Introduction
Lung cancer is a respiratory system malignancy with
high mortality and its incidence has increased in recent years
(1). Despite the number of novel
agents specifically targeting oncogenic pathways that have been
developed for lung cancer treatment, and a combination of
histomorphological, immunohistochemical and genetic analysis
currently employed in routine lung cancer diagnosis to stratify
patients into clinically relevant subgroups for tailored treatment
algorithms (2), metastatic lung
cancer and the development of drug resistance to target therapy and
chemotherapy mean that lung cancer remains incurable, and has poor
patient outcomes with a 5-year survival rate of <20% (3,4).
The platinum-based doublet chemotherapy has been
recommended as the first-line therapy for advanced non-small cell
lung cancer (NSCLC) and has a 20% response rate in patients with
NSCLC (5). However, the prognosis
of this treatment in patients with aggressive NSCLC remains poor,
mainly owing to the development of multidrug resistance, in
particular against cisplatin regimens (6,7).
Mechanistically, the cisplatin therapy induces DNA interstrand and
intrastrand crosslinks in tumor cells, sequentially inhibiting cell
replication and transcription (8).
Therefore, understanding the mechanisms underlying the development
of cisplatin therapy resistance may lead to the development of
efficient therapeutic strategies for NSCLC.
The Wnt/β-catenin signaling pathway has been
recognized as an oncogenic pathway with pivotal roles in numerous
types of cancer, and aberrant activation of Wnt signaling was
detected in 50% of human NSCLC cell lines and resected lung cancer
samples (9), where it was
associated with the increased proliferation and metastatic
properties of lung cancer cells, in addition to resistance to
conventional chemo-radiotherapies and targeted therapies, and a
poor prognosis in patients with lung cancer (10–12).
In this regard, mounting evidence has demonstrated that the
Wnt/β-catenin signaling pathway is an important factor in cancer
stem cell (CSC) fate determination. A previous study (13) revealed an association between CSCs
and the resistance to chemotherapeutic and/or targeted therapeutic
agents. Indeed, previous studies in cancer cell lines (14,15)
have demonstrated that cancer cells with elevated expression of
Wnt1 were resistant to therapy-induced apoptosis. In addition,
platinum-based chemotherapy was reported to induce stem cell-like
properties and therapeutic resistance via the β-catenin signaling
pathway in NSCLC cells (16).
The sex-determining region Y box-containing (Sox)
family of transcriptional factors have emerged as potent modulators
in embryonic development, stem cell maintenance, tissue homeostasis
and carcinogenesis in numerous processes. A previous study
(17) demonstrated that members of
Sox family were important in the development and maintenance of the
lung, and in the tumorigenesis of lung cancer. The Sox genes share
the non-canonical 79 amino acid DNA-binding, high mobility group
(HMG) domain, termed the HMG box domain. To date, 20 different Sox
genes have been identified in mammals (18). Among them Sox2 is the most
extensively studied, due to its crucial roles in embryonic
development, stem cell maintenance and involvement in
carcinogenesis, including in lung cancers (19–21).
Sox2 is intricately involved in numerous cancer-associated
processes including cell proliferation, evading cellular apoptosis
and metastasis, via interactions with other oncogenic/developmental
pathways and processes, and its expression has been demonstrated to
be heavily associated with patient survival rates and prognosis in
clinical settings (21). In
addition, increasing evidence has revealed that Sox2 is involved in
chemoresistance to conventional lung cancer therapies (16,22–24).
A previous study (25) demonstrated that Sox2 gene
amplification was associated with a favorable prognosis in several
types of lung cancers, including NSCLC. Other studies (25,26)
have demonstrated that Sox2 may also exert its roles in
carcinogenesis via the Wnt/β-catenin signaling pathway in breast,
colorectal and prostate cancers and in osteosarcomas. In lung
cancer, the functions of Sox2 in pathogenesis and chemoresistance
have been involved in the mitogen-activated protein kinase kinase
kinase kinase 4/Survivin, epidermal growth factor receptor (EGFR),
SRC proto-oncogene non-receptor tyrosine kinase/Akt
serine/threonine kinase 1 and bone morphogenetic protein 4
signaling pathways (21). However,
the role of Sox2 in the chemoresistance of lung cancer and
Wnt/β-catenin signaling activity in lung cancer has not yet been
identified.
The present study therefore attempted to investigate
the potential role of Sox2 in Wnt/β-catenin signaling and the
chemoresistance of NSCLC cells to cisplatin. The results suggest
that Sox2 may be involved in the chemoresistance of NSCLS to
platinum-based doublet chemotherapy.
Materials and methods
Cell lines and reagents
A549 lung cancer cell line (cat. no. CCL-185) was
purchased from American Type Culture Collection (Mannasas, VA,
USA). The cisplatin-resistant A549/DDP cell line was purchased from
the Bank of Cancer Cell Lines of the Chinese Academy of Medical
Science (Beijing, China) and its drug resistance phenotype was
maintained in a medium containing 10 nM cisplatin (Cayman Chemical
Company, Ann Arbor, MI, USA). The cells were cultured and
maintained at 37°C in a humidified atmosphere of 5%
CO2/95% air in Dulbecco's modified Eagle medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.).
Construction and transfection of
plasmids
To generate a plasmid capable of overexpressing Sox2
in mammalian cells, human Sox2 cDNA (NM_003106) was cloned into the
pcDNA3.1 backbone plasmid downstream of a cytomegalovirus (CMV)
promoter (Invitrogen; Thermo Fisher Scientific, Inc.), which was
referred to as pCDNA-Sox2. To construct a plasmid able to inhibit
Sox2 expression, a small hairpin RNA (shRNA) construct was
generated by annealing the sense oligonucleotide,
5′-CCGGCGCTCATGAAGAAGGATAACTCGAGTTATCCTTCTTCATGAGCGTTTTTG-3′, and
the anti-sense
oligonucleotide5′-AATTCAAAAACGCTCATGAAGAAGGATAACTCGAGTTATCCTTCTTCATGAGCG-3′.
The resulting double stranded shRNA was cloned into a GV248 vector
(Shanghai GenePharma Co., Ltd., Shanghai, China). The canonical Wnt
reporter plasmid carrying a tandem of 7 T cell factor (TCF) binding
sites upstream of a minimal c-fos promoter driving the firefly
luciferase gene (BATflash) and its control plasmid (BOTflash,
containing mutated TCF binding sites) were produced by EMD
Millipore (Billerica, MA, USA). The transfection of control plasmid
expressing Renilla luciferase (RL) from (Promega Corporation,
Madison, WI, USA) was used for assessing the transfection
efficiency. The plasmid DNA transfection was performed using
Lipofectamine LTX reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
Cells transfected with plasmid pcDNA3.1 served as the untreated
control. To investigate the effect of Sox2 on the chemoresistance
of lung cancer cells to cisplatin, the transfected A549 or A549/DDP
cells were then exposed to culture medium containing cisplatin at a
final concentration of 10 µM for 24 h prior to being harvested for
analysis. Control cells were untreated with cisplatin. The pcDNA3.1
plasmid (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was always included as a control.
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
(MTT) assay
Cell proliferation was determined by using an MTT
cell proliferation kit (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China). A549 or A549/DDP cells were cultured in
a 6-well plate and transfected with pcDNA3.1 control plasmid or
plasmids expressing Sox2 or Sox2 shRNA (shSox2) for 12 h, then the
cells were divided and seeded into a 96-well plate at a density of
2×104 per well and allowed to adhere overnight. The cells were then
used for MTT assay at indicated time points following the
manufacturer's protocol (Beijing Solarbio Science & Technology
Co., Ltd.).
