Introduction
Combinations of drugs that have similar effects have
been employed clinically. Of the three types of drug interactions
that can occur (synergism, additivity and antagonism), synergism is
preferred as it allows lower doses of each drug to be used, which
reduces the risk of any potential side effects is reduced (1). Clinically, combinations of opioid
analgesics and intravenous anesthetics are used to establish
balanced anesthesia and to reduce side effects, including earlier
recovery and less postoperative nausea and vomiting. Opioids have
long been used for the treatment of pain and are one of the most
commonly prescribed drugs for pain management. They act through
three receptors, termed µ, κ and δ opioid receptors. Opioid
receptors are enabled by endogenously produced peptides, such as
morphine, and opioid drugs, including fentanyl. Of these, the µ
opioid receptor has an important role as the mediator of the
majority of the effects of most clinically used opioids (2). Alfentanil is a short-acting synthetic
opioid analgesic drug that targets µ opioid receptors and is
~4-fold faster than fentanyl in terms of the onset of effects
(3). Opioid agonists are effective
analgesic drugs and are irreplaceable in the treatment of
inflammatory and severe pain (4).
Propofol is a widely used intravenous general
anesthetic. It is reported to function at a specific site in
γ-aminobutyric acid-A receptors (GABAAR) and functions
as a positive allosteric modulator or an agonist (5). It is indistinct from the analgesic
actions of propofol despite its anesthetic effects. Gilron et
al (6) reported that propofol
reduced hind paw formalin-induced expression of fos-like
immunoreactivity in spinal neurons. The results of the study
indicated the analgesic effect of propofol. Our previous research
demonstrated the analgesic effects of propofol in several animal
models (7). Sun et al
(8) previously demonstrated the
peripheral antinociceptive effect of propofol in an inflammatory
pain model.
Extracellular signal-regulated kinase (ERK) is a
mitogen-activated protein kinase (MAPK) subfamily member. It is
activated in spinal dorsal horn neurons in response to injury and
inflammation-induced hyperalgesia of the peripheral tissue, with
similar results in a murine model of visceral pain (9,10).
Furthermore, it is known that c-fos is an immediate early gene and
a downstream target of ERK. C-fos is recognized as a marker for
stimulus-induced changes in the activity of neurons. It is induced
in the central nervous system under various conditions, including
in response to inflammatory and painful stimuli (11). ERK and c-fos are expressed in the
spinal cord and are important for nociception.
Alfentanil combined with propofol is a promising
combination for total intravenous anesthesia. It is well
established that propofol and alfentanil synergistically suppress
pain in clinical and animal experiments (7,12).
However, to the best of our knowledge, no study has investigated
the synergistic antinociceptive effects and potential mechanism of
alfentanil and propofol on tonic inflammatory pain. The present
study proposed that formalin-triggered behavioral responses may be
synergistically inhibited by a combination of alfentanil and
propofol, and that this would be associated with the ERK and c-fos
signaling pathway. The type of interaction (synergism, additivity
or antagonism) between alfentanil and propofol was evaluated by
isobolographic analysis.
Materials and methods
Animals
All experiments were performed in adherence with the
guidelines for the care and use of laboratory animals (13) and were approved by the Fourth
Military Medical University Committee on Animal Care (Xi'an,
China). Animal treatments were performed according to the ethical
guidelines of the International Association for the Study of Pain
(14) for the investigation of
experimental pain in conscious animals. Male Sprague Dawley rats
(n=24; 7 weeks, 150–220 g) were obtained from the animal research
center at the Fourth Military Medical University. The rats were
raised in individual standard cages and maintained on a 12 h
light/dark cycle (lights on at 07:00 a.m.) at 22°C. Testing was
performed during the light cycle. Food and water were available ad
libitum. Each animal was restrainer-trained and habituated to the
test environment for 1 week prior to testing.
Reagents and antibodies
Alfentanil (cat. no. 20151009; Hubei Gedian
Humanwell Pharmaceutical Co., Ltd., Wuhan, China) was suspended in
propylene glycol (XiLu Chemical Co., Ltd., Guangzhou, China) and
diluted with propofol (10 mg/ml; cat. no. p028104; diprivan;
AstraZeneca, London, UK) to obtain the desired concentrations of
the drug. Rabbit p44/42 MAPK (ERK1/2; 137F5) antibody (cat. no.
4695; 1:1,000), rabbit phospho-p44/42 MAPK [phosphorylated ERK1/2
(pERK); Thr202/Tyr204] antibody (cat. no. 4376; 1:1,000) and rabbit
c-fos antibody (cat. no. 2250; 1:1,000) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Unless otherwise
stated, all other chemicals were purchased from Sigma-Aldrich
(Merck Millipore, Darmstadt, Germany).
