Introduction
Ovarian cancer is a common malignancy of the female
reproductive organs (1,2). The incidence of ovarian cancer ranks
third, after cervical cancer and uterine body cancer; however, the
mortality rate is the highest compared with all other types of
gynecological tumor, and it is therefore a serious threat to
survival (3–5). In the United States, it was estimated
that 22,240 cases of ovarian cancer were newly diagnosed, and an
estimated 14,030 cases of mortality were associated with ovarian
cancer in 2013 (6,7). The major underlying cause for the
high mortality rate is that ~75% of women had metastases throughout
the peritoneal cavity and were diagnosed with ovarian cancer at an
advanced stage. Although combined chemotherapy may improve the
overall survival rate in patients with advanced stage ovarian
cancer, novel therapeutic paradigms are required. Furthermore, the
underlying molecular mechanism remains to be clearly defined, since
the pathology differs in various types of ovarian cancer.
Klotho is a recently discovered anti-aging protein,
and Klotho-deficient mice exhibit a premature aging-like syndrome
(8). Klotho is predominantly
distributed in the kidneys and brain, and has an essential role in
protecting against dysfunction of the kidney-brain axis during the
aging process (9). There are two
types of Klotho protein: Transmembrane and secreted forms of Klotho
(10,11). Both of these proteins exert
distinct functions, which may collectively affect aging processes
in mammals. The single-pass transmembrane protein forms a complex
with numerous fibroblast growth factor receptors, whereas secreted
Klotho protein regulates the activity of several ion channels and
growth factors, including insulin, insulin-like growth factor 1
(IGF-1) and Wnt (12). Klotho has
been studied and reported to act as a tumor suppressor in various
human malignancies (13–15). Previous studies have focused on the
role of Klotho in tumorigenesis, cancer progression and prognosis.
Klotho has been reported to exert antitumor effects by inhibiting
insulin/IGF1, p53/p21 and Wnt signaling, and silencing Klotho
expression was mediated by promoter hypermethylation and histone
deacetylation in the progression of tumors (13,16).
In addition, Lee et al examined epigenetic silencing of
Klotho in human cervical carcinoma, and the functional loss of
Klotho as a secreted Wnt antagonist contributed to aberrant
activation of the canonical Wnt pathway in cervical carcinoma
(17).
To the best of our knowledge, there are currently
two main research groups that have reported on the role of Klotho
in human ovarian cancer. Lu et al demonstrated that Klotho
expression was associated with epithelial ovarian cancer
progression and the protein could serve as an independent marker
for the prognosis of ovarian cancer (18). Lojkin et al reported that
Klotho acted as an inhibitor of the IGF-1 pathway in cancer cells;
and restoring its expression slowed the proliferation of epithelial
ovarian cancer cells and inhibited transcriptional activity of the
estrogen receptor (13). The
present study aimed to clarify the association between the
expression levels of Klotho in human ovarian cancer tissues and the
progression of ovarian cancer. Furthermore, the molecular mechanism
underlying the effects of Klotho on ovarian cancer cell lines was
explored. The present study provided novel evidence regarding the
molecular mechanism underlying human ovarian cancer.
Materials and methods
Patients
Patients were recruited from the Zhujiang Hospital
of Southern Medical University (Guangzhou, China). A total of 120
patients with ovarian cancer and 78 normal controls were recruited
to the present study. The current study was conducted over a period
of 36 months, between March 2012 and March 2015. All patients were
diagnosed with ovarian cancer and underwent surgery following their
diagnosis. The median age of the patients was 56.8 years (range,
26–82 years). The paired paracancerous tissues were collected as
normal controls. The patient was diagnosed with ovarian cancer
using a pathological diagnosis. They have not received chemotherapy
or irradiation prior to tissue collection. Fresh tumor tissues were
collected from each patient during surgery. One fresh tissue sample
from each patient was frozen at −80°C for western blotting. Another
tissue sample from each patient was fixed in 10% formalin, and
paraffin-embedded sections were prepared and cut into 4 µm
sections. The research carried out on humans was in compliance with
the Helsinki Declaration, and the present study was approved by the
Ethics Committee of Zhujiang Hospital of Southern Medical
University. The subjects were well informed of the details and
signed relevant contracts prior to the study.
Cell lines and reagents
The A2780, SKOV-3, OVCA 432, OVCAR-5 OVCAR-8, CaOV4
and CaOV3 human ovarian cancer cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA). Dulbecco's
modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were
purchased from Hyclone (GE Healthcare Life Sciences, Logan, UT,
USA). Total RNA extraction kit (cat. no. MK700) and cDNA reverse
transcription kit (cat. no. 6110) were obtained from Takara Bio,
Inc. (Kusatsu, Japan). Lipofectamine 2000 (cat. no. 11668-019) was
obtained from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The stock concentration of short hairpin (sh)RNA was 20
µM and the working concentration was 50 nM. Klotho shRNA Plasmid
and control shRNA Plasmid-A were obtained from Santa Cruz
Biotechnology Inc. (Dallas, TX, USA). A2780 cells were plated into
a 24-well plate at a density of 2×104 cells/well. The
cells were grown to 70–80% confluence. Klotho shRNA plasmid (2 µl)
or control shRNA plasmid (2 µl) and 5 µl Lipofectamine 2000 were
diluted into 100 µl Opti-MEM, respectively. The shRNA were gently
mixed and maintained at room temperature for 5 min. Next, the 100
µl of Opti-MEM containing Klotho shRNA and 100 µl Opti-MEM
containing lipofectamin 2000 were gently mixed together and kept
for 15 min at room temperature. Finally, the mixture was added into
the medium of the human ovarian cancer cells to knockdown the
Klotho expression levels for indicated time.
Antibodies
Rabbit polyclonal anti-Klotho antibody (cat. no.
ab18131) was purchased from Abcam (Cambridge, MA, USA) for use in
western blotting. Anti-Klotho antibody (E-21; cat. no. sc-22220)
was obtained from Santa Cruz Biotechnology, Inc. for use in
immunohistochemistry. Mouse monoclonal anti-β-actin antibody (cat.
no. TA310155) was obtained from OriGene Technologies, Inc.
(Beijing, China). The secondary antibodies, including goat
anti-rabbit immunoglobulin (Ig) G-horseradish peroxidase (HRP)
(cat. no. sc-2004) and goat anti-mouse IgG-HRP (cat. no. sc-2005)
were obtained from Santa Cruz Biotechnology, Inc.
