Introduction
As the most common primary tumor of the central
nervous system, glioma accounts for 46% of all intracranial tumors
(1–3). The incidence of cerebral glioma is
3–10 in 100,000, comprising 1–3% of systemic malignant tumors. The
median survival time of glioma patients who undergo surgery plus
radiation and chemotherapy is only 8–11 months (4). Drug resistance to chemotherapy
contributes to this poor prognosis (5); therefore, it is important to identify
the mechanisms underlying drug resistance.
Integrin-linked kinase (ILK) was identified by
Hannigan et al (6) using
yeast two-hybrid screening. ILK cDNA is 1.8 kb long and encodes 452
amino acid residues, containing 3 structural units of Ser/Thr. ILK
is normally expressed at low levels; ILK is activated upon
adherence of extracellular matrix (ECM) to cells or by growth
factors via the phosphoinositide 3-kinase (PI3K)-dependent
signaling pathway. A previous study demonstrated that ILK
overexpression in cells resulted in the activation of numerous
signal transduction pathways (7).
Overexpression of ILK in epithelial cells may lead to increased
phosphorylation of protein kinase B (Akt) and glycogen synthase
kinase 3 (8).
Previous studies have revealed that ILK may induce
epithelial-mesenchymal transition, and promote the development of
malignant tumor cells in bladder and pancreatic cancer (9–12).
In addition, research performed by Duxbury et al (13) suggested that inhibition of the
expression of ILK through RNA interference may enhance the
sensitivity of pancreatic cancer cells to gemcitabine chemotherapy,
This indicated that ILK expression may be associated with drug
resistance of tumor cells. Overexpression of ILK has been detected
in numerous human malignant tumors, and this over expression has
been associated with poor prognosis (14–18).
Few studies have investigated the association between ILK and drug
resistance in glioma. The present study therefore investigated
whether overexpression of ILK affected drug resistance to
temozolomide in glioma cells.
Materials and methods
Cell line selection
U87 MG (Cell Bank of Type Culture Collection,
Chinese Academy of Sciences, Shanghai, China), U373 (Shanghai
Bioleaf Biotech Co., Ltd., Shanghai, China), U251 (Cell Bank of
Type Culture Collection, Chinese Academy of Sciences), SHG-44 (Cell
Bank of Type Culture Collection, Chinese Academy of Sciences) and
T98 G (Co Bioer Biosciences Co., Ltd., Nanjing, China) human
glioblastoma cell lines were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C and 5% CO2. These cell lines were used for analysis
of ILK protein expression levels by western blotting, to select a
cell line with low expression levels for subsequent
experiments.
Western blotting
Total proteins were extracted from cells using NP-40
(Beyotime Institute of Biotechnology, Shanghai, China) and the
protein concentration was determined using the Bicinchoninic Acid
assay. Total proteins (50 µg) were subjected to 12% SDS-PAGE and
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% skimmed milk
for 1 h, and subsequently incubated with the following primary
antibodies at 4°C overnight: Goat anti-ILK (1:5,000; clone, C-19;
catalog no. sc-7516; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-multidrug resistance-associated protein (MRP; 1:5,000;
cat. no. BA3340-2; rabbit anti human; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), anti-multidrug resistance protein
(MDR; 1:1,000; cat. no. PB0162; rabbit anti human; Wuhan Boster
Biological Technology, Ltd.), anti-B-cell lymphoma 2 (Bcl-2;
1:5,000; cat. no. BA0412; rabbit anti human; Wuhan Boster
Biological Technology, Ltd) and anti-Bcl-2-associated X protein
(Bax; 1:1,000; mouse anti human; cat. no. BA0315-2; Wuhan Boster
Biological Technology, Ltd.). Membranes were washed three times
with TBS and incubated with agoat anti rabbit, IgG-horseradish
peroxidase (HRP; 1:5,000; cat. no. BA1054; Wuhan Boster Biological
Technology, Ltd.) and goat anti mouse, IgG-HRP (1:5,000; cat. no.
BA1050; Wuhan Boster Biological Technology, Ltd.) at 37°C for 45
min. The proteins were visualized by RapidStep™ enhanced
chemiluminescence reagent (cat. no. 345818-100ML; Merck Millipore).
The Odyssey® Imaging system (LI-COR Biosciences,
Lincoln, NE, USA) was used for semi-quantitative analysis (Quantity
One image analysis system (BioRad Laboratories, Inc., Hercules, CA,
USA). β-actin (1:10,000; cat. no. BM0626; Wuhan Boster Biological
Technology, Ltd.) served as the control.
Establishment of ILK stable
transfected cell line
The commercial vector is the ILK Human cDNA ORF
clone (NM_004517), which was purchased from OriGene Technologies,
Inc. (Rockville, MD, USA; cat. no. RC20154L1). The primer sequences
are as follows: Forward, 5′-AAGGTACTCGAGCTATGGACGACATTTAAG0AAG1-3′
and reverse, 5′-ATCCAAGAATTCTCTACTTGTCCTGCAATC0ATC1-3′. PCR was
performed in a Mx3000 machine using the following thermocycling
conditions: An initial denaturation step at 95°C for 5 min,
followed by 30 cycles of 95°C for 30 sec and 55°C for 30 sec, and a
final step of 72°C for 90 sec and 4°C for 5 min. The amplified ILK
gene was separated and detected by 1% agarose gel electrophoresis.
The target DNA fragment was purified and obtained using a DNA
Recovery kit (catalog no. DP1702; BioTeke Corporation, Beijing,
China).
TA cloning
The obtained ILK gene was inserted into thepUM-T
vector (BioTeke Corporation). Briefly, 0.3 pmol ILK DNA was added
to 1 µlpUM-T vector (0.03 pmol), and double-distilled
H2O was added to a final volume of 5 µl. Subsequently, 5
µl Solution I (BioTeke Corporation; cat. no. DP6814) was added and
incubated at 16°C for 30 min. The mixed solution was added to 100
µl JM109 competent cells (2×109 cell/ml; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany), and cultured for 30 min on ice,
followed by 42°C for 90 sec and 2 min on ice. Super Optimal Broth
culture medium (800 µl; BioTeke Corporation) was added, and cells
were cultured for 1 h at 37°C with oscillation. The cells were
cultured on Fast-Media® Ampicillin Agar (InvivoGen, San
Diego, CA, USA) plates containing X-gal and isopropyl
β-D-1-thiogalactopyranoside. White colonies containing ILK were
selected, the inserted ILK fragment was confirmed by PCR, and
positive clones were selected and sequenced.
Plasmid extraction and genetic
recombination
Targeted plasmids [T-ILK and p enhanced green
fluorescent protein (EGFP)-C1 (Clontech Laboratories, Inc.,
Mountainview, CA, USA)] were extracted using a Plasmid DNA
Maxi-Preparation kit (catalog no. DP1008, BioTeke Corporation)
according to the manufacturer's protocol and plasmid concentration
was determined by ultraviolet spectrophotometry. Double enzyme
digestion for T-ILK and pEGFP-C1 was performed at designated
cutting sites as described previously (19), using Fast Digest EcoRI and Fast
Digest XholI restriction enzymes (Thermo Fisher Scientific, Inc.).