Formalin test
The paw formalin test is a well-characterized
experimental model of nociception and was performed as described
previously (15). Following
intraplantar injection of formalin solution (5%; 50 µl) into the
right hind paw, 24 rats (n=6 per group) were placed in individual
clear plastic cages (22×12×12 cm). Before the start of the
experiments, the animals were acclimatized to the laboratory
environment for at least one week. Flinching behavior was
considered to be an expression of nociception. Time courses of
antinociceptive responses resulting from the administration of
different drugs were constructed by plotting the mean number of
flinches as a function of time. The typical time course of the
response to formalin is biphasic, with an early and short-lasting
first phase followed, after a quiescent period, by a second,
prolonged (tonic) phase. While phase I is considered to reflect
acute nociceptive pain due to a direct stimulation of the nerve by
formalin, phase II is attributed to the combination of ongoing
inflammatory-associated afferent input from peripheral tissue and
functional changes in the spinal dorsal horn (central
sensitization) (16). Flinching
was defined as rapid and brief withdrawal or flexing of the
injected paw, lifting, licking and rubbing behavior. The
time-response data were presented as the total number of flinches.
To determine the ED50 values of each drug, the number of flinches
was converted to the percentage of maximum possible effect (% MPE)
according to the formula:
MPE (%)=100-(Sum of flinching count with drug/Sum of
control flinching count)x100.
Isobolographic analysis
The up-and-down method was used to determine the
median effective analgesic dosages of alfentanil and propofol
administered either alone, or in combination. Certain groups of
animals (n=6 per group) were treated intravenously with various
concentrations of either alfentanil (5, 10, 20 and 40 mg/kg) or
propofol (2.5, 5, 10 and 20 mg/kg) 10 min prior to formalin
injection. The alfentanil-propofol combination was also
administered intravenously at increasing doses (0.5, 1, 2 and 4
mg/kg). Control animals were given saline and were interspersed
concurrently with the drug-treated animals. The volumes
administered were 4 ml/kg. Rats in all groups were observed for
changes in behavior that may have been induced by the treatments.
In the present study, the initial dosages of alfentanil and
propofol were determined according to preliminary experiments and
literature values (7,17). Dose-response curves were
constructed using least-squares linear regression using the Pharm
Tools Pro software (version no. 1.1.27; The McCary Group, Inc.,
Lehigh Valley, PA, USA). Following the assessment of
antinociception for each drug, experimental median effective dose
(ED50) values for each drug were determined.
Subsequently, a dose-response curve was obtained by concurrent
delivery of alfentanil and propofol in a fixed-ratio mixture
(1:150), which was based on the ED50 values of each
individual agent. The ratio of the two drugs was obtained from our
previous study (18).
To construct the experimental antinociceptive
effect-dose curve, each group of rats received one of the following
doses of the drug combination: Alfentanil ED50 (25.3
µg/kg)+propofol ED50 (8.7 mg/kg); alfentanil
ED50/2 (12.65 µg/kg)+propofol ED50/2 (4.35
mg/kg); alfentanil ED50/4 (6.33 µg/kg)+propofol
ED50/4 (2.18 mg/kg); alfentanil ED50/8 (3.17
µg/kg)+propofol ED50/8 (1.09 mg/kg) or alfentanil
ED50/16 (1.59 µg/kg)+propofol ED50/16 (0.55
mg/kg). The experimental ED50 value for the
alfentanil-propofol combination was calculated from this curve. For
drug combinations, experimental ED50 (ED50
mix) and its associated 95% confidence intervals (CIs) were
determined by linear regression analysis of the log dose-response
curve and compared with a theoretical additive ED50
(ED50 add) obtained from the calculation: ED50
add=fxED50 alfentanil + (1-f)xED50
propofol, where f denotes a fraction of the
corresponding ED50 in drug mixture (in the present
study, f=0.5). In this equation (17,18),
ED50 add is the total dose, and the variance of
ED50 add was calculated as follows: Var ED50
add=f2 × Var ED50 alfentanil+ (1
-f)2x Var ED50 propofol.
From these variances, CIs were calculated and
resolved according to the ratio of the individual drug in the
combination. When the drug combination gives an experimental
ED50 not statistically different from the theoretical
calculated ED50, the combination has an additive effect.