Immunohistochemical analysis
Immunohistochemical analysis was performed as
described previously (19,20). Briefly, paraffin-embedded sections
were dewaxed, rehydrated, blocked and incubated overnight at 4°C
with the primary antibody specific to Klotho. After three washes
with phosphate-buffered saline (PBS), the sections were incubated
with secondary antibody for 1 h at room temperature. Subsequently,
the slides were dehydrated, mounted in Permount and visualized
under a Nikon Eclipse Ti microscope (Nikon Corporation, Tokyo,
Japan). Positive and negative images were captured using a camera
attached to the microscope at 400x magnification.
Western blot analysis
Frozen cancerous and paracancerous tissues were
homogenized using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) using the PowerGen 125 homogenizer (Thermo Fisher
Scientific, Inc.). The samples were washed with PBS and lysed with
radioimmunoprecipitation assay buffer [50 mmol/l Tris, 1% NP-40,
150 mmol/l NaCl, 1 mmol EDTA, 0.1% sodium dodecyl sulfate (SDS),
0.25% SDC]. Whole protein concentrations were quantified using the
Bradford assay. Proteins (20 µg per lane) were then separated by
10% SDS-polyacrylamide gel electrophoresis and were transferred
electrophoretically to a nitrocellulose membrane at 400 mA for 1 h.
The membrane was blocked with 5% non-fat milk in Tris-buffered
saline containing Tween (TBST; 50 mmol/l Tris-HCl, 150 mmol/l NaCl
and 0.1% Tween). Subsequently, the membranes were incubated with
primary antibodies at 4°C overnight and secondary antibodies for 40
min at room temperature. Between incubations the membranes were
washed with TBST for 5 min. The bands were detected using an
enhanced chemiluminescence western blotting detection system,
according to the manufacturer's protocol. β-actin was used as an
internal reference. The bands were developed by ECL
chemiluminescence kit and visualized by gel imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Image J version 1.49
(National Institutes of Health- Bethesda, MD, USA) was used to
analyze the results form western blotting.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cells were cultured in DMEM, supplemented with
10% FBS, 37°C in 5% CO2 atmosphere. An MTT assay was
performed as described previously (21,22).
Briefly, the human ovarian cancer cell lines were transfected with
pCMV6-Klotho or pCMV6 vector (both obtained from OriGene
Technologies, Inc.) for 48, 72 and 96 h. The cancer cells
(1×105 cells/well) were seeded into a 48-well plate. The
cells were grown to 70–80% confluence. The vector (100 ng) and 5 µl
Lipofectamine 2000 were diluted into 100 µl of Opti-MEM which were
maintained for 5 min at room temperature. They were mixed gently
for 15 min. The mixture was added into the medium of human ovarian
cancer cells and cultured for the aforementioned times. Cell
proliferation in each group was detected by MTT assay.
Enzyme-linked immunosorbent assay
(ELISA)
Whole blood samples (0.2 ml) were obtained from the
tail veins of the mice and were maintained at room temperature for
2 h, and the supernatant was obtained following centrifugation at
1,000 × g for 20 min at room temperature. The concentration of
inflammatory cytokines was determined using human IL-6 and IL-1β
ELISA kits (NeoBioscience, Beijing, China) according to the
manufacturer's protocols. Absorbance was measured at 450 nm using a
Benchmark Microplate Reader (Bio-Rad Laboratories, Inc., Hercules,
CA). All of the samples were analyzed in duplicate for cytokine
levels. The concentrations of interleukin (IL)-6 and IL-1β in the
samples were determined from standard curves.
Reverse transcription-polymerase chain
reaction
Total RNA was isolated from the tissues or cultured
cells using TRIzol RNA regent (Tiangen Biotech Co., Ltd., Beijing,
China). RNA (1 µg) was reverse transcribed by reverse transcriptase
SuperScript III (Invitrogen; Thermo Fisher Scientific, Inc.). The
primer sequence of Klotho was sense 5′-ACCTGGTGGCGCACAACC-3′ and
antisense 5′-TTGGCAAACCAACCTAGTACA-3′. The PCR reaction conditions
were as follows: 94°C for 5 min, followed by 30 cycles of 94°C for
30 sec, 55°C for 30 sec and 72°C for 30 sec and finally, elongation
at 72°C for 10 min.
Animals
Male C57BL/6 mice (n=18; weight, 18–20 g; age, 6–8
weeks) were obtained from the Laboratory Animal Center of Peking
University Health Science Center (Beijing, China) and were
maintained in the specific pathogen-free conditions. The
temperature was controlled at 18–22°C, humidity of 50- 60%. Food
and water was provided ad libitum. The light:dark cycle was 12:12
h. The mice were challenged subcutaneously with 4×104 A2780 cells
in the flank area; each group contained six mice. For the in
vivo antitumor experiment, the mice were randomly divided into
three groups: i) Klotho group; ii) control plamid group and iii)
negative control group. Klotho−/− mice (n=18; weight,
18–20 g; age, 8 weeks) were obtained from CasGene Biotech Co., Ltd.
(Beijing, China) and housed in specific pathogen-free conditions.
The temperature was controlled at 18–22°C, humidity of 50- 60%.