Following conjugation and transfection, JM109 competent cells were
cultured in kanamycin-containing agar plates, single clones
containing pEGFP-C1-ILK were selected and the inserted fragment
length was determined by PCR.
Recombined pEGFP-C1-ILK clones were verified by
automated sequencing using an ABI Prism 3730 DNA Sequencer (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and transfected into
glioma cells. Stably transfected cells were selected using
geneticin. Transfection of the empty pEGFP-C1 plasmid served as a
control.
Reverse transcription-PCR (RT-PCR) for
ILK mRNA expression levels
Total RNA was extracted from transfected cells using
TRIzol® reagent (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's protocol. RNA quality was
detected by ultraviolet spectrophotometry and agarose gel
electrophoresis. Total RNA (1 µg) was reverse-transcribed to cDNA
using the Takara RNA PCR kit (Takara Bio. Inc., Dalian, China; cat.
no. RR019), and the ILK gene was amplified by PCR (Takara PCR
Amplification kit (cat. no. RO11; Takara Bio. Inc.) with β-actin
serving as a control. The primer sequences were as follows:
Forward, 5′-ATGGAACCCTGAACAAACACT-3′ and reverse,
5′-AGCACATTTGGATGCGAGAAA-3′ for ILK; and forward,
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′ for β-actin. The thermocycling
conditions were as follows: An initial denaturation step at 95°C
for 5 min, followed by 30 cycles of 95°C for 20 sec and 60°C for 20
sec, and a final step at 72°C for 30 sec and 4°C for 5 min. The
amplified products were separated by 1.5% agarose gel and imaged
using the Odyssey®Imaging system (LI-COR
Biosciences).
MTT assay
Stably transfected (pEGFP-C1-ILK) cells were
cultured in RPMI-1640 medium containing 10% FBS, and seeded into
96-well plates at a density of 3×103 cells per well. TMZ
(Sigma-Aldrich; Merck Millipore; dissolved in DMSO (10 mg in 0.5
ml) and then diluted to 10 umol/l prior to experiment) was
subsequently added. Cells were divided into five groups (5
wells/group): pEGFP-C1-ILK, pEGFP-C1+0.1% dimethyl sulfoxide
(DMSO), pEGFP-C1+100 µM TMZ, pEGFP-C1-ILK+ 0.1% DMSO,
pEGFP-C1-ILK+100 µM TMZ. An MTT assay was performed at 12, 24, 48
and 72 h after treatment. The medium was removed from each well and
100 µl MTT (0.2 mg/ml in PBS) was added in the absence of light;
formazan crystals were produced over a 4-h incubation period at
37°C. To dissolve crystals, 200 µl DMSO was added to each well and
the optical density (OD) at a wavelength of 490 nm was measured on
a Tecan Spectra Fluor spectrophotometer (Tecan, Männedorf,
Switzerland).
Hoechst staining
Hoechst staining was performed on the SHG44,
pEGFP-C1+0.1% DMSO, pEGFP-C1+100 µM TMZ, pEGFP-C1-ILK +0.1% DMSO
and pEGFP-C1-ILK +100 µM TMZ groups 72 h following treatment. Cells
were fixed on glass slides and stained with Hoechst for 5 min.
Cells were subsequently washed twice with PBS, treated with
fluorescence quenching liquid, coverslipped and immediately
observed under a fluorescence microscope (magnification, ×400). A
total of 5 random fields of vision were selected, and, the number
of normal cells in each were counted, allowing for the average
number to be calculated. Staining results were analyzed (the nuclei
were pale blue and uniformly arranged in normal cells) and then
analyzed in conjunction with flow cytometry results.
Flow cytometry
Flow cytometry was performed in the SHG44,
pEGFP-C1+0.1% DMSO, pEGFP-C1+100 µM TMZ, pEGFP-C1-ILK + 0.1% DMSO
and pEGFP-C1-ILK +100 µM TMZ groups. The cells were inoculated in a
T25 culture flask until the cells adhered to the wall, following
which TMZ and DMSO were added. After 72 h of treatment, cells were
washed twice with PBS, digested by trypsin and centrifuged at 800 ×
g, 24°C for 5 min. All components of the Annexin V-FITC/PI
apoptosis double staining kit; cat. no. LHK601-050; JiaMay Biotech,
Ltd., Beijing, China) were added sequentially, before incubating at
room temperature for 15 min, and analyzed within 1 h by flow
cytometer (FACSCalibur; cat. no. FACS101; BD Biosciences, Franklin
Lakes, NJ, USA) using FlowJo software (version, 7.6.1; FlowJo, LLC,
Ashland, OR, USA).
Measurement of caspase-3 activity
A Caspase-3 Detection kit (Beyotime Institute of
Biotechnology) was used to detect the activity of caspase-3. The
transfected cells were transferred to a 6-well plate at a density
of 1×106/ml, and LAMD3100 (25 µl) was added when cell confluence
reached 65%. Following culture for 12 h, cells were washed in PBS,
homogenized, lysed by NP-40 (Beyotime Institute of Biotechnology)
and incubated on ice. Ac-DEVD-AMC (5 µl; AAT Bioquest, Inc.,
Sunnyvale, CA, USA) was added to a reaction solution containing 50
µl reaction buffer and 50 µl protein solution, and incubated at
37°C for 4 h. The absorption was measured at a wavelength of 405 nm
using an enzyme standard meter (ELX-800; BiotekInstruments,
Winooski, USA) andcaspase-3 activity was calculated using a
standard curve.
Statistical analysis
SPSS software version 18.0 (SPSS, Inc., Chicago, IL,
USA) was used to analyze the data. Data are presented as the mean ±
standard deviation. Analysis of variance analysis (along with
Bonferroni adjustment as post-hoc analysis) and independent t-tests
were performed to compare groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cell selection
Western blot analysis revealed that the protein
expression levels of ILK were lowest in the SHG-44 cell line
(Fig. 1); therefore, subsequent
experiments were performed on SHG-44 cells.
