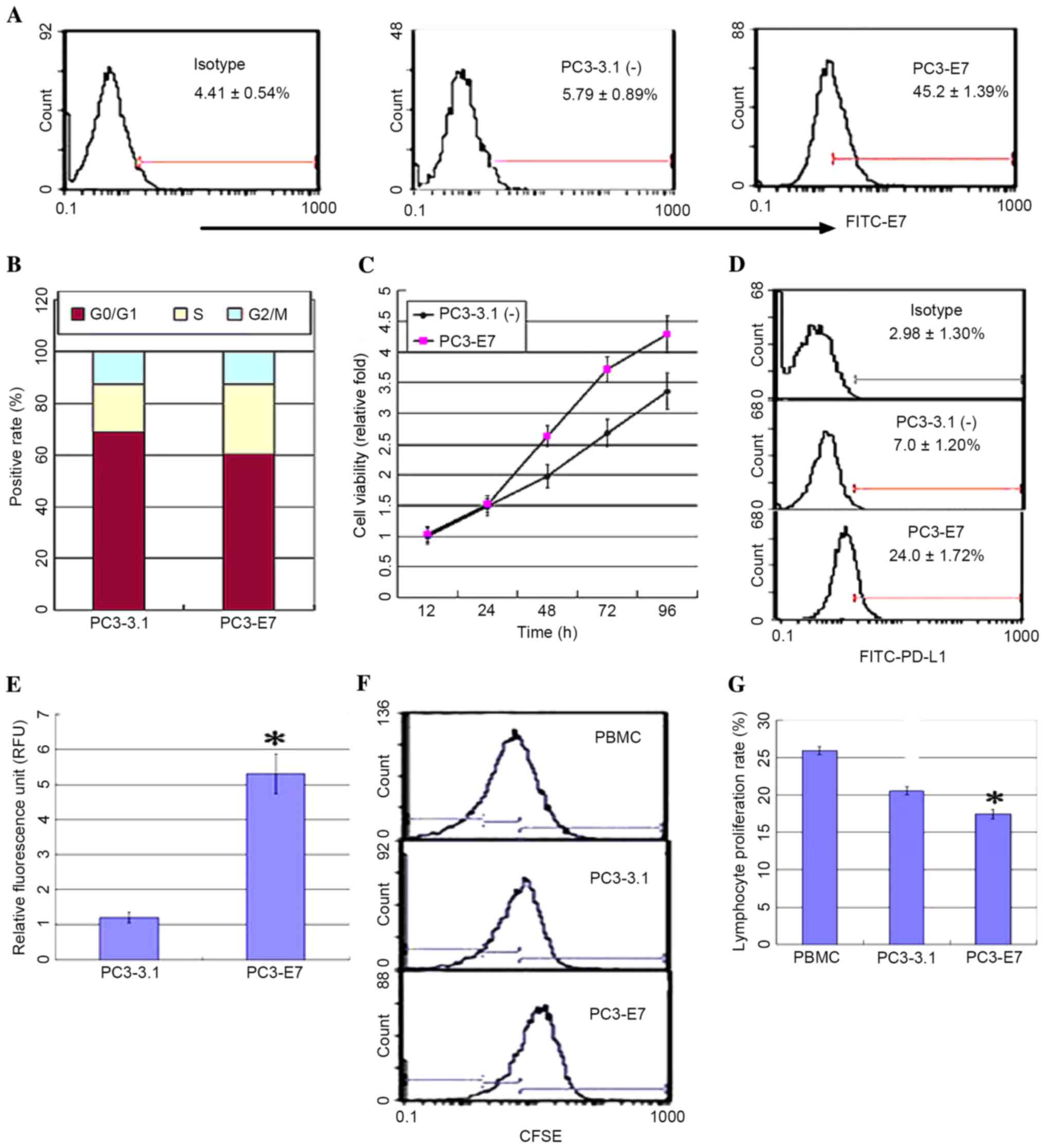
further explore this correlation, a PC3 cell line stably expressing
HPV16E7 (PC3-E7) was established by transfecting the pcDNA3.1
(−)-E7 plasmid or an empty vector control (PC3-3.1), and the
expression levels of PD-L1 were measured. PC3-E7 clones
demonstrated the oncogenic characteristics of enhanced viability, a
significant reduction in the number of cells at the G0/G1 phase, an
accumulation of cells at S phase, and no obvious difference at G2/M
phase when compared with the empty vector controls (Fig. 2A-C). Flow cytometry analysis and a
luciferase reporter system were used to detect PD-L1 expression in
order to investigate whether PD-L1 expression may be regulated by
HPV16E7. The results demonstrated that PD-L1 was overexpressed in
the PC3-E7 clones when compared with the empty vector control cells
(Fig. 2D). The luciferase assay
results demonstrated that fluorescence values in the PC3-E7 cells
were significantly higher than the control cells (P=0.022; Fig. 2E). These results suggest that
HPV16E7 promoted PD-L1 expression in PC3-E7 cells. PD-L1 expressed
on the surface of tumor cells inhibits lymphocyte activity through
binding to PD-1 on lymphocytes (13). Consistent with these observations,
the proliferation rate of lymphocytes was significantly reduced
following co-culture with PC3-E7 cells compared with PC3-3.1 cells
(Fig. 2F and G).
