Introduction
Liver fibrosis is a result of chronic or sustained
liver injury, which may be attributed to viral infections, alcohol
abuse, drugs, autoimmune reactions and other causes (1,2). The
severe and life-threatening complications of liver fibrosis have
made it a global challenge (3).
However, limited agents are available to treat liver fibrosis, and
liver transplantation remains the only effective therapy,
particularly for end-stage liver disease (4).
Cyclooxygenase (COX) is the rate-limiting enzyme in
the biosynthesis of prostaglandins and thromboxanes (5). There are two major types of COXs:
COX-1 and COX-2. COX-1 is constitutively expressed in multiple
tissues, while COX-2 is inducible by a variety of stimuli. Previous
studies have reported the upregulation of COX-2 in cirrhotic
livers, which indicates that COX-2 may be involved in the process
of liver cirrhosis (6,7). However, the involvement of COX-2 in
the progression of liver fibrosis is controversial. It has
previously been demonstrated that long term administration of a
COX-2 inhibitor, celecoxib, efficiently ameliorated liver cirrhosis
via dual inhibitive effects on the intrahepatic angiogenesis and
epithelial-to-mesenchymal transition of hepatocytes (8,9).
However, other previous studies have observed that celecoxib failed
to prevent liver fibrosis (10,11).
These conflicting results may be due to the dosage of drugs, the
experimental duration, and the differential induction of COX-2
according to the site and kinetics of the liver injury (12). Therefore, it is necessary to
further investigate the involvement of COX-2 in the liver
fibrosis.
Long non-coding RNAs (lncRNAs) are generally defined
as endogenous RNA molecules without protein-coding potential,
ranging from 200 bp to 100 kbp in length (13,14).
Additionally, lncRNAs are involved in regulating gene expression,
including chromatin remodeling, transcription and
post-transcriptional processes (15,16).
An increasing amount of research demonstrates that lncRNAs are
involved in liver diseases, particularly in liver carcinomas
(17,18). LncRNA-COX-2, which originates close
to the COX-2 gene and does not code for COX-2 protein, has been
observed to regulate the transcription of inflammatory genes in
intestinal epithelial cells (19).
Carpenter et al (20)
demonstrated that lncRNA-COX-2 may act as a regulatory component of
certain specific immune genes that further mediate inflammatory
signaling. Furthermore, previous studies have also demonstrated
that lncRNAs affect the activation of hepatic stellate cells (HSCs)
and liver fibrogenesis (21,22).
However, the involvement of lncRNA-COX-2 in liver fibrogenesis
remains to be elucidated.
The present study aimed to investigate the
expression of COX-2 and lncRNA-COX-2 in a carbon tetrachloride
(CCl4)-induced cirrhotic mouse model. Correlations
between COX-2 expression, lncRNA-COX-2 expression and liver
fibrosis were also determined.
Materials and methods
Animals and experimental groups
Specific pathogen-free male BALB/c mice (n=36; age,
8 weeks; weight, 22–25 g) were obtained from the West China
Experimental Animal Center, Sichuan University (Chengdu, China).
Mice were housed under 12-h light/dark cycles in an environmentally
controlled room (22–24°C, 50% humidity) with access to food and
water ad libitum. Liver fibrosis was induced by intraperitoneal
injection of CCl4 (4 ml/kg; dissolved in olive oil, 1:1)
twice weekly. The mice were randomly divided into 3 groups (n=12
per group) as follows: i) Control group received an injection of
normal saline (100 µl, twice a week); ii) the CCl4-2M
group received intraperitoneal injections of CCl4 for 2
months; and iii) the CCl4-3M group received
intraperitoneal injections of CCl4 for 3 months. Mice
were anesthetized by intraperitoneal administration of 4% chloral
hydrate (1 ml/100 g), and the mice were sacrificed by
exsanguination, 72 h following the final CCl4 injection
at 8 or 12 weeks, respectively. The livers were rapidly excised and
washed with saline. A part of each liver was immediately stored at
−80°C and the remainder was immersed in 4% paraformaldehyde
solution. All procedures performed in the course of the present
study were approved by the Animal Use and Care Committee of Sichuan
University and were conducted according to the regulations of
Sichuan University.
Histopathological evaluation
Liver tissues were fixed in 4% paraformaldehyde and
embedded in paraffin, cut into 4-µm thick sections and stained with
hematoxylin and eosin (HE) and Masson's trichrome (MT) staining.