Food and water was provided ad libitum. The light:dark cycle was
12:12 h. Wild type mice with the same genetic background were used
as a negative control. After 2 weeks, cytokine levels in the serum
were detected by ELISA. The animals used in the present study
received humane care in compliance with the Guide to the Care and
Use of Experimental Animals formulated by the Medical Ethical
Committee on animal experiments of Zhujiang Hospital of Southern
Medical University. The mice were sacrificed by cervical after 1
month of tumor injections. The mice were disinfected with 75%
alcohol. The tumors in nude mice were weighed and compared between
the different groups.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM SPSS, Armonk, NY, USA). data are presented as the
mean ± standard deviation. Comparisons between Klotho expression
levels and clinical indicators were performed by χ2 test. Analysis
of variance (ANOVA), followed by a post-hoc test was used for the
comparison of multiple groups. A repeated measures ANOVA was used
to compare tumor size in mice over time. Kaplan-Meier survival
estimates were used to evaluate survival rate. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression levels of Klotho in human
ovarian cancer tissues and normal ovarian tissues
In order to identify the role of Klotho in the
progression of human ovarian cancer, the expression levels of
Klotho were detected by western blotting in patients with human
ovarian cancer and normal controls. A total of 120 ovarian cancer
specimens and 78 normal ovarian specimens were collected from the
hospital; the median age of the patients was 56.8 years (range,
26–86 years). It was essential to use an appropriate internal
reference in the experiment; therefore, β-actin was used as the
internal reference, since this housekeeping gene exhibits stable
expression in various types of cells and tissues. The data were
normalized to β-actin and are presented as the mean ± standard
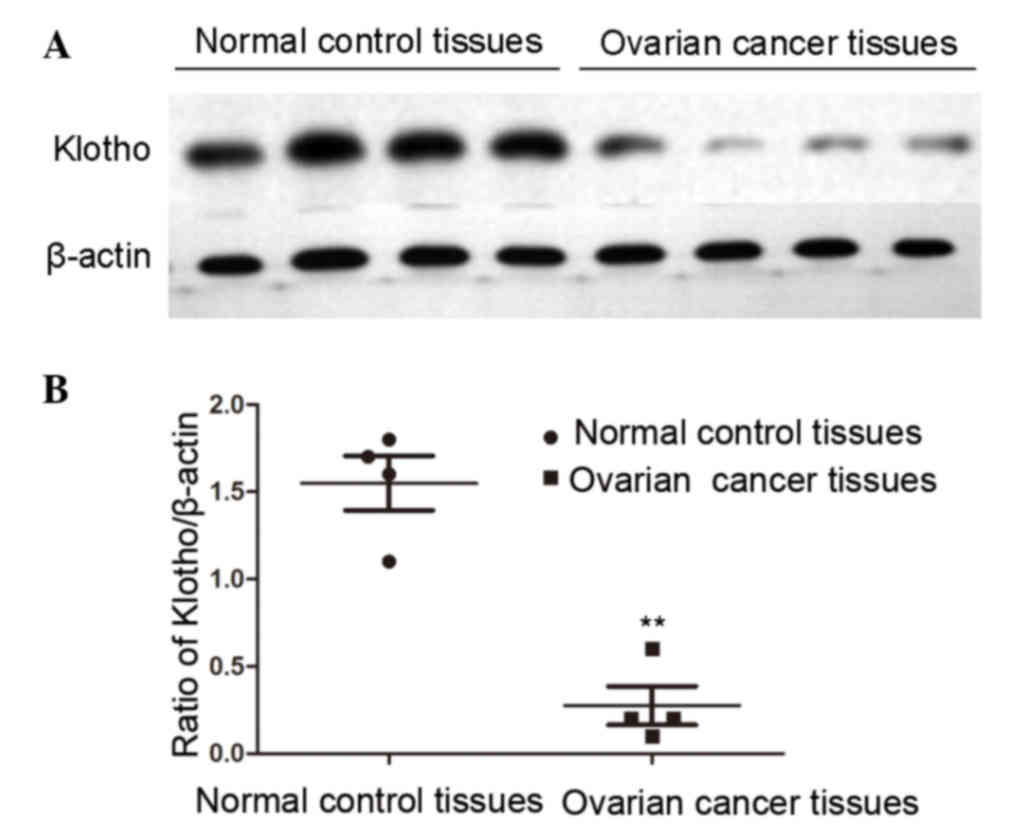
deviation. As presented in Fig. 1,
the expression levels of Klotho were significantly decreased in
human patients with ovarian cancer compared with in the normal
control group (P<0.01).
Survival rate is positively correlated
with Klotho levels in patients with ovarian cancer
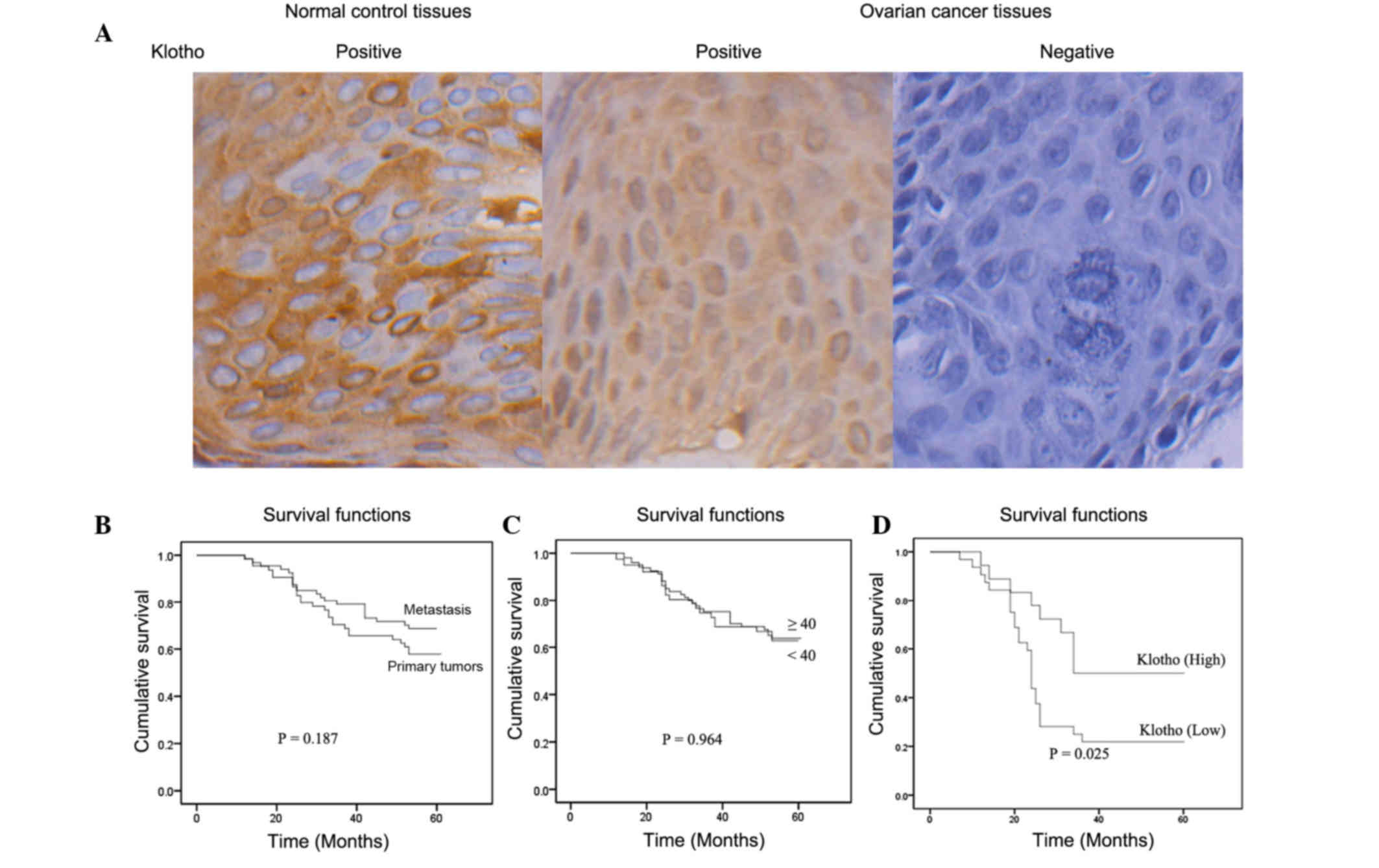
As shown in Fig.