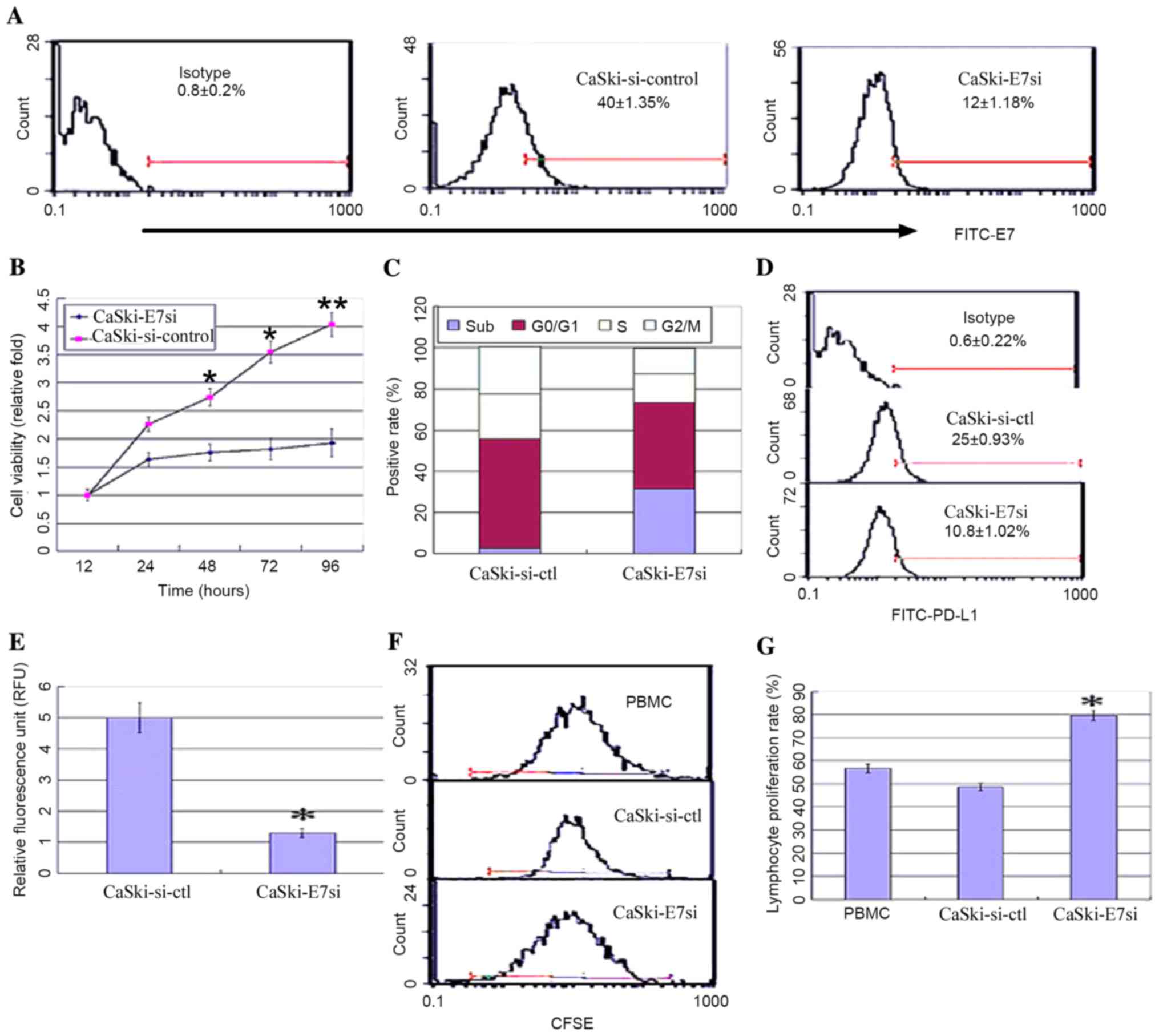
In order to further investigate the correlation
between HPV16E7 and PD-L1, HPV16E7 was knocked down using RNA
interference in the CaSki cell line, which had undergone
integration of HPV16 genes, and PD-L1 expression levels were
detected. A pSilencer™ 2.1-U6-E7siRNA plasmid was constructed and
stably transfected into CaSki cells. Positive clones were
identified by flow cytometry and denoted as CaSki-E7si cells. Empty
vector control-transfected cells were denoted as CaSki-si-ctl.