The morphological changes were examined under a microscope (CX41;
Olympus Corporation, Tokyo, Japan) equipped with a digital camera
(DP72; Olympus Corporation) and analyzed by Image-Pro Plus 4.0
software (Media Cybernetics, Rockville, MD, USA). A total of 3
images per section (x100 magnification) were captured, in order to
calculate the total percentages of the fibrotic areas of each
group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for mRNA expression
Total RNA was isolated from all mice liver tissues
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The RNA was then reverse transcribed using a
first-strand cDNA kit (Fermentas; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The expression of RNA was
detected by RT-qPCR. The qRT-PCR was performed using the SsoFast
EvaGreen PCR master mix (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Reactions were incubated at 95°C for 5 min, followed by 39
cycles at 95°C for 10 sec and 58°C for 30 sec. All samples were run
in triplicate using a CFX96 real-time PCR detection system (Bio-Rad
Laboratories, Inc.). The primers were synthesized by TSINGKE
(Chengdu, China). The primer sequences are listed as follows:
COX-2, forward 5′-GGGTGTGAAGGGAAATAAGG-3′, reverse
5′-TGTGATTTAAGTCCACTCCATG-3′; lncRNA-COX-2, forward
5′-CAAAAGTGGGAAGGAAGGT-3′, reverse 5′-TCCAGGAAGGGAAATATACAT-3′) and
β-actin forward 5′-TGACGTTGACATCCGTAAAG-3′ and reverse
5′-GAGGAGCAATGATCTTGATCT-3′. The relative expression levels of the
target genes were calculated using the comparative
2-∆∆Cq method (23) and
normalized to the expression of β-actin.
Immunohistochemical staining
Liver sections were deparaffinized in xylene for 15
min and sequentially rehydrated in 100, 95 and 90% ethanol
solutions. Following washing in phosphate-buffered saline (PBS) for
15 min, the heat-induced antigen retrieval procedure was performed.
The sections were incubated with 3% H2O2 for
15 min to quench the endogenous peroxidase. A primary mouse
polyclonal COX-2 antibody (1:200; ab15191; Abcam, Cambridge, UK)
was added to the sections and incubated overnight at 4°C.
Subsequent to rinsing with PBS, slides were incubated with
horseradish peroxidase-conjugated secondary antibody kits (SP-9001;
ZSGB-BIO, Beijing, China) for 1 h at 37°C. Liver sections were
stained with diaminobenzidine and counterstained with hematoxylin.
