Introduction
The guinea pig (Cavia porcellus) is a rodent
species in the Caviidae family (1,2).
Guinea pigs are a valuable animal model for the study of
developmental biology and the pathogenesis of multiple diseases due
to similarities between their biological characteristics and those
of humans. These similarities appear in hormonal and immunologic
responses, pulmonary physiology, exogenous vitamin C requirements
and delayed-type hypersensitivity to infections such as
tuberculosis (3). Similarities in
the sensitivity of the respiratory system and susceptibility to
infectious agents results in the broad use of this species as a
model of respiratory diseases (4).
With respect to the pathogenesis and the immune response to these
diseases, guinea pigs are more representative of a human than
models using other rodent species.
The lung is an organ directly exposed to the
environment, resulting in continuous contact with a diverse array
of environmental insults. To respond to the internal and/or
external environment, resident myeloid cells and stromal cells of
the lung express a full complement of Toll-like receptors (TLRs),
which are a class of signaling pattern-recognition receptors (PRRs)
that contribute to innate and adaptive immunity by recognizing
pathogen-associated molecular patterns (PAMPs) and endogenous
danger-associated molecular patterns (DAMPs) (5,6). In
addition, TLRs are implicated in the pathogenesis of non-infectious
pulmonary diseases, including pulmonary fibrosis, chronic
obstructive pulmonary disease (COPD) and acute lung injury (ALI)
(7). TLRs were originally
identified in Drosophila melanogaster, and revealed to be
transmembrane receptor proteins that are highly conserved between
D. melanogaster and humans (8,9).
Although a large body of studies have demonstrated the association
of TLRs with various pulmonary disorders in adult animal models and
adult patients (7), several
studies on fetal animals, including fetal murine and preterm lamb
lung tissue specimens, have suggested that steady-state TLR mRNA
expression levels increase with gestational development (10,11).
Distinct characteristics and expression patterns of TLRs have also
been reported between mammalian species, tissues and developmental
stages (12–17). Therefore, in order to improve
understanding of the mechanisms involved in cell specificity and
regulation of TLR signaling transduction pathways, there is a need
to investigate the nature of TLRs and ligands in different
species.
Given the importance of TLRs in the development and
homeostasis of tissues, the present study examined the
differentiation of guinea pig lung epithelial cells during
embryonic development and maturation following birth, and profiled
the expression of TLRs (TLR-1, TLR-1, TLR-3, TLR-4, TLR-6, TLR-7,
TLR-8, TLR-9 and TLR-10), as well as adaptors of the TLR pathway:
Myeloid differentiation factor 88 (MyD88) and tumor necrosis factor
receptor associated factor 6 (TRAF-6).
Materials and methods
Animals and tissue processing
All experimental procedures were carried out
according to ethical guidelines established by Ningxia University
and were approved by the Ethnic Committee for Scientific Research
of General Hospital of Ningxia Medical University (NXMU-H-2014-250;
Yinchuan, China). A total of 51 three months old outbred
Hartley-Duncan guinea pigs of both sexes (45 females and 6 males;
300±50 g) were obtained from the Animal Facility of Ningxia Medical
University (Yinchuan, China). Total of 51 animals (45 females and 6
males) were used. Individuals were housed in the animal facility
under clean, non-specific pathogen free conditions with a constant
temperature of 24–26°C and humidity of 30–50% (12/12 h light/dark
cycle), provided a proper balance of pellets, hay and fresh
vegetables and free access to water, according to the Housing and
Husbandry Guidelines for Laboratory Animals of Ningxia Medical
University. The appearance of a vaginal plug in the morning
following breeding was designated the first day of gestation (dGA).
Animals were terminally euthanized by intraperitoneal injection of
sodium pentobarbital (150 mg/kg) in the facility. Embryos were
harvested from pregnant animals for lung isolation at 45, 52 and 59
dGA (embryos implanted in uterus with normal development were
confirmed as alive, and those only showing implantation sites but
without an embryo or a normally developed embryo were defined as
dead), and lungs were harvested directly from pups 0, 7, 14 and 28
days following parturition (dPN). Lungs were also harvested from
mature animals (6 weeks old). The trachea and lungs were removed
and fixed in 10% neutral buffered formalin, then processed for
embedding in a Sakura Tissue Tek Paraffin Tissue Processor (Model
VIP E150; Sakura Finetek USA, Inc., Torrance, CA, USA).
Immunohistochemistry (IHC)
Immunohistochemical staining was performed on 6 µm
thick paraffin sections. Paraffin sections were dewaxed in xylene
and incubated in methanol containing 0.3%
H2O2 for 30 min to inactivate endogenous
peroxidase. Sections were subsequently rehydrated with graded
ethanol according to histological standards. The sections were
boiled in in citrate buffer (pH 6.0) at 95°C for 20 min for antigen
retrieval followed by slow cooling for 2 h at room temperature.
Sections were then blocked using a blocking buffer (5% horse serum
in PBS; Vector Laboratories, Inc., Burlingame, CA, USA) at room
temperature for 2 h prior to incubation with a primary antibody
(diluted in blocking buffer; Table
I) in a humid chamber at 4°C overnight. The primary antibodies
used in the present study are listed in Table I. Following washing three times for
5 min in PBS, a mixture of biotinylated horse anti-rabbit IgG or
anti-mouse IgG (cat. no. PK-6100; 1:200; Vector Laboratories, Inc.)
secondary antibodies (Table I for
detail) was applied at room temperature for 2 h. The antigen was
detected with an elite ABC kit (Vector Laboratories, Inc.) and
developed with a DAB peroxidase substrate kit (Vector Laboratories,
Inc.). The stained sections were counterstained with hematoxylin
for 10 sec (Gill's formula; Vector Laboratories, Inc.). Sections
were subsequently rinsed in running tap water for 5 min, dehydrated
with gradually increased concentration of ethanol (30–100% v/v),
cleared sections by submerging slides in xylene for 1 min during
mounting sections with Permount (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Hematoxylin and eosin (H&E) staining was
performed using H&E staining kit from Thermo Fisher Scientific,
Inc.. Periodic Acid Schiff's staining was conducted using Schiff's
reagents from Sigma-Aldrich; Merck Millipore. Staining was
visualized under a light microscope (TCS SP8; Leica, Wetzlar,
Germany) at magnifications of ×200, ×400 and ×630, and images were
captured using a Leica DFC300 F camera (Leica Microsystems, Inc.,
Buffalo Grove, IL, USA). For all antibodies tested, mouse positive
and negative tissues were utilized as positive and negative
controls, respectively.
 | Table I.Primary antibodies used for
immunohistochemical staining and western blotting. |
Table I.