Dual-luciferase reporter assay
Wnt/β-catenin signaling was assessed using a dual
luciferase reporter assay, which was determined using a Dual-Glo
Luciferase Assay System (Promega Corporation, Madison, WI, USA) on
a 20/20n Luminometer (Turner Designs, Sunnyvale, CA, USA) according
to the manufacturer's protocols. The A549 or A549/DDP cells were
cultured in a 24-well plate and transfected with plasmid BATflash
or BOTflash for 24 h. The activity of Wnt/β-catenin was assessed by
determining the relative activity of firefly luciferase. The
transfection efficiency was assessed by luciferase activity of the
co-transfected RL plasmid, pCMV-RL (Promega Corporation).
Cell scratch assay
The A549 or A549/DDP cells transfected with a
plasmid expressing Sox2 or shSox2 were seeded at 80% confluence and
exposed to cisplatin for 24 h (cells were cultured to confluence)
in 6-well culture plates. The cells were then scratched with a 200
µl pipette tip. The resultant unattached cells were removed by
washing with pre-warmed PBS three times and the wounded monolayers
were cultured for an additional 24 h prior to staining with 0.1%
crystal violet solution. The closure of the wounded areas was
observed under a light microscope at ×40 magnification and images
were captured. The distance of closure and unrecovered area were
quantified with the NIH Image J image processing program version
1.46 (National Institutes of Health, Bethesda, MD, USA). The
experiments were performed in triplicate. Each condition was tested
in duplicate and each experiment was repeated at least three
times.
Clonogenic assay
A clonogenic assay was used for assessing the degree
of stemness of the A549 and A549/DDP cells. For clonogenicity,
1–2×103 of cells treated under the different conditions were seeded
onto separated 35 mm dishes. Cells were continuously cultured for
10 days with a refreshment of an appropriate medium (e.g.,
containing 10 nM cisplatin for cisplatin-resistant cells or regular
medium for cisplatin-sensitive cells) at intervals of 3 days. For
colony counting, the medium was removed and the cells were rinsed
with PBS prior to being fixed with 4% paraformaldehyde at room
temperature for 5 min. Following the removal of the fixative, the
cells were then stained with 0.5% crystal violet solution and
incubated at room temperature for 30 min. The staining solution was
carefully removed, and the cells were rinsed with H2O to
remove residual staining solution, prior to air-drying the sample
at room temperature for up to a day. The number of colonies were
counted and calculated under a light microscope. Each condition was
tested in duplicate and each experiment was repeated three
times.
Transwell assay
In order to assess the effect of Sox2 on
cisplatin-resistant lung cancer cells, the invasive capacity of
A549 or A549DDP cells transfected with the plasmid either
expressing Sox2 or shSox2 was ascertained by a Transwell assay
using Transwell migration chambers (BD Biosciences, Franklin Lakes,
NJ, USA). The 8-µm filters were coated with 100 µl Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA), which was diluted to 1:2 of
concentration using serum-free 1640 medium (Invitrogen; Thermo
Fisher Scientific, Inc.), and incubated at 37°C in a 5%
CO2 atmosphere for 30 min for gelling. A total of 104
cells resuspended in 100 µl DMEM basal medium were seeded in the
upper chamber, and 700 µl of DMEM medium supplemented with 10% FBS
was added in the lower chamber. The culture was then incubated at
37°C in a 5% CO2 atmosphere for 12 h. The medium was
then removed and the cells were washed twice with cool PBS. Cells
were then fixed with 4% paraformaldehyde for 20 min, prior to being
stained with 1% crystal violet for 20 min. The crystal violet was
removed from the top of the membrane with a pipette tip or cotton
tipped applicator, then the rinsed the Transwell membrane with
distilled water to remove the excess crystal violet and allowed to
dry for a day. The number of cells in 10 different fields of view
was counted under a Upright light microscope (Leica DM4B, equipped
with DFC450 camera, Leica, Shanghai, China) to obtain an average
sum of cells that had migrated from the top of membrane toward the
basolateral side of the membrane. The percentage of invasive cells
was calculated as (the average sum of cells attached on basolateral
membrane/the average sum of cells (attached on the top membrane +
attached on basolateral membrane) ×100%.
Apoptosis assay
Cell apoptosis was assessed by Annexin V analysis
using flow cytometry. For flow cytometry, cells were detached and
labeled using an Annexin V-FITC/propidium iodide (PI) apoptosis
detection kit (NeoBioscience Technology Co., Ltd., Beijing, China)
according to the manufacturer's protocol. Apoptotic and necrotic
cells were quantified using a flow cytometer (FACSCalibur; BD
Biosciences, San Jose, CA, USA) and the CellQuest software (BD
Biosciences, Franklin Lakes, NJ, USA). For each sample, ≥10,000
cells were analyzed. Cells negative for Annexin V and PI were
considered to be viable, and cells stained with Annexin V but not
PI were considered to be apoptotic.
Immunoblotting analysis
Whole cell lysates were prepared in a lysis buffer
(50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 150 mM NaCl, 0.5% NP-40), and
cell nuclear proteins were extracted with the NucBuster Protein
Extraction kit following the manufacturer's protocol (Novagen; EMD
Millipore, Billerica, MA, USA). Whole cell extract or nuclear
extract of cells (40 µg) were resolved by a 10% sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis, followed by being
transferred to a PVDF membrane (EMD Millipore). The membranes were
blocked with blocking buffer (5% fat-free milk in PBS-0.1%
Tween-20) at room temperature for 1 h prior to being probed with
the primary antibody at 4°C overnight, followed by being incubated
with the appropriate horseradish peroxidase-labeled secondary
antibody (Donkey anti-mouse immunoglobulin G, cat. no. 109415;
donkey anti-rabbit immunoglobulin G, cat. no. 108894; or donkey
anti-goat immunoglobulin G, cat. no. 109291. All secondary
antibodies were applied by 1:2,000 dilution in blocking buffer.
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at
room temperature for 2 h. The blots were developed using the
enhanced chemiluminescence (ECL) reagent (Advansta, Menlo Park, CA,
USA) after they were incubated with the appropriate peroxidase
labeled secondary antibodies. Antibodies against β-actin (cat. no.
sc-8432; 1:1,000 dilution), lymphoid enhancer-binding factor-1
(LEF-1; cat. no. sc-8592; 1:1,000 dilution) were obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA); the antibody to
phosphorylated (phos)-catenin (cat. no. ab75777; 1:1,000 dilution)
was purchased from Abcam (Cambridge, MA, USA); antibodies against
phos-glycogen synthase kinase (GSK)3β (cat. no. 05–413; 1:1,000
dilution), acetyl-Histone H3 (cat. no. 06–942; 1:1,000 dilution)
and active β-catenin (cat. no. 05–665; 1:10,000 dilution) were
purchased from EMD Millipore; the antibody to GSK3β (cat. no.
610202; 1:1,000 dilution) was purchased from BD Biosciences (San
Jose, CA, USA); antibodies against Sox2 (cat. no. 66411; 1:500
dilution), B-cell lymphoma 2 (Bcl-2) (ct. no. 12789; 1:500
dilution), caspase-3 (cat. no. 10380; 1:1,000 dilution), Bcl-2-like
protein 4 (Bax; cat. no. 15422; 1:1,000 dilution), myeloid cell
leukemia sequence 1 protein (Mcl-1; cat. no. 66026; 1:1,000
dilution) and apoptosis inducing factor (AIF; cat. no. 17984;
1:1,000 dilution) were purchased from Proteintech (Wuhan Sanying
Biotechnology, Wuhan, China). The expressions of proteins of
interest were semi-quantified by optical densitometry using Image J
software version 1.46 (National Institutes of Health, Bethesda, MD,
USA). The ratio between the net intensity of each sample divided by
the respective internal controls (β-actin or Histone H3) was
calculated as densitometric arbitrary units, which served as an
index of the relative expression of a protein of interest, the fold
of induction for a specific protein under an indicated condition
was calculated by comparing its relative expression over the
control (27).