Amplification and transfection of
ILK
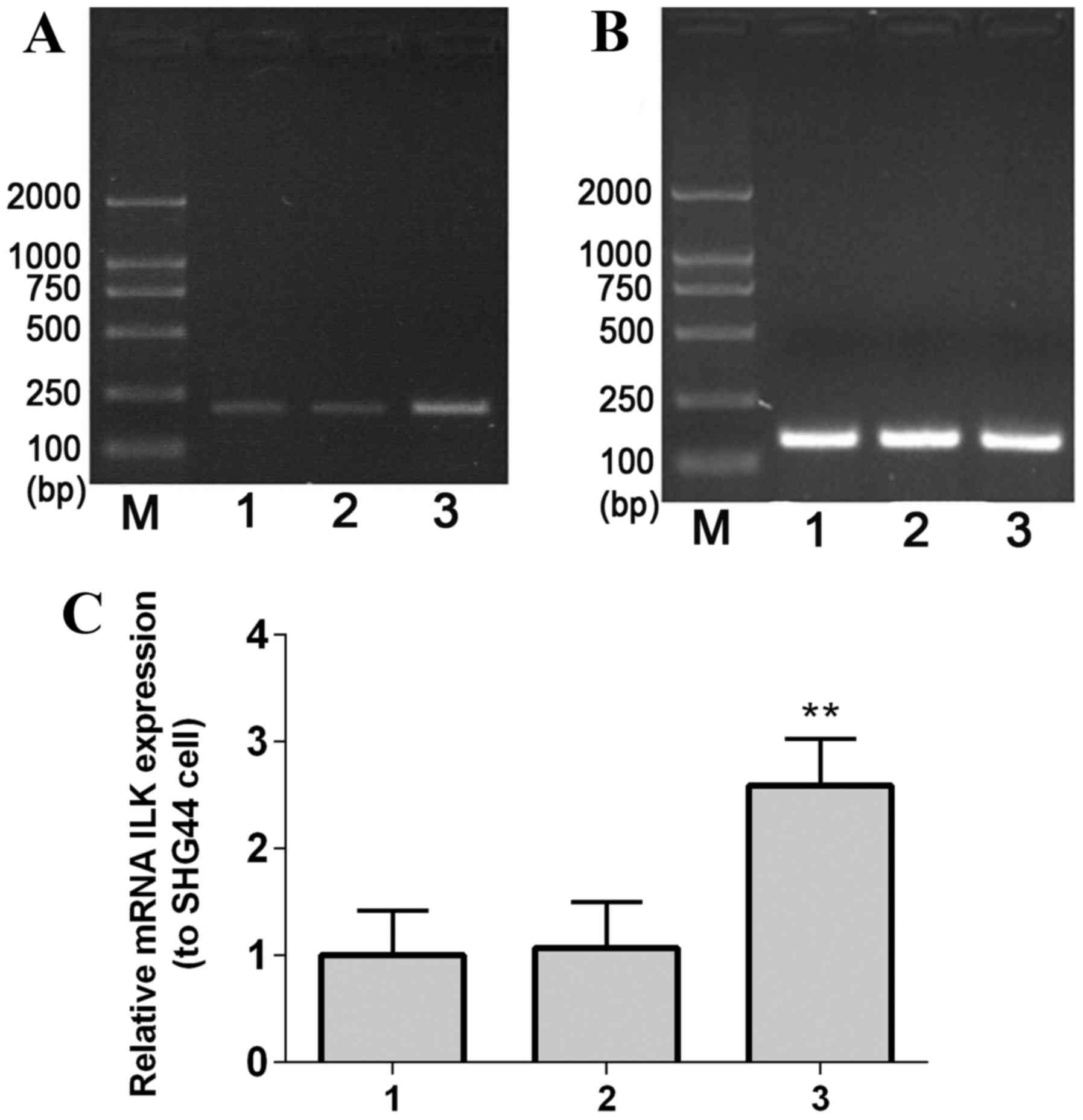
The complete ILK gene fragment (length, 1.8 kb) was
amplified by PCR, and agarose gel electrophoresis results
demonstrated that the size of the PCR product was consistent with
the expected size of the ILK gene (Fig. 2A), indicating that the ILK gene was
amplified.
 | Figure 2.Identification of the ILK gene using
PCR. PCR was performed on (A) purified ILK, (B) positive clones
following transfection of ILK and (C) recombined T-ILK clone. M,
marker; P, PCR product; E, enzyme-digested fragment; 1–6, selected
positive clones; Rp, recombinant plasmid; Rd, double
enzyme-digested recombinant plasmid; Rm, mono-enzyme-digested
recombinant plasmid. ILK, integrin-linked kinase; PCR, polymerase
chain reaction. |
The recombinant plasmid pEGFP-C1-ILK was identified
by PCR, and the obtained PCR fragments were consistent with the
expected size of 1.8 kb (Fig. 2B).
In addition, analysis confirmed that the inserted fragment length
presented no gene mutation in recombinant colonies (Fig. 2C). Furthermore, gene sequencing was
performed to confirm the ILK gene segment in the ILK plasmid
vector.
mRNA expression levels of ILK in
SHG-44 transfected cells
RT-PCR results demonstrated that ILK mRNA expression
levelsin stable transfected SHG-44 cellswere significantly greater
compared with the controls (mock-transfected and empty
vector-transfected SHG-44 cells; P=0.0067; Fig. 3).
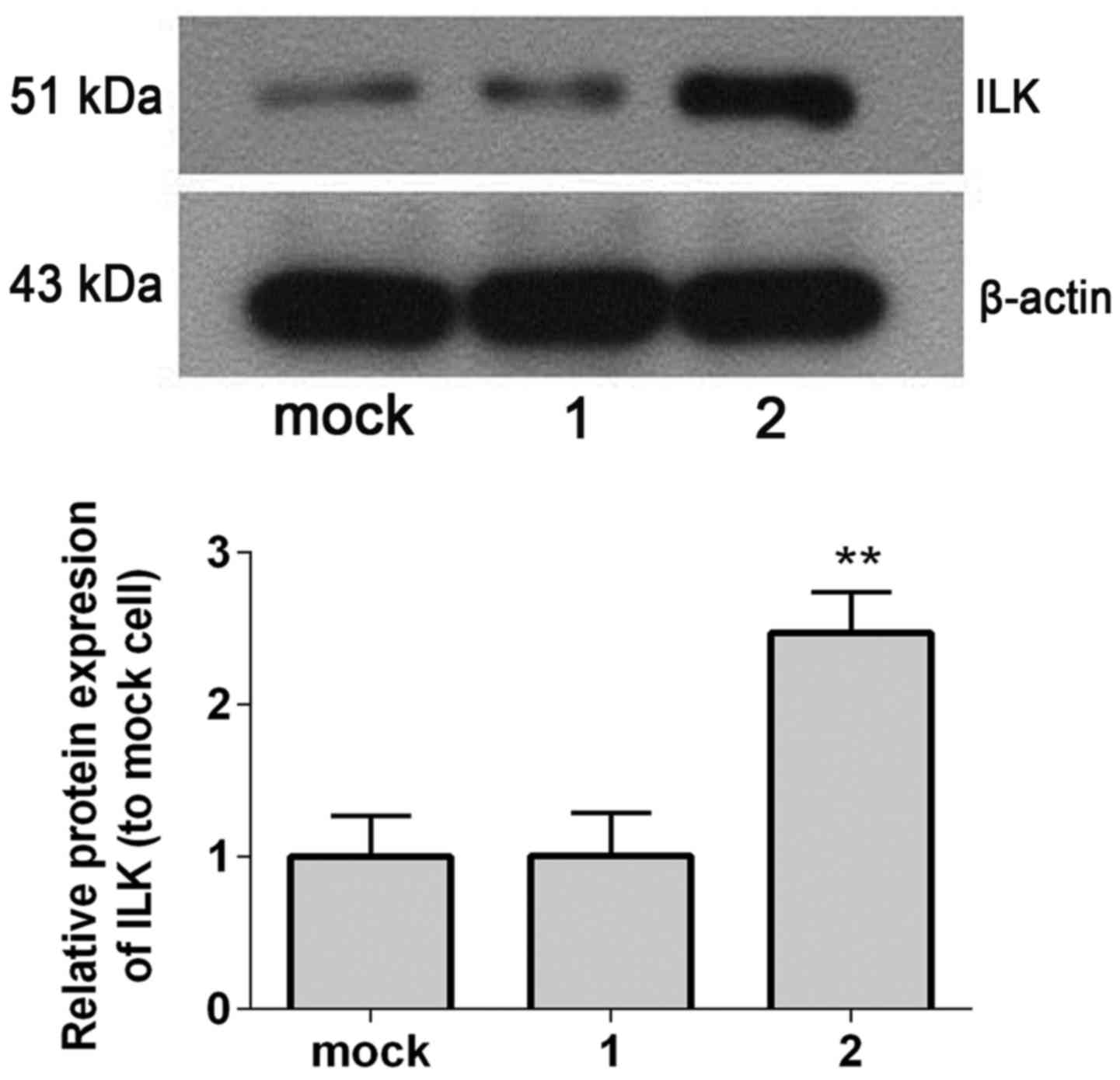
Protein expression levels of ILK in
SHG-44 transfected cells
Western blot analysis revealed that ILK protein
expression levels were significantly increased in ILK stable
transfected cells compared with controls (mock-transfected and
empty vector-transfected SHG-44 cells; P=0.005; Fig. 4).