Five random fields were selected from each section to evaluate
COX-2 expression. Quantification of positive staining was
accomplished by using Image-Pro Plus 4.0 software to score
integrated optical density at ×400 magnification under a light
microscope (CX41; Olympus Corporation).
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical significance among three groups was analyzed
by one-way analysis of variance followed by the
Student-Newman-Keuls test. Pearson correlation coefficients were
used to describe the strength of correlation. Statistical analysis
was conducted using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Accumulation of hepatocyte injury and
fibrosis following CCl4 administration
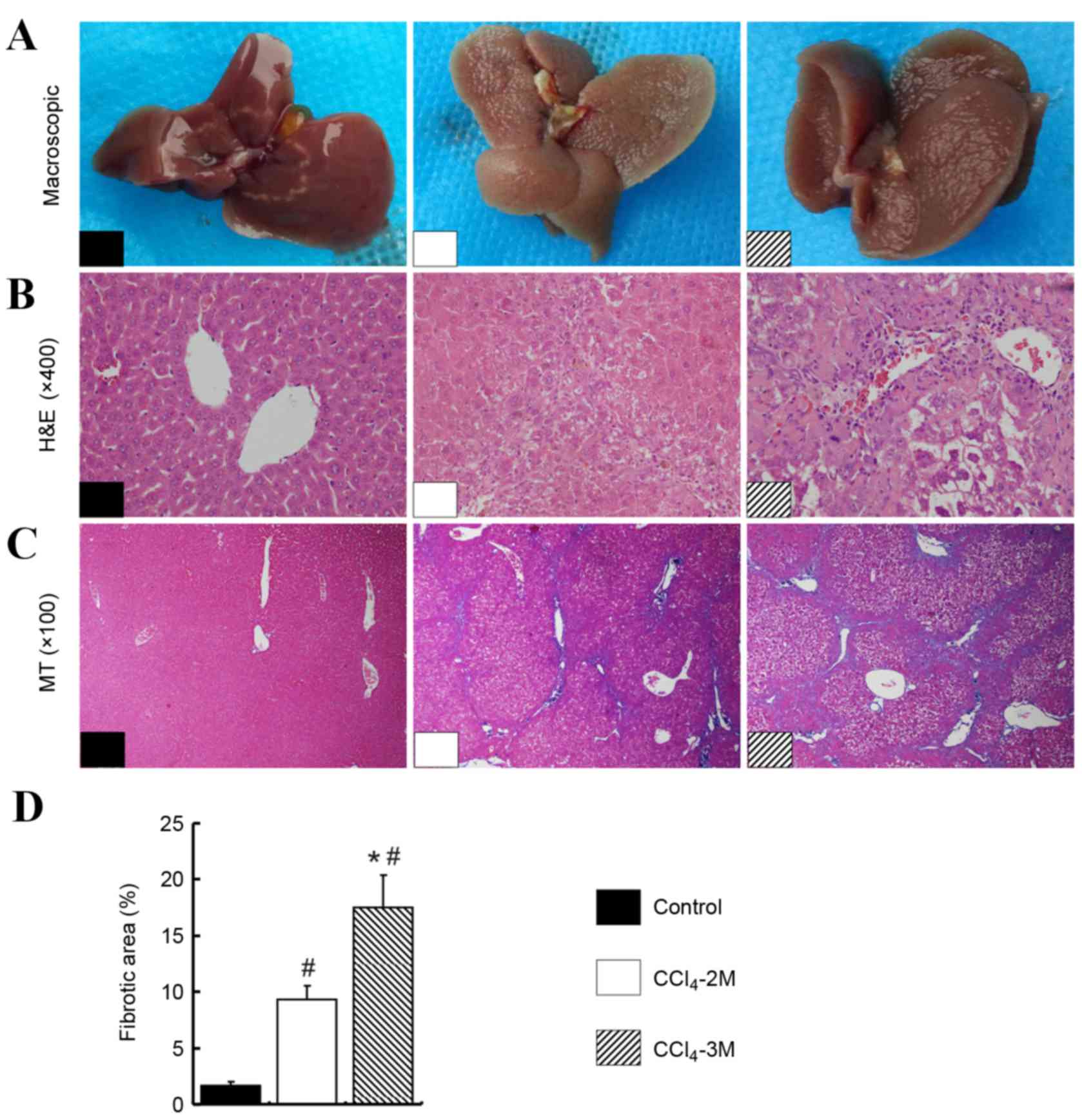
Extensive nodular formation was observed following
treatment with CCl4 in the livers of the
CCL4-2M and the CCL4-3M groups compared with
that in the control group. The most marked changes in color and
hardness were observed in the livers of the CCl4-3M
group (Fig. 1A). Liver tissues
stained with HE revealed mild intrahepatic inflammation and partial
destruction of the normal architecture in the CCl4-2M
group when compared with that in the control group (Fig. 1B). In addition, increased
inflammation and hepatocyte necrosis was observed in the
CCl4-3M group, with severe damage to the normal
architecture and the formation of septa and nodules (Fig. 1B). MT staining further demonstrated
the destruction of hepatic structure and reorganized fibrotic septa
in the CCL4-2M and the CCL4-3M groups
(Fig. 1C). The mean fibrotic areas
of liver tissues in the CCl4-2M group and
CCl4-3M group were increased by 4 and 9 times,
respectively, compared with the control group (P=0.001 and P=0.001,
respectively; Fig. 1D). In
addition, mean fibrotic areas of the CCl4-3M group were
twice those of the CCl4-2M group (P=0.012; Fig. 1D).
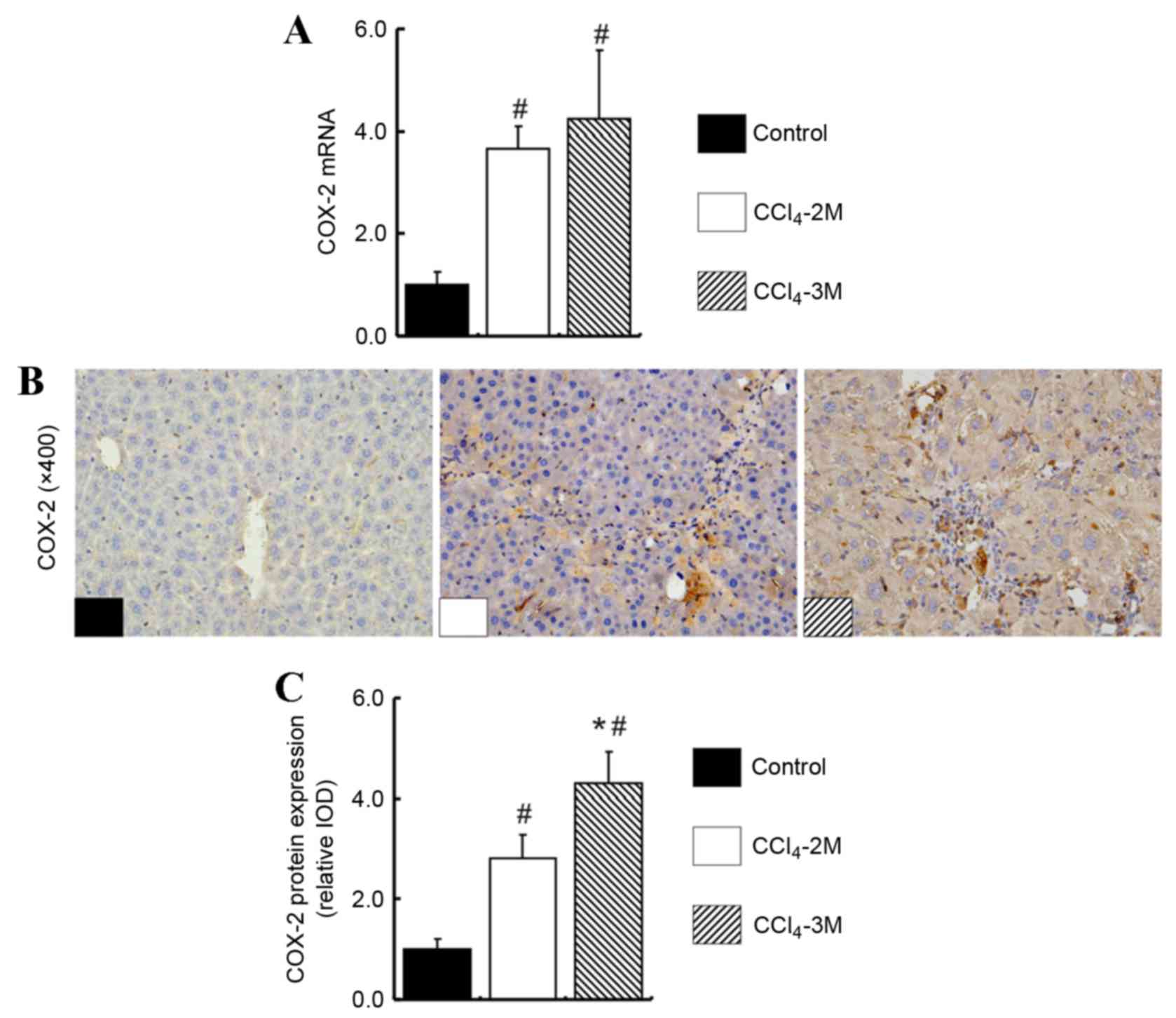
Expression of COX-2 is increased in
cirrhotic liver
The expression levels of COX-2 mRNA were increased
in the CCl4-2M group and the CCl4-3M group
compared with the control group (P=0.001 and P=0.001, respectively;
Fig. 2A). Although there was no
significant difference in COX-2 mRNA expression levels between the
CCl4-2 M group and the CCl4-3M group
(P=0.452; Fig. 2A), COX-2 protein
expression levels were increased in the CCl4-3M compared
with the CCl4-2M group (P=0.042; Fig. 2B and C).