Statistical analysis
All data collected in this study were obtained from
at least three independent experiments for each set of
circumstances. SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) and
PRISM 5 (GraphPad Software, Inc., La Jolla, CA, USA) were used for
statistical analysis. Statistical evaluation of the data was
performed by one-way analysis of variance when more than two groups
were compared with a single control, and by t-test for comparison
of differences between the two groups. P<0.05 was considered to
indicate a statistically significant difference Data was presented
as the mean ± standard deviation.
Results
Sox2 suppresses the Wnt/β-catenin
signaling activity in lung cancer cells
In order to examine the potential role of Sox2 in
canonical Wnt signaling, lung cancer A549 and cisplatin-resistant
A549/DDP cells, enforced expression of Sox2 or shSox2, and the
Wnt/β-catenin signaling activity was ascertained in terms of a dual
luciferase Wnt reporter assay, in addition to the expression of key
components in the Wnt/β-catenin signaling cascade. The results
demonstrated decreased Wnt activity in cells transfected with Sox2
compared with control cells (P<0.01), and marginally increased
activity of Wnt signaling in A549 and A549/DDP cells which
overexpressed shSox2 in comparison with cells transfected with
TOPflash and pcDNA3.1 plasmids (Fig.
1A). However, no change of luciferase activity was detected
between BOTflash-transfected cells co-transfected with pcDNA3.1,
Sox2 or shSox2 plasmids (data not shown). Molecular analysis by
immunoblotting assay furthermore revealed that the quantities of
active β-catenin, transcription factor LEF-1 and the Wnt signaling
target gene, cyclin D1, were decreased, whereas the quantities of
GSK3β and phos-β-catenin protein were increased in cells
transfected with Sox2 (Fig. 1B),
suggesting that Sox2 may inhibit Wnt/β-catenin signaling activity
in lung cancer cells.
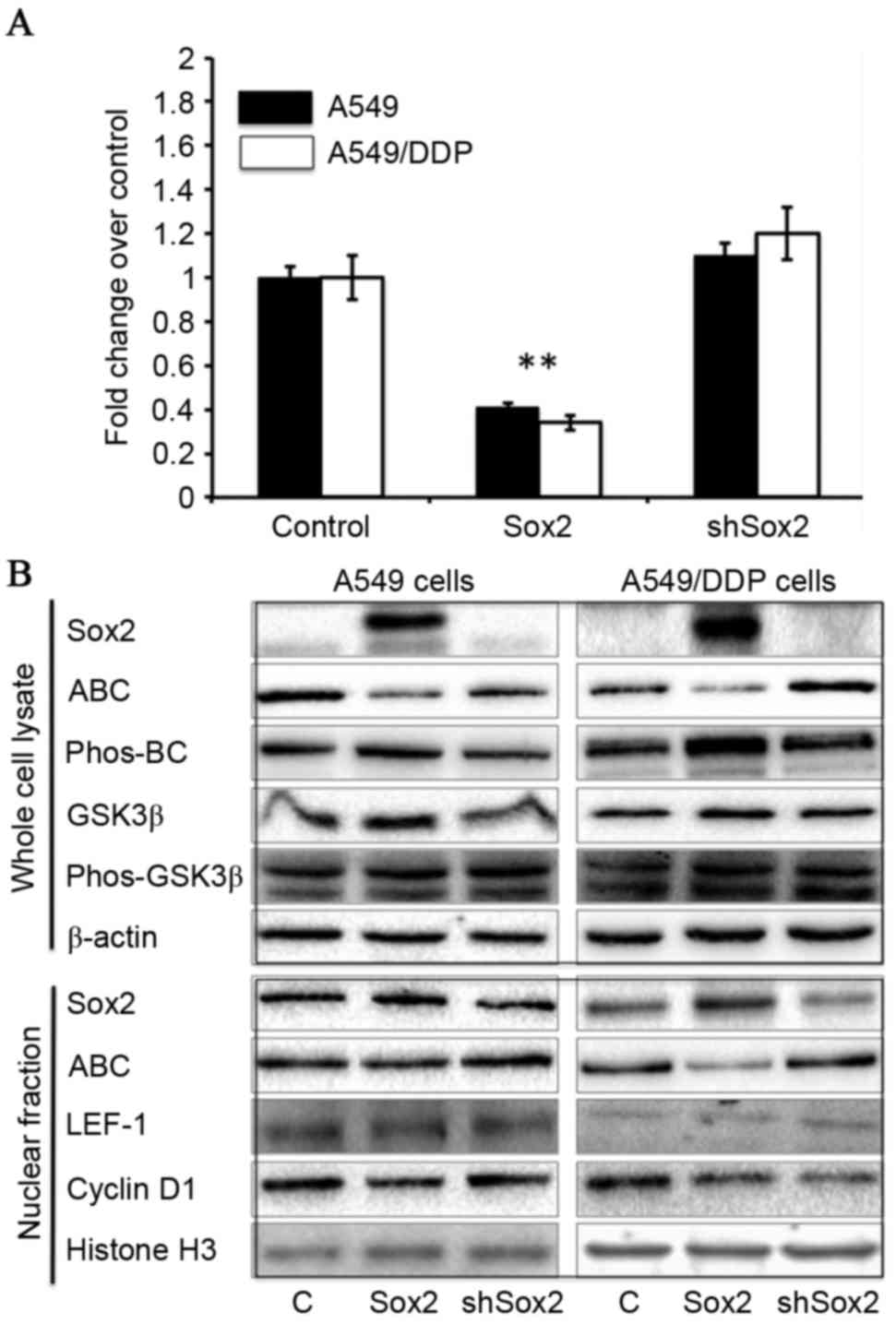 | Figure 1.Sox2 suppresses the Wnt/β-catenin
signaling activity in A549 and A549/DDP cells. A549 and A549/DDP
were transfected with canonical Wnt signaling reporter BATflash and
a plasmid expressing Renilla luciferase, along with a plasmid
expressing Sox2 or shSox2, or a pcDNA3.1 plasmid for 24 h. The
cells were then harvested for analysis of luciferase activity and
the expression of key components of Wnt/β-catenin signaling
cascade. (A) Wnt/β-catenin signaling luciferase reporter
demonstrates that Sox2 may inhibit Wnt signaling activity in A549
and A549/DDP cells (P<0.01), whereas the cells transfected with
shSox2 exhibited a moderately enhanced luciferase activity, as
compared with cells transfected with BATflash and pcDNA3.1 plasmids
(n=9). (B) Molecular analysis by immunoblotting demonstrated a
decreased expression of indicated Wnt signaling activators
including ABC, LEF-1 and cyclin D1, and an increased expression of
Wnt signaling inhibitor, GSK3β, and phos-BC in Sox2-transfected
cells. All data are presented as the mean ± standard deviation of
at least three independent triplicated experiments. **P<0.01 vs.
