Introduction
Osteoarthritis (OA) is a degenerative and
inflammatory disease of joints that affects an estimated 10% of men
and 18% of women >60 years of age. The condition causes severe
symptoms, including impaired mobility, joint deformities, and
disability (1). Pathologically, OA
is characterized by cartilage degeneration and osteophyte formation
at the affected joints (2).
Currently available treatment consists of pain management and joint
replacement in patients with end-stage disease; however, therapies
to control the progression of OA are in their early stages. In
addition to the limited lifespan of prostheses, arthroplasty for
osteoarthritic joints may be associated with adverse outcomes
(3).
The causation of OA is thought to be multifactorial,
with factors including age, body weight, gender, bone density,
trauma and genetic susceptibility hypothesized to be involved
(4). The pathogenesis of OA is not
completely understood. Emerging evidence suggests the involvement
of immunological factors in the development and progression of OA.
Shen et al (5) demonstrated
that CD4+T cells may serve a role in inducing inflammation in the
early stages of OA, as well as being instrumental in causing
inflammatory damage to the articular cartilage in the latter
stages. According to Da et al (6), approximately half of all cases of OA
manifest mild-to-moderate B lymphocytic infiltration in the
synovial tissues, and the degree of B cell infiltration is directly
correlated with the severity of local inflammation. Anti-cyclic
citrullinated peptide (anti-CCP) antibodies have also been shown to
be involved in the autoimmune processes of early-stage knee OA
(7).
CD4+T cells, particularly T follicular helper (TFH)
cells, are known to regulate B cell activation and functional
differentiation (8). Although the
identification of TFH cells remains controversial, a previous study
identified that CXCR5+CD4+ T cells shared the functional properties
of TFH cells. Therefore, CXCR5+CD4+ T cells are considered to be
TFH cells (9). Chemokine (C-X-C
motif) receptor 5 (CXCR5), inducible costimulator (ICOS),
programmed death (PD)-1, CD40 ligand, and the transcription factor,
Bcl-6, are known to be expressed on the surface of TFH cells, and
mediate the TFH cell-mediated activation of B cells within the
lymphoid germinal centers (10,11).
Furthermore, interleukin-21 (IL-21), secreted by TFH cells, is
known to modulate B cell differentiation and proliferation. In a
previous study, increased levels of anti-CCP antibodies were
demonstrated to be associated with a high frequency of TFH cells in
patients with new-onset rheumatoid arthritis (RA) (12). Dysfunction of TFH cells and IL-21
is also known to be involved in the pathogenesis of systemic lupus
erthymatosus and ankylosing spondylitis (13,14).
All these conditions are characterized essentially as chronic
inflammatory joint diseases. However, the role of TFH cells in the
pathogenesis of OA has yet to be fully elucidated.
The present study examined the frequency of
peripheral blood TFH cells and the concentration of serum IL-21 in
40 patients newly diagnosed with OA and 13 healthy controls. The
study also analyzed the frequency of different TFH cell subsets in
the peripheral blood of patients with different grades of OA, and
assessed the potential association with clinical characteristics.
The present study was aimed at assessing the immunopathological
roles and correlates of TFH cells in OA.
Materials and methods
Patients and controls
A total of 40 newly diagnosed OA patients were
enrolled at the inpatient service of the First Hospital of Jilin
University (Changchun, China) and 13 gender, age, and
ethnicity-matched healthy controls were also recruited. The
diagnosis of OA was made according to the clinical and radiographic
criteria of the American College of Rheumatology (15). Knee radiographs were evaluated
according to the Kellgren and Lawrence (KL) classification criteria
(16). OA patients were defined as
having radiographic knee OA of KL grade ≥2 in at least one knee,
whereas controls were having KL grades of 0. None of the patients
had been administered steroids, nonsteroidal anti-inflammatory
drugs or other immunosuppressants one month prior to the blood
sample collection. The severity of the disease in individual
patients was measured using the Western Ontario and McMaster
Universities Osteoarthritis Index (WOMAC) using a questionnaire
containing three sections: i) Pain assessment (five criteria); ii)
stiffness assessment (two criteria); and iii) functional assessment
(seventeen criteria). Patients were rated against each criterion on
a 5-point Likert Scale (0, none; 1, slight; 2, moderate; 3, severe;
4, extreme) (17). Patients with
RA, traumatic arthritis, multiple sclerosis, type 1 diabetes,
immune deficiency, chronic inflammatory diseases, and those with
recent infection were excluded from the present study. The
demographic and clinical characteristics of the study population
are summarized in Table I. Written
informed consent was obtained from all subjects. The study protocol
was approved by the Ethics committee at the First Hospital of Jilin
University (Changchun, China).
 | Table I.Demographic and clinical
characteristics according to study group. |
Table I.
Demographic and clinical
characteristics according to study group.
|
| Group |
|---|
|
|
|
|---|
| Variable | Healthy controls | OA |
|---|
| Number of subjects
(n) | 13 | 40 |
| Age (years) | 61
(55–65) | 65
(53–73) |
| Gender female, n
(%) | 10 (76%) | 29 (72%) |
| KL grade |
|
|
| II
(%) | NA | 12 (30%) |
| III
(%) | NA | 15
(37.5%) |
| IV
(%) | NA | 13
(32.5%) |
| WBC
(109/l) | 5.88
(4.2–8.9) | 5.76 (4.5–9.2) |
| ESR (mm/h) | 7
(2–14) | 12 (3–22) |
| CRP (mg/dl) | 1.5
(1.3–2.7) | 2.31
(0.79–5.14)a |
| Fibrae sanguis
(mg/µl) | 251
(150–378) | 265 (211–496) |
| WOMAC | NA | 52
(37–69) |
| Pain | NA | 12 (8–15) |
|
Stiffness | NA | 4 (2–6) |
| Physical
function | NA | 40
(30–48) |
Laboratory examinations
Fasting venous blood samples (10 ml) were obtained
from individual subjects, and their sera were prepared by
density-gradient centrifugation using Ficoll-Paque Plus at 468 × g
for 15 min at 37°C (GE Healthcare Life Sciences, Uppsala, Sweden).
