Introduction
Intracerebral hemorrhage (ICH), estimated to affect
over 1 million people worldwide each year, accounts for 10–15% of
all strokes and is associated with high mortality and morbidity
(1). To date, no effective
therapeutic strategy exists to improve the quality of life of
patients with ICH. ICH is a rapidly evolving process that causes
necrosis, followed by apoptotic cell death and altered gene
expression in the surrounding brain tissue (2). Thus, the identification of novel
therapeutic agents for preventing ICH is crucial.
Glucose-regulated protein 75 (GRP75), a member of
the heat shock protein 70 family, is located in multiple
organelles, but predominantly in mitochondria (3). A growing body of evidence has
demonstrated the role of GRP75 in regulating cellular stress
responses, mitochondrial homeostasis, intracellular trafficking,
antigen presenting, cell proliferation, differentiation, and
tumorigenesis (4). A potential
protective effect of GRP75 has also been demonstrated towards
diseases of the central nervous system. Zhang et al
(5) reported that GRP75 was
implicated in the process of B cell lymphoma 2 apoptosis regulator
(Bcl-2)- and caspase-dependent apoptosis of retinal ganglion cells
(RGCs) following optic nerve crush. Overexpression of GRP75 in PC12
cells has also been demonstrated to prevent apoptosis by reducing
the expression of Bcl-2 associated X apoptosis regulator (Bax) and
delaying the release of cytochrome c (6). In addition, overexpression of GRP75
attenuates the lipopolysaccharide-induced oxidative and metabolic
responses, and suppresses the inflammatory response in microglial
BV-2 cells (7). However, to date,
the biological function of GRP75 in ICH remains unclear. Thus, the
purpose of the present study was to evaluate the effects of GRP75
in a rat model of ICH. A distinct neuroprotective strategy has been
identified that prevents an inflammatory response and neuron
apoptosis following ICH.
Materials and methods
Animals and rat model of ICH
All animals were purchased from the laboratory
animal center of Tianjin Nankai Hospital (Tianjin, China). Sixty 5
week old adult male Sprague-Dawley rats, with body weight of
350–400 g were maintained at constant temperature (21±2°C) and
humidity in a holding facility under a 12-h light-dark cycle, with
free access to food and water. Animal experiments conformed to the
guidelines issued by Tianjin Nankai Hospital for laboratory
animals. Rats were anesthetized intraperitoneally with sodium
pentobarbital (50 mg/kg). Anesthetized rats were placed in a
stereotactic apparatus (David Kopf Instruments, Tujunga, CA, USA).
A 26-gauge needle was then implanted into the caudate-putamen at
the following coordinates: 0.8 mm posterior, 5.5 mm ventral, 3.5 mm
lateral to the bregma (8). ICH was
induced with an infusion of 0.7 µl saline and 0.14 U collagenase IV
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) by using a
microinfusion pump (Harvard Apparatus, Holliston, MA, USA) over a
duration of 7 min. Rats with sham surgery were received 0.7 µl
saline only.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the whole brain tissues
using the RNA plus kit (Fermentas; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Reverse transcription of 5 µg of the total RNA
into cDNA was performed using M-MLV reverse transcriptase (Clontech
Laboratories, Inc., Mountainview, CA, USA). The levels of mRNA
transcripts were analyzed by qPCR using Power SYBR-Green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) on an ABI
Prism 7500 sequence detector (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The gene-specific primer sequences used for
detection of GRP75 were 5′-CGGCTACCACATCCAAGGAA-3′ (forward) and
5′-GCTCGAATTACCGCGGCT-3′ (reverse), and for β-actin were
5′-AAATCGTGCGTGACATCAAAGA-3′ (forward), and
5′-GGCCATCTCCTGCTCGAA-3′ (reverse). Thermocycling conditions were
as follows: 94°C for 4 min, 94°C for 20 sec, 58°C for 30 sec and
72°C for 20 sec, 2 sec for plate reading for 40 cycles and melting
curve from 65 to 95°C. β-actin was used as a quantitative and
qualitative control to normalize the gene expression. Data were
analyzed by the 2-ΔΔCt method (9).
Western blotting
Total protein extracts were prepared from the whole
brain using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's protocol. The protein concentration in the lysates
was evaluated using a bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology). For protein separation, a
total of 20 µg of protein was loaded on a 12.5% sodium dodecyl
sulfate gel and separated by polyacrylamide gel electrophoresis,
followed by transfer to a nitrocellulose membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Membranes were subsequently
blocked with 2.5% non-fat milk for 1 h at 37°C, then probed with
anti-rabbit GRP75 (1:3,000; catalog no. sc-13967), caspase 3
(1:3,000; catalog no. sc-98785), Bcl-2 (1:2,500; catalog no.
sc-783), Bax (1:2,500; catalog no. sc-6236), Akt (1:2,500; catalog
no. sc-24500), p-Akt (1:2,500; catalog no. sc-135650) and GAPDH
(1:1,500; catalog no. sc-367714). The primary antibodies were
obtained from Santa Cruz Biotechnology, Santa Cruz, CA, USA) and
incubation occurred overnight at 4°C. The membrane was then
incubated with a chicken anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:30,2,500; catalog no.
sc-516087; Santa Cruz Biotechnology) diluted in blocking buffer for
1 h at room temperature. Blots were developed using an enhanced
chemiluminescence system (Pierce; Thermo Fisher Scientific, Inc.).