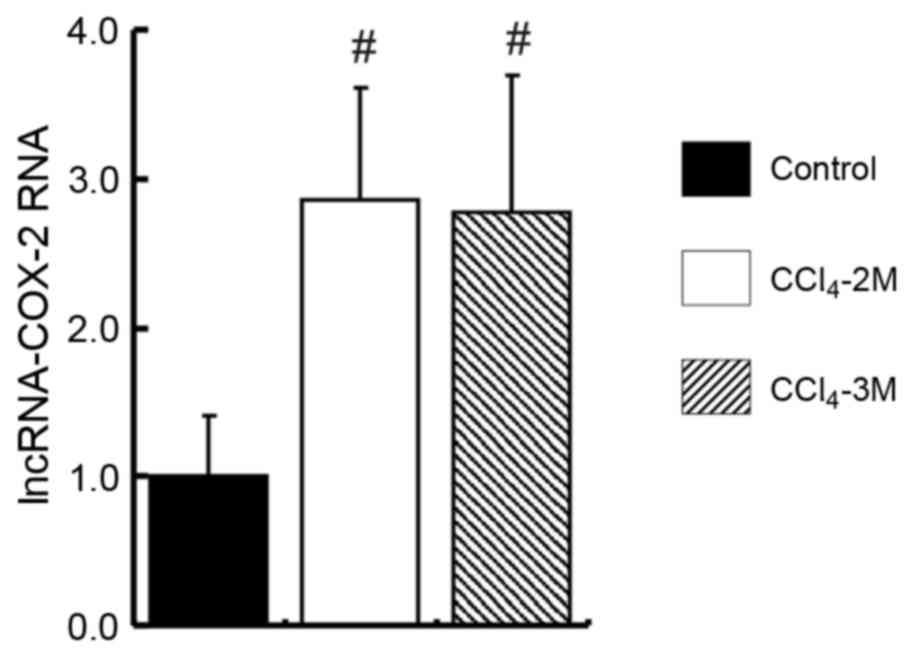
Upregulation of lncRNA-COX-2 is
observed in the cirrhotic liver
The RNA levels of lncRNA-COX-2 in the
CCl4-2M group and CCl4-3M group were 2 times
higher than in the control group (P=0.015 and P=0.008,
respectively; Fig. 3). However,
there was no significant difference in levels of lncRNA-COX-2 RNA
between the CCl4-2 M group and the CCl4-3 M
group (P=0.385; Fig. 3).