If the mixture ED50 lies on the theoretical additive
ED50 line, or the confidence intervals overlap that
line, then the mixture is considered to be additive. If the mixture
ED50 lies below the theoretical additive ED50
line and the confidence intervals do not overlap that line, the
mixture is considered to be synergistic. The theoretical and
experimental ED50 values of the combinations were also
contrasted by calculating the interaction index (γ). This was
calculated as ED50 mix/ED50
add. If the value is ~1, the interaction is additive.
Values <1 are an indication of the magnitude of supra-additive
or synergistic interactions, and values >1 correspond to
sub-additive or antagonistic interactions (19–22).
Protein preparation and
measurement
The behavioral test was performed on the following 5
groups of rats following formalin injection (5%; 50 µl; n=6 per
group): Group FA (rats received formalin and an equal volume of
saline vehicle); group Alf (rats received formalin and alfentanil
at dose of 25.3 µg/kg); group Pro (rats received formalin and
propofol at dose of 8.7 mg/kg); group Alf + Pro (rats received
formalin, alfentanil-propofol combination at dose of 2.51 mg/kg);
control group rats received an equal volume of saline vehicle only.
The dosage of alfentanil or propofol alone was ED50. The
dosages of combination of alfentanil and propofol were
ED5.
To evaluate pERK protein levels, rats were
sacrificed 30 min after drug treatment. For the measurement of
c-fos protein expression, rats were sacrificed 60 min after drug
treatment. Rats were anesthetized intraperitoneally with 40 mg/kg
sodium pentobarbital, and perfused with fresh 4% paraformaldehyde
in 0.1 M phosphate buffer (pH 7.4). Following decapitation, after a
brief 20 sec exposure to CO2, rat spinal cord L5-L6
segments were excised, spinal meninges removed and tissue
dissociated mechanically, and stored at −80°C until detection.
Tissue samples were homogenized by using a Polytron handheld
homogenizer (Kinematica, Luzern, Switzerland) in lysis buffer (50
mM Tris-HCl; 150 mM NaCl; 2 mM EDTA; 10% glycerol; 1% Triton X-100;
1% Igepal CA-630; 1% sodium deoxycholic acid). After 30 min
incubation, homogenates were centrifuged at 12,000 × g for
15 min at 4°C. Supernatants were then collected and stored at −80°C
until used. Total protein content was determined using a Bradford
kit from Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
Western blot analysis
Western blot assay was performed as previously
described (21). Aliquots of total
protein samples (50 µg) were separated on a 10% SDS-PAGE gel and
transferred onto a nitrocellulose membrane. The filter membranes
were blocked with 5% non-fat milk for 1 h at room temperature and
incubated with the primary antibody specific for pERK1/2 or total
ERK1/2 (1:1,000 dilution) and c-Fos (1:1,000 dilution). The
membrane was washed with 0.05% TBS Tween buffer and incubated for 1
h with the secondary antibody conjugated with horseradish
peroxidase (Goat anti-horseradish peroxidase; cat. no. 123-005-021,
1:1,000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA) for 1 h at room temperature and visualized in ECL solution
(cat. no. 1705060; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
for 1 min, followed by film exposure for 1–10 min. The loading and
blotting of equal amounts of proteins were verified by re-probing
the membrane with antibody against β-actin (1:1,000; cat. no.
sc-47778, Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
intensity of each immunoblot assay band was quantified using a
VersaDoc Imaging System (Bio-Rad Laboratories, Inc.). The
experiments were repeated twice. Quantification of immunoreactivity
corresponding to the total and phosphorylated bands was performed
by densitometric analysis using Multi Gauge Version 3.0 (Fujifilm,
Tokyo, Japan).
Statistical analysis
Results were presented as the mean ± standard error
of the mean or as ED50 values with 95% CIs. The
statistical significance of dose-responses was determined by
one-way analysis of variance followed by the Tukey's post hoc test.
Isobolographic calculations were performed by using the Pharm Tools
Pro software (version no. 1.1.27, The McCary Group, Inc.).