control. Sox2, sex-determining region Y box 2; shSox2, Sox2 short
hairpin RNA; ABC, active β-catenin; phos-, phosphorylated; BC,
β-catenin; GSK3β, glycogen synthase kinase 3β; LEF-1, lymphoid
enhancer-binding factor-1. |
Sox2 reduces the cisplatin-induced
apoptosis of lung cancer cells
The present study also investigated the effect of
Sox2 on cisplatin-mediated cell apoptosis in lung cancer cells. The
results from the MTT assay revealed that a transient expression of
Sox2 or shSox2 had no effect on cell proliferation, but
overexpression of Sox2 may increase the survival rate of A549 cells
in the presence of cisplatin, although it had no effect on
cisplatin-resistant A59/DDP cells. Notably, a suppression of Sox2
expression by transfection of shRNA led to an increase in the
cisplatin-induced cell death in A549/DDP cells (P<0.05; Fig. 2A). The cytometric analysis also
demonstrated that an inhibition of Sox2 by shSox2 significantly
enhanced cisplatin-induced apoptosis in A549 and A549/DDP cells
(P<0.05; Fig. 2B), despite the
evidence that the overall fraction of apoptotic cells was small. In
the absence of cisplatin, molecular analysis further indicated that
an overexpression of Sox2 tended to increase the expression of
pro-apoptotic proteins (caspase-3 and Bax), but decreased
expression of AIF and anti-apoptotic protein Bcl-2 and Mcl-1 in
A549 lung cancer cells; however, decreased caspase-3, AIF, Mcl-1
protein expression and increased BAX and Bcl-2 protein expression
were observed in A549/DDP cells overexpressing Sox2. Similarly in
the absence of cisplatin, an introduction of shSox2 led a reduced
abundance of caspase-3 and Mcl-1 proteins in both A549 and A549/DDP
cells. Decreased AIF and Bcl-1 protein expression was also detected
in A549 cells, but expression levels increased in
cisplatin-resistant A459/DDP cells. In contrast, in the presence of
cisplatin, a reduced expression of caspase-3, Bcl-2 and Mcl-1 was
observed, but increased BAX and AIF expression was observed in A549
cells transfected with either Sox2 expressed plasmid or shSox2.
Caspase-3 expression was increased in A549 cells ectopically
expressing shSox2; in A549/DDP cells, however, an overexpression of
Sox2 induced increased caspase-3 protein expression but decreased
expressions of BAX, AIF, Bcl-1 and Mcl-1, and an introduction of
shSox2 resulted in upregulated expression of caspase-3, AIF and
Bcl-2 but downregulated BAX and Mcl-1 protein expression (Fig. 3). Of note, certain controversial
results were also observed between the A549 cells and
cisplatin-resistant A549/DDP cells, as well as between cells with a
gain and loss of Sox2 function in the present study, which requires
further elucidation. Nonetheless, the data from the functional
studies implied that Sox2 may be a target for sensitizing
cisplatin-resistant lung cancer cells to chemopreventive agents,
including cisplatin.
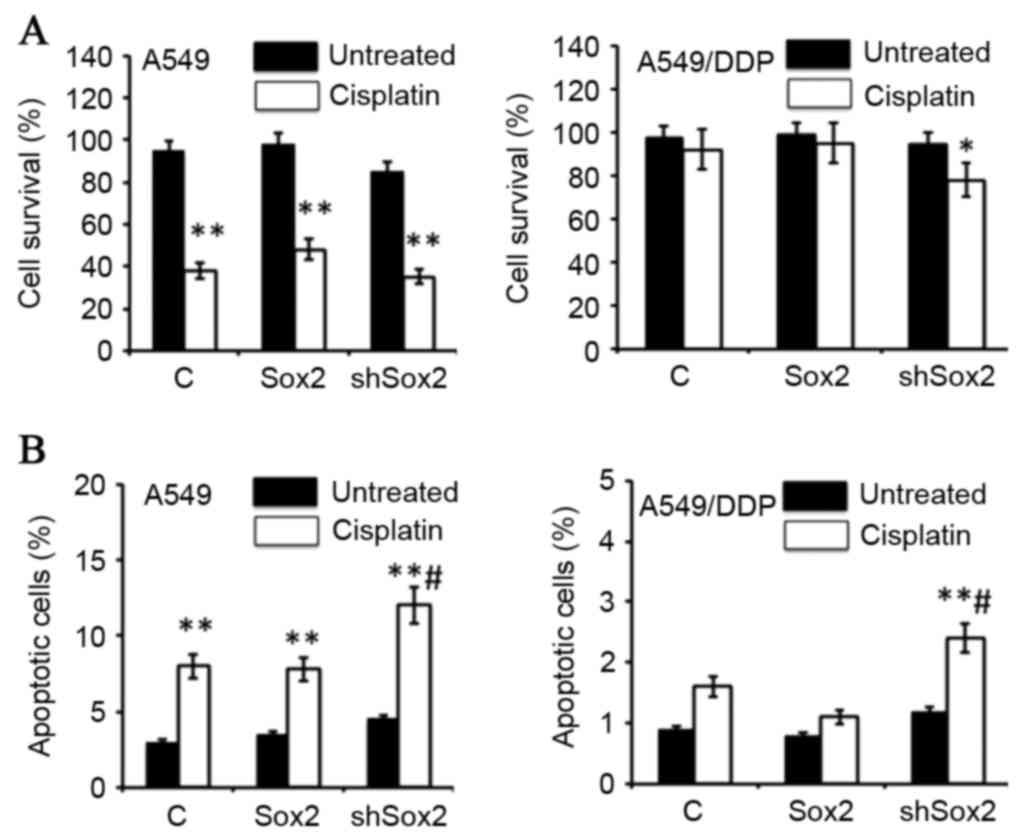 | Figure 2.Sox2 inhibits cisplatin-induced
apoptosis in lung cancer cells. A549 and A549/DDP cells were
transfected with a plasmid expressing Sox2 or shSox2, or a pcDNA3.1
plasmid for 12 h, and then cultured in medium containing 10 µM
cisplatin for an additional 24 h prior to being harvested for
analysis. (A) MTT assay determined the proliferation of cells in
the presence of cisplatin. The transient transduction of Sox2 or
shSox2 had no effect on cell proliferation. Overexpression of Sox2
increased the survival rate of A549 cells in the presence of
cisplatin, but had no effect on cisplatin-resistant A59/DDP cells.