2A, formalin-fixed, paraffin-embedded tissues from 120 patients
with ovarian cancer and 78 normal controls were analyzed by
immunohistochemistry to detect the protein expression of Klotho.
The results indicated that positive Klotho expression was detected
in all normal ovarian specimens; however, in human ovarian cancer
patients, the positive rate of Klotho was 61.6%, which was
significantly decreased compared with in the normal control group
(Table I).
 | Table I.Klotho expression in human ovarian
cancer tissues and normal ovarian tissues. |
Table I.
Klotho expression in human ovarian
cancer tissues and normal ovarian tissues.
|
|
| Klotho |
|
|---|
|
|
|
|
|
|---|
| Tissue type | n | + | − | Positive rate
(%) |
|---|
| Normal gastric
tissues | 78 | 78 | 0 | 100.0 |
| Gastric cancer
tissues | 120 | 74 | 46 | 61.6a |
In order to clarify the relevance of clinical
parameters in the prognosis of human ovarian cancer, the
relationship between clinical indicators, including age, metastasis
and Klotho expression, and the 5-year survival rates of patients
with ovarian cancer was determined. As shown in Fig. 2B, Kaplan-Meier curves demonstrated
the survival rate was higher in patients with primary tumors
compared with those with metastasis; however, there was no
statistical difference (P=0.187). Kaplan-Meier curves were also
generated to compare the survival rates of patients that were
<40 years old with those that were ≥40 years old; the results
demonstrated that there was no statistically significant difference
between the two groups (P=0.964; Fig.
2C). In addition, reduced Klotho expression was significantly
correlated with decreased survival rates in patients with ovarian
cancer (P=0.025; Fig. 2D). These
results indicated that Klotho expression was associated with a
higher survival probability in patients with human ovarian
cancer.
Klotho expression levels in ovarian
cancer cell lines
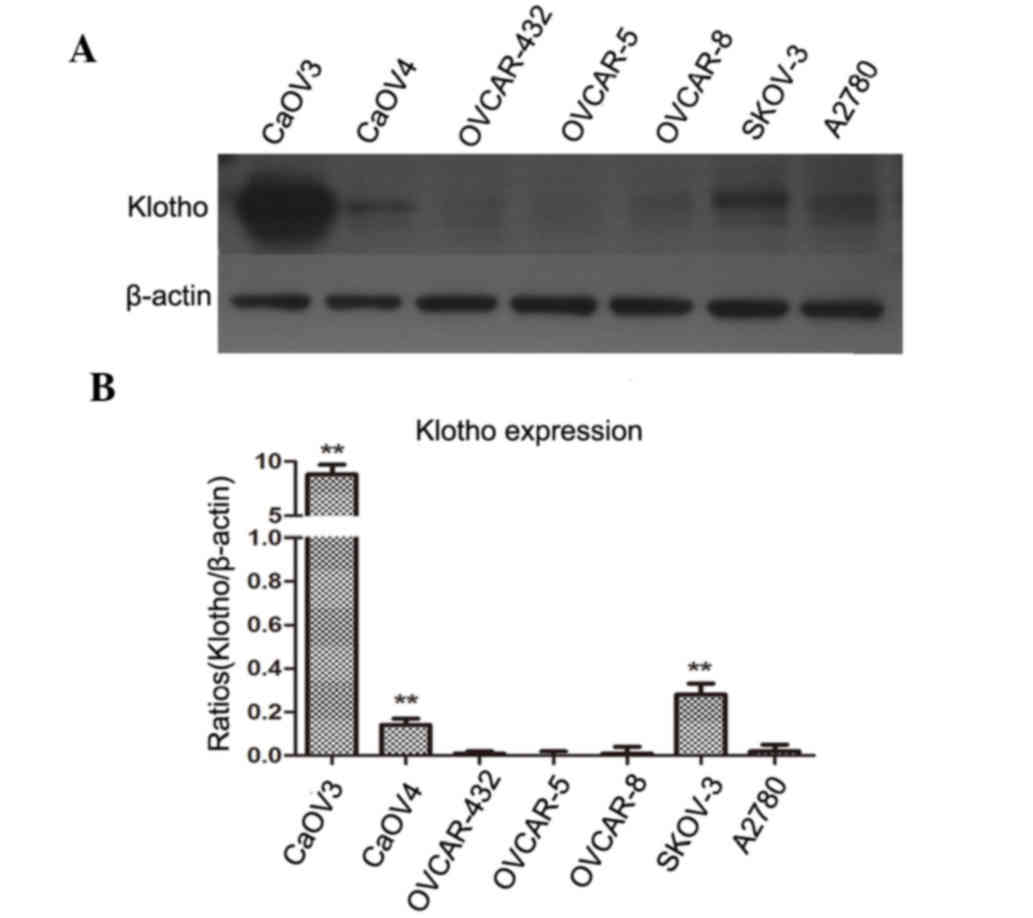
The expression levels of Klotho were detected in
several human ovarian cancer cell lines by western blotting. As
shown in Fig. 3, Klotho protein
was not expressed or was expressed at low levels in four cell
lines: OVCA 432, OVCAR-5, OVCAR-8 and A2780. Low expression levels
were observed in two cell lines: CaOV4 and SKOV-3. Notably, the
CaOV3 human ovarian cancer cell line expressed high levels of
Klotho compared with in the ovarian cancer cell lines where no
Klotho was detected (P<0.01).