Statistical analysis of the isobolograms was performed as
previously described (22) and
differences between experimental and theoretical values were
assessed by Student's unpaired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Antinociceptive effect of alfentanil
and propofol
Plantar injection of formalin produces nociceptive
behavior, including flinches of the paw. Flinching was defined as
rapid and brief withdrawal or flexing of the injected paw, lifting,
licking and rubbing behavior. In the present study, the number of
pain responses in 5 min intervals during phase I, for 20 min, and
10 min intervals during phase II, for 40 min, following formalin
injection was recorded. Saline-treated control rats exhibited
discrete biphasic behavioral responses consisting of an early
short-lasting response (phase I, 0–10 min post-injection), followed
by a late, prolonged response (phase II, ~16–60 min
post-injection). The duration of licking, lifting and rubbing were
considered to be nociceptive behaviors in the formalin model. The
mean number of flinches peaked around 0–10 min and 30–50 min after
formalin intraplantar injection, which was followed by a gradual
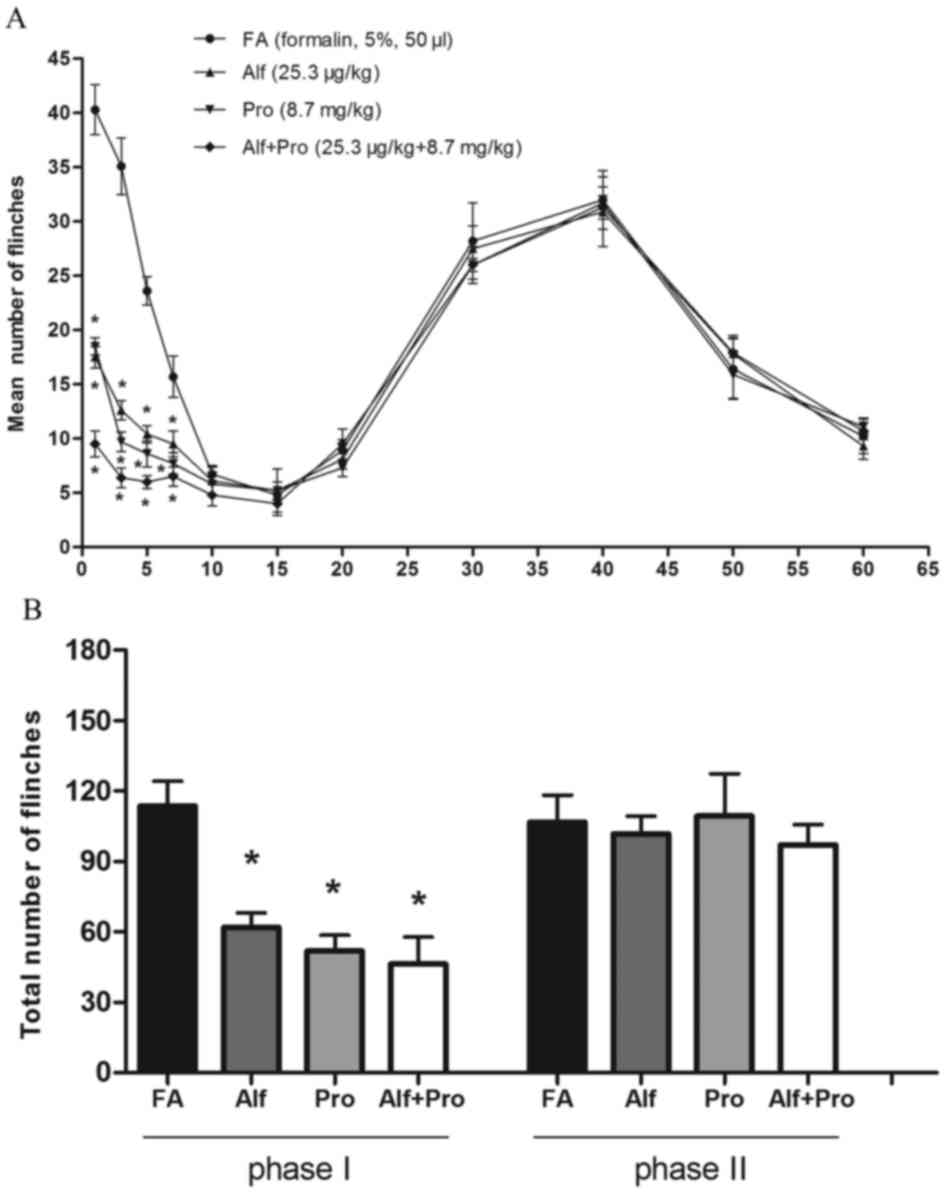
decline in all groups (Fig. 1A).
Nociceptive behavior, the mean number of flinches, between saline
and drug-treated groups was compared. There were no significant
differences between any of the groups during phase II of the
behavioral response. However, the amount of licking and lifting
behavior was reduced in the alfentanil or propofol alone groups,
and alfentanil-propofol combination group for the first 5 min after
formalin injection, compared with the saline group during phase I
(P<0.05; Fig. 1A). The total
numbers of flinches during phase I and phase II following formalin
injection in the saline treatment group was 121.4±8.8 and
100.5±12.1, respectively. However, compared with the saline
treatment group, the total number of flinches was significantly
decreased in groups Alf (56.1±5.3), Pro (50.3±5.0) and Alf+Pro
(33.2±4.6) during phase I (P<0.05; Fig. 1B). There were no significant
differences between any of the groups during phase II.