Protein expression was analyzed using BandScan 5.0 software (Glyko
Biomedical Ltd., Novato, CA, USA).
GRP75 expression vector and in vivo
injection
The cDNA encoding GRP75, obtained from PCR
amplification, was amplified and subcloned into the adenoviral
shuttle vector pAd-CMV (Invitrogen; Thermo Fisher Scientific,
Inc.). Rat green fluorescent protein (GFP; Invitrogen, Thermo
Fisher Scientific, Inc.) was used as a non-specific control. The
adenoviral shuttle vector pAd-CMV and adenoviral gene expression
vector pAdEasy-1 were recombined in Escherichia coli strain BJ5183.
The recombined plasmid Ad-GRP75 was then propagated in 293T cells
(American Type Culture Collection, Manassas, VA, USA). For in
vivo injection, 5 µl of Ad-GFP or Ad-GRP75 dissolved in 30 µl
i-Fect transfection reagent (Neuromics, Edina, MN, USA) was
administered intrathecally for 1 day, from 1 day prior to ICH and
rats were sacrificed 2 days subsequent to ICH for analysis of
neuron apoptosis.
Enzyme-linked immunosorbent assay
(ELISA)
The brain tissues were rapidly removed and
homogenized in lysis buffer. Following centrifugation (6000 × g) at