Suppression of HPV16E7 expression in CaSki-E7si cells was confirmed
by flow cytometry analysis (Fig.
3A). The viability of CaSki-E7si cells was significantly
suppressed compared with the controls [P=0.041 (48 h); P=0.028 (72
h); P=0.008 (96 h); Fig. 3B]. Flow
cytometry analysis of cell cycle was conducted subsequent to E7
siRNA treatment. It was demonstrated that the cell cycle
distribution was altered in the CaSki-E7si cells. The percentage of
cells in the sub-G1 phase increased from 2.1% in the untreated
control to 33.6% following E7 siRNA expression (P=0.018; Fig. 3C). Both flow cytometry and
luciferase reporter system analysis demonstrated that PD-L1
expression was significantly reduced in CaSki-E7si cells (P=0.041;
Fig. 3D; P=0.027; Fig. 3E). Following co-culture with
lymphocytes, CaSki-E7si cells significantly stimulated lymphocyte
proliferation when compared with CaSki-si-ctl cells (P=0.039
Fig. 3F and P=0.035; Fig. 3G). According to the results
presented in the current study, HPV16E7 overexpression in cervical
cancer may lead to PD-L1 upregulation and mediate the escape of
tumors from the immune system.
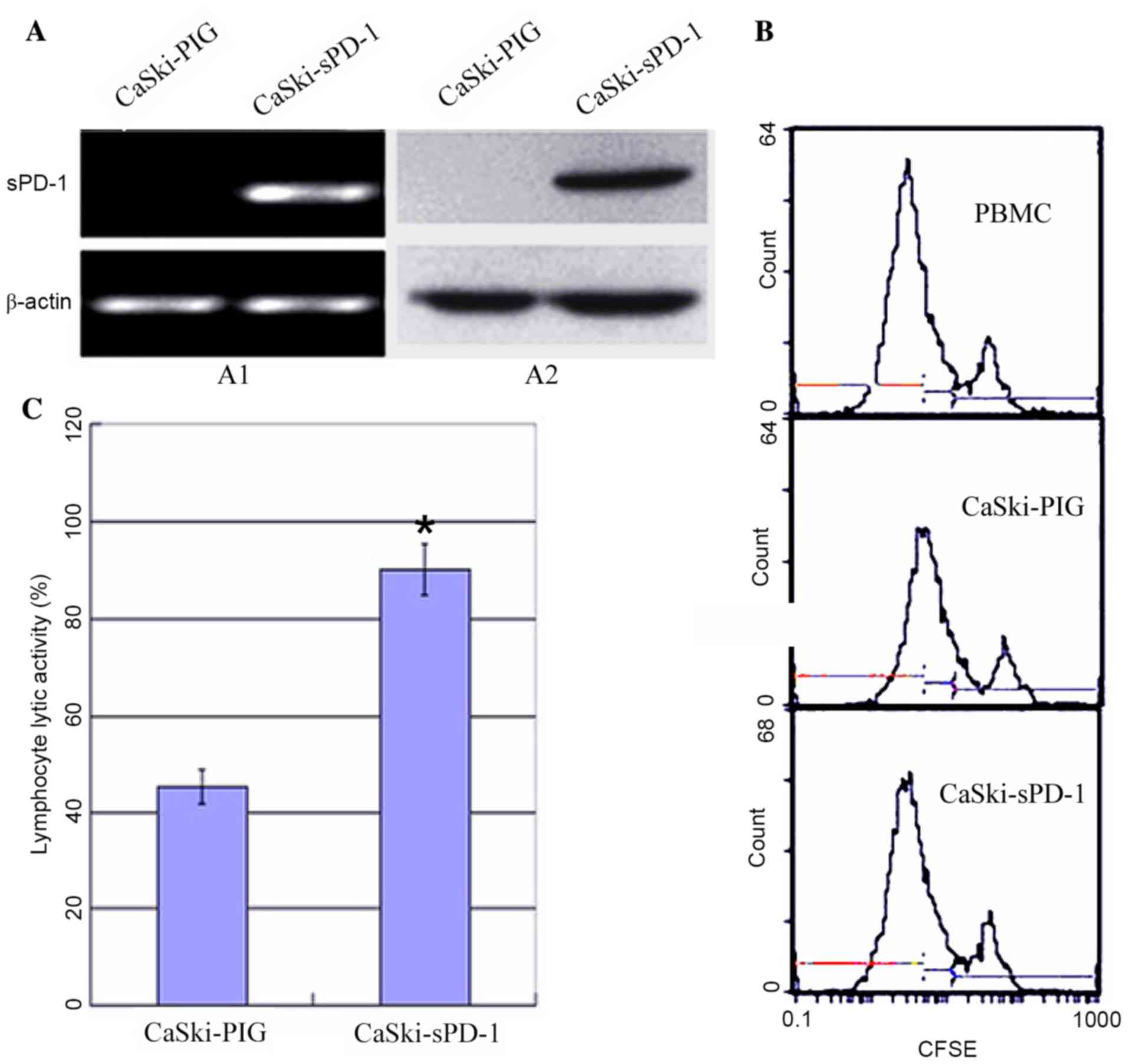
Soluble PD-1 (sPD-1) promotes
lymphocyte proliferation and cytotoxic activity
Due to the observation that HPV16E7 suppressed
lymphocyte proliferation and cytotoxic activity potentially via
upregulating PD-L1 expression in cervical cancer, the authors
hypothesized that inhibition of the PD-L1/PD-1 signaling pathway
may improve immunotherapy in cervical cancer. PD-1 expressed on
lymphocytes binds to PD-L1 on tumor cells and mediates negative
regulation of the immune response (13). Therefore, the recombinant plasmid
sPD-1 was constructed and stably transfected into CaSki cells.
Empty vector controls were denoted as CaSki-PIG. CaSki-sPD-1 cells