Isobolographic analysis for drug
combination
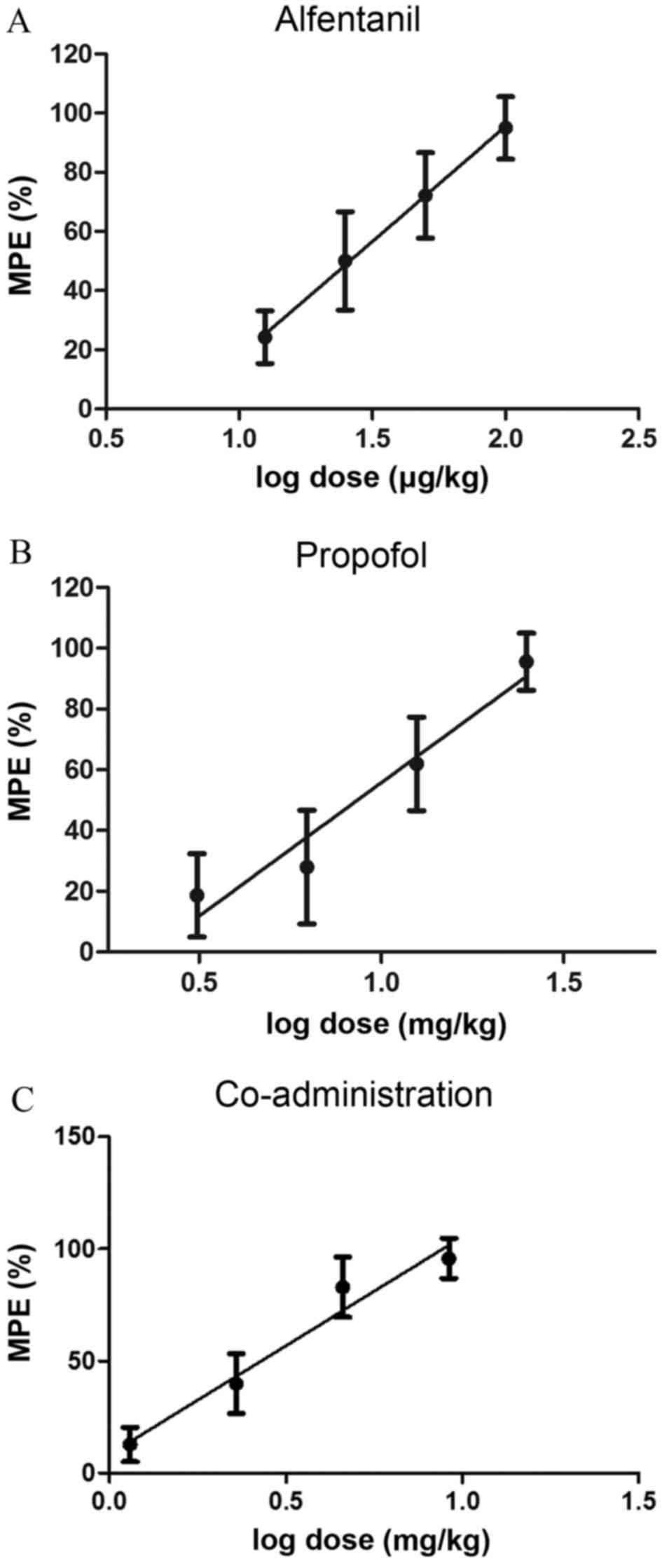
In phase I, alfentanil, propofol and
alfentanil-propofol combination groups led to a dose-dependent
antinociceptive effect. Linear regressions for alfentanil
(Y=186.0X-24.7; R2=0.9861), propofol (Y=153.7X-10.92;
R2=0.9728) and co-administration (Y=91.97X+8.55;
R2=0.9962) were calculated by plotting MPE (%) against
the log dose (Fig. 2). The
ED50 of individual administration of alfentanil and
propofol were 25.3±2.21 µg/kg and 8.7±1.344 mg/kg, respectively
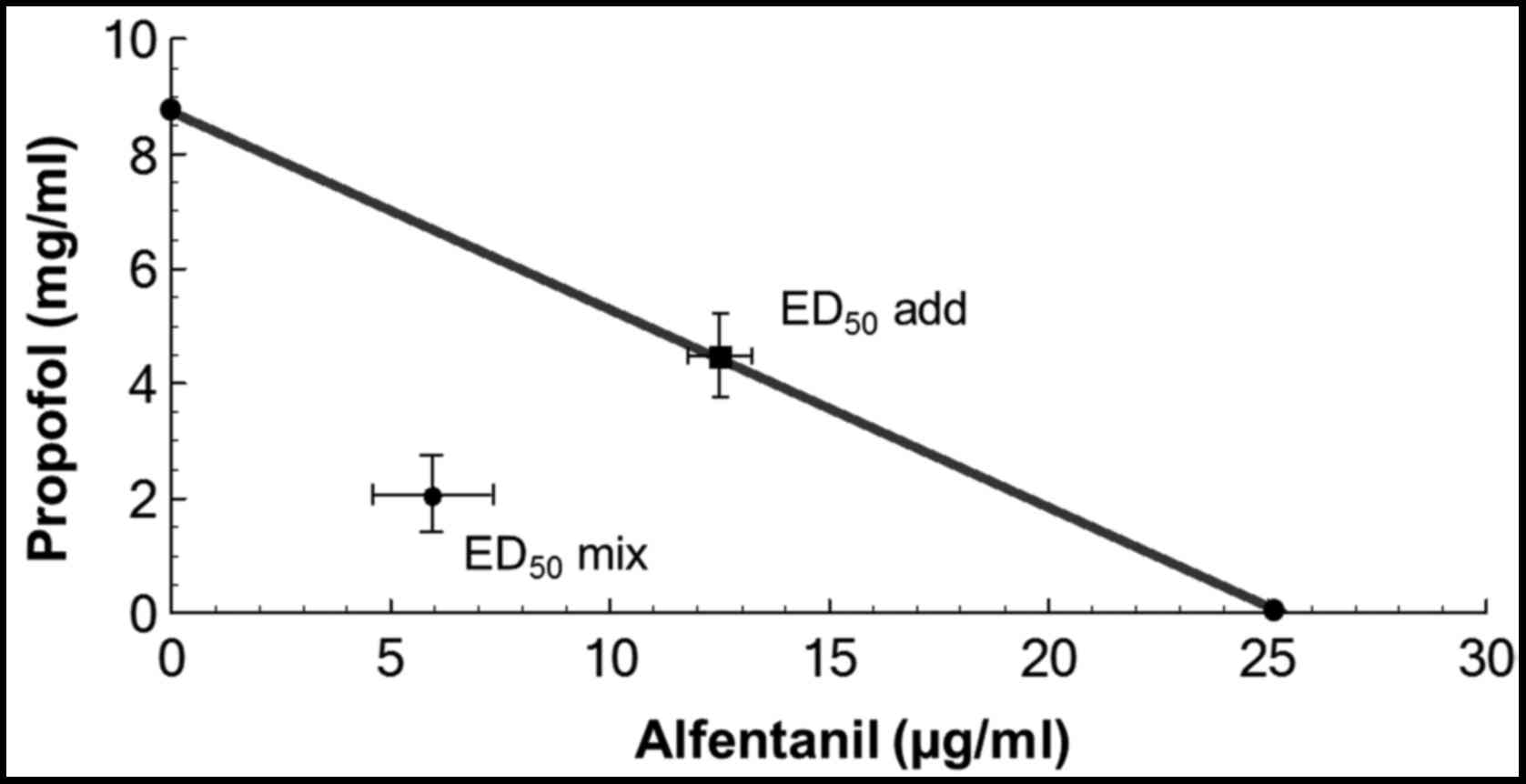
(Table I). The isobologram was
constructed by connecting the ED50 of alfentanil on the
abscissa with the ED50 of propofol on the ordinate to
obtain the additive line (Fig. 3).
For the drug combination, the ED50 mix and
the 95% CI of the mixture were computed by linear regression of the
log dose-response curve. The ED50 mix and
ED50 add were plotted in the isobologram
(Fig. 3). The fixed drug-dose
ratio based on mass quantity for alfentanil and propofol is 1:344.
The total ED50 mix for the
alfentanil-propofol combination is 2.51±0.56 mg/kg (Table I), representing 7.27 µg/kg
alfentanil and 2.50 mg/kg propofol. By isobolographic analysis, the
ED50 add=4.36 mg/kg [(0.5xED50
alfentanil) + (0.5xED50 propofol)] (Table I), representing 12.64 µg/kg
alfentanil plus 4.35 mg/kg propofol. The ED50
mix was <ED50 add (Table I). The γ value was 0.57, which
suggests a synergistic interaction between alfentanil and propofol
during phase I of the formalin test (Table I).
 | Table I.ED50 and interaction index
of alfentanil, propofol, and alfentanil-propofol combination in the
formalin test in rats. |
Table I.