Notably, inhibition of Sox2 expression by short hairpin RNA
increased the cisplatin-induced cell death in A549/DDP cells. (B)
Cell apoptosis analyzed by a cytometric assay. An inhibition of
Sox2 by shSox2 significantly enhanced cisplatin-induced apoptosis
in A549 and A549/DDP cells (P<0.05). All data are presented as
the mean ± standard deviation of three independent triplicated
experiments (n=9). *P<0.05, **P<0.01 vs. the corresponding
non-cisplatin-treated group, #P<0.05 vs. the
cisplatin-treated pcDNA3.1-transfected cells. Sox2, sex-determining
region Y box 2; shSox2, Sox2 short hairpin RNA; MTT,
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide. |
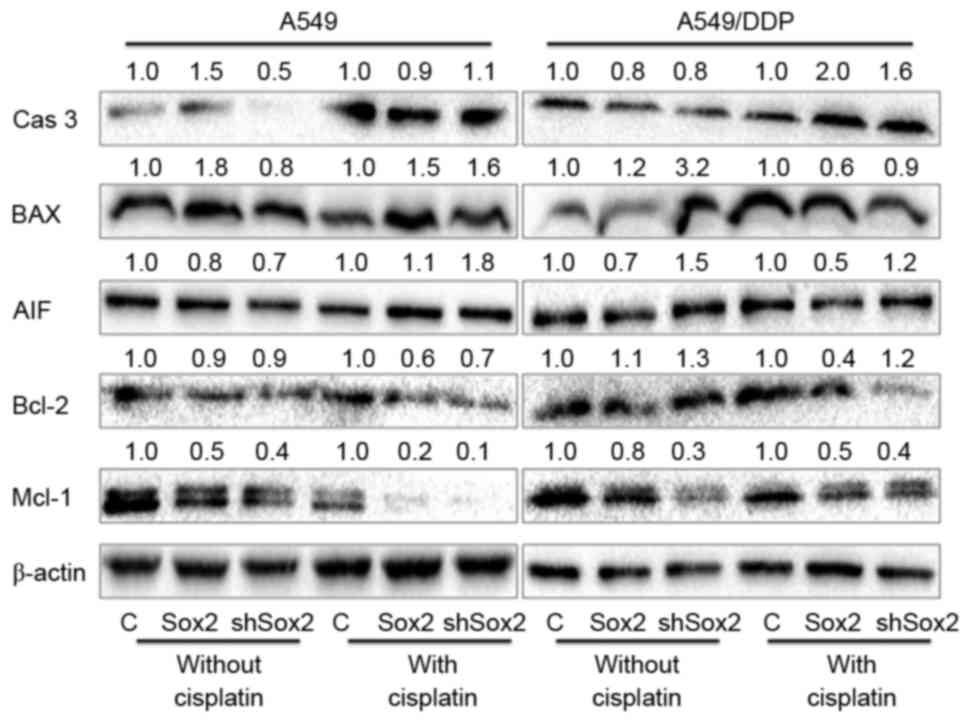 | Figure 3.Apoptosis associated proteins
determined by an immunoblotting analysis. A549 and A549/DDP cells
were transfected with plasmid expressing Sox2 or shSox2, or control
pcDNA3.1 plasmid for 12 h, and then cultured in medium containing
10 µM cisplatin for additional 24 h prior to being harvested for
immunoblotting analysis for indicated proteins. The values labeled
on the top of each bands represented the relative expression levels
of proteins over their respective pcDNA3.1 control as determined by
a densitometric assay. Overexpression of Sox2 demonstrated a trend
to reduce the expression of pro-apoptotic proteins (caspase-3,
Bax), but increased the expression of anti-apoptotic proteins Bcl-2
in lung cancer cells. Cas 3: caspase-3; Bax, Bcl-2-like protein 4;
AIF, apoptosis inducing factor; Bcl-2, B-cell lymphoma 2; Mcl-1,
myeloid cell leukemia sequence 1 protein; C, control; Sox2,
sex-determining region Y box 2; shSox2, Sox2 short hairpin RNA. |
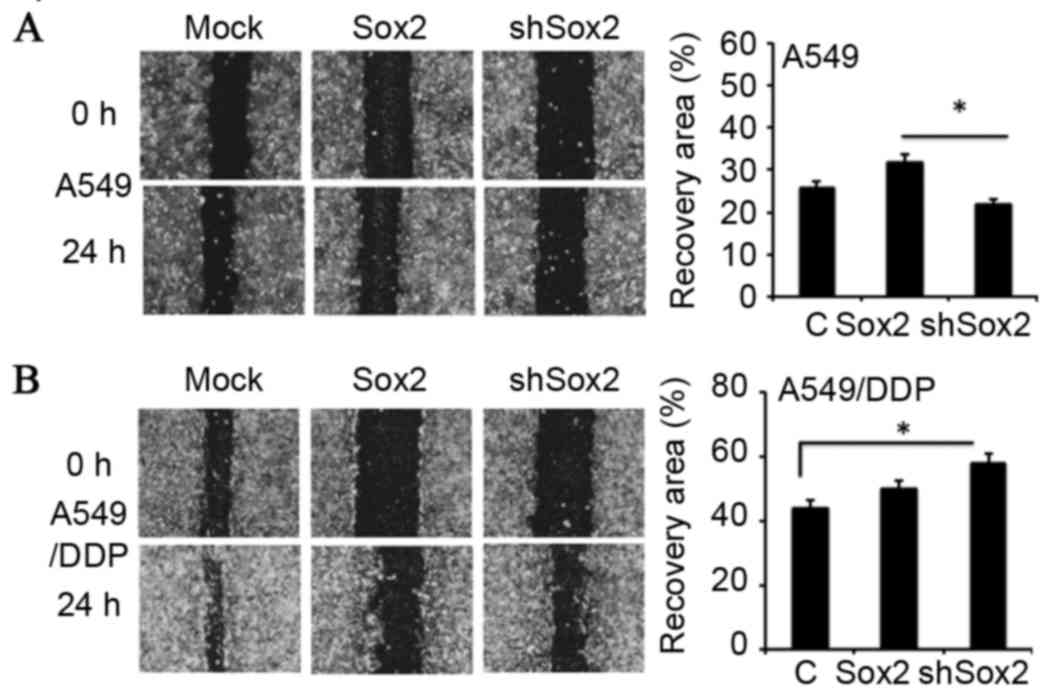
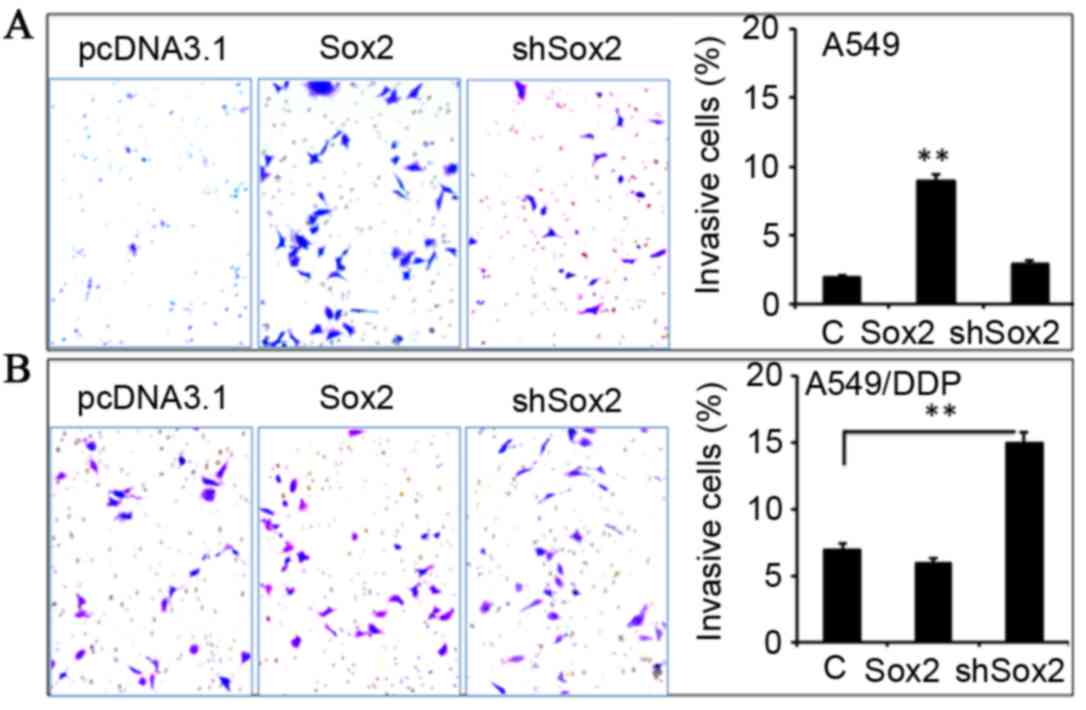
Effect of Sox2 on the migration and
invasion of lung cancer cells in vitro
In order to investigate whether Sox2 has an effect
on the metastatic properties of lung cancer cells, the capability
of migration and invasion in A549 and A549/DDP cells introduced
with a plasmid expressing Sox2 or shSox2 was evaluated by scratch
assay (Fig. 4) and Transwell
analysis (Fig. 5), respectively.