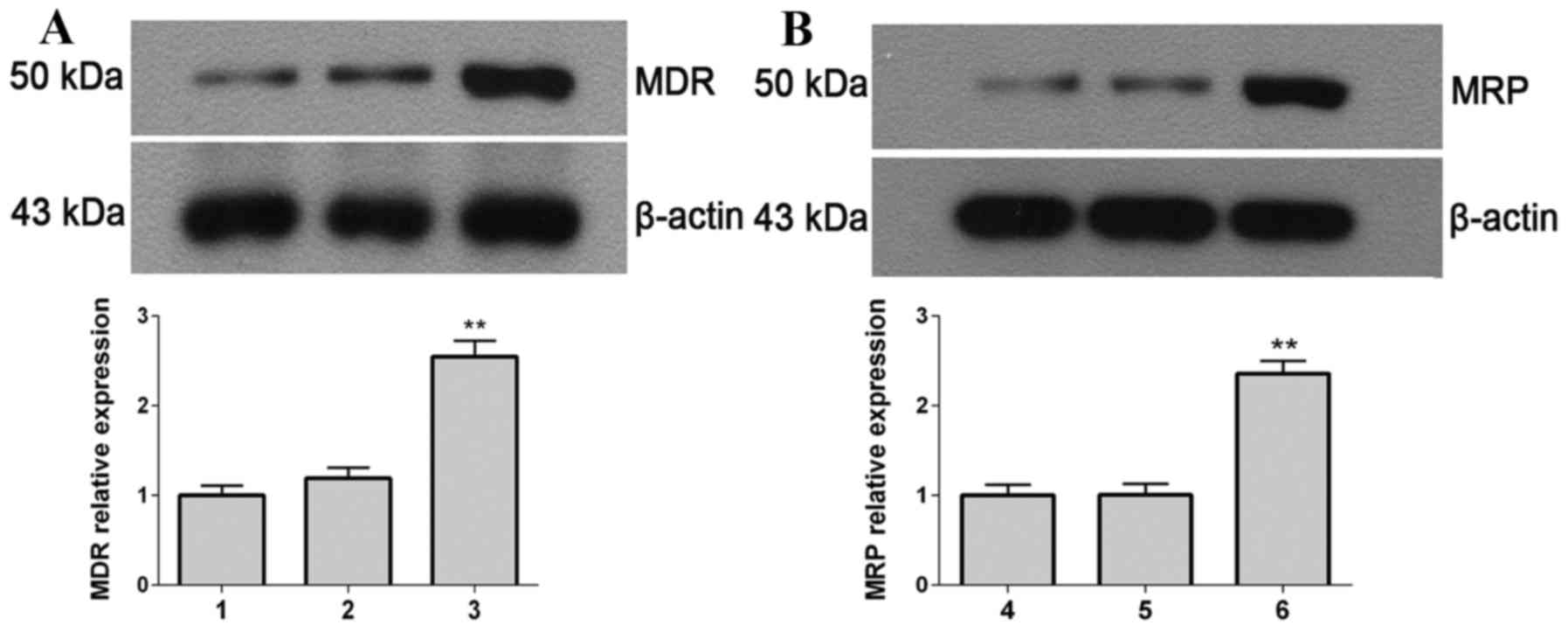
Protein expression levels of MDR and
MRP
The protein expression levels of MDR (Fig. 5A) and MRP (Fig. 5B) were detected by western blot
analysis. The protein expression levels of MDR and MRP in the
stable transfected cells were significantly greater compared with
the controls (mock-transfected and empty vector-transfected SHG-44
cells; P=0.0082). This demonstrated that the transfected cells
increase their resistance ability to TMZ through promoting the
expression of MDR and MRP.
Cell growth measured by MTT
Cell proliferation was evaluated using an MTT assay;
results are presented in Table I.
In thepEGFP-C1-ILK transfected group (subsequently abbreviated to
ILK), the OD values at 24, 48 and 72 h following TMZ treatment
group were significantly greater compared with the pEGFP-C1+TMZ
group (P=0.0074); at the same time points, the OD values in the
ILK+DMSO group were increased compared with the pEGFP C1+DMSO group
(P=0.0063). These results suggested that overexpression of ILK
promotesSHG-44 human glioma cell proliferation.
 | Table I.Proliferation of cells. |
Table I.