4°C for 10 min, the supernatants were analyzed for cytokine
production by ELISA. ELISA was performed as per the manufacturer's
instructions (Dakewe Biotech Co., Ltd., Shenzhen, China) to assess
the concentrations of tumor necrosis factor-α (TNF-α; catalog no.
DKW12-2720-048) and interleukin (IL)-1β (catalog no.
DKW12-2012-096) in the culture supernatant.
Statistical analysis
Data was expressed as the mean ± standard deviation.
Statistical analysis was performed with SPSS software version 13
(SPSS, Inc., Chicago, IL, USA). Multiple comparisons were evaluated
by one-way analysis of variance followed by Tukey's post hoc test.
Two-group comparisons were analyzed by Student's t test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of GRP75 in a rat model of
ICH
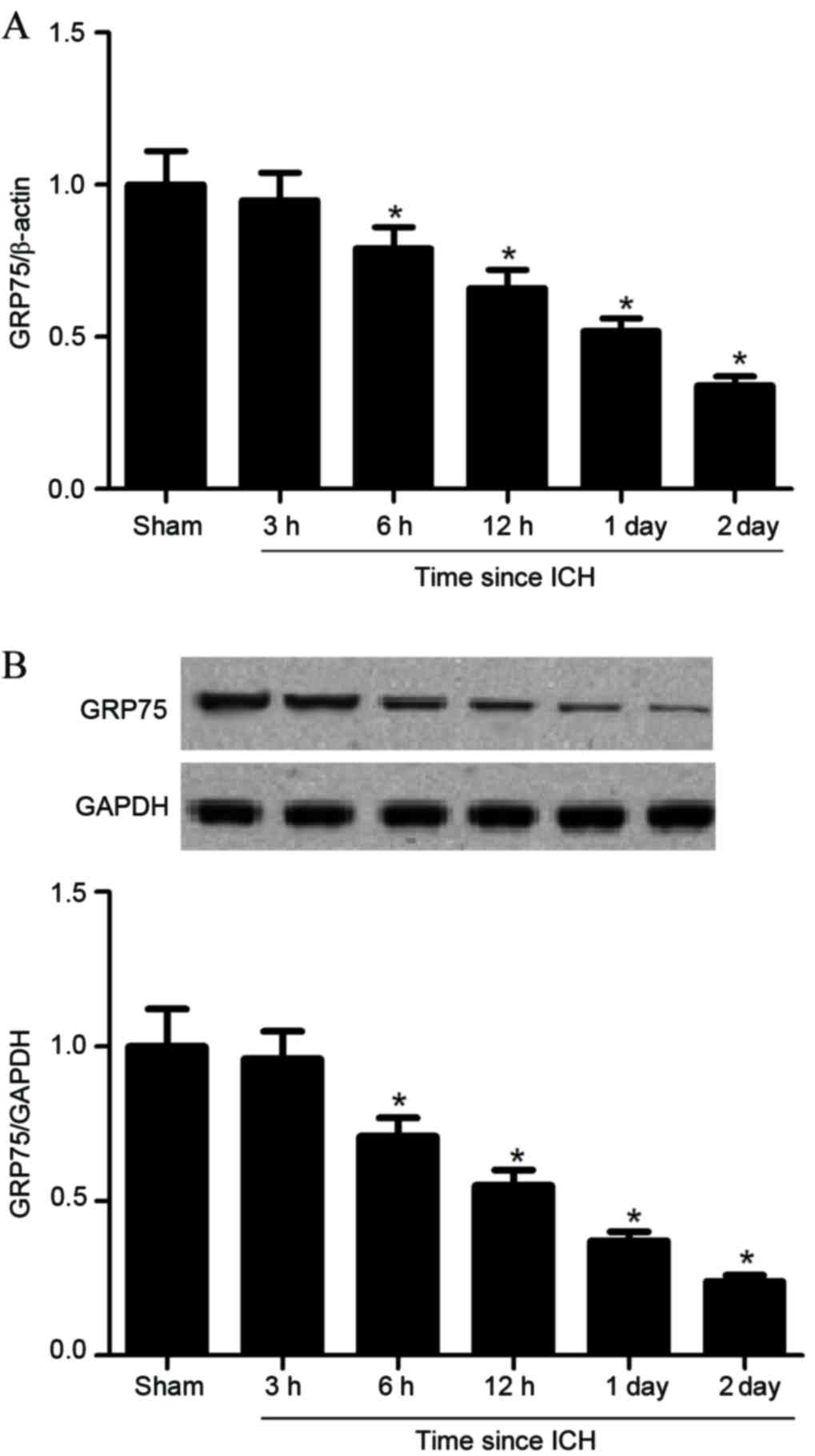
GRP75 expression was investigated in brain tissues
at different time points subsequent to ICH. Compared with the
sham-operated group, GRP75 mRNA expression levels were
significantly decreased from 6 h following ICH (P=0.038; Fig. 1A), with progressive decreases at