High levels of Klotho inhibit the
proliferation of human ovarian cancer cells
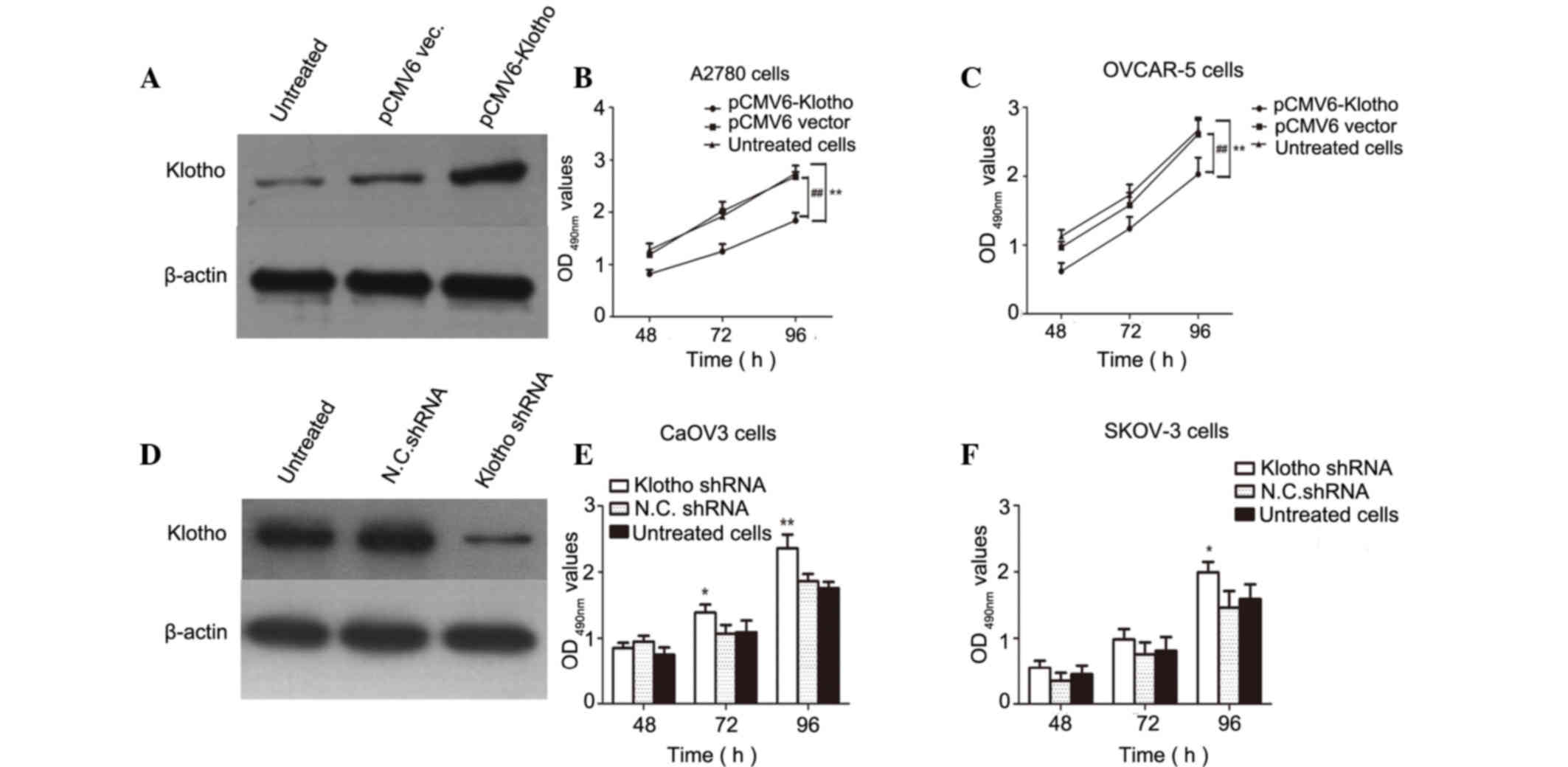
In order to clarify the relationship between Klotho
expression and the proliferation of ovarian cancer cells, two human
ovarian cancer cell lines were selected as a cell model. A2780
cells exhibited hardly any detectable Klotho expression, and CaOV3
cells exhibited high Klotho levels. In Fig. 4A, Klotho was overexpressed in A2780
cells and the results demonstrated that Klotho levels were markedly
increased compared with in the negative control cells.
Subsequently, proliferative activity was determined by MTzT assay
in pCMV6-Klotho and pCMV6 vector-transfected ovarian cancer cells.
The data revealed that overexpression of Klotho in A2780 and
OVCAR-5 cells contributed to the inhibition of human ovarian cancer
cell proliferation compared with in untreated and pCMV6
vector-transfected cells (P<0.01; Fig. 4B and C). In addition, endogenous
Klotho expression was suppressed by Klotho-specific shRNA. As
expected, Klotho expression was suppressed in CaOV3 cells
transfected with the shRNA, as detected by western blotting.
Results of the MTT assay demonstrated that Klotho shRNA-transfected
optical density (490 nm) values were increased compared with
negative control shRNA-transfected cells or untreated cells
(P<0.05 and P<0.01, compared with untreated cells; Fig. 4D-F). These data indicated that high
levels of Klotho inhibited the proliferation of human ovarian
cancer cells, and inhibiting the endogenous expression of Klotho
may promote tumor cell growth in human ovarian cancer cells.
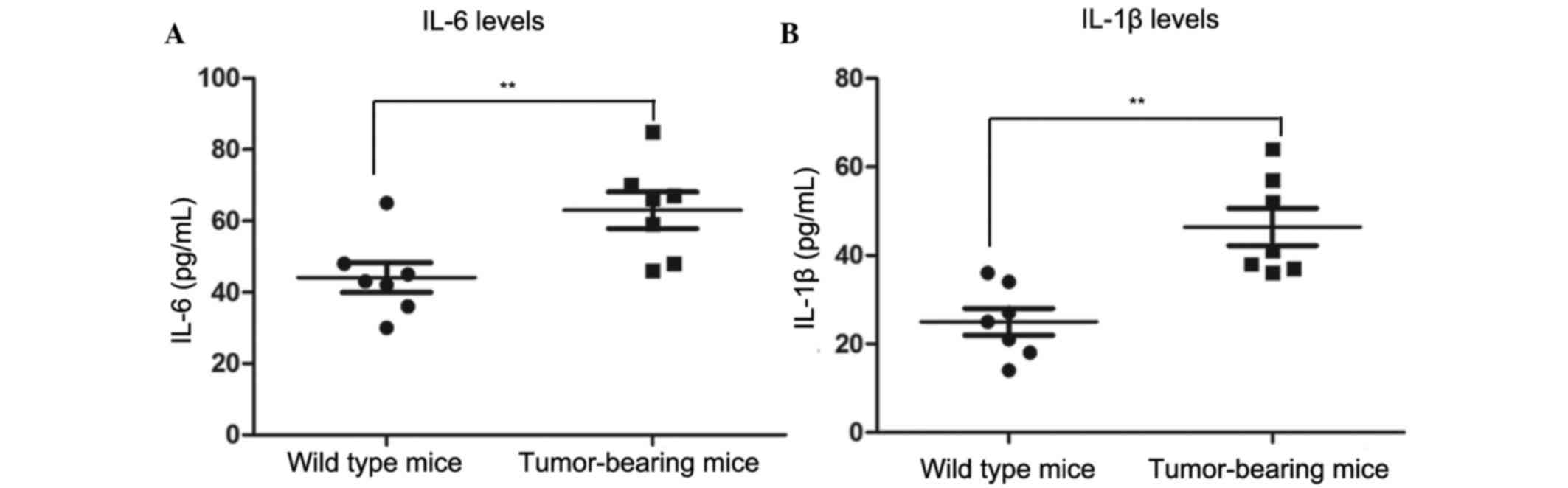
Plasma IL-6 and IL-1β levels are
elevated in tumor-bearing mice
In order to detect the inflammatory responses in
normal and tumor-bearing mice, inflammatory cytokine levels in the
plasma were detected. The concentrations of plasma IL-6 and IL-1β
were detected by ELISA. As shown in Fig. 5, mean plasma IL-6 concentration was
63.0 pg/ml in tumor-bearing mice, which was significantly increased
compared with in the wild type mice (39.9 pg/ml; P<0.01). The
levels of IL-1β showed a similar trend to IL-6. The concentration
of IL-1β in the plasma of tumor-bearing mice was 46.43 pg/ml, which
was significantly increased compared with the mean value in wild
type mice (25.0 pg/ml; P<0.01). These results indicated that
plasma IL-6 and IL-1β levels are elevated in tumor-bearing mice,
thus suggesting that the systemic inflammatory response was severe
in tumor-bearing mice.