The number of white blood cells (WBCs), erythrocyte sedimentation
rate (ESR), and the concentration of serum C-reactive protein (CRP)
were measured using Siemens special protein analysis instrument
(Siemens AG, Munich, Germany).
Peripheral blood mononuclear cell
(PBMC) stimulation
PBMCs were isolated by density-gradient
centrifugation using Ficoll-Paque Plus at 800 × g for 30 min at
37°C (GE Healthcare Life Sciences). PBMCs (4×106 cells/ml) were
cultured in RPMI-1640 medium with 10% fetal calf serum (Hyclone™;
GE Healthcare Life Sciences Waltham, MA, USA) in 24-well U-bottom
tissue-culture plates (Corning Costar Inc., Corning, NY, USA).
Cells were stimulated with or without 50 ng/ml phorbol myristate
acetate and 2 g/ml ionomycin (Sigma-Aldrich, St. Louis, MO, USA) at
37°C in a humidified incubator containing 5% CO2 for 1
h. Cells were cultured with Brefeldin A (10 g/ml; GolgiStop™; BD
Biosciences, San Jose, CA, USA) for 5 h, and subjected to
intraplasmic staining and flow cytometric analyses.
Flow cytometry
Human PBMCs (5×105 cells/tube) were stained with
PerCP/Cy5.5 anti-CXCR5 (cat. no. 562781), fluorescein
isothiocyanate (FITC) anti-CD4 (cat. no. 555346), phycoeryrthrin
(PE) anti-CD278 (cat. no. 557802), and Brilliant Violet 421 (BV421)
anti-CD279 (cat. no. 562516) antibodies (BD Pharmingen, San Diego,
CA, USA) at room temperature in the dark for 30 min. Control
staining was performed using FITC anti-IgG1 (cat. no. 556649), PE
anti-IgG1 (cat. no. 551436), PerCP/Cy5.5 anti-IgG1 (cat. no.
550795), and BV421 anti-IgG1 (cat. no. 562438) antibodies (BD
Pharmingen). Cell gating was set to isolate CD4+ cells. The number
of CXCR5+CD4+ (TFH) cells per sample was analyzed using FlowJo
software version 7.6.2 (TreeStar, Ashland, OR, USA) (18).
Stimulated PBMCs were harvested and stained
simultaneously with PerCP/Cy5.5 anti-CXCR5 and FITC anti-CD4
antibodies at room temperature in the dark for 30 min; the
antibodies were diluted with TF Diluent Buffer (1:100; cat. no.
51-9008101; BD Pharmingen). Subsequently, cells were fixed,
permeabilized, and stained with Alexa Fluor 647 anti-IL-21 antibody