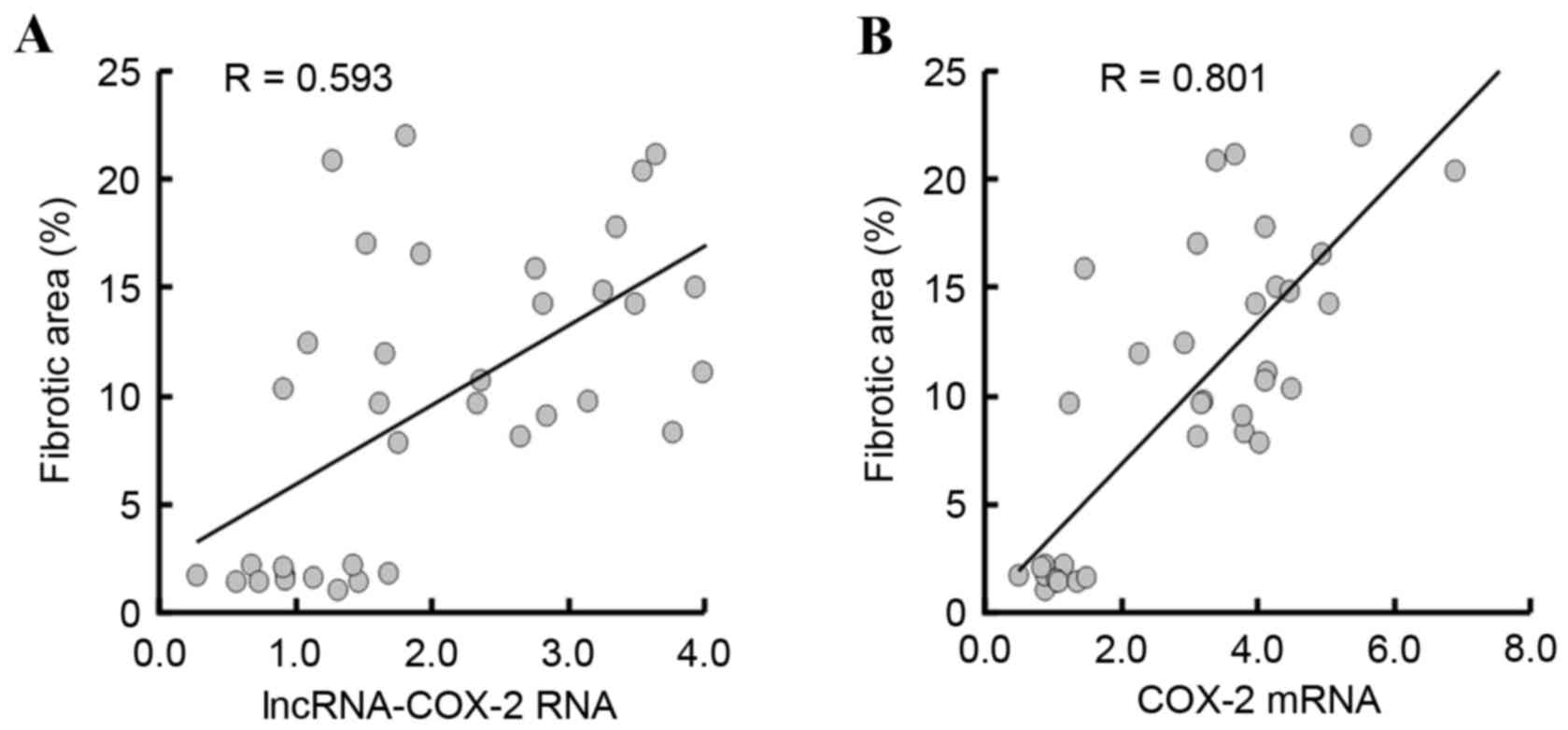
COX-2 mRNA and lncRNA-COX-2 expression
are correlated with fibrosis
By analyzing all the data from the three groups, a
significant positive correlation between lncRNA-COX-2 RNA
expression and fibrotic area was demonstrated (R=0.593, P=0.001;
Fig. 4A). Furthermore, COX-2 mRNA
expression was also significantly positively correlated with
fibrotic area (R=0.801, P=0.001; Fig.
4B).
Elevation of COX-2 mRNA is associated
with increased lncRNA-COX-2 RNA expression
Consistently, by analyzing all the data from the
three groups, the expression of COX-2 mRNA was significantly
positively correlated with increased lncRNA-COX-2 RNA expression in
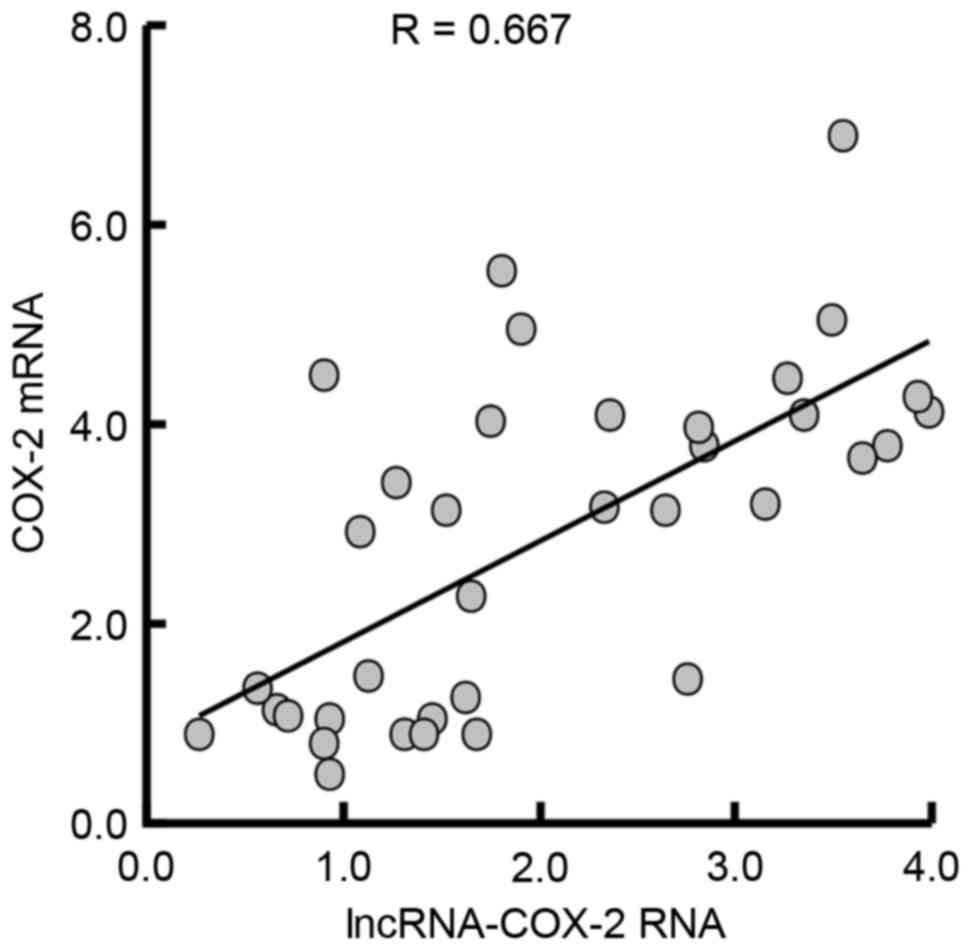
the cirrhotic liver (R=0.667, P=0.001; Fig. 5).