Primary antibodies used for
immunohistochemical staining and western blotting.
| Antibody | Host | Dilution | Supplier | Catalog number | IHC or IB |
|---|
| Keratin 14 | Rabbit | 1:500 | Thermo Sci | RB-9020 | Both |
| Tubulin IV | Mouse | 1:2,000 | BioGenex | MU178-UC | Both |
| pro-SPC | Rabbit | 1:5,000 | Millipore | AB3786 | Both |
| CCSP | Rabbit | 1:5,000 | USBiological | C5828–03 | Both |
| TLR-3 | Rabbit | 1:1,000 | Millipore | NG1593494 | IB |
| TLR-6 | Rabbit | 1:1,000 | Abcam | AB37072 | IB |
| TLR-9 | Rabbit | 1:1,000 | Millipore | AB10011 | IB |
| MyD88 | Rabbit | 1:1,000 | Abcam | AB2068 | IB |
| TRAF-6 | Rabbit | 1:1,000 | Millipore | 04–451 | IB |
| GAPDH | Goat | 1:1,000 | Santa Cruz | Sc-20357 | IB |
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from each tissue sample
using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's instructions. RNA quality was
assayed by calculation of the RNA integrity number (RIN) (18). High quality RNA (RIN value >7.5)
was used to synthesize first strand cDNA using M-MLV reverse
transcriptase (Takara Bio, Inc., Otsu, Japan). qPCR was performed
in the Roche LightCycler 2.0 (Roche Diagnostics, Basel,
Switzerland) using a SYBR-Green I kit (Takara Bio, Inc.). The
thermal cycling conditions were as follows: 95°C for 30 sec, 40
cycles of 95°C for 5 sec, 60°C for 20 sec and 72°C for 20 sec,
followed by 40°C for 20 min. The primer sets used for β-actin
control, TLRs, MyD88 and TRAF-6 are listed in Table II. To assess primer efficiency,
standard curves for each primer pair were generated using serially
diluted transcribed cDNA samples. PCR efficiency was calculated
from the slope of the standard curves. β-actin internal controls
were always included to normalize each reaction with respect to RNA
integrity, sample loading and inter-PCR variations. The relative
expression ratio was calculated from the real-time PCR efficiencies
and the crossing point deviation of experimental samples vs.
controls (19). The resulting
threshold cycle (Cq) values were normalized to the endogenous
control, β-actin (ΔCq=Cq value of target gene-Cq value of β-actin).
Dissociation analysis of amplification products was performed at
the end of each PCR to confirm the specificity of the amplicon. The
fold change in TLR gene expression during lung development was
calculated by the 2-ΔΔCq method (20).
 | Table II.Sequences and parameters of reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Sequences and parameters of reverse
transcription-quantitative polymerase chain reaction.
| Genes | Sequence
(5′-3′) | Anneal
temperature | Product size | Ensembl ID |
|---|
| TLR1 | F:
GTCCCAAGTTAGCCCATTTTTA | 60.0°C | 240 bp |
ENSCPOT00000005143 |
|
| R:
CTTTCAGCCCAAGAGCAAGTAT |
|
|
|
| TLR2 | F:
AGGAAAAGTTGGAGCGGTTT | 60.0°C | 225 bp |
ENSCPOG00000025374 |
|
| R:
GGCAAAGGCAAAGAAAGTGA |
|
|
|
| TLR3 | F:
GCAACAACAACATAGCCAACA | 60.0°C | 133 bp |
ENSCPOT00000004982 |
|
| R:
AGAAAATGAACAGGACCACCA |
|
|
|
| TLR4 | F:
GTTATCGTTGTGGTGTCTCAGC | 60.0°C | 198 bp |
ENSCPOT00000015306 |
|
| R:
TTCCCATTCCAGGTAAGTGTTC |
|
|
|
| TLR6 | F:
TTTGTCAGGGCTCAACATTCT | 60.0°C | 147 bp |
ENSCPOT00000005143 |
|
| R:
AAACTCACCACAGGGTAGCAG |
|
|
|
| TLR7 | F:
ACTCCTTGGGGATAGATGGTTT | 60.0°C | 154 bp |
ENSCPOT00000015254 |
|
| R:
AATAGTGAGGGTGAGGTTGGTG |
|
|
|
| TLR8 | F:
AGATGTGATTTGTGCCAGTCC | 60.0°C | 233 bp |
ENSCPOT00000004812 |
|
| R:
GGGATGTGGAAAGAGACCTGT |
|
|
|
| TLR9 | F:
GGGAAGCAGTGACCAGAAC | 60.0°C | 121 bp | XM003476720 |
|
| R:
TCCAAGGTGAATGGGATGTT |
|
|
|
| TLR10 | F:
GAGGAGATGGGCTTCGACTAC | 60.0°C | 169 bp |
ENSCPOT00000015432 |
|
| R:
CTCAGTTCCAACAGCACGTC |
|
|
|
| MyD88 | F:
GAGGAGATGGGCTTCGACTAC | 60.0°C | 164 bp |
ENSCPOT00000027931 |
|
| R:
CTCAGTTCCAACAGCACGTC |
|
|
|
| TRAF6 | F:
ATCACTTGGCACGACACCTAC | 60.0°C | 138 bp |
ENSCPOT00000004556 |
|
| R:
GGGACAGAGAGGCAGAATCAT |
|
|
|
| β-actin | F:
GGACTTTGAGCAGGAGATGACT | 60.0°C | 127 bp |
ENSCPOT00000011124 |
|
| R:
CTGGAAAATGGCTTCAGGAC |
|
|
|
Western blot analysis
Tissue extracts were prepared by homogenizing the
cells in a lysis buffer (50 mM Tris-HCl, 5 mM EDTA, 150 mM NaCl,
0.5% NP-40; pH 7.5) for 60 min on ice. Lysates were subsequently
centrifuged at 10,000 × g for 10 min at 4°C, and the supernatants
were collected as whole-cell extracts. The soluble protein
concentration was measured with the Bio-Rad Protein Assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using bovine serum albumin
(BSA; Thermo Fisher Scientific, Inc.) as a standard. The cell
extracts (50 µg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE) and transferred to a
polyvinylidene difluoride membrane (Merck Millipore). The membrane
was blocked with 4% fat free dry milk in PBS containing 0.2%
Tween-20 at room temperature for 2 h and incubated with primary
antibodies (Table I) at 4°C
overnight, followed by appropriate peroxidase-labeled secondary
antibodies (cat. no. 108894; 1:2,000; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) at room temperature for 1
h. The blots were then developed using an enhanced
chemiluminescence reagent (GE Healthcare, Chalfont, UK).