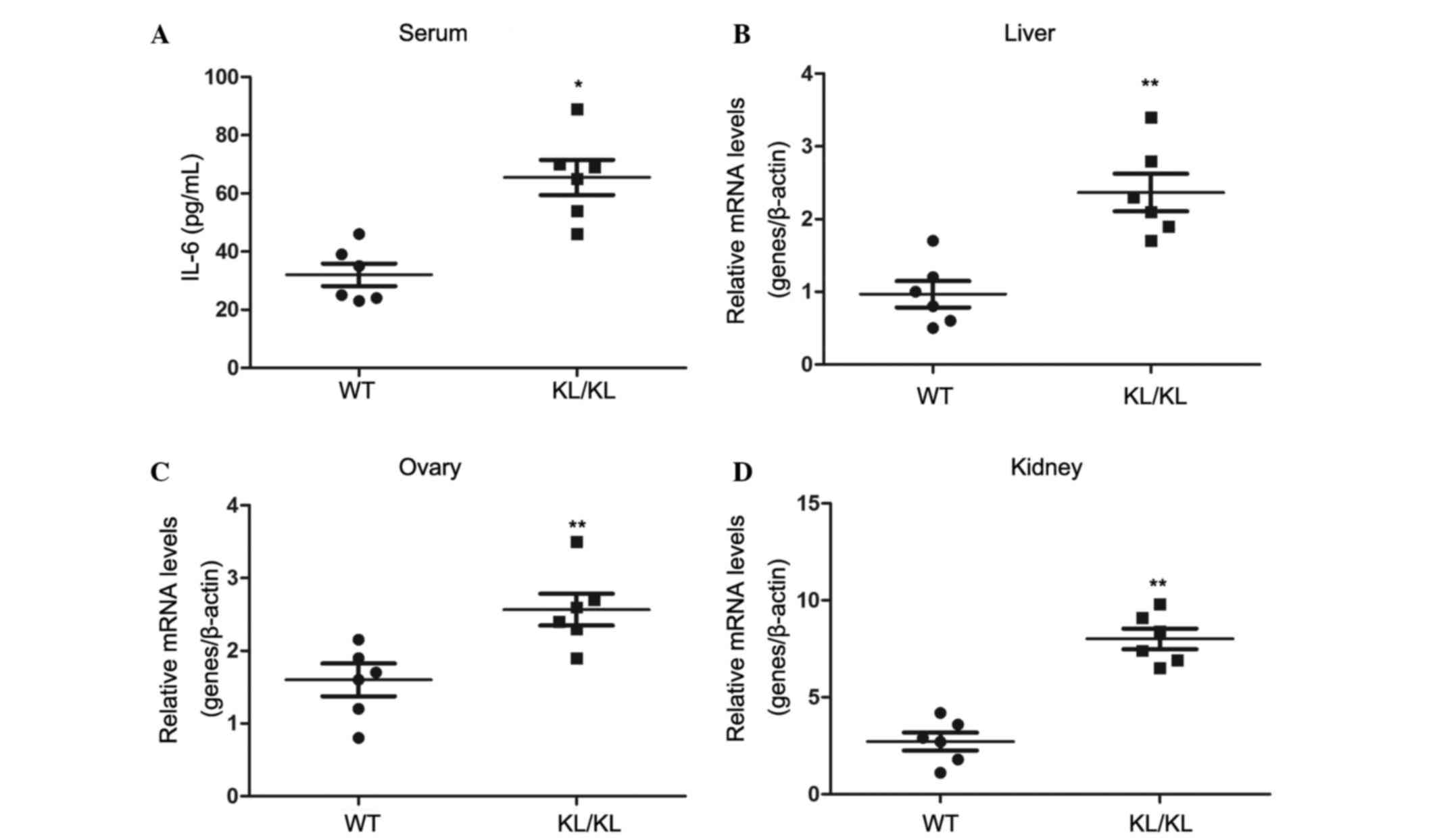
Aberrant Klotho expression contributes
to systemic chronic inflammation in Klotho−/− mice
In order to determine whether aberrant Klotho
expression would induce or suppress systemic inflammation in mice,
8-week-old Klotho−/− mice and wild type mice with the
same genetic background were used as an animal model. As presented
in Fig. 6A, plasma IL-6 levels in
Klotho−/− mice were 65.0 pg/ml, whereas plasma IL-6
concentration was 32.0 pg/ml in the wild type mice with the same
genetic background (P<0.05).
The mRNA expression levels of IL-6 were detected in
various organs, including the liver, ovaries and kidneys (Fig. 6B-D). The results demonstrated that
the expression levels of IL-6 were significantly elevated in the
liver, ovaries and kidneys of the Klotho−/− mice
compared with in the wild type mice (P<0.01). These data
demonstrated that aberrant Klotho expression may contribute to
systemic inflammation in Klotho−/− mice.