The results demonstrated that A549 cells overexpressing Sox2
exhibited enhanced capacities of migration (Fig. 4A) and invasion compared with the
control cells (Fig. 5A;
P<0.05), although a similar effect was not observed in A549/DDP
cells (Figs. 4B and 5B). Notably, a reduced expression of Sox2
by shSox2 demonstrated the potential to promote cell migration
(Fig. 4B) and invasion (Fig. 5B) in A549/DDP cells (P<0.05),
but not in A549 cells (Figs. 4A
and 5A). These results suggest
that Sox2 may play a regulatory role in the migration and invasion
of lung cancer cells in a cell-context-dependent manner.
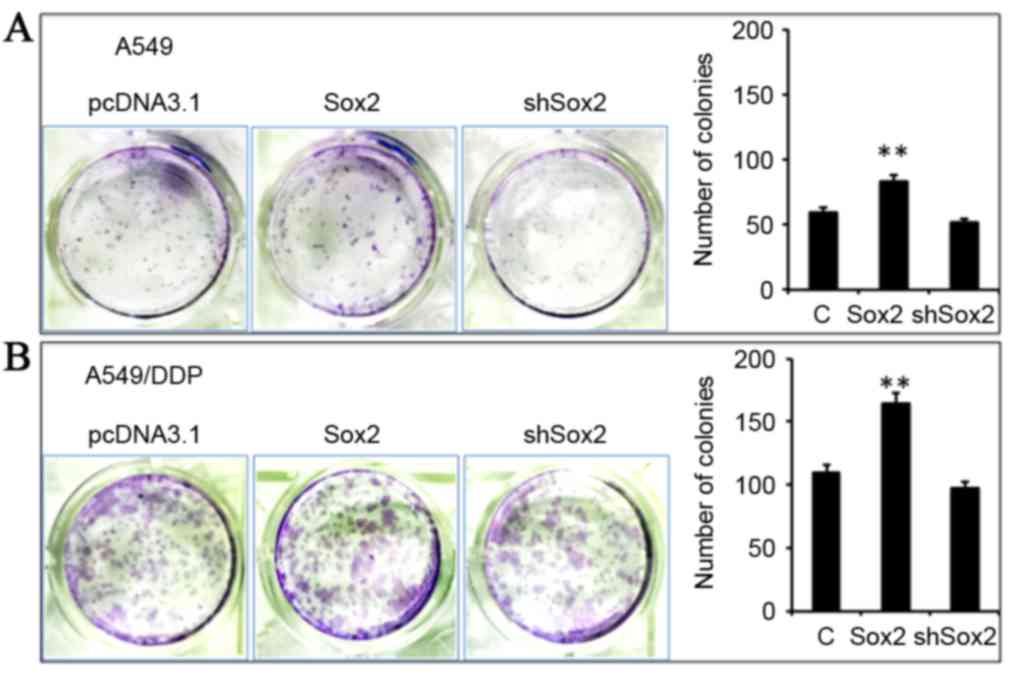
Sox2 enhances the stemness of lung
cancer cells
Since Sox2 is a well-characterized marker of
pluripotency of stem cells and CSCs (24,28,29),
the capacity for clone formation of lung cancer cells altered by an
overexpression of Sox2 was using a clonogenic assay (Fig. 6). Notably, cells overexpressing
Sox2 demonstrated an enhanced clonogenicity in A549 and A549/DDP
cells compared with control cells (P<0.05), although a decreased
expression of Sox2 by shSox2 only marginally reduced the
clonogenicity in A549 and A549/DDP cells, regardless of their
resistance to cisplatin (Fig. 6).
Notably, the clonogenicity of A549/DDP cells was markedly increased
compared with A549 cells (Fig. 6A and
B), indicating that a more abundant lung CSC population may
exist in the cisplatin-resistant A549/DDP cells compared with their
parent cells.
Discussion
Chemotherapy is a common treatment for lung cancer
and regimens containing cisplatin remain the main treatment in
clinical settings (30). However,
the development of resistance to chemotherapeutic agents eventually
leads the failure of lung cancer chemotherapy (5,31).
Therefore, an improved understanding of mechanisms underpinning the
chemoresistance of lung cancer may aid the identification of novel
targets for reversing drug-resistance in cancer therapy. The
present study investigated the potential roles of the Sox2 gene in
cisplatin-resistant lung cancer cells. The results demonstrated
that Sox2 may inhibit Wnt/β-catenin signaling activity and effect
the proliferation, metastasis and chemoresistance of lung cancer
cells. In this context, Sox2 repressed Wnt/β-catenin signaling,
promoted cell proliferation and clonogenicity, and inhibited
cisplatin-induced cell apoptosis in lung cancer cells. Notably,
cell context-dependent Sox2-promoted cell migration and invasion
were also observed, that is, Sox2 may enhance the migratory and
invasive capacity of A549 cells; by contrast, a reduced expression
of Sox2 by shRNA increased the migration and invasion of A549/DDP
cells. More importantly, targeting Sox2 using shRNA demonstrated a
potential to sensitize A549/DDP cells to cisplatin, suggesting that
Sox2 may be a novel target for chemotherapy in lung cancer.
Wnt signaling has been recognized as serving
multiple functions in cell proliferation and migration,
organogenesis and tissue homeostasis (32). Increasing evidence suggests an
interaction between the Sox2 and Wnt signaling pathways (33–35).
For example, Sox2 has been identified as being able to bind to
β-catenin and inhibit the differentiation of stem cells into
osteoblast lineage by attenuating Wnt signaling through
post-transcriptional and transcriptional mechanisms. Sox2 regulates
the expression of Wnt signaling inhibitors dickkopf-1, adenomatous
polyposis coli and GSK3β, enhances the stemness of cancer stem
cells and increases the tumorigenic capacity of osteosarcoma
(26,36). In agreement with this finding, an
inhibitory role of Sox2 in Wnt/β-catenin signaling was also
identified in A549 cells and A549/DDP cells, partially by
regulating the expression of GSK3β. Conversely, Sox2 may
synergistically act with β-catenin to transcriptionally regulate
cyclin D1 gene expression and promote cell proliferation and
tumorigenesis by facilitating the G1/S transition in breast cancer
cells (37). Results from these
studies and the present study may imply a cell context-dependent
bifunctional role of Sox2 in regulation of Wnt/β-catenin signaling
activity.
As an important pluripotent marker of stem cells,
Sox2 has been recognized as serving a crucial role in maintaining
the properties of cancer stem cells that contribute to resistance
to therapeutic agents (20,24,28,38,39).
Therefore, inhibition of Sox2 may result in decreased metastatic
characteristics of cancer cells and an increased sensitivity of
these cells to chemotherapeutic and/or targeted therapeutic agents
(21). Recently, the role of Sox2
and its mechanisms in CSC maintenance and regulation have prompted
an increased interest (13,15,34,40,41).
In this way, the regulatory role of Sox2 in CSC self-renewal and
maintenance has been investigated in numerous types of cancer,
including breast, prostate, gastric, ovarian, pancreatic and lung
cancers (24,42–44).