Statistical analysis
All data collected in the present study were
obtained from at least three independent experiments for each
condition. SPSS 18.0 analysis software (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Statistical evaluation of
the data was performed by one-way ANOVA and Student's t-tests to
compare differences between groups. Tukey's honestly significant
difference test was employed for post hoc testing for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference and P<0.01 was considered to indicate a
highly statistically significant difference. Data were expressed as
the mean ± standard deviation.
Results
Development of the guinea pig lung
during embryogenesis
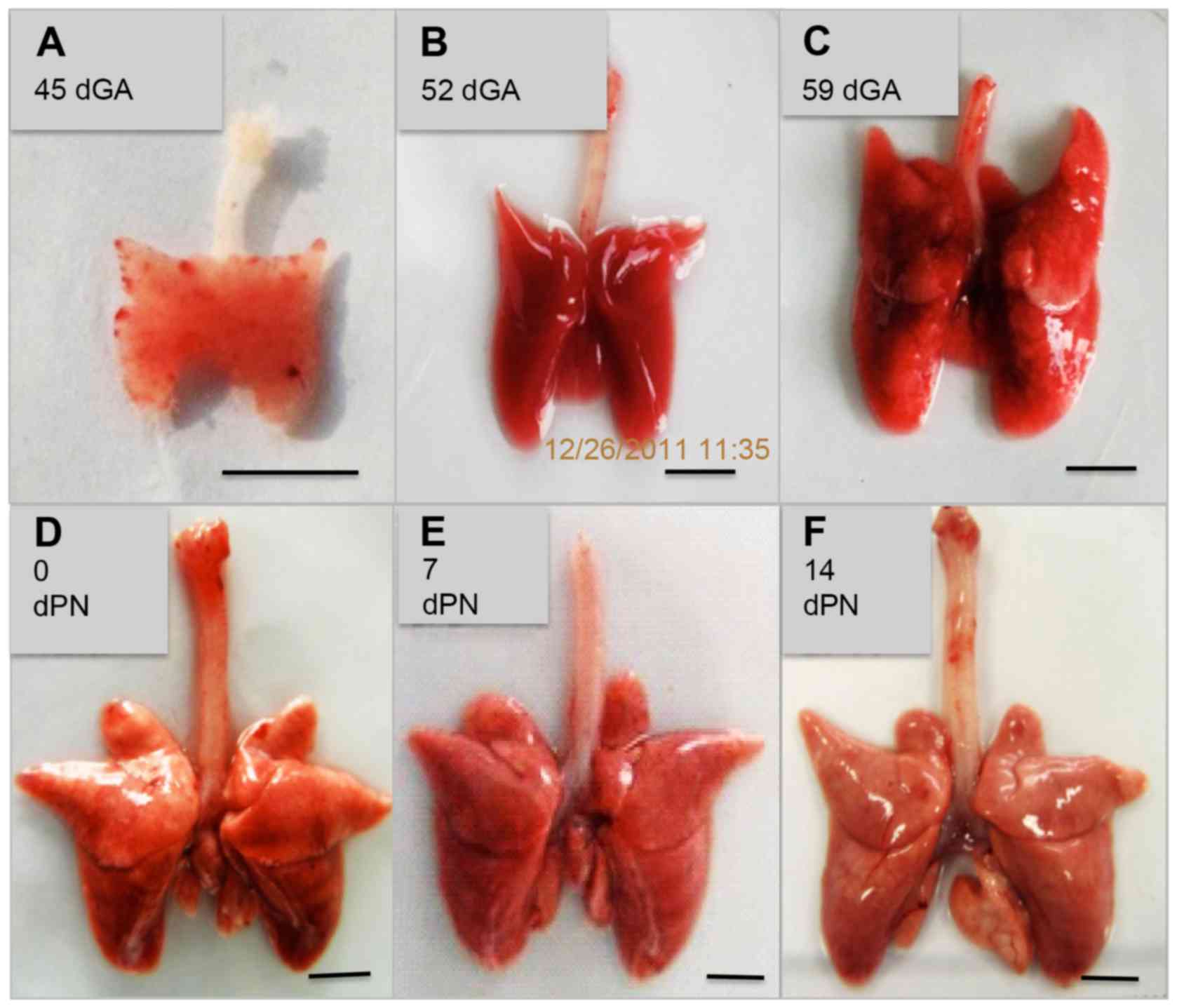
The guinea pig gestation period spans 59–73 days,
with an average of 66±2 days recorded in the present study. Lobe
formation in the lung was displayed at 45 dGA, with all 7 lobes (4
lobes in the right, 3 lobes in the left) formed at 59 dGA, as