Proliferation of cells.
|
| Time following
treatment (h). |
|---|
|
|
|
|---|
| Group | 0 | 12 | 24 | 48 | 72 |
|---|
| SHG-44 | 0.2290±0.0125 | 0.3065±0.0164 | 0.4448±0.0354 | 0.5728±0.0342 | 0.7953±0.0186 |
| pEGFP-C1+DMSO | 0.2293±0.0144 | 0.3083±0.0152 | 0.4583±0.0295 | 0.5723±0.0373 | 0.8213±0.0867 |
| pEGFP-C1+TMZ | 0.2235±0.0138 | 0.3150±0.0274 | 0.3720±0.0307 | 0.4845±0.0456 | 0.5798±0.0610 |
| pEGFP-C1-ILK+
DMSO | 0.2303±0.0150 | 0.4183±0.038 |
0.7113±0.0480a |
1.1238±0.0885a |
1.3480±0.1284a |
| pEGFP-C1-ILK +
TMZ | 0.2270±0.0138 | 0.3360±0.0214 |
0.5048±0.0128b |
0.8478±0.0836b | 1.0450±0.0910 |
Apoptosis of cells detected by Hoechst
staining
Under a fluorescence microscope, apoptotic cells
revealed nuclear dense staining or chunky dense staining; apoptosis
occurred to the greatest extent in the pEGFP-C1+TMZ group. No
significant differences were observed in the percentage of
apoptosis between the SHG-44 and pEGFP-C1+DMSO groups; the
percentage of apoptotic cells in the ILK+TMZ group was
significantly reduced compared with the pEGFP-C1+TMZ group
(Fig. 6). These results indicated
that overexpression of ILK reduced apoptosis in glioma cells,
reducing their sensitivity to TMZ.
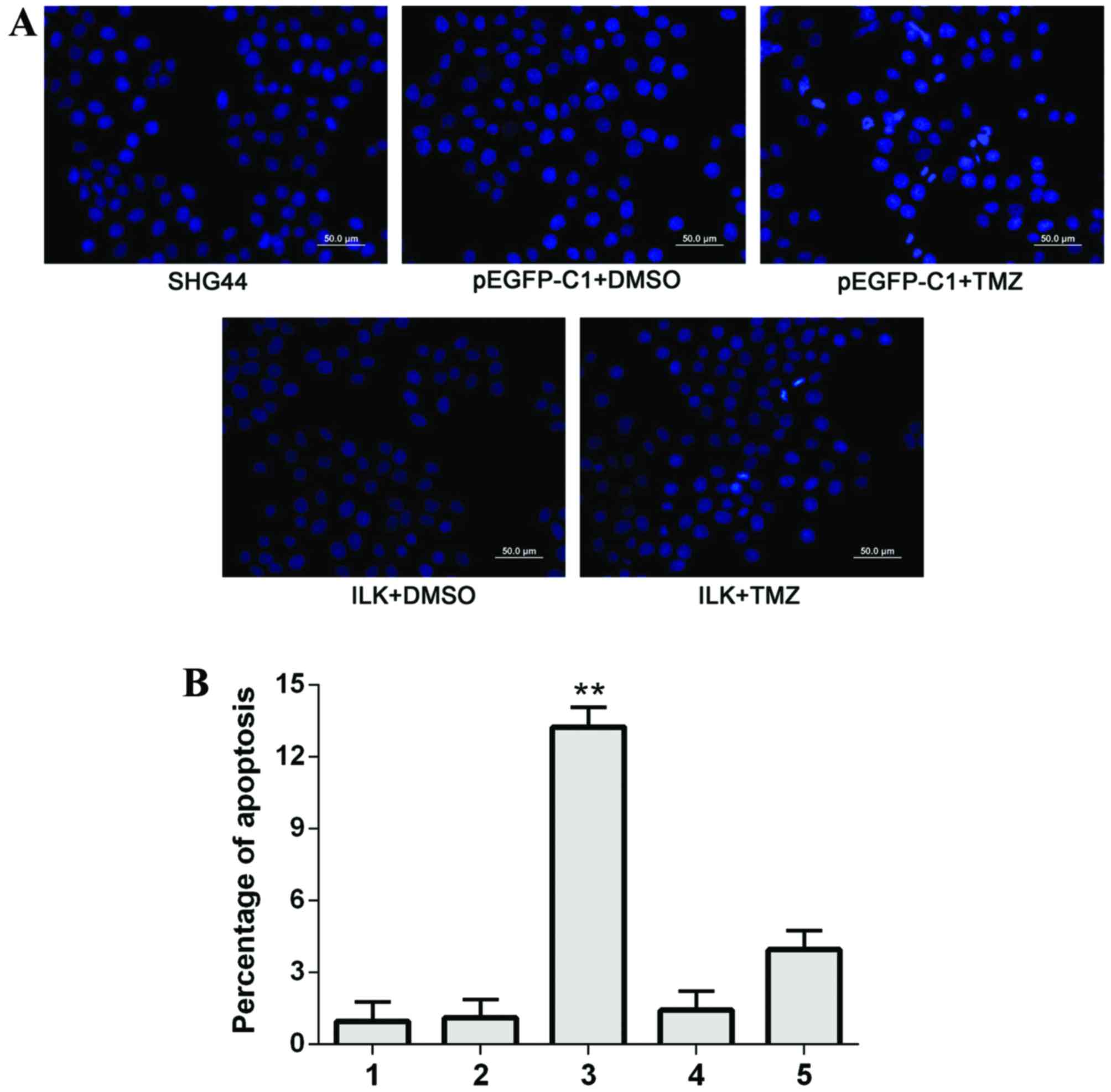 | Figure 6.Apoptosis of SHG-44 transfected cells,
as detected by Hoechst staining. (A) Representative images of
Hoechst staining in the different groups. Scale bar=50 µm. (B)
Quantification of Hoechst staining. 1, SHG-44 cells; 2,
pEGFP-C1+DMSO; 3, pEGFP-C1+TMZ; 4, pEGFP-C1-ILK+DMSO; 5,
pEGFP-C1-ILK+TMZ. Data are expressed as the mean ± standard
deviation. **P<0.01 vs. other groups. EGFP, enhanced green
fluorescent protein; DMSO, dimethyl sulfoxide; TMZ, temozolomide;
ILK, integrin-linked kinase. |
Flow cytometry
Flow cytometric analysis demonstrated that the
percentage of early apoptotic cells (lower right quadrant) was
greatest in the pEGFP-C1+TMZ group; the percentage of these cells
was significantly reduced in the ILK+TMZ group (P=0.001; Fig. 7). These results suggested that the
overexpression of ILK may decrease apoptosis in glioma cells
treated with TMZ, and therefore decrease the sensitivity of cells
to TMZ.