were determined to express sPD-1 by RT-PCR and western blotting
analyses (Fig. 4A). Following
co-culture of lymphocytes with CaSki-sPD-1 cells, lymphocyte
proliferation was significantly elevated when compared with
CaSki-PIG cells (P=0.038; Fig.
4B). The activated lymphocytes exhibited significantly enhanced
cytotoxic activity when they were co-cultured with CaSki-sPD-1
cells compared with CaSki-PIG cells (P=0.042; Fig. 4C). These results demonstrated that
inhibition of the PD-L1/PD-1 signaling pathway may partially
restore the T cell response and enhance antitumor immunity.
Discussion
Persistent oncogenic HPV infection is a leading
cause of cervical cancer (14).
Viral oncoproteins E6 and E7 serve important roles in the
initiation and malignant progression (15). In the present study, HPV16E7
expression in normal and cancerous cervical tissues was examined.
Higher levels of HPV16E7 expression were observed in cervical
cancer tissues, when compared with normal cervical tissues. In
addition, the study demonstrated that HPV16E7 promoted cell cycle
progression and cell growth, whilst HPV16E7 knockdown increased sub
G1 percentage which would indicate apoptosis of CaSki cells
(Fig. 3C). The results indicated
that the conversion from normal cervical epithelial cells to
cervical cancer cells is a gradual process and that E7 expression
level is positively correlated with cell transformation and tumor
progression (16).
HPVs perturb cell growth, apoptosis and
differentiation, however, they are additionally involved in
affecting host anti-virus/tumor immune responses (17). Previous studies have reported that
HPVE7 affects the innate immune response by downregulating the
expression of interferon-responsive genes, which affects activation
of the subsequent adaptive immune response (18,19).