determined by whole mount anatomic lung morphological examination
(Fig. 1). The lung continued to
grow and mature, increasing in weight and size, with body growth
(Fig. 1 and Table III). Histological analysis by
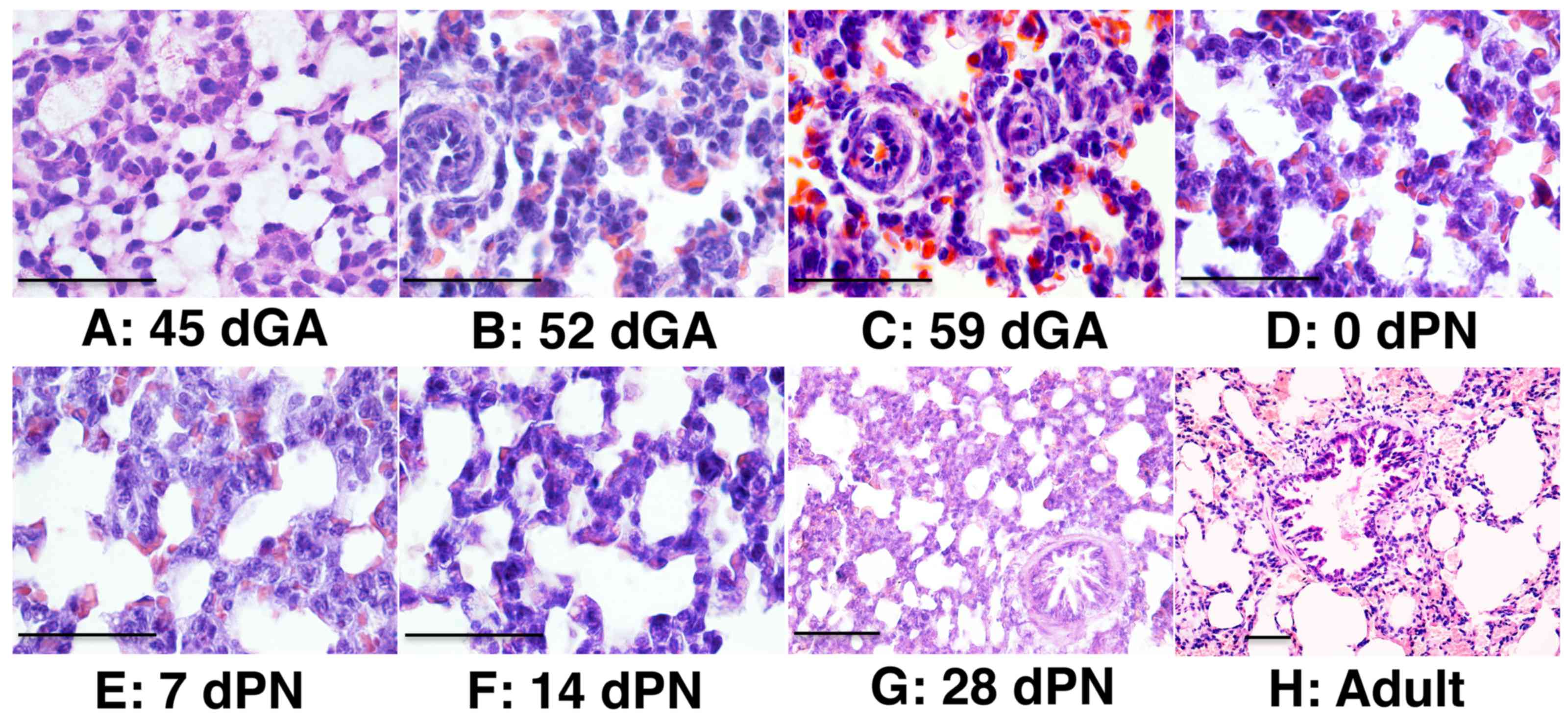
H&E staining revealed that primary alveolar sacs had formed by
45 dGA, and bronchiolar structure was first observed in the distal
lung of 52 dGA embryos (Fig. 2).
Well-structured alveolar epithelia and bronchiolar epithelia were
observed at 52 dGA and onward (Fig.
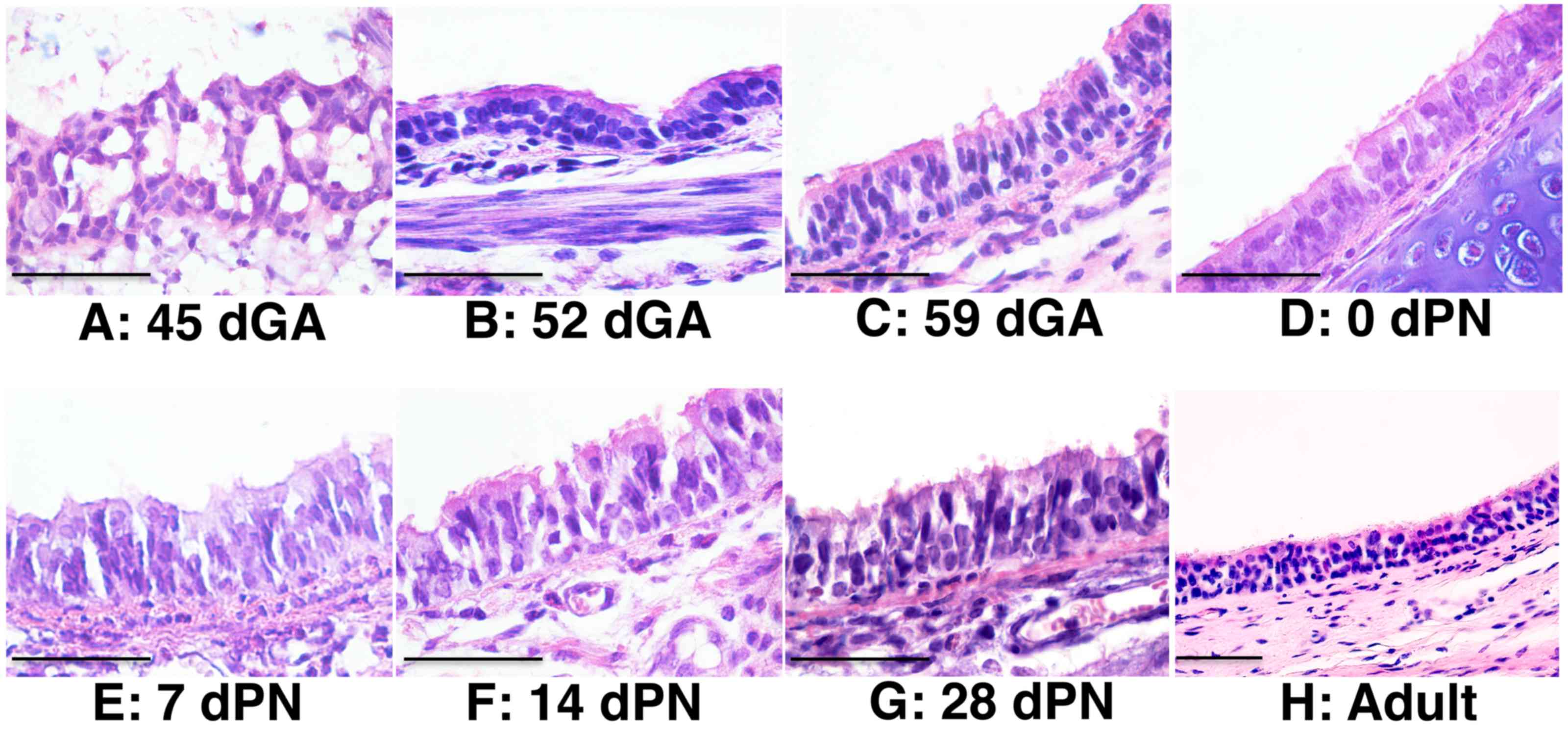
2). Epithelial cells in the trachea of 45 dGA embryos were
poorly differentiated, despite the formation of a primary
epithelial structure at this stage (Fig. 3). Abundant epithelial cells were
observed on the surface of 52 dGA tracheas, but pseudostratified
epithelial layers with ciliated cells were only observed in 59 dGA
tracheas and onward (Fig. 3).
 | Table III.Gross parameters of the guinea pig
lung at different developmental stages. |
Table III.