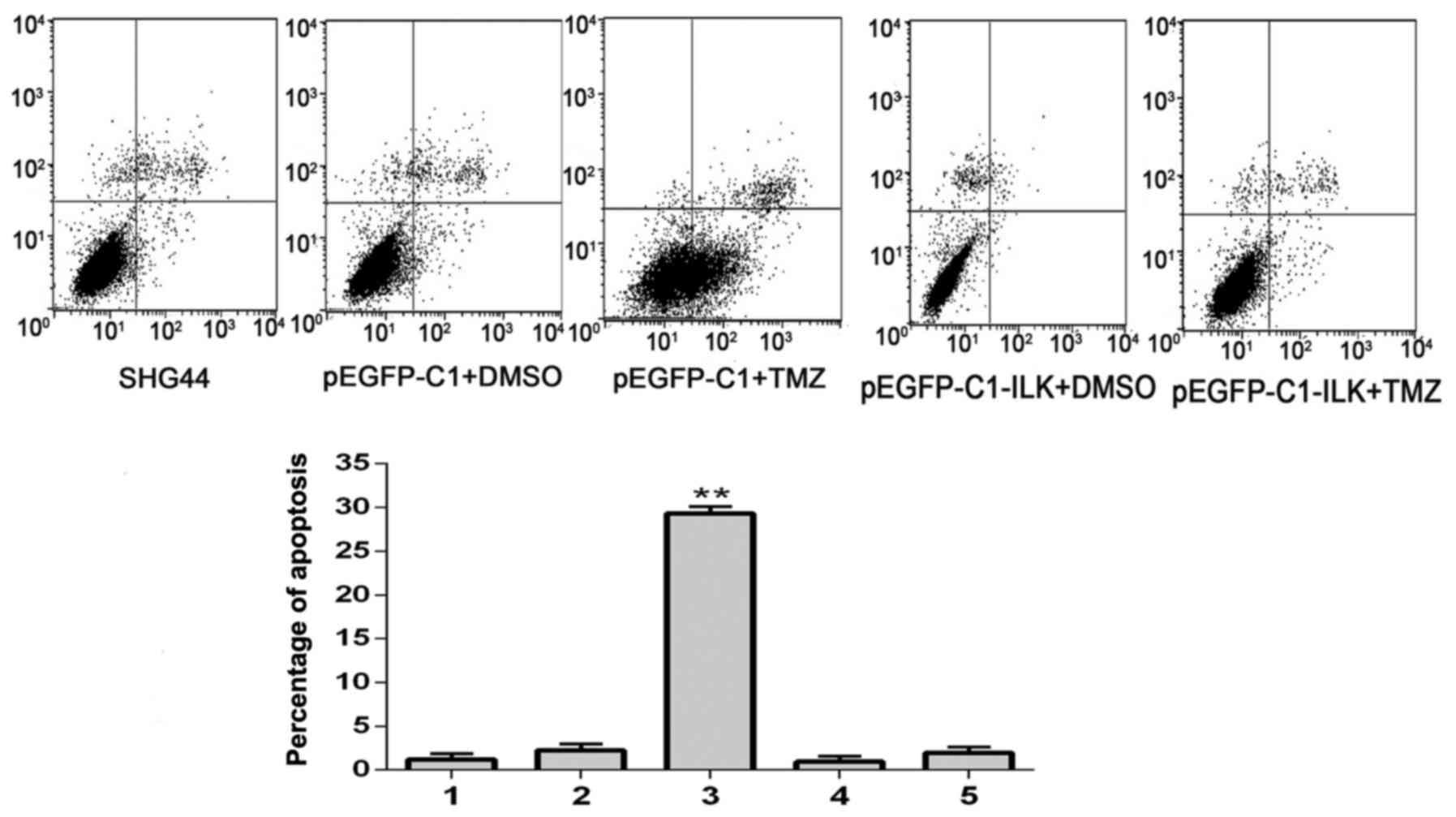 | Figure 7.Early apoptosis of SHG-44 transfected
cells, as detected by flow cytometric analysis. 1, SHG-44 cells; 2,
pEGFP-C1+DMSO; 3, pEGFP-C1+TMZ; 4, pEGFP-C1-ILK+DMSO; 5,
pEGFP-C1-ILK+TMZ. Data are expressed as the mean ± standard
deviation. **P<0.01 vs. other groups. EGFP, enhanced green
fluorescent protein; DMSO, dimethyl sulfoxide; TMZ, temozolomide;
ILK, integrin-linked kinase. |
Protein expression levels of Bcl-2 and
Bax
The protein expression levels of Bcl-2 (Fig. 8A) and Bax (Fig. 8B) were detected by western blot
analysis. The protein expression levels of Bcl-2, an anti-apoptotic
protein, were significantly increased (P=0.0093) in the ILK+TMZ
group compared with the pEGFP-C1+TMZ group; whereas the protein
expression levels of the pro-apoptotic protein Bax were
significantly decreased (P=0.0068) in the ILK+TMZ group compared
with the pEGFP-C1+TMZ group. These results indicated that ILK
increased expression of anti-apoptotic proteins, and decreased
expression of pro-apoptotic proteins, thus reducing apoptosis in
glioma cells and their sensitivity to TMZ.
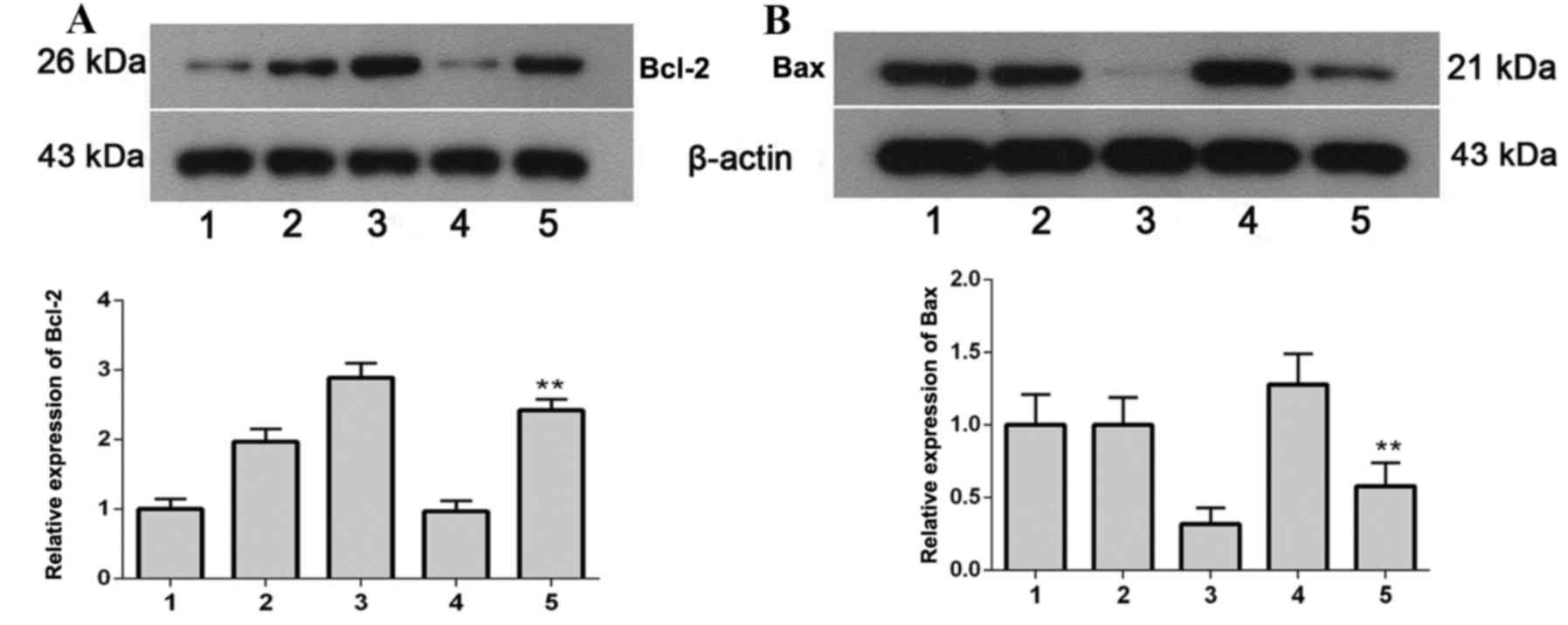 | Figure 8.Bcl-2 and Bax protein expression
levels in SHG-44 transfected cells, as determined by western blot
analysis. (A) Bcl-2 and (B) Bax protein expression levels. 1,
SHG-44 cells; 2, pEGFP-C1+DMSO; 3, pEGFP-C1-ILK+DMSO; 4,
pEGFP-C1+TMZ; 5, pEGFP-C1-ILK+TMZ. Data are expressed as the mean ±
standard deviation. **P<0.01 vs. pEGFP-C1+TMZ. Bcl-2, B-cell
lymphoma 2; Bax, B-cell lymphoma 2-associated X protein; EGFP,
enhanced green fluorescent protein; DMSO, dimethyl sulfoxide; TMZ,
temozolomide; ILK, integrin-linked kinase. |
Caspase-3 activity
The activity of caspase-3 in the ILK+TMZ group was
significantly reduced compared with the pEGFP-C1+TMZ group
(P=0.0078; Table II). These
results suggested that ILK decreased the activity of caspase-3,
thus decreasing apoptosis in glioma cells and their sensitivity to
TMZ.