subsequent time points, to a minimum level at 2 days (P=0.014;
Fig. 1A). Furthermore, western
blot analysis demonstrated that GRP75 protein expression levels
were also significantly inhibited by ICH in the same progressive
manner (Fig. 1B).
Overexpression of GRP75 inhibits the
inflammatory response in a rat model of ICH
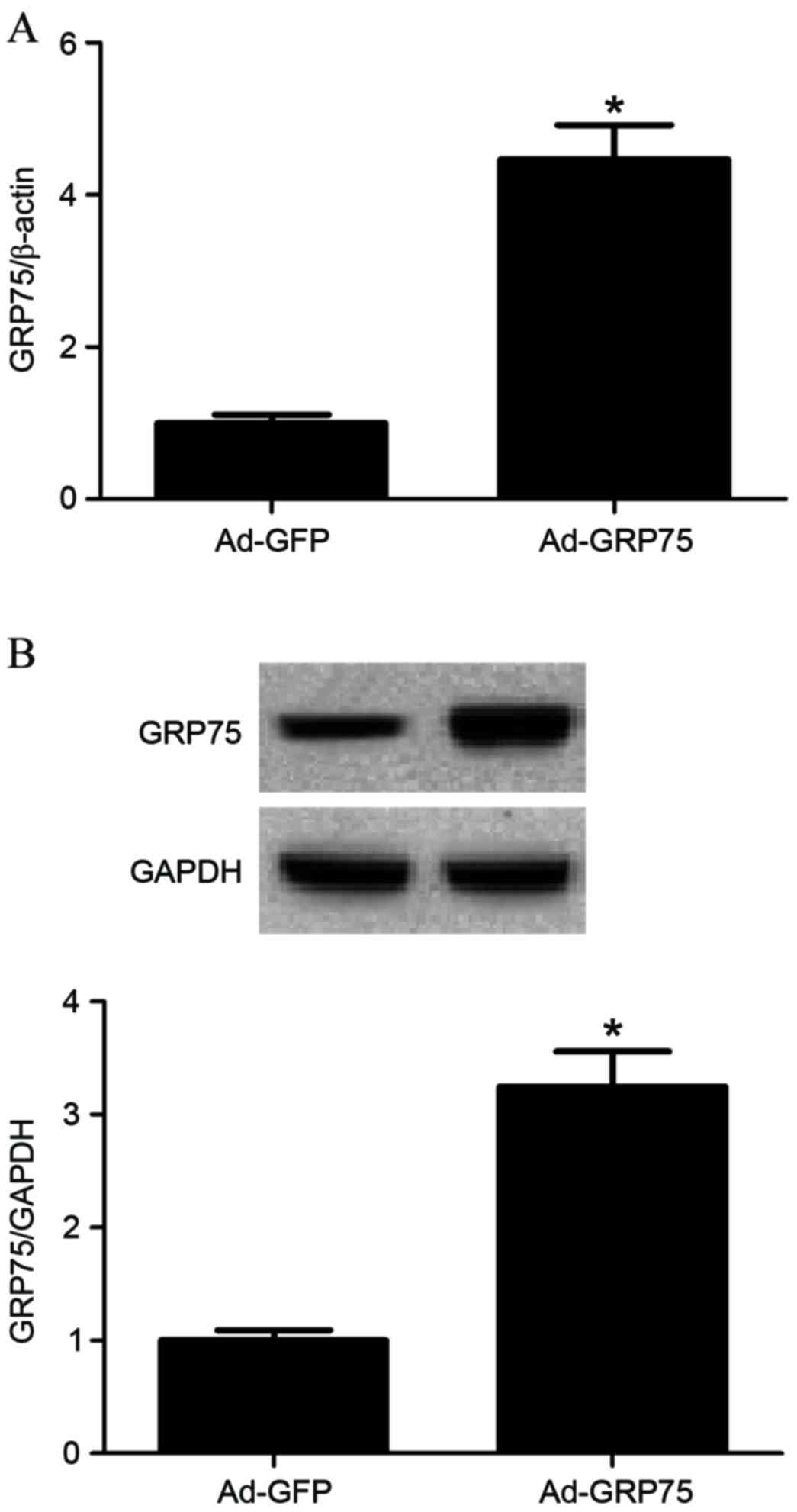
To investigate the role of GRP75 in the progression
of ICH, 5 µl of Ad-GFP or Ad-GRP75 dissolved in 30 µl i-Fect
transfection reagent (Neuromics, Edina, MN, USA) was administered
intrathecally once daily for 1 days. Subsequent GRP75 expression
levels were determined by RT-qPCR and western blot. GRP75 mRNA and
protein expression levels were confirmed to be significantly
increased in brain tissues that received Ad-GRP75 compared with
tissues in the Ad-GFP control group (P=0.021 and P=0.036; Fig. 2A and B, respectively). In addition,
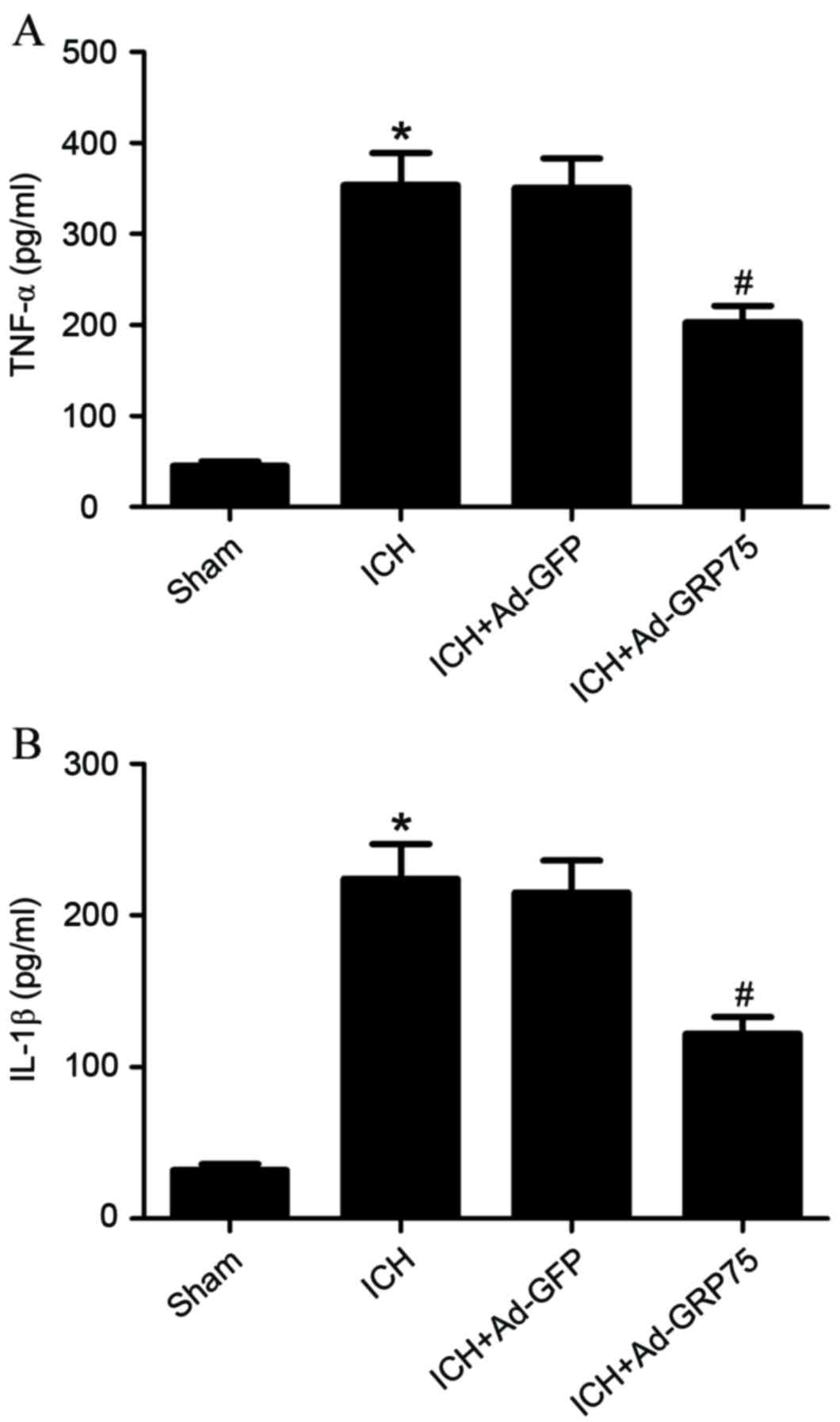
overexpression of GRP75 in brain tissues of rats with ICH
significantly suppressed the production of TNF-α and IL-1β compared
with the ICH group (P=0.026 and P=0.032; Fig. 3A and B, respectively).