Gross parameters of the guinea pig
lung at different developmental stages.
| Ages | Body length
(cm) | Body weight
(g) | Lung length
(cm) | Lung weight
(g) | Trachea length
(cm) |
|---|
| 45 dGA |
4.27±0.05 |
4.88±0.10 | 1.33±0.03 | 0.13±0.01 |
0.7±0.00 |
| 52 dGA |
7.70±0.09 |
37.63±3.68 | 3.08±0.13 | 0.78±0.09 | 1.58±0.13 |
| 59 dGA | 10.55±0.12 |
79.97±2.83 | 3.83±0.13 | 1.90±0.06 | 1.85±0.07 |
| 0 dPN | 11.38±0.26 |
96.32±5.01 | 3.70±0.12 | 1.47±0.06 | 1.82±0.05 |
| 7 dPN | 13.03±0.25 | 110.22±3.47 | 3.75±0.18 | 1.28±0.05 | 2.08±0.11 |
| 14 dPN | 15.23±0.87 | 177.58±21.15 | 4.28±0.19 | 1.72±0.21 | 2.23±0.17 |
| 28 dPN | 17.73±0.65 | 239.47±19.07 | 4.77±0.08 | 2.16±0.22 | 2.57±0.03 |
Expression of epithelial cell
type-specific markers in the developing guinea pig lung
The expression of epithelial cell markers (21) in developing guinea pig lungs was
examined using IHC analysis. At 45 dGA, keratin 14 (K14) was
expressed in the basal layer of the tracheal epithelia (Fig. 4Aa) and pro-surfactant protein C
(SPC) of alveolar type II cells was expressed in the lung (Fig. 4Ba), but there was no expression of
β-tubulin IV (T4) of ciliary axonemes in the tracheal ciliated
cells (Fig. 4C) or secretoglobin
family1A member 1 (CCSP) of cube cells in the distal airway
(Fig. 4D) at this gestation age
(21). However, these 4
cell-specific markers were expressed in the lung of 52 dGA embryos
and onward, particularly the CCSP was mainly expressed in
epithelial cells of small airways in distal lung (Fig. 4), indicating that differentiation
of varied epithelial cell types occurs during lung development in
the guinea pig.
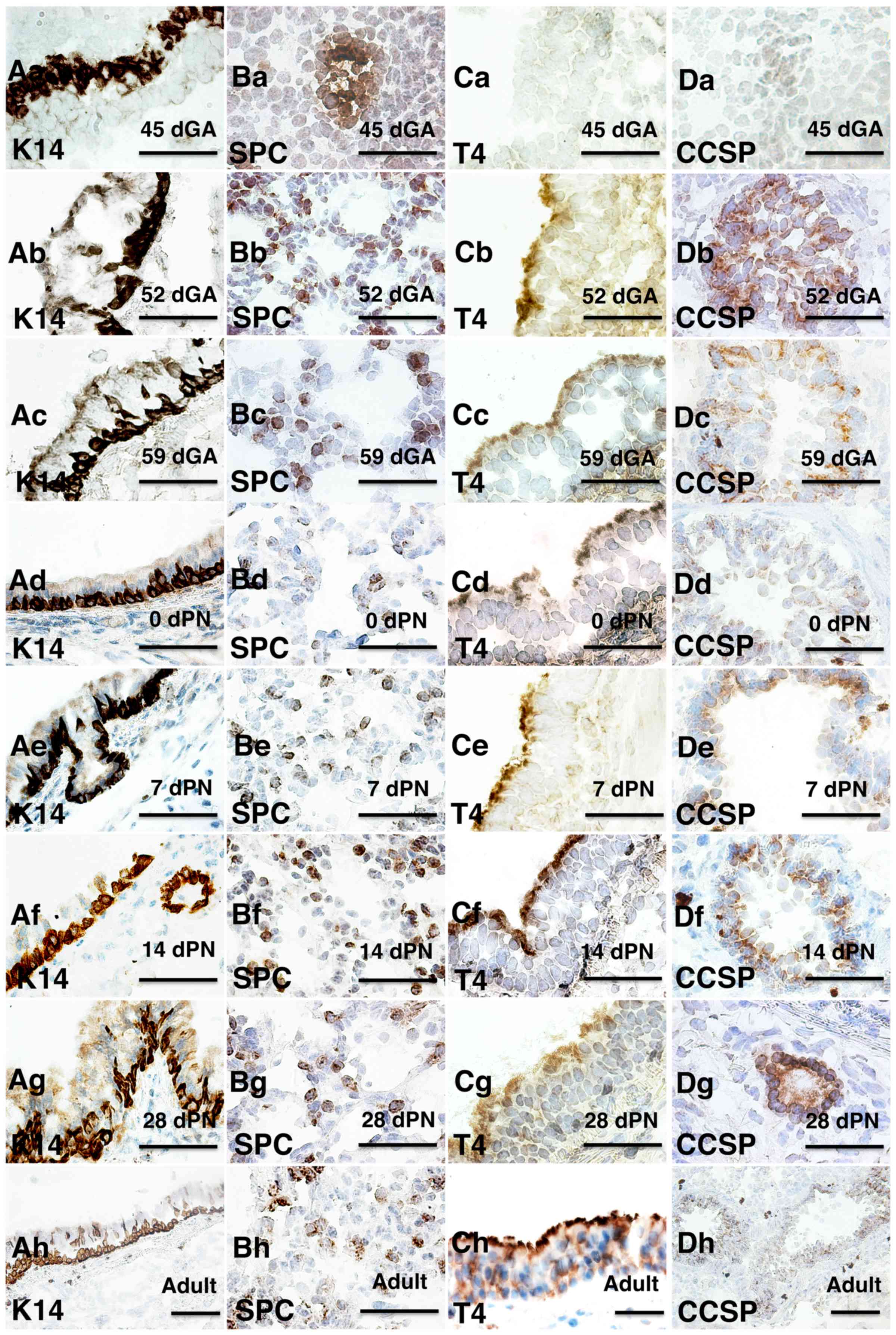 | Figure 4.Immunohistochemical staining of major
epithelial cell markers at different developmental stages in the
guinea pig lung. Epithelial markers and developmental stages are
indicated on the images. (A) K14 staining of tracheal epithelium;
(B) SPC staining of lung tissue; (C) T4 staining of tracheal
epithelium and (D) CCSP staining of lung tissue at developmental
stages of (a) 45 dGA, (b) 52 dGA, (c) 59 dGA, (d) 0 dPN, (e) 7 dPN,
(f) 14 dPN, (g) 28 dPN and (h) adult. Bar=50 µm. dGA, days of
gestation; dPN, days following parturition; K14, keratin 14; T4,
β-tubulin IV; CCSP, secretoglobin family1A member 1; SPC,
pro-surfactant protein C. |
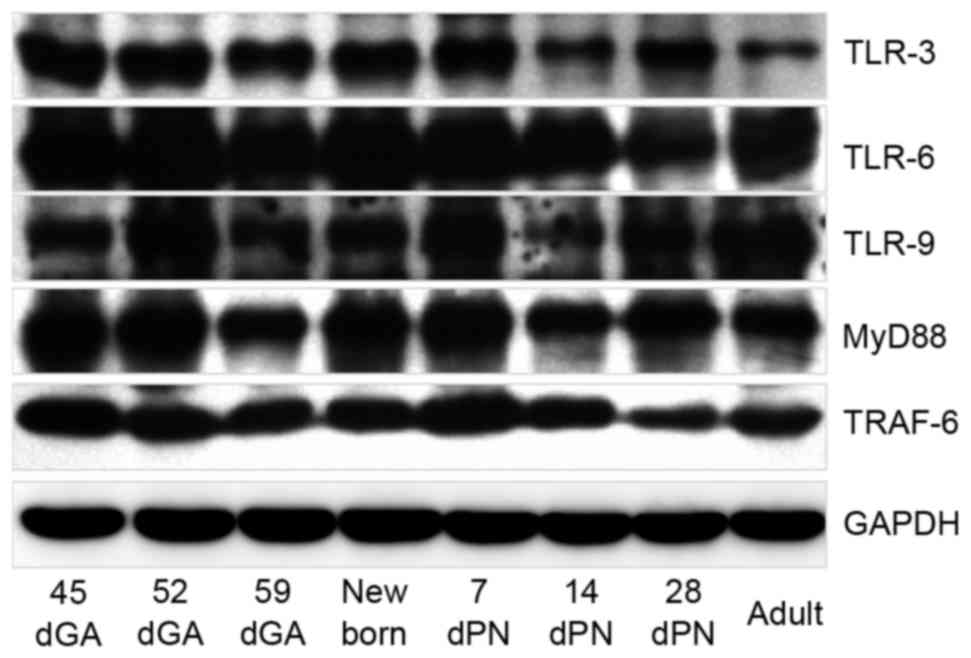
Developmental expression of TLRs in
the lung of guinea pig
In order to evaluate changes of mRNA and protein
expression levels of TLR signaling components during organogenesis
and maturation of the lung in guinea pigs, the relative abundance
of TLRs (−1, −2, −3, −4, −6, −7, −8, −9 and −10), MyD88 and TRAF-6
were investigated by RT-qPCR and western blotting, respectively, in
distal lung tissues at 45, 52 and 59 dGA, 0, 7, 14 and 28 dPN, and
in adult animals. RT-qPCR analysis demonstrated that the mRNA
expression levels of all tested TLR signaling components was
dynamic, with a similar expression pattern, in which transcripts
were more abundant in the 45 dGA and 52 dGA lungs compared with
later stages (Fig. 5A-D). Notably,
TLR-2, −3, −6, −7, −8, −9 and −10 mRNA expression levels were
higher in 45 dGA lungs compared with 52 dGA lungs (Fig. 5A and C), while TLR-1, −4, MyD88 and
TRAF-6 mRNA expression levels were higher in 52 dGA lungs compared
with 45 dGA lungs (Fig. 5A, B and
D). In addition, increased TLR-7 mRNA expression levels were