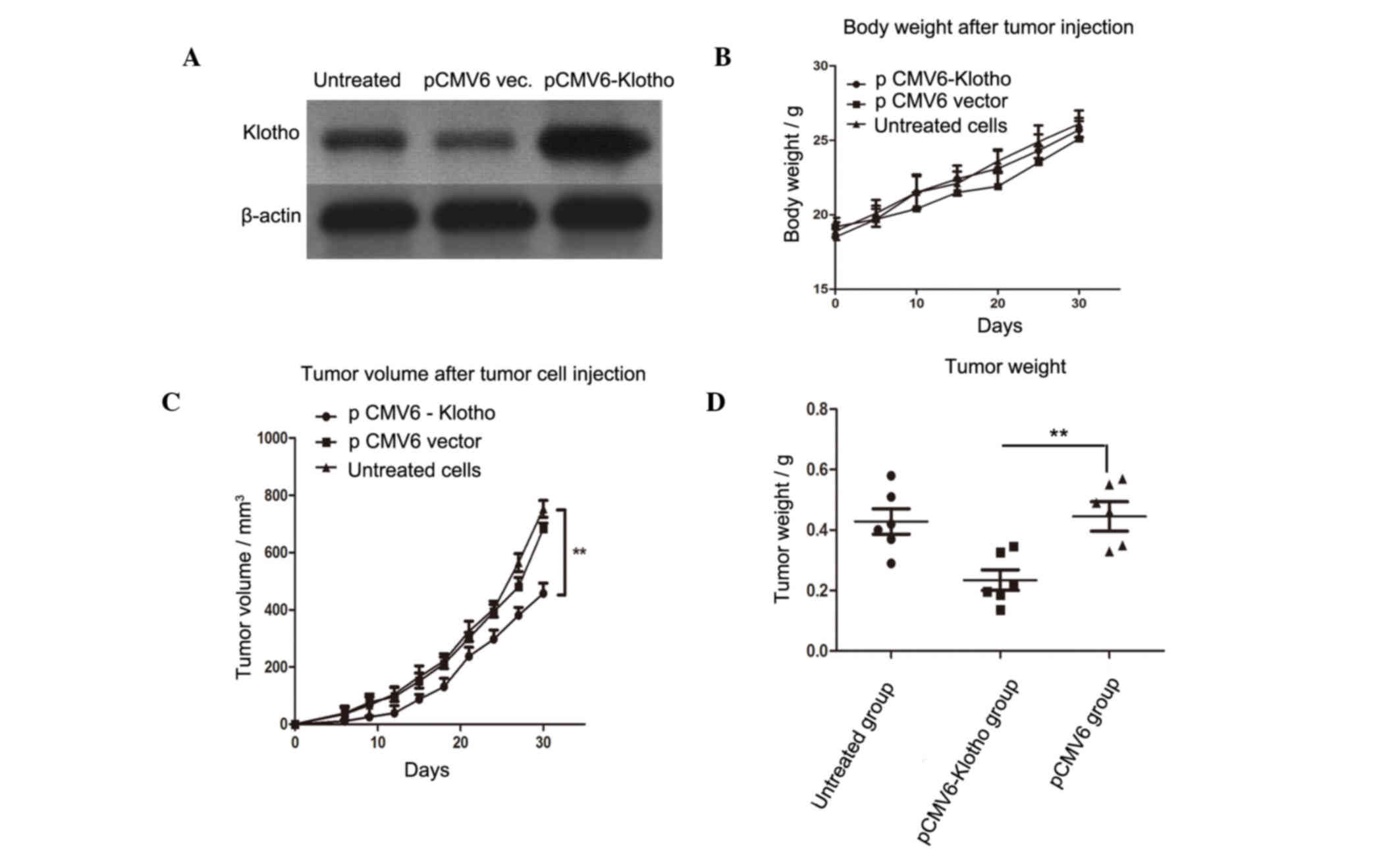
Overexpression of Klotho suppresses
tumor growth in a murine model
To assess the in vivo antitumor role of
aberrant Klotho expression, A2780 human ovarian cancer cells were
transfected with pCMV6-Klotho, and the stably transfected cells
were screened. As shown in Fig.
7A, the expression of Klotho was markedly elevated in
pCMV6-Klotho-transfected A2780 cells compared with in the PCMV6
vector-transfected cells or untreated cells. Mice were randomly
divided into three groups (n=6/group). The mice were subcutaneously
challenged with 3×105 live stably transfected A2780 cells in the
rear leg flank. A total of 10 days after the injection, tumors
became gradually evident. The mice in each group were observed
daily to monitor tumor volume. A total of 30 days after tumor
injection, the mice were sacrificed and tumor weight was determined
in each group. There was no significant difference in body weight
between the three groups (Fig.
7B); however, tumor volume and tumor weight were significantly
decreased in the PCMV6-Klotho group compared with in the pCMV6
vector group (P<0.01).
Discussion
Ovarian cancer is a common malignancy that affects
the ovaries (23–25). Previous studies have demonstrated
that Klotho is involved in the development and progression of
several types of human tumor (26,27);
however, the effects of Klotho on ovarian cancer have not been
clearly reported. The present study explored the role of Klotho in
the progression of ovarian cancer, and investigated the molecular
mechanism underlying the effects of Klotho during the progression
of human ovarian tumors.
The present study detected Klotho expression in 120
ovarian cancer specimens and 78 normal ovarian specimens by western
blotting and immunohistochemical analysis. The results demonstrated
that Klotho acted as a tumor suppressor in human ovarian cancer.
Notably, Klotho was highly expressed in normal control specimens;
however, its expression was significantly reduced in 38.4% of
specimens with ovarian cancers. Furthermore, reduced levels of
Klotho were correlated with lower survival rates in patients with
ovarian cancer (P=0.025). In addition, survival rate was not
associated with age or metastasis. These results indicated that
Klotho may serve as an indicator for the prognosis of patients with
ovarian cancer.
The expression levels of Klotho were detected in
seven human ovarian cancer cell lines by western blotting. Notably,
CaOV3 cells were shown to have the highest levels of Klotho, CaOV4
and SKOV-3 cells had medium levels of Klotho protein, and four
ovarian cancer cell lines, OVCA 432, OVCAR-5, OVCAR-8 and A2780,
had almost no detectable levels of Klotho. Therefore, the ovarian
cell lines with the highest and the lowest expression of Klotho
served as a cell model. The results of an MTT assay demonstrated
that overexpression of Klotho inhibited the proliferation of
ovarian cancer cells, whereas suppression of Klotho promoted the
growth of CaOV3 cells. These data suggested that Klotho may act as
a tumor suppressor in human ovarian cancer cells.