(BD Pharmingen). Percentages of IL-21+TFH cells were determined by
flow cytometric analysis.
Cytometric bead array (CBA) analysis
of serum cytokines
The concentrations of serum cytokines [IL-21, IL-4,
IL-17A and interferon-γ (INF-γ)] were determined using a CBA kit,
according to the manufacturer's protocol (CBATM; BD Biosciences).
Individual samples were quantified in duplicate on a
fluorescence-activated cell sorting (FACS) Calibur cytometer (BD
Biosciences), and the data were acquired using the CellQuestPro
software, and subsequently analyzed using the CBA software (BD
Biosciences) (19).
Statistical analysis
Data are expressed as the median and range, or as
individual values. Inter-group differences were analyzed by the
Mann-Whitney U nonparametric test with IBM SPSS software, version
19.0 (IBM SPSS, Armonk, NY, USA). The association between variables
was evaluated using the Pearson rank correlation test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The frequency of TFH cells in the blood samples
obtained from 40 newly diagnosed OA patients and 13 HCs was
assessed. No significant intergroup differences were observed in
the distribution of TFH cells across age- and gender-matched
subgroups.
Significantly higher levels of CRP were observed in
OA patients compared with those in the HCs, and considerable
variability in WOMAC values was observed among OA patients
(Table I).
High frequency of peripheral blood TFH
cells in patients with OA
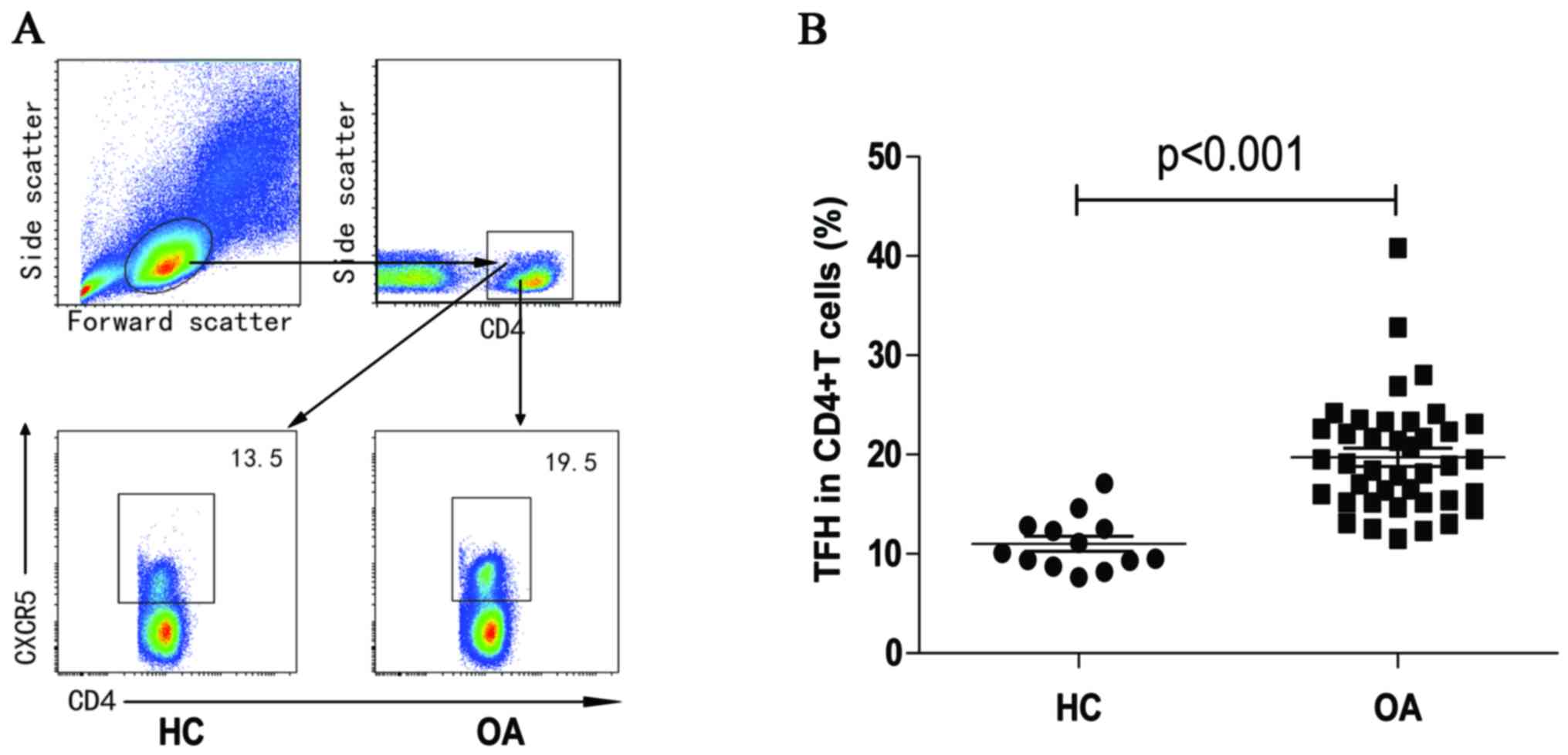
The frequency of peripheral blood CD4+ cells was
assessed using flow cytometry (Fig.
1A). No significant inter-group differences were observed in
the frequency of CD4+T cells (data not shown). Percentages of
CXCR5+CD4+TFH cells in OA patients were significantly higher than
those in the HC group (P<0.001, Fig. 1B).
Altered expression of TFH cell subsets
in OA patients
Flow cytometry was also performed for quantitation
of subsets of TFH cells (Fig. 1A).
Significantly higher percentages of PD-1+CXCR5+CD4+,
ICOS+CXCR5+CD4+ and IL-21+CXCR5+CD4+ T cells were observed in OA
patients compared with those in HCs (P<0.001, P<0.001, and