observed in 7 dPN lungs as compared with TLR-8, 9 and 10 at this
stage (P=0.000; Fig. 5C). The
results of western blotting demonstrated similar expression
patterns to the RT-qPCR analysis for TLR-3, −6, −9, MyD88 and
TRAF-6 proteins, with differential expression in guinea pig lungs
during different developmental stages (Fig. 6). These data imply that TLR
expression in guinea pig lungs is developmentally regulated.
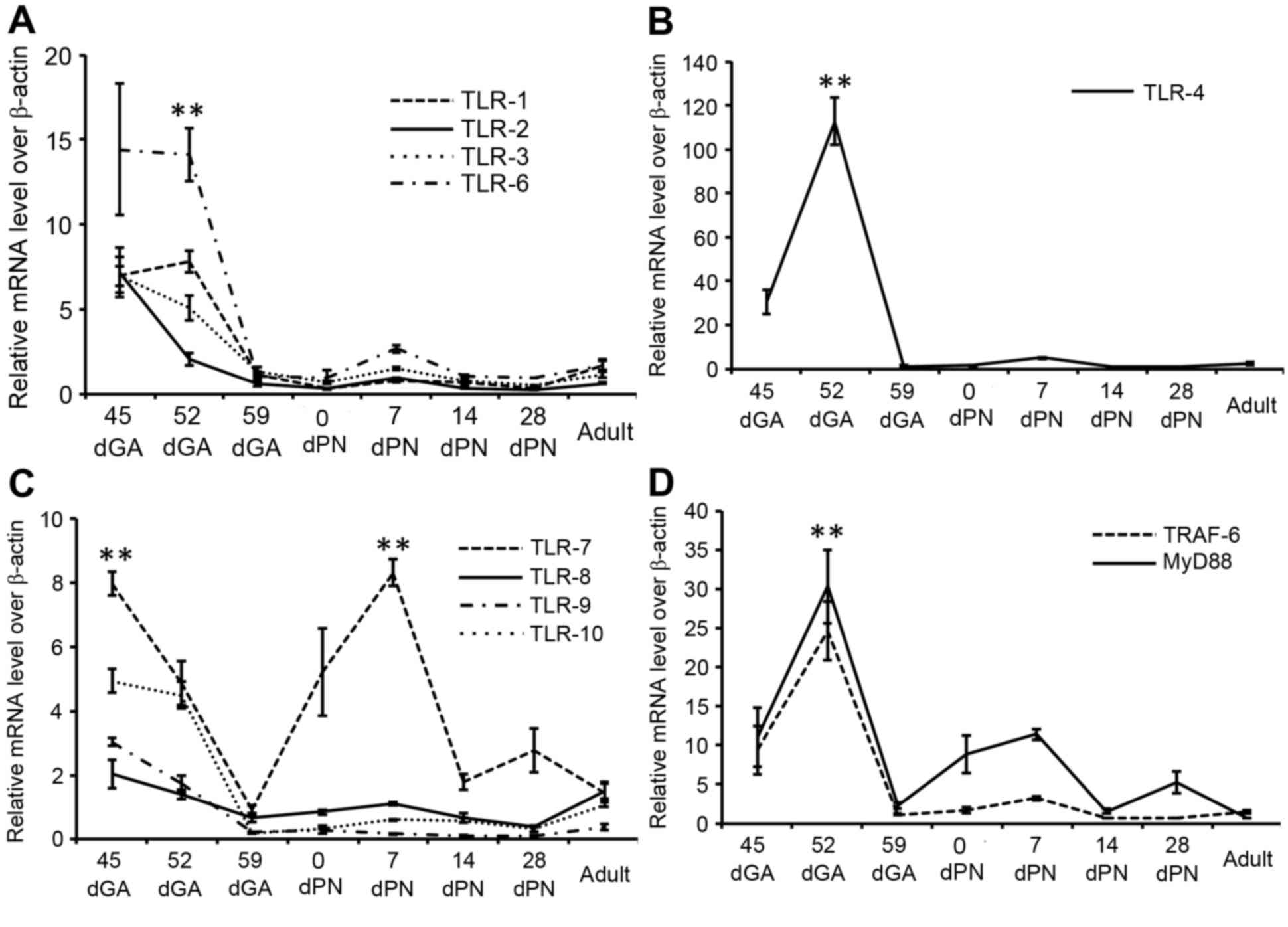 | Figure 5.Differential expression of TLR mRNA in
the guinea pig lung. The relative expression levels of TLR mRNAs in
lung tissues at the indicated gestation and postnatal ages were
determined by reverse transcription-quantitative polymerase chain
reaction assays. (A) Fold-changes of TLR-1, −2, −3 and −6 mRNA
expression levels. (B) Fold-changes of TLR-4 mRNA expression
levels. (C) Fold-changes of TLR-7, −8, −9 and −10 mRNA expression
levels. (D) fold-changes of MyD88 and TRAF-6 mRNA expression
levels. Data are expressed as the mean ± standard deviation of
fold-changes over the internal control (β-actin) from 6 animals for
each time point. **P<0.01: TLR-1 vs. TLR-2, −3 and −6 at 56 dGA
in A; 52 dGA vs. other developmental stages for TLR-4 in B; TLR-7
vs. TLR-8, −9 and −10 at 45 dGA and 7dPN in C; 52 dGA vs. other
developmental stages for MyD88 and TRAF-6 in D. dGA, days of
gestation; dPN, days following parturition, MyD88, myeloid
differentiation factor 88; TRAF-6, tumor necrosis factor receptor
associated factor 6. |
Discussion
In the present study, the morphogenesis and
maturation of the guinea pig lung and the expression of TLR ligands
and TLR signaling adaptors MyD88 and TRAF-6 were investigated from
45 dGA to maturation. Lung development was observed at as early as
45 dGA, along with expression of the basal cell marker K14 and
alveolar type II cell marker SPC, but the cube cell marker CCSP and
ciliated cell marker T4 were only observed in the lungs from 52 dGA
onwards. Notably, the expression of all 9 examined TLRs and the TLR
signaling adaptors MyD88 and TRAF-6 were detected at the
transcriptional level in all lung tissues harvested from 45, 52 and
59 dGA, 0, 7, 14 and 28 dPN, and in adult animals. Transcript
levels of all TLR signaling components formed similar dynamic
expression patterns with gestation age and maturation time
following parturition, with TLRs significantly more abundant in the
lungs of 45 and 52 dGA embryos compared with later stages. These
data imply that TLR expression in guinea pig lungs may be
developmentally regulated.
TLRs are a family of mammalian proteins first
observed in D. melanogaster (5). To date, 11 TLRs have been identified
in humans (5). Different TLR
ligands have been demonstrated to be involved in in the regulation
of both innate and adaptive immune responses, in part through
binding to different specific ligands. TLR-2 forms heterodimers
with TLR-1 and TLR-6 upon binding to different lipopeptide and
lipoprotein structures (22);
TLR-2 and TLR-4 recognize lipids including lipopolysaccharide
(LPS); TLR-3 and TLR-7-9 are activated by nucleic acids derived
from viruses or bacteria, and TLR-5 primarily recognizes the
protein flagellin from flagellated bacteria (23). In particular, TLR-9 recognizes
various types of unmethylated cytosine-guanine repeat
dideoxynucleotides (24). TLR-10,
an orphan receptor with an unidentified ligand, is the most recent