Overexpression of GRP75 inhibits
expression of neuronal apoptosis markers in a rat model of ICH
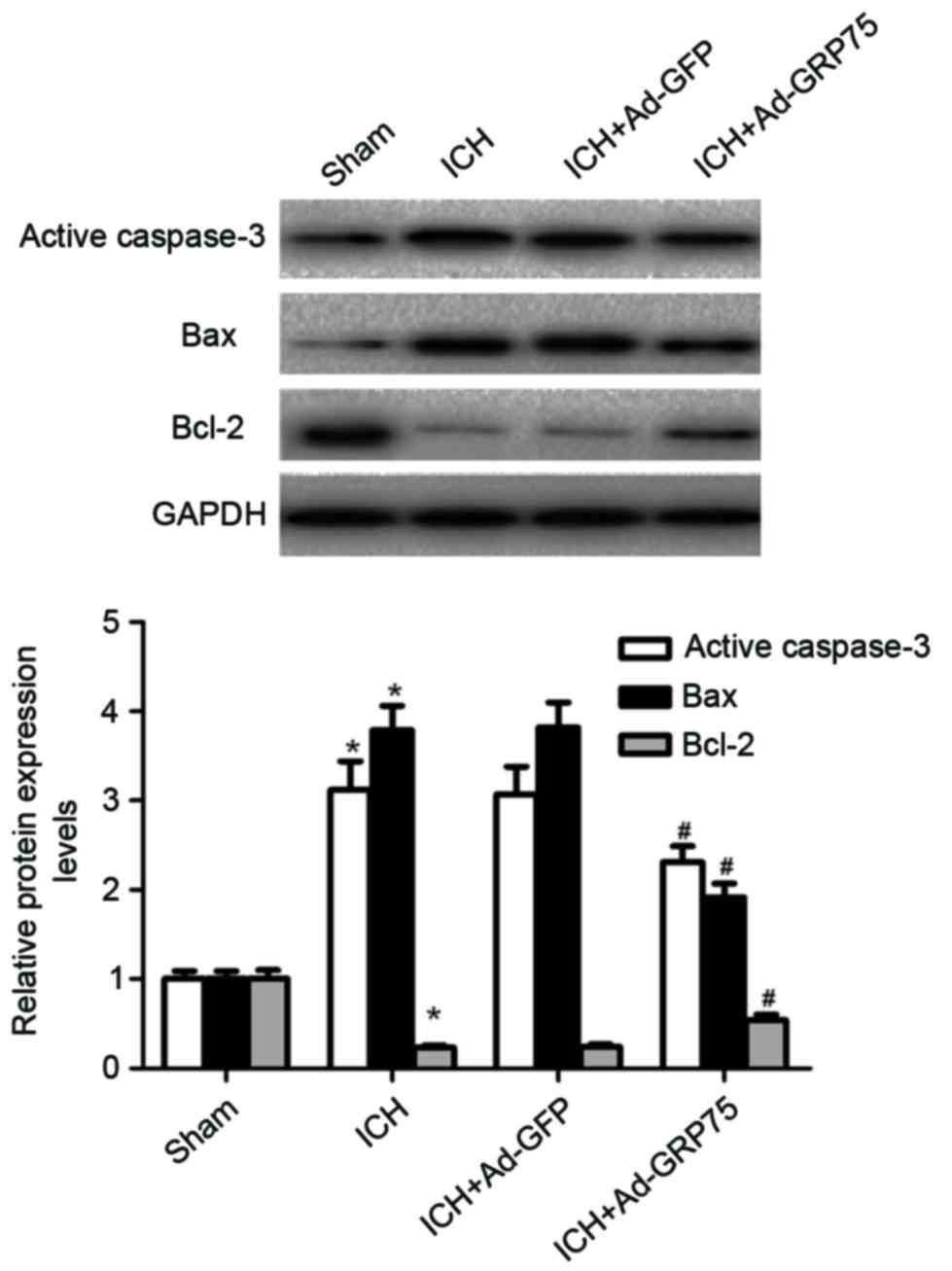
Neuronal apoptosis is a significant event
surrounding hematoma in ICH (10).