Notably, a knockdown of Sox2 gene expression by small interfering
RNA (siRNA) or shRNA demonstrated abilities to reduce CSC
properties in several types of cancer. For example, siRNA-mediated
Sox2 knockdown in gastric cancer cells led to a reduced spheroid
colony formation and increased apoptosis within sphere cells
(24). In human glioma cells,
siRNA of Sox2 demonstrated an ability to attenuate S-phase entry
and induce a RhoA-dependent switch to protease-independent amoeboid
migration (45). Another example
is the enhanced self-renewal capacity of prostate CSCs induced by
EGFR-mediated Sox2 expression (46). With respect to lung cancer, a
siRNA-mediated Sox2 knockdown in NSCLC cells also exhibited a
significant reduction of sphere formation (47). In the current study, an increased
expression of Sox2 demonstrated the potential to enhance
clonogenicity in A549 lung cancer cells, although the
shRNA-mediated Sox2 knockdown only moderately reduced the clone
formation. It was hypothesized that the inefficient inhibition of
shSox2 in A549 clone formation may be due to the expression of
shRNA being transiently introduced rather than persistently
expressed, in addition to the effect of transfection efficacy. More
pronounced clonogenic capacity was observed in A549/DDP cells
compared with the parent A549 cells, partially due to that
cisplatin-resistant A549 cell populations may be selected fractions
of cells with CSC potentials. These studies thus emphasize the
importance of the Sox2 gene in the maintenance of the stemness of
CSCs.
Increasing evidence has suggested that Sox2
expression is associated with the cancer hallmarks of sustained
proliferative signaling, activation of invasion and metastasis, and
evasion of cell death (48). In
this respect, Sox2 has been reported to promote cellular
proliferation in breast, prostate, pancreatic and cervical cancers
(39), evade apoptosis in prostate
and gastric cancer, and NSCLC (39,42),
and promote invasion, migration and metastasis in melanoma,
colorectal, glioma, gastric and ovarian cancers, and in
hepatocellular carcinoma (49).
Notably, the involvement of Sox2 in cancer cell physiology was
demonstrated to vary between different types of cancer cell
(21). In the present study, we
also identified that Sox2 may promote migration and invasion in
A549 cells but not in A549/DDP cells. By contrast, a knockdown of
Sox2 increased the migration and invasion in A549/DDP cells but not
in A549 cells, although the underlying mechanism remains to be
elucidated. Together with the present study and others, these data
suggest that the Sox2 gene serves a cell context-dependent role in
maintaining the physiological phenotype of cancer cells.
Aside from its role in cancer cell migration and
invasion, Sox2 also serves an important role in evading apoptotic
signals. In this context, an overexpression of Sox2 may induce the
increased apoptotic resistance in prostate cancer cells and
xenograft models (39). Equally
noteworthy, a knockdown of Sox2 may induce apoptosis in NSCLC cell
lines (42). For example, the
shRNA-mediated knockdown of Sox2 in EGFR mutated lung cancer HCC827
cells exhibited a decreased proliferation and an increased
sensitivity of cells to erlotinib (50). In agreement with these findings,
the present study also identified that a knockdown of Sox2
expression demonstrated the potential to enhance the sensitivity of
A549/DDP cells to cisplatin. Therefore, targeting Sox2 gene in lung
cancer may be therapeutically beneficial.
In summary, the present study demonstrated that an
overexpression of the Sox2 gene led to the decreased activity of
Wnt/β-catenin signaling in lung adenocarcinoma A549 cells and the
cisplatin-resistant A549/DDP cells through an upregulation of the
Wnt/β-catenin signaling negative regulator GSK3β. Notably, the
increased expression of the Sox2 gene was able to promote cell
migration and invasion, in addition to enhancing clonogenic
capacity in A549 cells. Conversely, a knockdown of Sox2 expression
by shRNA led to an enhanced susceptibility of A549 and A549/DDP
cells to cisplatin, along with an increased cisplatin-induced
apoptosis of cancer cells. The present study therefore suggests
that the Sox2 gene may be a novel target for the treatment of
chemoresistant lung cancers.
Acknowledgments
This study was supported by a grant from The Natural
Science Foundation of Ningxia (grant no. NZ15277) to Jinxi He, and
a starting grant (grant no. XM2015093) from the Ningxia Medical
University to Juan Shi.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chuang JC, Neal JW, Niu XM and Wakelee HA:
Adjuvant therapy for EGFR mutant and ALK positive NSCLC: Current
data and future prospects. Lung Cancer. 90:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naidu S and Garofalo M: microRNAs: An
emerging paradigm in lung cancer chemoresistance. Front Med
(Lausanne). 2:772015.PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rose MC, Kostyanovskaya E and Huang RS:
Pharmacogenomics of cisplatin sensitivity in non-small cell lung
cancer. Genomics Proteomics Bioinformatics. 12:198–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stinchcombe TE: Recent advances in the
treatment of non-small cell and small cell lung cancer. F1000prime
Rep. 6:1172014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brabec V and Kasparkova J: Molecular
aspects of resistance to antitumor platinum drugs. Drug Resist
Updat. 5:147–161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akiri G, Cherian MM, Vijayakumar S, Liu G,
Bafico A and Aaronson SA: Wnt pathway aberrations including
autocrine Wnt activation occur at high frequency in human
non-small-cell lung carcinoma. Oncogene. 28:2163–2172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartis D, Csongei V, Weich A, Kiss E,
Barko S, Kovacs T, Avdicevic M, D'Souza VK, Rapp J, Kvell K, et al:
Down-regulation of canonical and up-regulation of non-canonical Wnt
signalling in the carcinogenic process of squamous cell lung
carcinoma. PLoS One. 8:e573932013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakata A, Yoshida R, Yamaguchi R, Yamauchi
M, Tamada Y, Fujita A, Shimamura T, Imoto S, Higuchi T, Nomura M,
et al: Elevated β-catenin pathway as a novel target for patients
with resistance to EGF receptor targeting drugs. Sci Rep.
5:130762015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Connor ML, Xiang D, Shigdar S, Macdonald
J, Li Y, Wang T, Pu C, Wang Z, Qiao L and Duan W: Cancer stem
cells: A contentious hypothesis now moving forward. Cancer Lett.
344:180–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen S, Guttridge DC, You Z, Zhang Z,
Fribley A, Mayo MW, Kitajewski J and Wang CY: Wnt-1 signaling
inhibits apoptosis by activating beta-catenin/T cell
factor-mediated transcription. J Cell Biol. 152:87–96. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/beta-catenin signaling regulates cancer stem cells in lung
cancer A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barr MP, Gray SG, Hoffmann AC, Hilger RA,
Thomale J, O'Flaherty JD, Fennell DA, Richard D, O'Leary JJ and
O'Byrne KJ: Generation and characterisation of cisplatin-resistant
non-small cell lung cancer cell lines displaying a stem-like
signature. PLoS One. 8:e541932013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Li Y, Jun Wei JW and Liu X: The
role of Sox genes in lung morphogenesis and cancer. Int J Mol Sci.