P<0.001, respectively; Fig.
2B-D). However, no significant difference was observed in the
frequency of PD-1+ICOS+CXCR5+CD4+ T cells between the two groups
(data not shown).
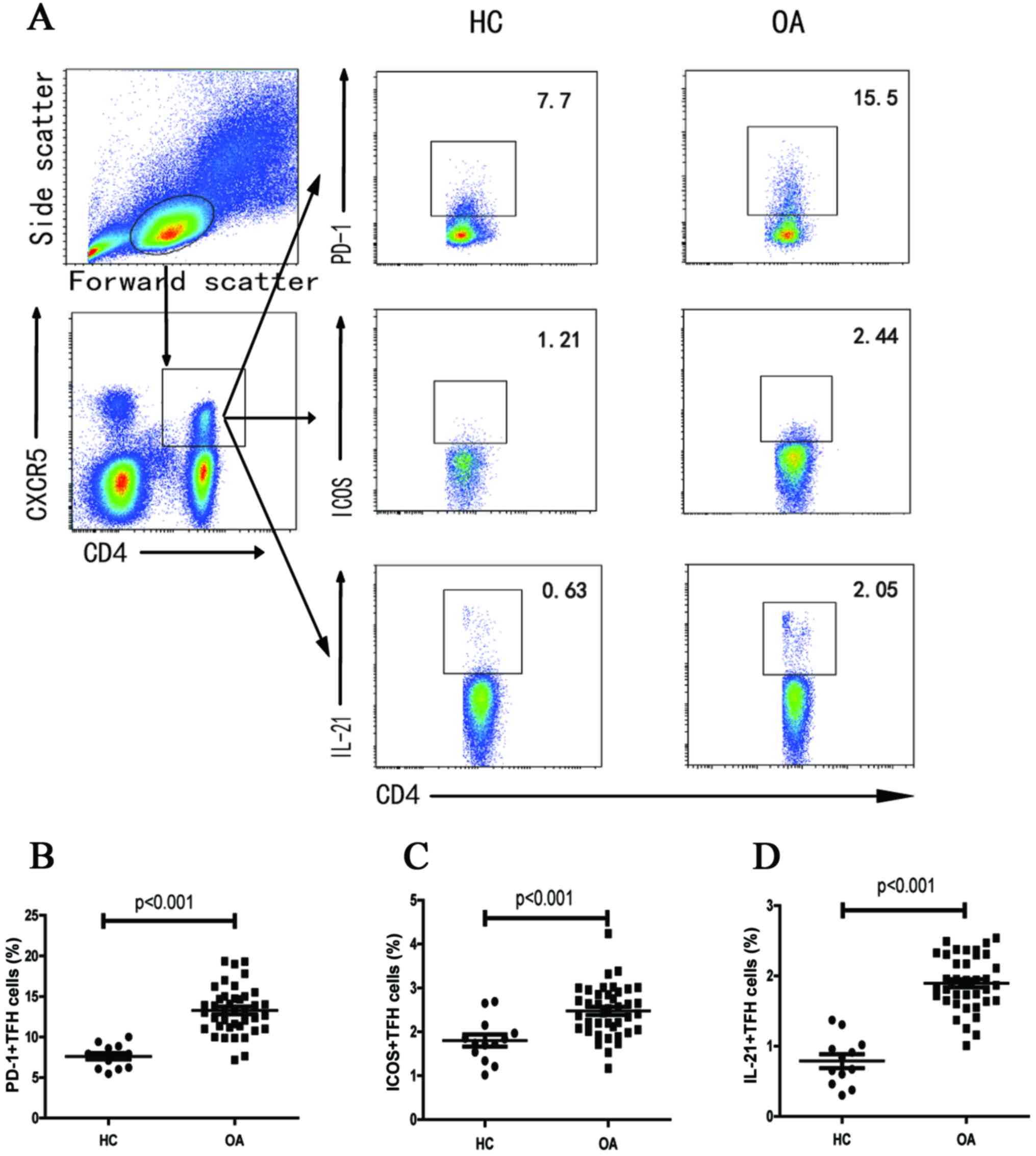 | Figure 2.Flow cytometry for quantitation of
subsets of TFH cells. Fluorescence-activated cell sorting analysis
of the numbers of different subsets of TFH cells in individual
subjects. Peripheral blood mononuclear cells were isolated from
individual subjects and were stained in duplicate with anti-CD4,
anti-CXCR5, anti-ICOS, anti-PD-1 and intracellular anti-IL-21 or
isotype-matched IgG antibodies, respectively. The cells were
characterized using flow cytometry with gating, initially on living
lymphocytes, and then on CD4+CXCR5+TFH cells. Subsequently, the
frequency of ICOS+, PD-1+ and IL-21+TFH in total TFH was analyzed,
and a minimum of 30,000 events were analyzed for each sample. Data
are expressed as the mean values of individual participants from
two separate experiments. (A) Flow cytometry analysis. (B-D) The
numbers of (B) CD4+CXCR5+PD-1+, (C) CD4+CXCR5+ICOS+ and (D)
CD4+CXCR5+IL-21+TFH cells. The horizontal lines indicate the median
values for each group. OA: All OA patients (n=40), HC: healthy
control group (n=13). TFH, follicular helper T; IL-21,
interleukin-21; OA, osteoarthritis; (ICOS)+, inducible
costimulator; (PD-1), programmed death 1; CXCR5, chemokine (C-X-C
motif) receptor 5. |
Frequency of different subsets of TFH
cell in different grades of OA
To assess the potential association of different TFH
cell subsets with progression of OA, TFH cell subsets were analyzed
by different KL grades of OA and a higher frequency of TFH cells,
and PD-1+ TFH cells in stage III OA were compared with those in
grade II patients (P=0.03 and P=0.002, respectively; Fig. 3A and B). In addition, no
significant difference was identified in expression of the TFH
cells and PD-1+ TFH cells between patients with advanced grade (III
and IV) OA (P=0.07 and P=0.52, respectively; Fig. 3A and B). In addition, IL-21+TFH
cells were significantly higher in patients with stage IV disease