The effects of GRP75 on neuronal apoptosis were, therefore,
investigated in a rat model of ICH. Active caspase-3 protein
expression was increased following ICH compared with the sham group
(P=0.019; Fig. 4), while
overexpression of GRP75 in brain tissues of rats with ICH
significantly inhibited the expression of active caspase-3 compared
with the ICH group (P=0.041; Fig.
4). To further examine the effects of GRP75 on neuronal
apoptosis, the expression profiles of Bax and Bcl-2 were
investigated. Overexpression of GRP75 in brain tissues of rats with
ICH significantly decreased the expression of Bax and increased the
expression of Bcl-2 compared with the ICH group (P=0.023 and
P=0.036 respectively; Fig. 4).
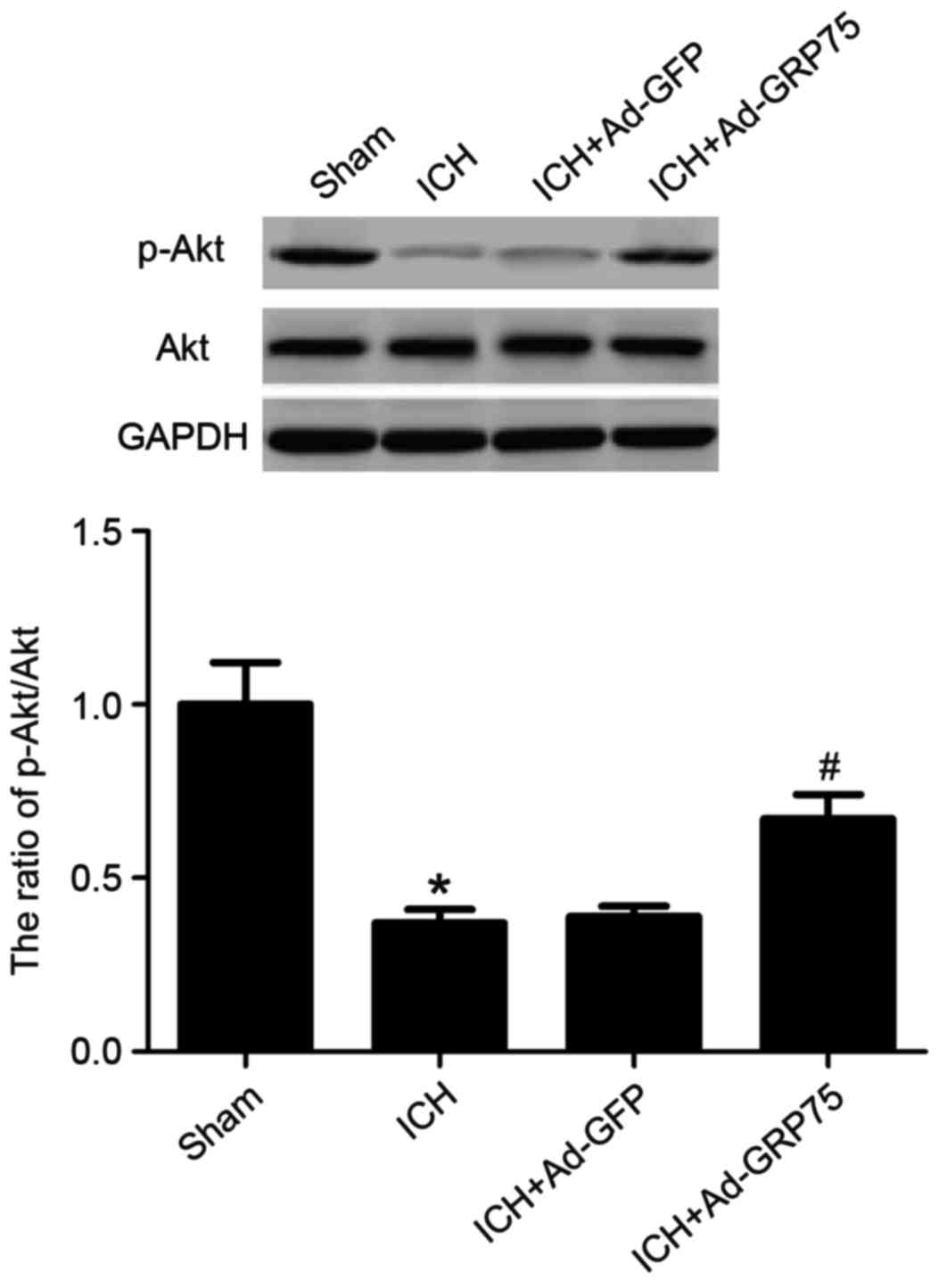
Overexpression of GRP75 upregulates
the level of p-Akt in a rat model of ICH
To examine whether GRP75 promotes neuronal apoptosis
via the PI3K/Akt signaling pathway, the effect of GRP75
overexpression on Akt phosphorylation was examined. The level of
Akt Ser473 phosphorylation was demonstrated to be significantly
diminished 2 days subsequent to ICH compared with the sham group
(P=0.017; Fig. 5), whereas
overexpression of GRP75 in brain tissues of rats with ICH
significantly upregulated p-Akt levels compared with the ICH group
(P=0.036; Fig. 5).