 | Table II.Caspase-3 activity. |
Table II.
Caspase-3 activity.
| Group | Caspase-3 activity
(µM/gprotein) |
|---|
| SHG-44 | 17.77±0.89 |
| pEGFP-C1+DMSO | 17.49±2.00 |
| pEGFP-C1+TMZ | 24.19±3.13 |
|
pEGFP-C1-ILK+DMSO |
10.91±1.71a |
|
pEGFP-C1-ILK+TMZ | 17.48±2.50 |
Discussion
The occurrence and development of tumors requires
cells to perform various functions, including proliferation,
independent growth, anchoring, apoptosis, angiogenesis, invasion
and metastasis. ILK, a unique intracellular regulator, may
contribute to these functions. In addition, ILK interacts with the
protein kinase of cell adhesion receptors, as well as integrin and
actin cytoskeleton growth factors. Evidence indicates that ILK may
be a cancer gene, and that it may promote tumor cell survival or
tumor progression by regulating various signaling pathways
(20).
ILK serves a complex role in numerous cellular
functions, including proliferation, motility, invasion, metastasis
and angiogenesis (21,22). A study by Lee et al
(23) demonstrated that ILK
regulates phosphorylation of Akt in phosphatase and tensin homolog
deficient prostate cancer and breast cancer cells. Overexpression
of ILK has been associated with tumor progression and poor
prognosis in patients with rectal cancer (24). Monferran et al (25) revealed that the sensitivity of
glioma cells to ionizing radiation was regulated by RhoB and ILK.
Lanvin et al (26)
demonstrated that ILK altered the radiation sensitivity of glioma
via the regulation of the hypoxia-inducible factor 1α and survivin
pathway and the mediation of mitochondrial death. These findings
indicated that ILK may be a potential target for the treatment of
glioma (20). The aim of the
present study was to investigate biological behaviors, including
proliferation and apoptosis, in ILK stable transfected SHG-44 cells
in vitro, and investigate the sensitivity of transfected
glioma cells to TMZ.
In the present study, western blot analysis and
RT-PCR revealed that the expression levels of ILK protein and mRNA
in the stable transfected ILK cell line was significantly greater
compared with the mock- and empty vector-transfected groups, which
indicated the successful construction of the ILK stable transfected
cell line. The protein expression levels of MDR and MRP were
significantly increased in ILK-transfected cells, which made their
resistance ability to TMZ increased.
Bcl-2 family proteins are important regulatory
factors in the endogenous mitochondrial-dependent apoptosis
pathway. In the past 20 years, in vitro and in vivo
studies have revealed that Bcl-2 family proteins serve important
roles in the drug resistance of tumor cells. The endogenous
apoptosis pathway may be activated by numerous factors, including
chemotherapy agents, growth deprivation, mitochondria membrane
breakdown and apoptosis factors, particularly the release of
cytochrome C from mitochondria into the cytoplasm (27). The mitochondrial apoptosis pathway
is mediated by a complex network composed of pro- and
anti-apoptotic proteins of the Bcl-2 family (28). Through upregulating anti-apoptotic
proteins or downregulating pro-apoptotic proteins, the
mitochondrial apoptotic pathway may determine whether gliomas are
sensitive to radiation and chemotherapy (29). Western blot analysis demonstrated
that ILK increased the expression of the anti-apoptotic protein
Bcl-2 significantly and decreased the expression of the
pro-apoptotic protein Baxin human glioma cells.
MTT assays revealed that following 24, 48 and 72 h
of treatment with TMZ or DMSO, OD values in the ILK stable
transfected group were significantly greater compared with the
empty vector group, which indicated that the overexpression of ILK
may promote the proliferation of TMZ-treated glioma cells. Hoechst
staining revealed that the percentage of apoptotic cells in the ILK
stable transfected group was significantly decreased compared with
the empty vector group. Therefore, ILK may promote proliferation
and decrease apoptosis in TMZ-treated cells.
Song et al (30) indicated that ILK influenced the MDR
gene expression via the PI3K-Akt and mitogen-activated protein
kinase-extracellular signal-regulated kinase signaling pathways,
regulating the progress of gastric cancer. It is well known that
caspase activation is a typical marker of early and late apoptosis.
The activity of caspase-3 is an important indicator to determine
whether apoptosis is occurring in cells or tissues (31). Duxbury et al (13) demonstrated that ILK increased
gemcitabine resistance in pancreatic cancer cells, and that
knockdown of ILK increased the sensitivity of pancreatic cancer
cells to gemcitabine via increasing caspase-3-mediated apoptosis.
Therefore, ILK may regulate the sensitivity of glioma cells to TMZ.
The results of the present study revealed that the activity of
caspase-3 was significantly reduced in the ILK stable transfected
cells compared with the empty vector group. The expression of ILK
in glioma cells may decrease apoptosis in tumor cells via the
downregulation of caspase-3 activity, thereby reducing the
sensitivity of tumor cells to TMZ.
In conclusion, the results of the present study
demonstrated that overexpression of ILK in SHG-44 glioma cells
downregulated the expression of the anti-apoptotic protein Bcl-2,
increased the expression of Bax, and decreased the activity of the
key enzyme caspase-3, thus reducing the apoptosis of tumor cells,
promoting their proliferation, and reducing the sensitivity of
SHG-44 glioma cells to TMZ. The present results suggested that ILK
may serve as a potential therapeutic target for glioma.