compared with that in patients with stage III (P=0.005; Fig. 3C), in which the percentage of
IL-21+ TFH cells was also significantly higher compared with that
in stage II disease (P=0.004; Fig.
3C). Furthermore, the frequency of ICOS+TFH cells associated
with different grades of OA were significantly higher compared with
that in HCs (P=0.01; Fig. 3D).
However, no significant difference was observed in the frequency of
ICOS+TFH cells between grades II and III, and between grades III
and IV OA patients. (P=0.96 and P=0.32, respectively; Fig. 3D).
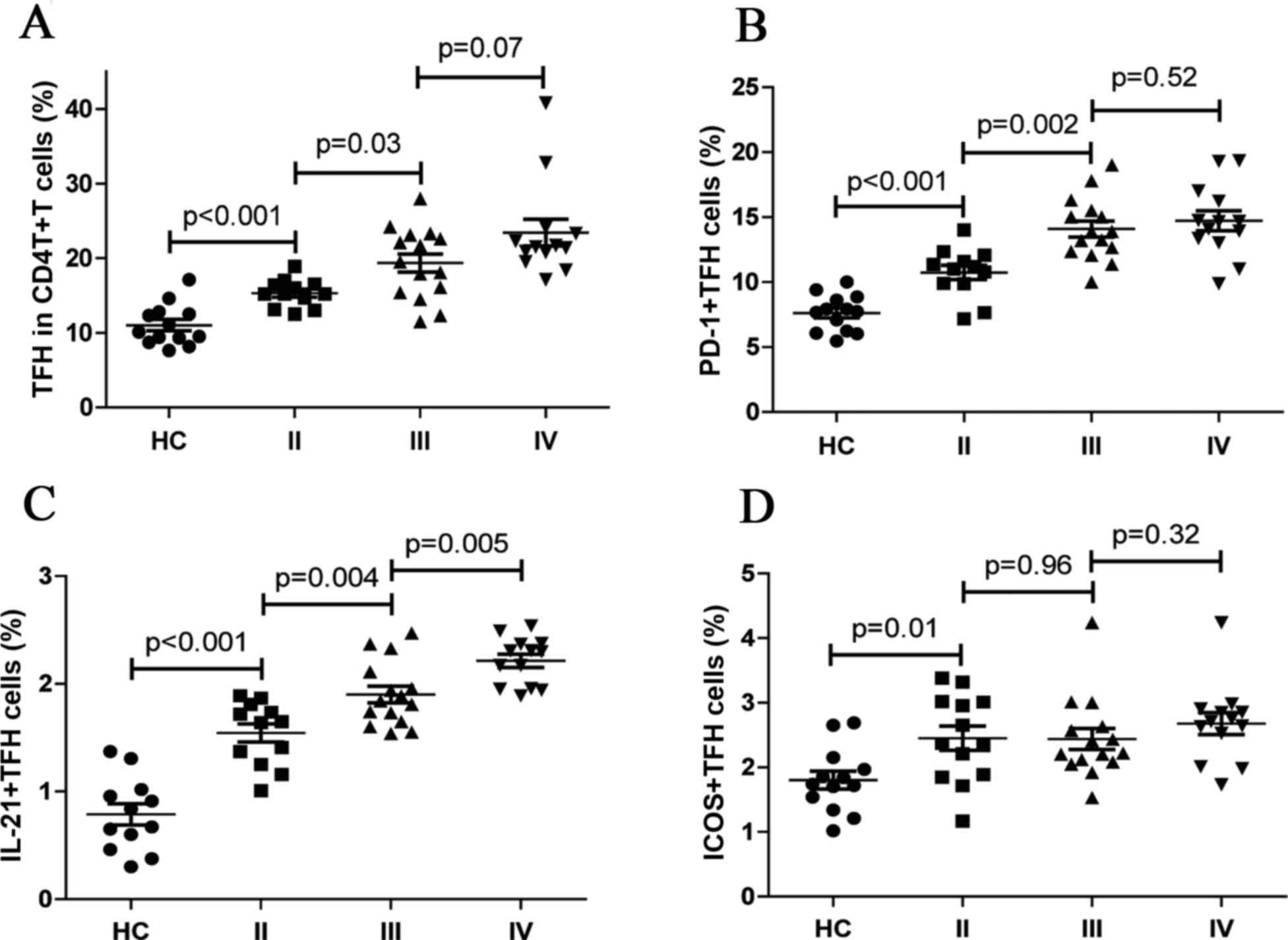 | Figure 3.Quantification of peripheral blood TFH
cell subsets disaggregated by OA clinical grade. The frequency of
CD4+CXCR5+, PD-1+CD4+CXCR5+ IL-21+CD4+CXCR5+TFH and ICOS+CD4+CXCR5+
cells for stages II–IV of OA was analyzed. Knee radiographs were
evaluated according to the KL classification criteria. Data are
expressed as the mean values of individual participants from two
separate experiments. (A-D) The frequency of (A) CD4+CXCR5+, (B)
PD-1+CD4+CXCR5+, (C) IL-21+CD4+CXCR5+TFH and (D) ICOS+CD4+CXCR5+
cells in stage II–IV of OA. The horizontal lines indicate the
median values for each group. OA patients with stage II (n=12), OA
patients with stage III (n=15), OA patients with stage IV (n=13),
and HC: healthy control group (n=13). OA, osteoarthritis; KL,
Kellgren and Lawrence; IL-21, interleukin-21; PD-1, programmed
death 1; TFH, follicular helper T. |
Variability in serum inflammatory
cytokine levels in OA
Serum levels of the inflammatory cytokines, IL-21,
IL-4, IL-17A, and IFN-γ were measured by CBA (Fig. 4). No significant differences in the
levels of serum IL-4 were observed between OA patients and HCs
(P=0.07, Fig. 4A). Furthermore,
the concentrations of serum IL-21 (P<0.001; Fig. 4B), IFN-γ (P<0.001; Fig. 4C) and IL-17A (P<0.001; Fig. 4D) in OA patients were significantly
higher than that in the HCs. Thus, increased levels of serum IL-21,
IL-17A, and IFN-γ may serve a crucial role in the development of
OA.
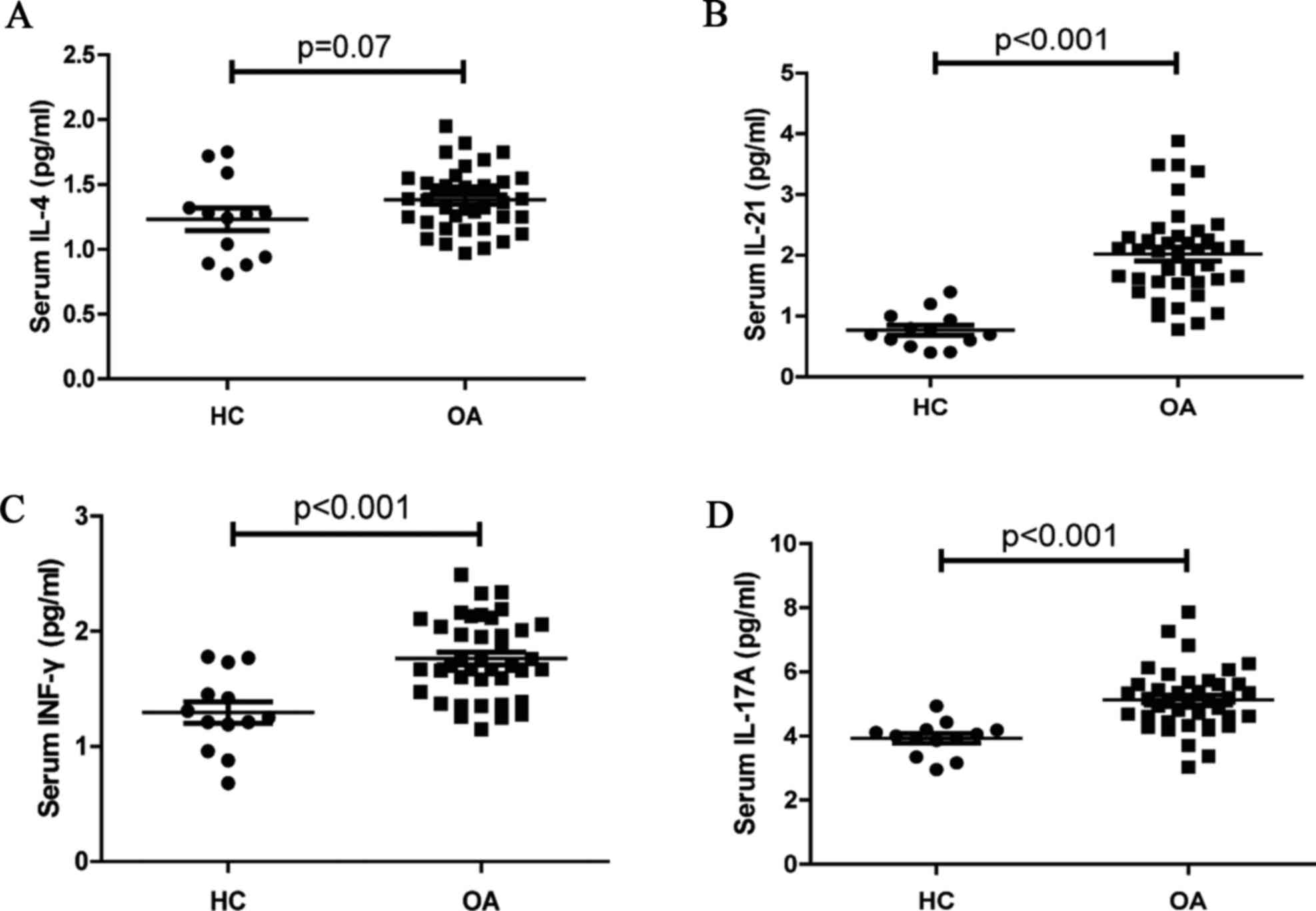 | Figure 4.Analysis of serum cytokines. The