An in vivo experiment in Klotho−/−
mice demonstrated that IL-6 plasma concentration was significantly
increased in Klotho−/− mice compared with in wild type
mice with the same genetic background. This result was consistent
with the mRNA expression levels of IL-6 detected in the liver,
ovaries and kidneys of Klotho−/− mice. These data
suggested that aberrant Klotho expression contributed to systemic
inflammation. Notably, overexpression of Klotho suppressed tumor
growth and tumor volume in a murine model, which was partly due to
the inhibition of systemic inflammation and other tumor
growth-related signaling pathways, such as Akt, ERK, insulin and
Wnt signaling pathways.
In conclusion, the present study determined that
aberrant Klotho expression contributed to systemic inflammation.
Overexpression of Klotho suppressed tumor growth and tumor volume
in a murine model, which was partly due to the inhibition of
systemic inflammation.
References
|
1
|
Yahata T, Banzai C and Tanaka K: Niigata
Gynecological Cancer Registry: Histology-specific long-term trends
in the incidence of ovarian cancer and borderline tumor in Japanese
females: A population-based study from 1983 to 2007 in Niigata. J
Obstet Gynaecol Res. 38:645–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maksimović M, Maksimović M, Gojnić M,
Maksimović Z, Petković S, Ljubić A, Stefanović A and Jeremić K:
Surgical treatment of ovarian cancer and early detection of venous
thromboembolism. Eur J Gynaecol Oncol. 32:415–418. 2011.PubMed/NCBI
|
|
3
|
Chen M, Jin Y, Bi Y, Li Y, Shan Y and Pan
L: Prognostic significance of lymphovascular space invasion in
epithelial ovarian cancer. J Cancer. 6:412–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lloyd KL, Cree IA and Savage RS:
Prediction of resistance to chemotherapy in ovarian cancer: A
systematic review. BMC Cancer. 15:1172015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bacalbaşa N and Popescu I: Ovarian cancer
liver metastases - should we apply the principle of optimal
cytoreduction to the liver? A review. Hepatogastroenterology.
62:355–357. 2015.PubMed/NCBI
|
|
6
|
Rooth C: Ovarian cancer: Risk factors,
treatment and management. Br J Nurs. 22:S23–S30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu XH, Man YN and Wu XZ: Recurrence
season impacts the survival of epithelial ovarian cancer patients.
Asian Pac J Cancer Prev. 15:1627–1632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dërmaku-Sopjani M, Kolgeci S, Abazi S and
Sopjani M: Significance of the anti-aging protein Klotho. Mol Membr
Biol. 30:369–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng Y, Wang PH, Zhang M and Du JR:
Aging-related renal injury and inflammation are associated with
downregulation of Klotho and induction of RIG-I/NF-κB signaling
pathway in senescence-accelerated mice. Aging Clin Exp Res.
28:69–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banerjee S, Zhao Y, Sarkar PS, Rosenblatt
KP, Tilton RG and Choudhary S: Klotho ameliorates chemically
induced endoplasmic reticulum (ER) stress signaling. Cell Physiol
Biochem. 31:659–672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang CL: Regulation of ion channels by
secreted Klotho. Adv Exp Med Biol. 728:100–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuro-o M: Klotho and aging. Biochim
Biophys Acta. 1790:1049–1058. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lojkin I, Rubinek T, Orsulic S,
Schwarzmann O, Karlan BY, Bose S and Wolf I: Reduced expression and
growth inhibitory activity of the aging suppressor klotho in
epithelial ovarian cancer. Cancer Lett. 362:149–157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolf I, Levanon-Cohen S, Bose S, Ligumsky
H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP
and Rubinek T: Klotho: A tumor suppressor and a modulator of the
IGF-1 and FGF pathways in human breast cancer. Oncogene.
27:7094–7105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X and Wang X: Klotho: A novel
biomarker for cancer. J Cancer Res Clin Oncol. 141:961–969. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie B, Chen J, Liu B and Zhan J: Klotho
acts as a tumor suppressor in cancers. Pathol Oncol Res.
19:611–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee J, Jeong DJ, Kim J, Lee S, Park JH,
Chang B, Jung SI, Yi L, Han Y, Yang Y, et al: The anti-aging gene
KLOTHO is a novel target for epigenetic silencing in human cervical
carcinoma. Mol Cancer. 9:1092010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu L, Katsaros D, Wiley A, de la Longrais
IA, Puopolo M and Yu H: Klotho expression in epithelial ovarian
cancer and its association with insulin-like growth factors and
disease progression. Cancer Invest. 26:185–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saleem M, Maddodi N, Abu Zaid M, Khan N,
bin Hafeez B, Asim M, Suh Y, Yun JM, Setaluri V and Mukhtar H:
Lupeol inhibits growth of highly aggressive human metastatic
melanoma cells in vitro and in vivo by inducing apoptosis. Clin
Cancer Res. 14:2119–2127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adhami VM, Siddiqui IA, Ahmad N, Gupta S
and Mukhtar H: Oral consumption of green tea polyphenols inhibits
insulin-like growth factor-I-induced signaling in an autochthonous
mouse model of prostate cancer. Cancer Res. 64:8715–8722. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spinner DM: MTT growth assays in ovarian
cancer. Methods Mol Med. 39:175–177. 2001.PubMed/NCBI
|
|
22
|
Sargent J, Elgie A, Taylor CG, Wilson J,
Alton P and Hill JG: The identification of drug resistance in
ovarian cancer and breast cancer: Application of the MTT assay.
Contrib Gynecol Obstet. 19:64–75. 1994.PubMed/NCBI
|
|
23
|
Ebell MH, Culp M, Lastinger K and Dasigi
T: A systematic review of the bimanual examination as a test for
ovarian cancer. Am J Prev Med. 48:350–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozga M, Aghajanian C, Myers-Virtue S,
McDonnell G, Jhanwar S, Hichenberg S and Sulimanoff I: A systematic
review of ovarian cancer and fear of recurrence. Palliat Support
Care. 13:1771–1780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iżycka N, Lubin J, Markowska A and
Markowska J: Late recurrence of ovarian cancer: A literature review
and description of two cases. Eur J Gynaecol Oncol. 36:351–353.
2015.PubMed/NCBI
|
|
26
|
Martín-Núñez E, Donate-Correa J,
Muros-de-Fuentes M, Mora-Fernández C and Navarro-González JF:
Implications of Klotho in vascular health and disease. World J
Cardiol. 6:1262–1269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Chen L, Huang G, He D, He J, Xu W,
Zou C, Zong F, Li Y, Chen B, et al: Klotho sensitizes human lung
cancer cell line to cisplatin via PI3k/Akt pathway. PloS One.
8:e573912013. View Article : Google Scholar : PubMed/NCBI
|