Acknowledgements
The present study was supported by the Doctor
Startup Foundation in Liaoning Province (grant no. 201501101) and
the Technology Startup Foundation of The First Affiliated Hospital
of Liaoning Medical University (grant no. FYK201214).
References
|
1
|
DeAngelis LM: Brain tumors. N Engl J Med.
344:114–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asklund T, Malmstrom A, Bergqvist M, Björ
O and Henriksson R: Brain tumors in Sweden: Data from a
population-based registry 1999–2012. Acta Oncol. 54:377–384. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Tonn JC, Brada M and
Pentheroudakis G: ESMO Guidelines Working Group: High-grade
malignant glioma: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 21:(Suppl 5). v190–v193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mrugala MM, Adair J and Kiem HP:
Temozolomide: Expanding its role in brain cancer. Drugs Today
(Barc). 46:833–846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hannigan GE, Leung-Hagesteijn C,
Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC and Dedhar
S: Regulation of cell adhesion and anchorage-dependent growth by a
new beta 1-integrin-linked protein kinase. Nature. 379:91–96. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dedhar S: Cell-substrate interactions and
signaling through ILK. Curr Opin Cell Biol. 12:250–256. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmitz M, Grignard G, Margue C, Dippel W,
Capesius C, Mossong J, Nathan M, Giacchi S, Scheiden R and Kieffer
N: Complete loss of PTEN expression as a possible early prognostic
marker for prostate cancer metastasis. Int J Cancer. 120:1284–1292.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gil D, Ciołczyk-Wierzbicka D,
Dulińska-Litewka J, Zwawa K, McCubrey JA and Laidler P: The
mechanism of contribution of integrin linked kinase (ILK) to
epithelial-mesenchymal transition (EMT). Adv Enzyme Regul.
51:195–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsui Y, Assi K, Ogawa O, Raven PA,
Dedhar S, Gleave ME, Salh B and So AI: The importance of
integrin-linked kinase in the regulation of bladder cancer
invasion. Int J Cancer. 130:521–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Serrano I, McDonald PC, Lock FE and Dedhar
S: Role of the integrin-linked kinase (ILK)/Rictor complex in
TGFβ-1-induced epithelial-mesenchymal transition (EMT). Oncogene.
32:50–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen D, Zhang Y, Zhang X, Li J, Han B, Liu
S, Wang L, Ling Y, Mao S and Wang X: Overexpression of
integrin-linked kinase correlates with malignant phenotype in
non-small cell lung cancer and promotes lung cancer cell invasion
and migration via regulating epithelial-mesenchymal transition
(EMT)-related genes. Acta Histochem. 115:128–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duxbury MS, Ito H, Benoit E, Waseem T,
Ashley SW and Whang EE: RNA interference demonstrates a novel role
for integrin-linked kinase as a determinant of pancreatic
adenocarcinoma cell gemcitabine chemoresistance. Clin Cancer Res.
11:3433–3438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai DL, Makretsov N, Campos EI, Huang C,
Zhou Y, Huntsman D, Martinka M and Li G: Increased expression of
integrin-linked kinase is correlated with melanoma progression and
poor patient survival. Clin Cancer Res. 9:4409–4414.
2003.PubMed/NCBI
|
|
15
|
Sawai H, Okada Y, Funahashi H, Matsuo Y,
Takahashi H, Takeyama H and Manabe T: Integrin-linked kinase
activity is associated with interleukin-1 alpha-induced progressive
behavior of pancreatic cancer and poor patient survival. Oncogene.
25:3237–3246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Zhang H, Wu J, Guan H, Yuan J, Huang
Z and Li M: Prognostic significance of integrin-linked kinase1
overexpression in astrocytoma. Int J Cancer. 126:1436–1444.
2010.PubMed/NCBI
|
|
17
|
Yu J, Shi R, Zhang D, Wang E and Qiu X:
Expression of integrin-linked kinase in lung squamous cell
carcinoma and adenocarcinoma: Correlation with E-cadherin
expression, tumor microvessel density and clinical outcome.
Virchows Arch. 458:99–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao D, Tang XF, Yang K, Liu JY and Ma XR:
Over-expression of integrin-linked kinase correlates with aberrant
expression of Snail, E-cadherin and N-cadherin in oral squamous
cell carcinoma: Implications in tumor progression and metastasis.
Clin Exp Metastasis. 29:957–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhuang X, Lv M, Zhong Z, Zhang L, Jiang R
and Chen J: Interplay between intergrin-linked kinase and
ribonuclease inhibitor affects growth and metastasis of bladder
cancer through signaling ILK pathways. J Exp Clin Cancer Res.
35:1302016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: A cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Persad S and Dedhar S: The role of
integrin-linked kinase (ILK) in cancer progression. Cancer
Metastasis Rev. 22:375–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McDonald PC, Fielding AB and Dedhar S:
Integrin-linked kinase-essential roles in physiology and cancer
biology. J Cell Sci. 121:3121–3132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SL, Chou CC, Chuang HC, Hsu EC, Chiu
PC, Kulp SK, Byrd JC and Chen CS: Functional role of mTORC2 versus
integrin-linked kinase in mediating Ser473-Akt phosphorylation in
PTEN-negative prostate and breast cancer cell lines. PLoS One.
8:e671492013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li R, Liu B, Yin H, Sun W, Yin J and Su Q:
Overexpression of integrin-linked kinase (ILK) is associated with
tumor progression and an unfavorable prognosis in patients with
colorectal cancer. J Mol Histol. 44:183–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Monferran S, Skuli N, Delmas C, Favre G,
Bonnet J, Cohen-Jonathan-Moyal E and Toulas C: Alphavbeta3 and
alphavbeta5 integrins control glioma cell response to ionising
radiation through ILK and RhoB. Int J Cancer. 123:357–364. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lanvin O, Monferran S, Delmas C, Couderc
B, Toulas C and Cohen-Jonathan-Moyal E: Radiation-induced mitotic
cell death and glioblastoma radioresistance: A new regulating
pathway controlled by integrin-linked kinase, hypoxia-inducible
factor 1 alpha and survivin in U87 cells. Eur J Cancer.
49:2884–2891. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hotchkiss RS, Strasser A, McDunn JE and
Swanson PE: Cell death. N Engl J Med. 361:1570–1583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang T and Saghatelian A: Emerging roles
of lipids in BCL-2 family-regulated apoptosis. Biochim Biophys
Acta. 1831:1542–1554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kouri FM, Jensen SA and Stegh AH: The role
of Bcl-2 family proteins in therapy responses of malignant
astrocytic gliomas: Bcl2L12 and beyond. ScientificWorldJournal.
2012:8389162012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song W, Jiang R and Zhao CM: Role of
integrin-linked kinase in multi-drug resistance of human gastric
carcinoma SGC7901/DDP cells. Asian Pac J Cancer Prev. 13:5619–5625.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mazumder S, Plesca D and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 414:13–21.
2008.PubMed/NCBI
|