concentrations of serum IL-21, IL-4, IFN-γ, and IL-17A in the HC
and OA patients were determined by CBA. Data are expressed as the
mean levels of serum (A) IL-4, (B) IL-21, (C) IFN-γ, and (D) IL-17A
in the HC and OA patients. HC, healthy controls (n=13); OA,
Osteoarthritis (n=40). IL-21, interleukin-21; HC, healthy controls;
OA, osteoarthritis; CBA, cytometric bead array; IFN-γ,
interferon-γ. |
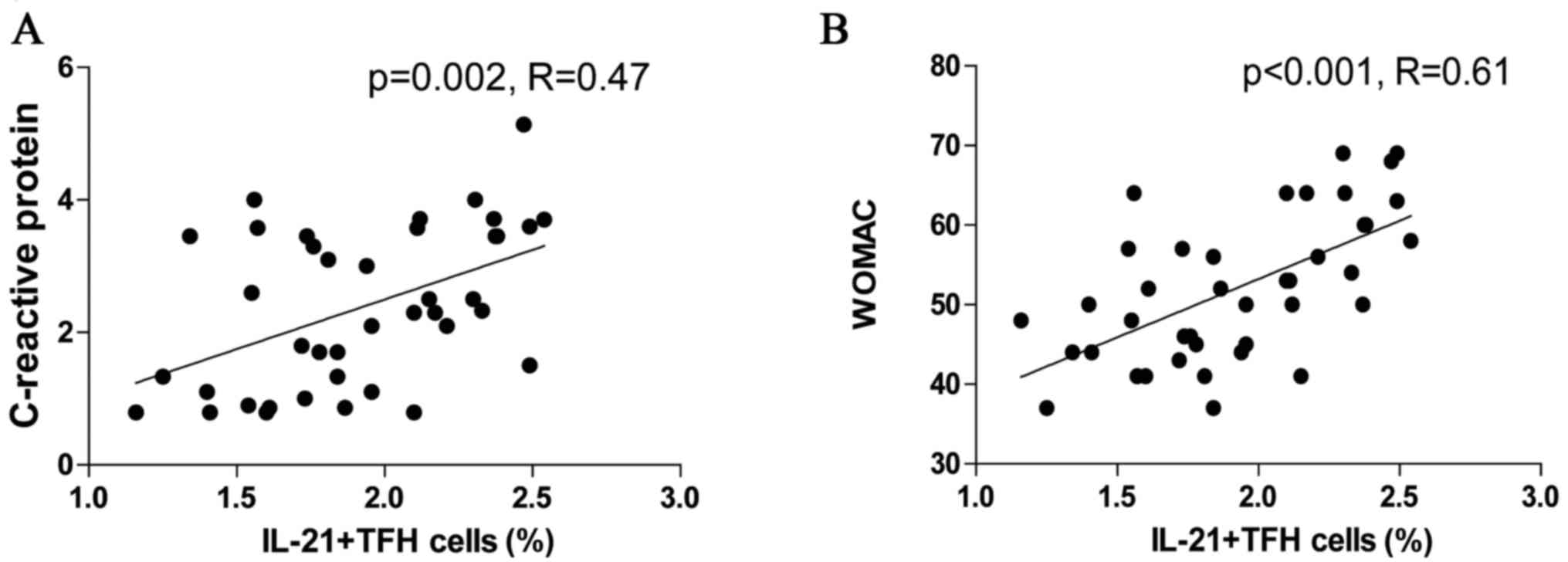
Correlation of peripheral blood
IL-21+TFH cells with CRP level and disease severity
The potential association of the frequency of TFH
cell subsets with clinical parameters and disease severity in OA
patients was assessed using Pearson's correlation analysis. The
percentage of IL-21+TFH cells correlated with CRP levels in OA
patients (R=0.47, P=0.002; Fig.
5A); however, no significant correlation between TFH cells and
the other clinical parameters was observed. The frequency of
peripheral blood IL-21+TFH cells also demonstrated a positive
correlation with WOMAC of OA patients (R=0.61, P<0.001; Fig. 5B). IL-21+TFH cells may be involved
in the inflammatory state of OA, as they correlated with the
symptoms and functionality score of OA patients.
Discussion
Although the pathophysiology of OA is poorly
understood, immunological factors are widely acknowledged as
serving an important role in the pathogenesis of OA. In the present
study, the frequency of different subsets of circulating TFH cells
was examined, and their association with the clinical
characteristics of OA patients was evaluated. A significantly
higher frequency of peripheral blood CXCR5+CD4+TFH cells was
identified in patients with OA compared with that in HC. Previous
studies have implicated CD4+T cells and B cells in the pathogenesis
of OA (5–7). An imbalance of the immune response is
known to be involved in the development of OA. TFH cells serve an
important role in B cell differentiation, antibody production, and
humoral immunity (8). Based on the
findings of the present study, TFH cells appear to contribute to
the development of an inflammatory environment that is
characteristic of OA.