Discussion
In the present study, significantly reduced
expression of GRP75 was demonstrated in the brain tissues of rats
with ICH. In addition, overexpression of GRP75 in the brain tissues
of rats with ICH significantly inhibited the production of
inflammatory cytokines and affected production of neuronal
apoptosis markers. Furthermore, overexpression of GRP75
significantly upregulated p-Akt in brain tissues following ICH.
GRP75 participates in neuronal processes (5,11,12).
It has been reported that the expression of GRP75 decreased
significantly in dopaminergic cells overexpressing A53T
α-synuclein, and downregulation of GRP75 attenuated the disrupted
mitochondrial dynamics by reducing α-synuclein translocation to
mitochondria (13). Liu et
al (14) reported that upon
exposure to glucose deprivation, GRP75-overexpressing PC12 cells
exhibited more moderate cell damage than control PC12 cells.
Consistent with these results, the present study demonstrated
reduced GRP75 expression in brain tissues following ICH. These data
suggest that GRP75 may be important in the progression of ICH.
Activation of the inflammatory cascade is a common
event in the ICH-induced brain (15). Previous studies have reported that
inflammatory cytokines, including TNF-α and IL-1β, were upregulated
following ICH (16–18). In the present study, overexpression
of GRP75 was observed to significantly suppress the production of
TNF-α and IL-1β in the brain tissues of rats with ICH.
Neuronal apoptosis is important in the progression
of ICH (2). Caspase-3, known as
the death enzyme, has is important in the controlled execution of
programmed cell death (19). In
the present study, overexpression of GRP75 was demonstrated to
significantly inhibit the expression of active caspase-3. The
apoptotic protein Bax is a Bcl-2 family cytoplasmic protein, which,
following activation by diverse stimuli, inserts into mitochondria,
leading to caspase-3 activation and cell apoptosis (20). In the present study, overexpression
of GRP75 was observed to significantly decrease the expression of
Bax and increase the expression of Bcl-2 in brain tissues of rats
with ICH.
Previous studies have demonstrated that the PI3K/Akt
pathway is an important signal transduction pathway that regulates
cell survival of neurons (21–23).
Various cellular stresses can activate Akt through phosphorylation,
which phosphorylates a range of Akt downstream targets such as
Bcl-2-associated death promoter (Bad), glycogen synthase kinase-3
beta (GSK3β) and forkhead box O-subclass (FoxO) proteins, leading
to the survival of target cells (24,25).
Previous studies demonstrated that Akt inhibits apoptosis by
phosphorylating and inactivating pro-apoptotic factors (such as
Bad) to maintain mitochondrial integrity through preventing the
inhibition of anti-apoptotic Bcl-2 by Bad (26); Yang et al (27) demonstrated that the activation of
Akt by GRP75 inhibited the Bax conformational change and,
subsequently, apoptosis. The present study observed that the level
of p-Akt was significantly downregulated in brain tissues following
ICH, and overexpression of GRP75 upregulates the level of p-Akt.
These results indicated that GRP75 promotes neuronal apoptosis
following ICH through modulation of the Akt-dependent pathway.
In conclusion, the present study demonstrated for
the first time (to the best of our knowledge) that GRP75 expression
is significantly decreased in brain tissues following ICH, and that
overexpression of GRP75 inhibits inflammation and neuronal
apoptosis in a rat model of ICH. Therefore, GRP75 may represent a
promising target for the treatment of ICH.
References
|
1
|
Schmidt LB, Geertz S, Wohlfahrt J, Melbye
M and Munch TN: Recurrent intracerebral hemorrhage: Associations
with comorbidities and medicine with antithrombotic effects. PLoS
One. 11:e01662232016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsushita K, Meng W, Wang X, Asahi M,
Asahi K, Moskowitz MA and Lo EH: Evidence for apoptosis after
intracerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab.
20:396–404. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ran Q, Wadhwa R, Kawai R, Kaul SC, Sifers
RN, Bick RJ, Smith JR and Pereira-Smith OM: Extramitochondrial
localization of mortalin/mthsp70/PBP74/GRP75. Biochem Biophys Res
Commun. 275:174–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wadhwa R, Taira K and Kaul SC: An Hsp70
family chaperone, mortalin/mthsp70/PBP74/Grp75: What, when, and
where? Cell Stress Chaperones. 7:309–316. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang G, Han M, Wang X and Xiao A: GRP75
Involves in retinal ganglion cell apoptosis after rat optic nerve
crush. J Mol Neurosci. 56:422–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Liu X, Hao J, Yang Y, Zhao M, Zuo
J and Liu W: Glucose-regulated protein 75 suppresses apoptosis
induced by glucose deprivation in PC12 cells through inhibition of
Bax conformational change. Acta Biochim Biophys Sin (Shanghai).