Lymphocyte-mediated cytotoxicity involving cytotoxic T lymphocytes
is the most effective mechanism for the control and clearance of
viral infections (20,21). In the current study, PC3-E7 or
CaSki-E7si cells were co-cultured with PBMCs, and the results
demonstrated that HPV16E7 modulated lymphocyte proliferation and
cytotoxic activity. This suggested that HPV16E7 may induce
HPV-infected cervical epithelial cells to circumvent the host
immune response, however the mechanisms involved remain
elusive.
T lymphocyte activation requires two signals, the
recognition of a peptide (presented by major histocompatibility
complex molecules on APCs) by a T cell receptor, and the second
signal provided by co-stimulatory molecules (22). The balance of positive and negative
signals is of central importance in maximizing the ability of the
adaptive immune response to defend the host and/or maintain
auto-tolerance. One of the previously identified T lymphocyte
inhibitory molecules is PD-L1 (23). PD-L1 on APCs binds to its receptor
PD-1 on activated T lymphocytes, resulting in inhibition of T
lymphocyte activity (8,9). Aberrant high expression of PD-L1 is
frequent in various tumor tissues and is correlated with tumor
progression (10,11). Fife and Pauken (24) demonstrated that, during chronic
viral infections and cancer, PD-1-expressed on the T cell membrane
surface encountered PD-L1 on infected or tumor cells and are
associated with functional exhaustion of virus-specific
CD8+ T cells. The results of the present study
demonstrated that PD-L1 expression in cervical cancer tissues was
significantly higher when compared with that in normal control
tissues. A positive correlation between HPVE7 and PD-L1 expression
was observed in cervical cancer tissues and the cell lines. Using a
luciferase assay, it was demonstrated that the increased expression
of PD-L1 was mediated by HPV16E7 in cervical cancer cells or cells
overexpressing E7. These results suggest that HPV16E7 may inhibit
lymphocyte proliferation and cytotoxic activity by upregulating
PD-L1, thus resulting in immune escape of tumor cells.
Increased PD-L1/PD-1 expression on the cell membrane
surface is the main cause of lymphocyte dysfunction during chronic
HPV infection, and inhibiting this pathway may be beneficial to
restore the function of tumor infiltrating lymphocytes (25,26).
Blockade of PD-1 using the specific anti-PD-1 antibody in
vivo has been demonstrated to restore the function of
virus-specific CD8+ T cells, which leads to enhanced
inflammatory cytokines and viral clearance (27,28).
In conclusion, overexpressed PD-L1 was positively correlated with
HPV16E7 in cervical cancer cells, which itself may have been
responsible for lymphocyte dysfunction. In the present study,
sPD-1-modified CaSki cells were co-cultured with PBMCs, which
significantly elevated lymphocyte proliferation and cytotoxic
activity. These results indicated that sPD-1 may restore lymphocyte
function by inhibiting the PD-L1/PD-1 pathway, and provides a novel
insight into immunotherapeutic approaches for the treatment of
cervical cancer.
Acknowledgements
The present study was supported by the Major
Research Plan of the Hubei Province Natural Science Foundation of
China (grant no. 2012FFA086) and Hubei Province's Youth Science and
Technology Innovation Team (grant no. T201203).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM and Bray F: Chapter 2: The
burden of HPV-related cancers. Vaccine. 24:(Suppl 3). S11–S25.
2006. View Article : Google Scholar
|
|
3
|
Scheffner M, Werness BA, Huibregtse JM,
Levine AJ and Howley PM: The E6 oncoprotein encoded by human
papillomavirus types 16 and 18 promotes the degradation of p53.
Cell. 63:1129–1136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dyson N, Howley PM, Münger K and Harlow E:
The human papilloma virus-16 E7 oncoprotein is able to bind to the
retinoblastoma gene product. Science. 243:934–937. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: Moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dudley ME and Rosenberg SA:
Adoptive-cell-transfer therapy for the treatment of patients with
cancer. Nat Rev Cancer. 3:666–675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selenko-Gebauer N, Majdic O, Szekeres A,
Höfler G, Guthann E, Korthäuer U, Zlabinger G, Steinberger P, Pickl
WF, Stockinger H, et al: B7-H1 (programmed death-1 ligand) on
dendritic cells is involved in the induction and maintenance of T
cell anergy. J Immunol. 170:3637–3644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zang X and Allison JP: The B7 family and
cancer therapy: Costimulation and coinhibition. Clin Cancer Res.