ICOS and PD-1 molecules are known to be expressed on
the TFH cell surface (10,11). Furthermore, ICOS+TFH cells are
known to have a positive regulatory effect on humoral responses,
whereas PD-1+TFH cells serve as negative regulators of TFH cell
activity (20,21). The frequency of the different
subsets of circulating TFH cells was analyzed, and significantly
higher percentages of ICOS+CXCR5+CD4+, PD-1+CXCR5+CD4+ and
IL-21+CXCR5+CD4+T cells were identified in OA patients compared
with those in HCs. However, no significant difference in the number
of PD-1+ICOS+CXCR5+CD4+T cells was observed between the two study
groups (data not shown). These findings appear to implicate
activated TFH cell subsets in the development of OA. The increased
frequencies of both PD-1+ and ICOS+TFH cells in the OA patients
appear to be paradoxical, as these molecules have
counter-regulatory effects on TFH cells. ICOS-mediated
co-stimulation is crucial for TFH cell differentiation. PD-1+TFH
cells may serve as negative regulators for the number and
functionality of TFH cells, and to minimize collateral damage
effected by the immune response. Notably, a similar phenomenon is
also known to be associated with RA (22).
OA is characterized by cartilage and disc
degeneration, and osteophyte formation at the joints (2). The morbid state in OA patients can be
approximately categorized into different grades based on
radiographic results according to the KL classification criteria
(16). To characterize the altered
dynamics of TFH cells in different grades of OA, the association of
different subsets of TFH cells with the clinical grade of OA was
studied. The stage III–IV OA patients were identified to have
higher frequencies of TFH cells and PD-1+TFH cells compared with
those in stage II patients, whereas no significant differences with
respect to TFH cells and PD-1+TFH cells between stage III and IV
patients were identified. Furthermore, no significant differences
in the expression of ICOS+TFH cells were observed between stage II,
III and IV OA patients. Thus, it is possible that the high
frequency of TFH cells may be a result of positive regulation
mediated through ICOS in early-stage OA, and the increased
population of PD-1+TFH cells in the later stages may limit the TFH
cell frequency as a negative regulator. Notably, a sustained
increase in the numbers of IL-21+TFH cells was observed during
disease progression, with high levels detected in the advanced
stages of OA. Similarly, an increase in IL-21 levels was also
observed in OA patients. Previous studies have reported elevated
IL-21 levels and transcription product in the synovial fluid of
both early- and advanced-stage knee OA (23,24).
Thus, IL-21+TFH cells appear to have an important role to serve in
the progression of OA. The present study also identified higher
levels of INF-γ and IL-17 in OA patients. These results are
consistent with those of previous studies, which indicated that OA
was a T-helper cell 1 (Th1)-mediated form of arthritis (25). Yamada et al (25) and Lùrati et al (26) reported elevated levels of Th17
cells in OA patients, which further illustrated OA as a disease
marked by an imbalance in the immune response towards a
pro-inflammatory state.
Immunological factors serve an important role in OA
pathogenesis (4–7). TFH cells are crucial regulators of B
cells, and IL-21 stimulates T and B cell proliferation (8,10).
IL-21 can increase the number of differentiated osteoclasts and
induce bone marrow cells to differentiate into mature osteoclasts
via upregulation of receptor activator of nuclear factor-κB ligand
(RANKL) expression (27). To study
the association between TFH cells and OA, the correlation between
different TFH cell subsets and WOMAC was analyzed. A positive
correlation between the frequency of IL-21+TFH cells and WOMAC was
observed in OA patients. Furthermore, a positive correlation was
observed between IL-21+TFH cells and the CRP level, which is an
inflammatory marker of OA patients. Based on these findings,
IL-21+TFH cells appear to be involved in the inflammatory state of
OA, and IL-21+TFH cells correlate with the symptoms and
functionality score of OA patients, and therefore may serve as
markers of OA disease activity.
The small sample size is a limitation of the present
study, as is the lack of evaluation of the functional aspects of
TFH cells. Therefore, further studies with larger sample sizes are
required to elucidate the pathogenic mechanism of TFH cells.
In conclusion, a high frequency of CXCR5+CD4+TFH
cells was observed in patients with OA, as compared with that in
the HCs. Furthermore, a correlation between IL-21+TFH cells and CRP
levels was observed. The findings of the current study indicate an
association between IL-21+TFH cells and disease activity. IL-21+TFH
levels may prove to be a useful marker of disease activity in OA
patients. However, the exact mechanism of OA pathogenesis mediated
by IL-21+TFH cells remains to be elucidated. In addition, further
studies are required to determine whether IL21+TFH cells and IL-21
could be novel therapeutic targets for OA.
Acknowledgements
This study was supported by grants received from the
National Natural Science Foundation of China (grant nos. 30972610
and 81273240), Jilin Province Science and Technology Agency (grant
no. 20110716), the Health Department Research Projects in Jilin
Province (grant no. 2009Z054) and the Norman Bethune Program of
Jilin University (grant no. 2012206).
References
|
1
|
Woolf AD and Pfleger B: Burden of major
musculoskeletal conditions. Bull World Health Organ. 81:646–656.