Discussion
Liver fibrosis is a reversible wound-healing process
characterized by excessive accumulation of extracellular matrix
components, followed by the distortion of normal liver
architecture. Without early intervention, this continuous process
progresses into liver cirrhosis, which has a poor prognosis and
high mortality (24). Multiple
anti-fibrotic treatments have been attempted in the past decades,
but at present none have succeeded (2). Thus, it is important to identify a
novel therapeutic target or biomarker for this condition. In the
present study, the expression of lncRNA-COX-2 RNA and COX-2 mRNA
was evaluated in CCL4-induced cirrhotic mice, and COX-2
mRNA and lncRNA-COX-2 RNA expression levels were demonstrated to
increase in liver fibrosis. COX-2 and lncRNA-COX-2 may, therefore,
be involved in the progression of liver fibrosis.
Previous studies have demonstrated that COX-2 is
upregulated in the cirrhotic liver (25,26).
The involvement of COX-2 involved in the pathology of liver
cirrhosis was also verified in previous studies, including
inflammation, activation of hepatic stellate cells, epithelial
mesenchymal transition and angiogenesis (9,27,28).
Although certain previous research has reported that COX-2
inhibitors exacerbate fibrogenesis, COX-2 inhibitors have also been
demonstrated to attenuate liver fibrosis in other studies (8,10,29).
Consistent with these latter findings, the present study
demonstrated that COX-2 mRNA expression levels increased with
progression of liver fibrosis. Furthermore, COX-2 mRNA expression
levels were positively correlated with the extent of the fibrotic
area, which suggested that COX-2 is involved in the progression of
liver cirrhosis (6).
LncRNAs have become a topic of interest with the
development of sequencing technology in transpriptome analysis
(30). In addition, lncRNAs have
been demonstrated to be involved in numerous biological processes,
including proliferation, differentiation, and apoptosis (31,32).
Multiple lncRNAs have been associated with the development of
hepatocellular carcinoma (33,34).
However, the involvement of lncRNAs in other liver diseases remains
largely unknown. It has been demonstrated in the present study that
lncRNA-COX-2 was upregulated in the murine CCl4-induced
cirrhotic liver. Furthermore, a significant correlation between
lncRNA-COX-2 RNA and the extent of the fibrotic area was revealed,
suggesting that lncRNA-COX-2 may be involved in the progression of
liver cirrhosis. Notably, Carpenter et al (20) demonstrated that lncRNA-COX-2 RNA
expression levels and COX protein expression levels were increased
following treatment with lipopolysaccharide in mouse bone
marrow-derived macrophages (BMDMs). Furthermore, whole
transcriptome analysis was conducted and it was determined that
lncRNA-COX-2 was the most highly induced lncRNA in BMDMs following
treatment with synthetic bacterial lipopeptide and the Toll-like
receptor 2 ligand Pam3CSK4 (20).
This suggests that upregulation of lncRNA-COX-2 RNA expression may
be involved in the inflammatory process of liver cirrhosis.
However, further studies are required to elucidate the underlying
cellular and molecular mechanisms.
COX-1 is constitutively expressed in normal tissues,
while the expression of COX-2 is inducible in different ways.
Inflammatory cytokines, growth factors and hormones, which are
increased during liver cirrhosis, result in the upregulation of
COX-2 (35). In, addition,
multiple studies have demonstrated that Kupffer cells and HSCs are
the predominant cells contributing to this increased expression of
COX-2 in liver cirrhosis (36,37).
Increased activation and proliferation of HSCs and Kupffer cells
was observed in liver cirrhosis (38), and in this manner the expression of
COX-2 is upregulated in the cirrhotic liver.