13:5271–5279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hino R, Kabashima K, Kato Y, Yaqi H,
Nakamura M, Honjo T, Okazaki T and Tokura Y: Tumor cell expression
of programmed cell death-1 Ligand 1 Is a prognostic factor for
malignant melanoma. Cancer. 116:1757–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tavassoli FA and Devilee P: World Health
Organization classification of tumours. Pathology and Genetics of
the breast an female genital organs Lyon: IARC Press; 2003
|
|
13
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Files DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muñoz N: Human papillomavirus and cancer:
The epidemiological evidence. J Clin Virol. 19:1–5. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Isaacson Wechsler E, Wang Q, Roberts I,
Pagliarulo E, Jackson D, Untersperger C, Coleman N, Griffin H and
Doorbar J: Reconstruction of human papillomavirus type 16-mediated
early-stage neoplasia implicates E6/E7 deregulation and the loss of
contact inhibition in neoplastic progression. J Virol.
86:6358–6364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Xue Y, Poidinger M, Lim T, Chew
SH, Pang CL, Abastado JP and Thierry F: Mapping of HPV transcripts
in four human cervical lesions using RNAseq suggests quantitative
rearrangements during carcinogenic progression. Virology.
462-463:14–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bahrami AA, Ghaeni A, Tabarraei A,
Sajadian A, Gorji A and Soleimanjahi H: DNA vaccine encoding HPV-16
E7 with mutation in L-Y-C-E pRb-binding motif induces potent
anti-tumor responses in mice. J Virol Methods. 206:12–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang YE and Laimins LA: Microarray
analysis identifies interferon-inducible genes and Stat-1 as major
transcriptional targets of human papillomavirus type 31. J Virol.
74:4174–4182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nees M, Geoghegan JM, Hyman T, Frank S,
Miller L and Woodworth CD: Papillomavirus type 16 oncogenes
downregulate expression of interferon-responsive genes and
upregulate proliferation-associated and NF-kappaB-responsive genes
in cervical keratinocytes. J Virol. 75:4283–4296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stanley MA: Immune responses to human
papilloma viruses. Indian J Med Res. 130:266–276. 2009.PubMed/NCBI
|
|
21
|
Bontkes HJ, de Gruijl TD, Walboomers JM,
van den Muysenberg AJ, Gunther AW, Scheper RJ, Meijer CJ and Kummer
JA: Assessment of cytotoxic T-lymphocyte phenotype using the
specific markers granzyme B and TIA-1 in cervical neoplastic
lesions. Br J Cancer. 76:1353–1360. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flies DB, Sandler BJ, Sznol M and Chen L:
Blockade of the B7-H1/PD-1 pathway for cancer immunotherapy. Yale J
Biol Med. 84:409–421. 2011.PubMed/NCBI
|
|
23
|
Keir ME, Liang SC, Guleria I, Latchman YE,
Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH and Sharpe
AH: Tissue expression of PD-L1 mediates peripheral T cell
tolerance. J Exp Med. 203:883–895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fife BT and Pauken KE: The role of the
PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad
Sci. 1217:45–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lyford-Pike S, Peng S, Young GD, Taube JM,
Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et
al: Evidence for a role the PD-1: PD-L1 pathway in immune
resistance of HPV-associated head and neck squamous cell carcinoma.
Cancer Res. 73:1733–1741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ukpo OC, Thorstad WL and Lewis JS Jr:
B7-H1 expression model for immune evasion in human
papillomavirus-related oropharyngeal squamous cell carcinoma. Head
Neck Pathol. 7:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barber DL, Wherry EJ, Masopust D, Zhu B,
Allison JP, Sharpe AH, Freeman GJ and Ahmed R: Restoring function
in exhausted CD8 T cells during chronic viral infection. Nature.
439:682–687. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Badoual C, Hans S, Merillon N, Van Ryswick
C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A,
Besnier N, et al: PD-1 expression tumor-infiltrating T cells are a
favorable prognostic biomarker in HPV-associated head and neck
cancer. Cancer Res. 73:128–138. 2013. View Article : Google Scholar : PubMed/NCBI
|