2003.PubMed/NCBI
|
|
2
|
Guccione AA, Felson DT, Anderson JJ,
Anthony JM, Zhang Y, Wilson PW, Kelly-Hayes M, Wolf PA, Kreger BE
and Kannel WB: The effects of specific medical conditions on the
functional limitations of elders in the Framingham study. Am J
Public Health. 84:351–358. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma L and Kapoor D: Epidemiology of
Osteoarthritis. Osteoarthritis: Diagnosis and Medical/Surgical
Management. 4th. Lippincott Williams & Wilkins; Philadelphia,
PA: 2007
|
|
4
|
Sowers M: Epidemiology of risk factors for
osteoarthritis: Systemic factors. Curr Opin Rheumatol. 13:447–451.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen PC, Wu CL, Jou IM, Lee CH, Juan HY,
Lee PJ, Chen SH and Hsieh JL: T helper cells promote disease
progression of osteoarthritis by inducing macrophage inflammatory
protein-1γ. Osteoarthritis Cartilage. 19:728–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Da RR, Qin Y, Baeten D and Zhang Y: B cell
clonal expansion and somatic hypermutation of Ig variable heavy
chain genes in the synovial membrane of patients with
osteoarthritis. J Immunol. 178:557–565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du H, Masuko-Hongo K, Nakamura H, Xiang Y,
Bao CD, Wang XD, Chen SL, Nishioka K and Kato T: The prevalence of
autoantibodies against cartilage intermediate layer protein,
YKL-39, osteopontin and cyclic citrullinated peptide in patients
with early-stage knee osteoarthritis: Evidence of a variety of
autoimmune processes. Rheumatol Int. 26:35–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yusuf I, Kageyama R, Monticelli L,
Johnston RJ, Ditoro D, Hansen K, Barnett B and Crotty S: Germinal
center T follicular helper cell IL-4 production is dependent on
signaling lymphocytic activation molecule receptor (CD150). J
Immunol. 185:190–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morita R, Schmitt N, Bentebibel SE,
Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S,
Sabzghabaei N, et al: Human blood CXCR5(+)CD4(+) T cells are
counterparts of T follicular cells and contain specific subsets
that differentially support antibody secretion. Immunity.
34:108–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nurieva RI, Chung Y, Hwang D, Yang XO,
Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q and Dong C:
Generation of T follicular helper cells is mediated by
interleukin-21 but independent of T helper 1, 2, or 17 cell
lineages. J Immunity. 29:138–149. 2008. View Article : Google Scholar
|
|
11
|
Kerfoot SM, Yaari G, Patel JR, Johnson KL,
Gonzalez DG, Kleinstein SH and Haberman AM: Germinal center B cell
and T follicular helper cell development initiates in the
interfollicular zone. J Immunity. 34:947–960. 2011. View Article : Google Scholar
|
|
12
|
Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao
C, Wu W, Chen J, Tong J, Yang M, et al: Increased frequency of
circulating follicular helper T cells in patientswith rheumatoid
arthritis. Clin Dev Immunol. 2012:8274802012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simpson N, Gatenby PA, Wilson A, Malik S,
Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, et
al: Expansion of circulating T cells resembling follicular helper T
cells is a fixed phenotype that identifies a subset of severe
systemic lupus erythematosus. Arthritis Rheum. 62:234–244. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao F, Zhang HY, Liu YJ, Zhao D, Shan YX
and Jiang YF: Higher frequency of peripheral blood interleukin 21
positive follicular helper T cells in patients with ankylosing
spondylitis. J Rheumatol. 40:2029–2037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg
M, et al: Development of criteria for the classification and
reporting of osteoarthritis. Classification of osteoarthritis of
the knee. Diagnostic and therapeutic criteria committee of the
American rheumatism Association. Arthritis Rheum. 29:1039–1049.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bellamy N, Buchanan WW, Goldsmith CH,
Campbell J and Stitt LW: Validation study of WOMAC: A health status
instrument for measuring clinically important patient relevant
outcomes to antirheumatic drug therapy in patients with
osteoarthritis of the hip or knee. J Rheumatol. 15:1833–1840.
1988.PubMed/NCBI
|
|
18
|
Jiang Y, Ma Z, Xin G, Yan H, Li W, Xu H,
Hao C, Niu J and Zhao P: Th1 and Th2 immune response in chronic
hepatitis B patients during a long-term treatment with adefovir
dipivoxil. Mediators Inflamm. 2010:1430262010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morgan E, Varro R, Sepulveda H, Ember JA,
Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, et al:
Cytometric bead array: A multiplexed assay platform with
applications in various areas of biology. Clin Immunol.
110:252–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deenick EK and Ma CS: The regulation and
role of T follicular helper cells in immunity. J Immunology.
134:361–367. 2011. View Article : Google Scholar
|
|
21
|
Rasmussen TK, Andersen T, Hvid M, Hetland
ML, Hørslev-Petersen K, Stengaard-Pedersen K, Holm CK and Deleuran
B: Increased interleukin 21 (IL-21) and IL-23 are associated with
increased disease activity and with radiographic status in patients
with early rheumatoid arthritis. J Rheumatol. 37:2014–2020. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma
L and Jiang Y: High frequencies of activated B cells and T
follicular helper cells are correlated with disease activity in
patients with new-onset rheumatoid arthritis. Clin Exp Immunol.
174:212–220. 2013.PubMed/NCBI
|
|
23
|
Scanzello CR, Umoh E, Pessler F,
Diaz-Torne C, Miles T, Dicarlo E, Potter HG, Mandl L, Marx R, Rodeo
S, et al: Local cytokine profiles in knee osteoarthritis: Elevated
synovial fluid interleukin-15 differentiates early from end-stage
disease. Osteoarthritis Cartilage. 17:1040–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamada H, Nakashima Y, Okazaki K, Mawatari
T, Fukushi J, Oyamada A, Fujimura K, Iwamoto Y and Yoshikai Y:
Preferential accumulation of activated Th1 cells not only in
rheumatoid arthritis but also in osteoarthritis joints. J
Rheumatol. 38:1569–1575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lùrati A, Laria A, Mazzocchi D, Re KA,
Marrazza M and Scarpellini M: Effects of hyaluronic acid (HA)
viscosupplementation on peripheral Th cells in knee and hip
osteoarthritis. Osteoarthritis Cartilage. 23:88–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwok SK, Cho ML, Park MK, Oh HJ, Park JS,
Her YM, Lee SY, Youn J, Ju JH, Park KS, et al: Interleukin-21
promotes osteoclastogenesis in humans with rheumatoid arthritis and
in mice with collagen-induced arthritis. Arthritis Rheum.
64:740–751. 2012. View Article : Google Scholar : PubMed/NCBI
|