human TLR to be identified, and has been demonstrated to form
heterodimers with TLR-1 and TLR-2 (22,25).
TLR-10 mRNA expression was detected in guinea pig lungs in the
present study.
In addition to immunoregulation, TLRs are involved
in development and homeostasis, sensing endogenously derived
materials during the processes of organogenesis and tissue
generation. For example, hyaluronan (HA) is a breakdown product of
extracellular matrix components, and the recognition of different
forms of HA is essential for the maintenance of homeostasis and
epithelial integrity during tissue regeneration following an injury
and the restoration of normal tissue architecture (26–28).
Several lines of evidence have demonstrated the developmental
involvement of TLRs in mammalian and non-mammalian species during
embryogenesis. For example, Kannaki et al (29) demonstrated differential expression
patterns of TLR mRNAs during chicken embryological development,
suggesting the potential involvement of TLRs in the regulation of
chicken embryo development. This is supported by the initial
discovery of Toll in D. melanogaster, the first member of
the TLR family, as it was initially characterized as a
developmental protein prior to the establishment of its
immunological involvement (8,15).
This is further supported by the discovery of the developmental
involvement of TLR-7 and TLR-9 in the vertebrate brain (16).
The immunological and developmental involvements of
TLRs in embryonic organogenesis have also been reported in
mammalian lungs and other epithelial tissues (17,30).
For example, in a study evaluating the developmental expression of
TLR-2 and TLR-4 in preterm baboon lungs, Awasthi et al
(30) demonstrated that the
expression of these TLRs was significantly lower at 125 and 140 dGA
compared with adult baboons, only reaching comparable levels at 175
dGA. This indicates that that TLR expression in baboon lungs is
developmentally regulated. This developmental expression pattern
was consistent with previous findings in fetal murine lung tissues
(10) and in preterm lamb lung
tissues (11), with TLR expression
increasing with the length of gestation. This expression pattern
may be different to that observed in the mature lung of adult
animals and humans, where the expression of TLRs is dependent on
immune cells including macrophages, dendritic cells and lung
epithelial cells (7,31). In addition, developmentally
regulated expression of TLRs was observed in fetal and neonatal
human and mouse colonic epithelium (17). The results of the present study
were consistent with these previous findings, with the expression
of TLRs also being developmentally regulated in the guinea pig
lung. Altered TLR signaling may lead to compromised structural
development in the lung, as previously demonstrated in mice
(32). An injection of LPS into
the amniotic fluid of 15 dGA mice led to increases in the luminal
volume density and decreases in distal branching of the lungs at 17
and 18 dGA, induced by activation of functional TLR-4 (32). Notably, in the present study, TLR
mRNA expression levels were highest in guinea pig lungs at 45 and
52 dGA, and dramatically decreased as lung organogenesis and
maturation progressed. Adult levels were reached by 59 dGA. The
implications of such a dramatic change in TLR expression in the
guinea pig lung warrants further investigation.