40:339–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voloboueva LA, Emery JF, Sun X and Giffard
RG: Inflammatory response of microglial BV-2 cells includes a
glycolytic shift and is modulated by mitochondrial
glucose-regulated protein 75/mortalin. FEBS Lett. 587:756–762.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang S, Song S, Hua Y, Nakamura T, Keep RF
and Xi G: Effects of thrombin on neurogenesis after intracerebral
hemorrhage. Stroke. 39:2079–2084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak K and Schmittgen T: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tao X, Xie L, Duan C, Dai S, Ren J, Yan Y,
Shen J, Lu H and Ge J: Up-regulation of interferon regulatory
factor 3 involves in neuronal apoptosis after intracerebral
hemorrhage in adult rats. Neurochem Res. 41:2937–2947. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shih YY, Lee H, Nakagawara A, Juan HF,
Jeng YM, Tsay YG, Lin DT, Hsieh FJ, Pan CY, Hsu WM and Liao YF:
Nuclear GRP75 binds retinoic acid receptors to promote neuronal
differentiation of neuroblastoma. PLoS One. 6:e262362011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin J, Hulette C, Wang Y, Zhang T, Pan C,
Wadhwa R and Zhang J: Proteomic identification of a stress protein,
mortalin/mthsp70/GRP75: Relevance to Parkinson disease. Mol Cell
Proteomics. 5:1193–1204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Ft Chen Y, Yang Yj, Yang L, Yu M, Zhao
J, Wu JJ, Huang F, Liu W, Ding ZT and Wang J: Involvement of
mortalin/GRP75/mthsp70 in the mitochondrial impairments induced by
A53T mutant α-synuclein. Brain Res. 1604:52–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Liu W, Song XD and Zuo J: Effect of
GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP
level, mitochondrial membrane potential and ROS accumulation
following glucose deprivation in PC12 cells. Mol Cell Biochem.
268:45–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Yang S, Xi G, Song S, Fu G, Keep RF
and Hua Y: Microglial activation and brain injury after
intracerebral hemorrhage. Acta Neurochir Suppl. 105:59–65. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu S, Fang CX, Kim J and Ren J: Enhanced
pulmonary inflammation following experimental intracerebral
hemorrhage. Exp Neurol. 200:245–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirashima Y, Nakamura S, Endo S, Kuwayama
N, Naruse Y and Takaku A: Elevation of platelet activating factor,
inflammatory cytokines, and coagulation factors in the internal
jugular vein of patients with subarachnoid hemorrhage. Neurochem
Res. 22:1249–1255. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abraham E, Bursten S, Shenkar R, Allbee J,
Tuder R, Woodson P, Guidot DM, Rice G, Singer JW and Repine JE:
Phosphatidic acid signaling mediates lung cytokine expression and
lung inflammatory injury after hemorrhage in mice. J Exp Med.
181:569–575. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tawa P, Hell K, Giroux A, Grimm E, Han Y,
Nicholson DW and Xanthoudakis S: Catalytic activity of caspase-3 is
required for its degradation: Stabilization of the active complex
by synthetic inhibitors. Cell Death Differ. 11:439–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smaili SS, Hsu YT, Sanders KM, Russell JT
and Youle RJ: Bax translocation to mitochondria subsequent to a
rapid loss of mitochondrial membrane potential. Cell Death Differ.
8:909–920. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leinninger GM, Backus C, Uhler MD, Lentz
SI and Feldman EL: Phosphatidylinositol 3-kinase and Akt effectors
mediate insulin-like growth factor-I neuroprotection in dorsal root
ganglia neurons. FASEB J. 18:1544–1546. 2004.PubMed/NCBI
|
|
22
|
Xu X, Chua CC, Gao J, Chua KW, Wang H,
Hamdy RC and Chua BH: Neuroprotective effect of humanin on cerebral
ischemia/reperfusion injury is mediated by a PI3K/Akt pathway.
Brain Res. 1227:12–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brunet A, Datta SR and Greenberg ME:
Transcription-dependent and-independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romashkova JA and Makarov SS: NF-κB is a
target of AKT in anti-apoptotic PDGF signalling. Nature. 401:86–90.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng WH, Kar S and Quirion R:
Insulin-like growth factor-1-induced phosphorylation of
transcription factor FKHRL1 is mediated by phosphatidylinositol
3-kinase/akt kinase and role of this pathway in insulin-like growth
factor-1-induced survival of cultured hippocampal neurons. Mol
Pharmacol. 62:225–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Chen Y, Jenkins LW, Kochanek PM
and Clark RS: Bench-to-bedside review: Apoptosis/programmed cell
death triggered by traumatic brain injury. Crit Care. 9:66–75.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang L, Guo W, Zhang Q, Li H, Liu X, Yang
Y, Zuo J and Liu W: Crosstalk between Raf/MEK/ERK and PI3K/AKT in
suppression of Bax conformational change by Grp75 under glucose
deprivation conditions. J Mol Biol. 414:654–666. 2011. View Article : Google Scholar : PubMed/NCBI
|