13:15767–15783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kormish JD, Sinner D and Zorn AM:
Interactions between SOX factors and Wnt/beta-catenin signaling in
development and disease. Dev Dyn. 239:56–68. 2010.PubMed/NCBI
|
|
19
|
Chou YT, Lee CC, Hsiao SH, Lin SE, Lin SC,
Chung CH, Chung CH, Kao YR, Wang YH, Chen CT, et al: The emerging
role of SOX2 in cell proliferation and survival and its crosstalk
with oncogenic signaling in lung cancer. Stem cells. 31:2607–2619.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakatsugawa M, Takahashi A, Hirohashi Y,
Torigoe T, Inoda S, Murase M, Asanuma H, Tamura Y, Morita R,
Michifuri Y, et al: SOX2 is overexpressed in stem-like cells of
human lung adenocarcinoma and augments the tumorigenicity. Lab
Invest. 91:1796–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weina K and Utikal J: SOX2 and cancer:
Current research and its implications in the clinic. Clin Transl
Med. 3:192014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Chen K, Li L, Li R, Zhang J and Ren
W: Overexpression of SOX2 is involved in paclitaxel resistance of
ovarian cancer via the PI3K/Akt pathway. Tumour Biol. 36:9823–9828.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun FF, Hu YH, Xiong LP, Tu XY, Zhao JH,
Chen SS, Song J and Ye XQ: Enhanced expression of stem cell markers
and drug resistance in sphere-forming non-small cell lung cancer
cells. Int J Clin Exp Pathol. 8:6287–6300. 2015.PubMed/NCBI
|
|
24
|
Tian T, Zhang Y, Wang S, Zhou J and Xu S:
Sox2 enhances the tumorigenicity and chemoresistance of cancer
stem-like cells derived from gastric cancer. J Biomed Res.
26:336–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toschi L, Finocchiaro G, Nguyen TT, Skokan
MC, Giordano L, Gianoncelli L, Perrino M, Siracusano L, Di Tommaso
L, Infante M, et al: Increased SOX2 gene copy number is associated
with FGFR1 and PIK3CA gene gain in non-small cell lung cancer and
predicts improved survival in early stage disease. PloS One.
9:e953032014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basu-Roy U, Seo E, Ramanathapuram L, Rapp
TB, Perry JA, Orkin SH, Mansukhani A and Basilico C: Sox2 maintains
self renewal of tumor-initiating cells in osteosarcomas. Oncogene.
31:2270–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Shi J, Yang J, Ma Y, Cheng L, Zeng
J, Hao X, Ma C, Wang Y and Liu X: A Wnt/β-catenin negative feedback
loop represses TLR-triggered inflammatory responses in alveolar
epithelial cells. Mol Immunol. 59:128–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SH, Oh SY, Do SI, Lee HJ, Kang HJ, Rho
YS, Bae WJ and Lim YC: SOX2 regulates self-renewal and
tumorigenicity of stem-like cells of head and neck squamous cell
carcinoma. Br J Cancer. 111:2122–2130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masui S, Nakatake Y, Toyooka Y, Shimosato
D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, et
al: Pluripotency governed by Sox2 via regulation of Oct3/4
expression in mouse embryonic stem cells. Nat Cell Biol. 9:625–635.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stinchcombe TE, Borghaei H, Barker SS,
Treat JA and Obasaju C: Pemetrexed with platinum combination as a
backbone for targeted therapy in non-small-cell lung cancer. Clin
Lung Cancer. 17:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tamashiro DA, Alarcón VB and Marikawa Y:
Ectopic expression of mouse Sry interferes with Wnt/beta-catenin
signaling in mouse embryonal carcinoma cell lines. Biochim Biophys
Acta. 1780:1395–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zakaria N, Yusoff NM, Zakaria Z, Lim MN,
Baharuddin PJ, Fakiruddin KS and Yahaya B: Human non-small cell
lung cancer expresses putative cancer stem cell markers and
exhibits the transcriptomic profile of multipotent cells. BMC
cancer. 15:842015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka K, Kumano K and Ueno H:
Intracellular signals of lung cancer cells as possible therapeutic
targets. Cancer Sci. 106:489–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seo E, Basu-Roy U, Zavadil J, Basilico C
and Mansukhani A: Distinct functions of Sox2 control self-renewal
and differentiation in the osteoblast lineage. Mol Cell Biol.
31:4593–4608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Y, Shi L, Zhang L, Li R, Liang J, Yu
W, Sun L, Yang X, Wang Y, Zhang Y and Shang Y: The molecular
mechanism governing the oncogenic potential of SOX2 in breast
cancer. J Biol Chem. 283:17969–17978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen S, Xu Y, Chen Y, Li X, Mou W, Wang L,
Liu Y, Reisfeld RA, Xiang R, Lv D and Li N: SOX2 gene regulates the
transcriptional network of oncogenes and affects tumorigenesis of
human lung cancer cells. PLoS One. 7:e363262012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jia X, Li X, Xu Y, Zhang S, Mou W, Liu Y,
Liu Y, Lv D, Liu CH, Tan X, et al: SOX2 promotes tumorigenesis and
increases the anti-apoptotic property of human prostate cancer
cell. J Mol Cell Biol. 3:230–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leis O, Eguiara A, Lopez-Arribillaga E,
Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R
and Martin AG: Sox2 expression in breast tumours and activation in
breast cancer stem cells. Oncogene. 31:1354–1365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiang R, Liao D, Cheng T, Zhou H, Shi Q,
Chuang TS, Markowitz D, Reisfeld RA and Luo Y: Downregulation of
transcription factor SOX2 in cancer stem cells suppresses growth
and metastasis of lung cancer. Br J Cancer. 104:1410–1417. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen S, Li X, Lu D, Xu Y, Mou W, Wang L,
Chen Y, Liu Y, Li X, Li LY, et al: SOX2 regulates apoptosis through
MAP4K4-survivin signaling pathway in human lung cancer cells.
Carcinogenesis. 35:613–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bareiss PM, Paczulla A, Wang H, Schairer
R, Wiehr S, Kohlhofer U, Rothfuss OC, Fischer A, Perner S, Staebler
A, et al: SOX2 expression associates with stem cell state in human
ovarian carcinoma. Cancer Res. 73:5544–5555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Herreros-Villanueva M, Zhang JS, Koenig A,
Abel EV, Smyrk TC, Bamlet WR, De Narvajas AA, Gomez TS, Simeone DM,
Bujanda L and Billadeau DD: SOX2 promotes dedifferentiation and
imparts stem cell-like features to pancreatic cancer cells.
Oncogenesis. 2:e612013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oppel F, Müller N, Schackert G, Hendruschk
S, Martin D, Geiger KD and Temme A: SOX2-RNAi attenuates S-phase
entry and induces RhoA-dependent switch to protease-independent
amoeboid migration in human glioma cells. Mol Cancer. 10:1372011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rybak AP and Tang D: SOX2 plays a critical
role in EGFR-mediated self-renewal of human prostate cancer
stem-like cells. Cell Signal. 25:2734–2742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Singh S, Trevino J, Bora-Singhal N,
Coppola D, Haura E, Altiok S and Chellappan SP: EGFR/Src/Akt
signaling modulates Sox2 expression and self-renewal of stem-like
side-population cells in non-small cell lung cancer. Mol Cancer.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hainaut P and Plymoth A: Targeting the
hallmarks of cancer: Towards a rational approach to next-generation
cancer therapy. Curr Opin Oncol. 25:50–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun C, Sun L, Li Y, Kang X, Zhang S and
Liu Y: Sox2 expression predicts poor survival of hepatocellular
carcinoma patients and it promotes liver cancer cell invasion by
activating Slug. Med Oncol. 30:5032013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dogan I, Kawabata S, Bergbower E, Gills
JJ, Ekmekci A, W III Wilson, Rudin CM and Dennis PA: SOX2
expression is an early event in a murine model of EGFR mutant lung
cancer and promotes proliferation of a subset of EGFR mutant lung
adenocarcinoma cell lines. Lung cancer. 85:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|