To conclude, morphogenesis and developmental
expression of TLR signaling components in the guinea pig lung were
examined in the present study. The dynamic expression of TLRs
during lung development and maturation in the guinea pig was
demonstrated, further confirming the developmental involvement of
TLRs in lung morphogenesis in this animal model. These data lay a
foundation upon which further understanding of the immunoregulatory
mechanisms in the guinea pig lung may be built.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31260615
and 31472191).
References
|
1
|
D'Erchia AM, Gissi C, Pesole G, Saccone C
and Arnason U: The guinea-pig is not a rodent. Nature. 381:597–600.
1996. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Graur D, Hide WA and Li WH: Is the
guinea-pig a rodent? Nature. 351:649–652. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Padilla-Carlin DJ, McMurray DN and Hickey
AJ: The guinea pig as a model of infectious diseases. Comp Med.
58:324–340. 2008.PubMed/NCBI
|
|
4
|
Kashino SS, Napolitano DR, Skobe Z and
Campos-Neto A: Guinea pig model of Mycobacterium tuberculosis
latent/dormant infection. Microbes Infect. 10:1469–1476. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jimenez-Dalmaroni MJ, Gerswhin ME and
Adamopoulos IE: The critical role of toll-like receptors-From
microbial recognition to autoimmunity: A comprehensive review.
Autoimmun Rev. 15:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kovach MA and Standiford TJ: Toll like
receptors in diseases of the lung. Int Immunopharmacol.
11:1399–1406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hashimoto C, Hudson KL and Anderson KV:
The Toll gene of Drosophila, required for dorsal-ventral
embryonic polarity, appears to encode a transmembrane protein.
Cell. 52:269–279. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Nardo D: Toll-like receptors:
Activation, signalling and transcriptional modulation. Cytokine.
74:181–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harju K, Glumoff V and Hallman M: Ontogeny
of Toll-like receptors Tlr2 and Tlr4 in mice. Pediatr Res.
49:81–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meyerholz DK, Kawashima K, Gallup JM,
Grubor B and Ackermann MR: Expression of select immune genes
(surfactant proteins A and D, sheep beta defensin 1, and toll-like
receptor 4) by respiratory epithelia is developmentally regulated
in the preterm neonatal lamb. Dev Comp Immunol. 30:1060–1069. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mestas J and Hughes CC: Of mice and not
men: Differences between mouse and human immunology. J Immunol.
172:2731–2738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schneberger D, Caldwell S, Suri SS and
Singh B: Expression of toll-like receptor 9 in horse lungs. Anat
Rec (Hoboken). 292:1068–1077. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh Suri S, Janardhan KS, Parbhakar O,
Caldwell S, Appleyard G and Singh B: Expression of toll-like
receptor 4 and 2 in horse lungs. Vet Res. 37:541–551. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kambris Z, Hoffmann JA, Imler JL and
Capovilla M: Tissue and stage-specific expression of the Tolls in
Drosophila embryos. Gene Expr Patterns. 2:311–317. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaul D, Habbel P, Derkow K, Krüger C,
Franzoni E, Wulczyn FG, Bereswill S, Nitsch R, Schott E, Veh R, et
al: Expression of Toll-like receptors in the developing brain. PLoS
One. 7:e377672012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng D, Zhu W, Shi HN, Lu L, Wijendran V,
Xu W and Walker WA: Toll-like receptor-4 in human and mouse colonic
epithelium is developmentally regulated: A possible role in
necrotizing enterocolitis. Pediatr Res. 77:416–424. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schroeder A, Mueller O, Stocker S,
Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M
and Ragg T: The RIN: An RNA integrity number for assigning
integrity values to RNA measurements. BMC Mol Biol. 7:32006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Wang J, He HY, Ma LJ, Zeng J, Deng
GC, Liu X, Engelhardt JF and Wang Y: Immunohistochemical
demonstration of airway epithelial cell markers of guinea pig.
Tissue Cell. 43:283–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ospelt C and Gay S: TLRs and chronic
inflammation. Int J Biochem Cell Biol. 42:495–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeda K and Akira S: Toll receptors and
pathogen resistance. Cell Microbiol. 5:143–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bauer S: Toll-like receptor 9 processing:
The key event in Toll-like receptor 9 activation? Immunol Lett.
149:85–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasan U, Chaffois C, Gaillard C, Saulnier
V, Merck E, Tancredi S, Guiet C, Brière F, Vlach J, Lebecque S, et
al: Human TLR10 is a functional receptor, expressed by B cells and
plasmacytoid dendritic cells, which activates gene transcription
through MyD88. J Immunol. 174:2942–2950. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang D, Liang J, Fan J, Yu S, Chen S, Luo
Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, et al:
Regulation of lung injury and repair by Toll-like receptors and
hyaluronan. Nature Med. 11:1173–1179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Neill LA: TLRs play good cop, bad cop in
the lung. Nature Med. 11:1161–1162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sabroe I, Parker LC, Dower SK and Whyte
MK: The role of TLR activation in inflammation. J Pathol.
214:126–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kannaki TR, Reddy MR, Verma PC and
Shanmugam M: Differential Toll-like receptor (TLR) mRNA expression
patterns during chicken embryological development. Animal
Biotechnol. 26:130–135. 2015. View Article : Google Scholar
|
|
30
|
Awasthi S, Cropper J and Brown KM:
Developmental expression of Toll-like receptors-2 and −4 in preterm
baboon lung. Dev Comp Immunol. 32:1088–1098. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Armstrong L, Medford AR, Uppington KM,
Robertson J, Witherden IR, Tetley TD and Millar AB: Expression of
functional toll-like receptor-2 and −4 on alveolar epithelial
cells. Am J Respir Cell Mol Biol. 31:241–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prince LS, Dieperink HI, Okoh VO,
Fierro-Perez GA and Lallone RL: Toll-like receptor signaling
inhibits structural development of the distal fetal mouse lung. Dev
Dyn. 233:553–561. 2005. View Article : Google Scholar : PubMed/NCBI
|