Unlike the traditional inflammatory regulation
pathway for the regulation of COX-2 expression, a novel mechanism
at the transcriptional level has been characterized. Matsui et
al (39) revealed that an
RNA-mediated mechanism activated gene transcription of COX-2. The
RNA sequence in the COX-2 promoter acted as a scaffold for
recognition of the RNA-AGO2-GW182 complex, which activates COX-2
gene expression. Carpenter et al (20) also demonstrated that lncRNA-COX-2
regulates target genes by interacting with various regulatory
complexes, including heterogeneous nuclear ribonucleoprotein A/B
and A2/B1. In addition, Krawczyk and Emerson (40) demonstrated that a lncRNA expressed
in the upstream region of COX-2, called p50-associated COX-2
extragenic RNA, activated COX-2 expression by inhibiting the
binding of the repressive nuclear factor-κΒ p50 subunit to the
promoter of COX-2. These studies suggest that lncRNA-COX-2
regulated the expression of COX-2 in different ways. Consistently,
the present study demonstrated that COX-2 mRNA expression levels
were significantly correlated with lncRNA-COX-2 RNA expression
levels.
In conclusion, the present study has demonstrated
that COX-2 mRNA expression levels and lncRNA-COX-2 RNA expression
levels were increased in the murine cirrhotic liver. In addition,
lncRNA-COX-2 RNA expression levels were significantly correlated
with COX-2 RNA expression levels and the extent of liver fibrosis.
These results indicate that lncRNA-COX-2 may be involved in the
development of liver fibrosis. In addition, lncRNA-COX-2 may
potentially be considered a novel therapeutic target for liver
fibrosis.
Acknowledgements
The authors would like to thank technicians: Mr.
Xian Li and Ms. Ou Qiang from the Division of Peptides Related with
Human Diseases, State Key Laboratory of Biotherapy, West China
Hospital, Sichuan University (Chengdu, China). The present study
was supported by the National Science Fund of China (grant nos.
81170413 and 81400637), the Chinese Postdoctoral Science Foundation
(grant nos. 2014M560721 and 2015T80984), the Chinesisch-Deutsches
Zentrum fṻr Wissenschaftsfὅrderung (grant no, GZ1065) and the
Science and Technology Support Program of Sichuan province (grant
no. 2016SZ0041).
References
|
1
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fallowfield JA: Therapeutic targets in
liver fibrosis. Am J Physiol Gastrointest Liver Physiol.
300:G709–G715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olson JC and Saeian K: Gastrointestinal
issues in liver disease. Crit Care Clin. 32:371–384. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pazirandeh S, Khettry U, Gordon FD,
Resnick RH, Murray JE and Sheth SG: Cyclooxygenase-2 expression in
hepatocellular carcinoma, cirrhosis and chronic hepatitis in the
United States. Dig Dis Sci. 52:220–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simmons DL, Botting RM and Hla T:
Cyclooxygenase isozymes: The biology of prostaglandin synthesis and
inhibition. Pharmacol Rev. 56:387–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeong SW, Jang JY, Lee SH, Kim SG, Cheon
YK, Kim YS, Cho YD, Kim HS, Lee JS, Jin SY, et al: Increased
expression of cyclooxygenase-2 is associated with the progression
to cirrhosis. Korean J Intern Med. 25:364–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou JY, Jiang ZA, Zhao CY, Zhen Z, Wang W
and Nanji AA: Long-term binge and escalating ethanol exposure
causes necroinflammation and fibrosis in rat liver. Alcohol Clin
Exp Res. 37:213–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao JH, Wen SL, Yang WJ, Lu YY, Tong H,
Huang ZY, Liu ZX and Tang CW: Celecoxib ameliorates portal
hypertension of the cirrhotic rats through the dual inhibitory
effects on the intrahepatic fibrosis and angiogenesis. PLoS One.
8:e693092013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wen SL, Gao JH, Yang WJ, Lu YY, Tong H,
Huang ZY, Liu ZX and Tang CW: Celecoxib attenuates hepatic
cirrhosis through inhibition of epithelial-to-mesenchymal
transition of hepatocytes. J Gastroenterol Hepatol. 29:1932–1942.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu J, Hui AY, Chu ES, Go MY, Cheung KF, Wu
CW, Chan HL and Sung JJ: The anti-inflammatory effect of celecoxib
does not prevent liver fibrosis in bile duct-ligated rats. Liver
Int. 29:25–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holt AP and Adams DH: Complex roles of
cyclo-oxygenase 2 in hepatitis. Gut. 56:903–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng W and Fan H: Long non-coding RNA
PANDAR correlates with poor prognosis and promotes tumorigenesis in
hepatocellular carcinoma. Biomed Pharmacother. 72:113–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hung T and Chang HY: Long noncoding RNA in
genome regulation: Prospects and mechanisms. RNA Biol. 7:582–585.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang KC, Yang YW, Liu B, Sanyal A,
Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta
RA, et al: A long noncoding RNA maintains active chromatin to
coordinate homeotic gene expression. Nature. 472:120–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guttman M, Donaghey J, Carey BW, Garber M,
Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al:
lincRNAs act in the circuitry controlling pluripotency and
differentiation. Nature. 477:295–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mourtada-Maarabouni M, Hedge VL, Kirkham
L, Farzaneh F and Williams GT: Growth arrest in human T-cells is
controlled by the non-coding RNA growth-arrest-specific transcript
5 (GAS5). J Cell Sci. 121:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Xie X, Xiao YF, Xie R, Hu CJ, Tang
B, Li BS and Yang SM: The emergence of long non-coding RNAs in the
tumorigenesis of hepatocellular carcinoma. Cancer Lett.
360:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun J, Bie B, Zhang S, Yang J and Li Z:
Long non-coding RNAs: Critical players in hepatocellular carcinoma.
Int J Mol Sci. 15:20434–20448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tong Q, Gong AY, Zhang XT, Lin C, Ma S,
Chen J, Hu G and Chen XM: LincRNA-Cox2 modulates TNF-α-induced
transcription of Il12b gene in intestinal epithelial cells through
regulation of Mi-2/NuRD-mediated epigenetic histone modifications.
FASEB J. 30:1187–1197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carpenter S, Aiello D, Atianand MK, Ricci
EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB,
et al: A long noncoding RNA mediates both activation and repression
of immune response genes. Science. 341:789–792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu WH, Zhang JB, Dang Z, Li X, Zhou T, Liu
J, Wang DS, Song WJ and Dou KF: Long non-coding RNA URHC regulates
cell proliferation and apoptosis via ZAK through the ERK/MAPK
signaling pathway in hepatocellular carcinoma. Int J Biol Sci.
10:664–676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Y, Wu YT, Huang C, Meng XM, Ma TT, Wu
BM, Xu FY, Zhang L, Lv XW and Li J: Inhibitory effects of long
noncoding RNA MEG3 on hepatic stellate cells activation and liver
fibrogenesis. Biochim Biophys Acta. 1842:2204–2215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method). Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giannitrapani L, Ingrao S, Soresi M,
Florena AM, La Spada E, Sandonato L, D'Alessandro N, Cervello M and
Montalto G: Cyclooxygenase-2 expression in chronic liver diseases
and hepatocellular carcinoma: An immunohistochemical study. Ann N Y
Acad Sci. 1155:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohammed NA, Abd E, l-Aleem SA, El-Hafiz
HA and McMahon RF: Distribution of constitutive (COX-1) and
inducible (COX-2) cyclooxygenase in postviral human liver
cirrhosis: A possible role for COX-2 in the pathogenesis of liver
cirrhosis. J Clin Pathol. 57:350–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Y, Wang Y, Wang Q, Liu Z, Liu Q and
Deng X: Hepatic stellate cells produce vascular endothelial growth
factor via phospho-p44/42 mitogen-activated protein
kinase/cyclooxygenase-2 pathway. Mol Cell Biochem. 359:217–223.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sacerdoti D, Pesce P, Di Pascoli M, Brocco
S, Cecchetto L and Bolognesi M: Arachidonic acid metabolites and
endothelial dysfunction of portal hypertension. Prostaglandins
Other Lipid Mediat. 120:80–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu H, Jing X, Zou X and Wu J: Role of
cyclooxygenase 2 and its inhibitor valdecoxib in liver fibrosis.
Zhonghua Yi Xue Za Zhi. 94:784–787. 2014.(In Chinese). PubMed/NCBI
|
|
30
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon JH, Abdelmohsen K and Gorospe M:
Posttranscriptional gene regulation by long noncoding RNA. J Mol
Biol. 425:3723–3730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kitagawa M, Kotake Y and Ohhata T: Long
non-coding RNAs involved in cancer development and cell fate
determination. Curr Drug Targets. 13:1616–1621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang F, Xie C, Zhao W, Deng Z, Yang H and
Fang Q: Long non-coding RNA CARLo-5 expression is associated with
disease progression and predicts outcome in hepatocellular
carcinoma patients. Clin Exp Med. Oct 3–2015.(Epub ahead of
print).
|
|
34
|
Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY,
Zhang F, Wu LM, Chen LM and Zheng SS: Long non-coding RNA MALAT-1
overexpression predicts tumor recurrence of hepatocellular
carcinoma after liver transplantation. Med Oncol. 29:1810–1816.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rouzer CA and Marnett LJ: Cyclooxygenases:
Structural and functional insights. J Lipid Res. 50(50 Suppl):
S29–S34. 2009.PubMed/NCBI
|
|
36
|
Efsen E, Bonacchi A, Pastacaldi S, Valente
AJ, Wenzel UO, Tosti-Guerra C, Pinzani M, Laffi G, Abboud HE,
Gentilini P and Marra F: Agonist-specific regulation of monocyte
chemoattractant protein-1 expression by cyclooxygenase metabolites
in hepatic stellate cells. Hepatology. 33:713–721. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hui AY, Cheng AS, Chan HL, Go MY, Chan FK,
Sakata R, Ueno T, Sata M and Sung JJ: Effect of prostaglandin E2
and prostaglandin I2 on PDGF-induced proliferation of LI90, a human
hepatic stellate cell line. Prostaglandins Leukot Essent Fatty
Acids. 71:329–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawada N: Evolution of hepatic fibrosis
research. Hepatol Res. 41:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsui M, Chu Y, Zhang H, Gagnon KT,
Shaikh S, Kuchimanchi S, Manoharan M, Corey DR and Janowski BA:
Promoter RNA links transcriptional regulation of inflammatory
pathway genes. Nucleic Acids Res. 41:10086–10109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Krawczyk M and Emerson BM: p50-associated
COX-2 extragenic RNA (PACER) activates COX-2 gene expression by
occluding repressive NF-αB complexes. Life. 3:e017762014.
|