ED50 and interaction index
of alfentanil, propofol, and alfentanil-propofol combination in the
formalin test in rats.
| Drug group | ED50
(confidence limits) | ED50
add | ED50
mix | γ |
|---|
| Alfentanil
(µg/kg) | 25.31±2.21
(23.11–27.51) | NA | NA | NA |
| Propofol
(mg/kg) | 8.73±1.34
(7.36–10.04) | NA | NA | NA |
| Alfentanil +
propofol (mg/kg) | NA | 4.36 |
2.51±0.56a | 0.57 |
Effects of alfentanil-propofol
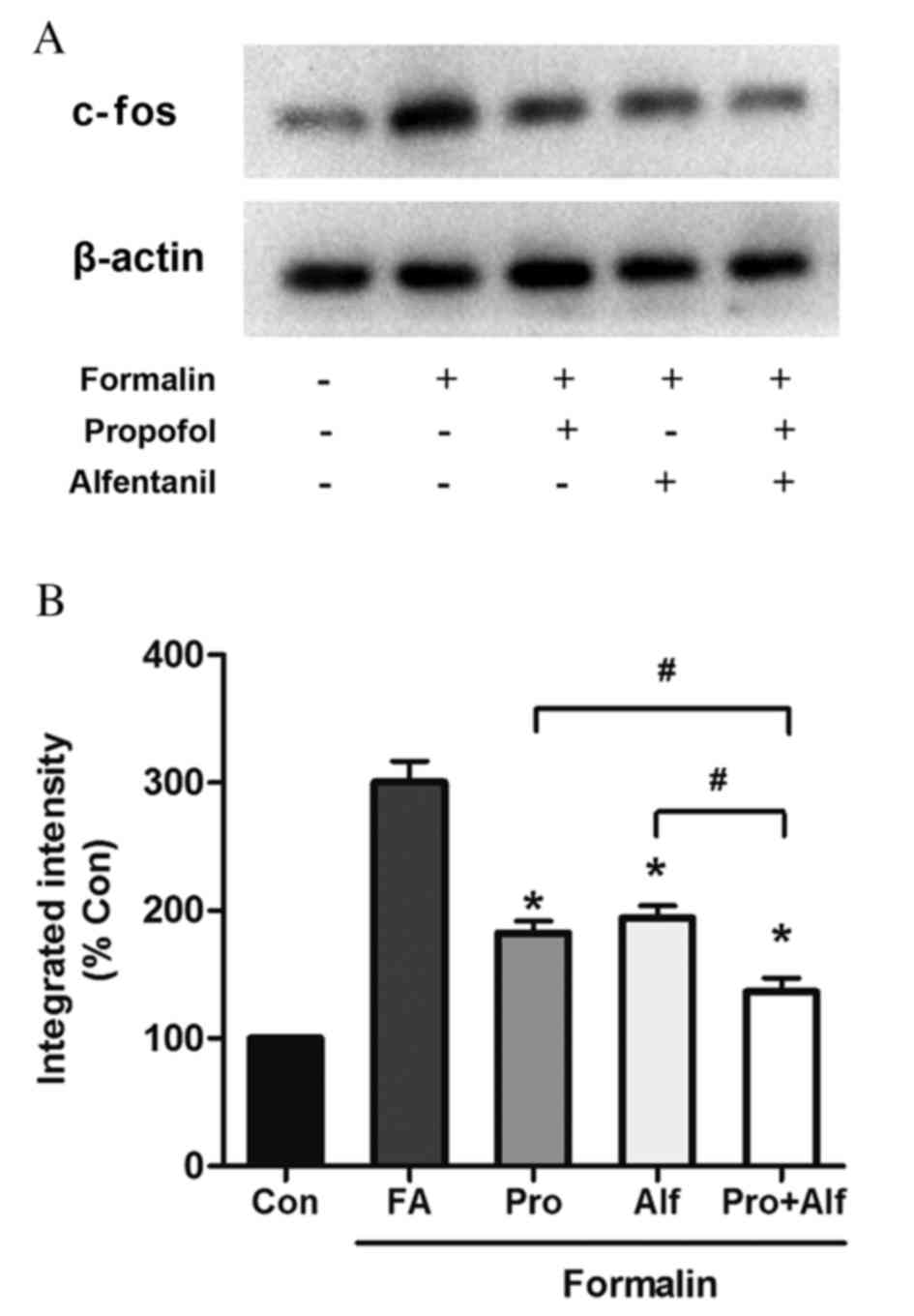
combination on ERK and c-fos expression
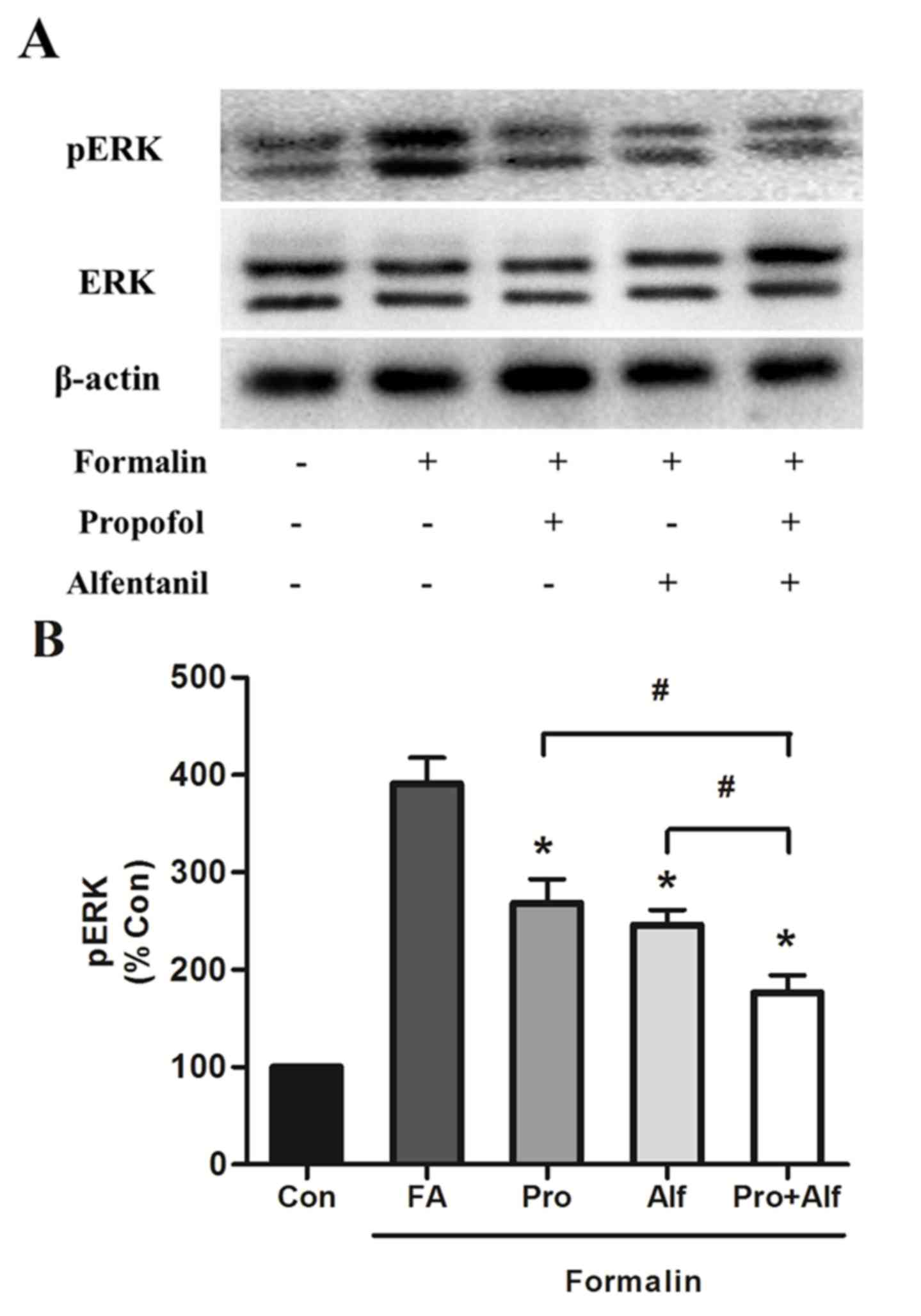
The expression of total and phosphorylated-ERK
(Fig. 4), and c-fos (Fig. 5) in control and drug-treated groups
was detected. Compared with the β-actin bands, the ratios of the
optical density of total ERK bands in all groups were not
significantly different. To investigate the expression level of the
two proteins, the ratio of the optical density of each band for
phosphorylated and total proteins were detected, and values were
normalized as a percentage of the control group. The effect of drug
treatment on total and pERK expression was determined in spinal
cord L4-L5 segments of the lumbar enlargement, areas that are
involved in the transmission of nociceptive inputs and the control
of sympathetic outflow (23). In
the present study, two bands, p42 and p44, represented ERK and were
analyzed. Concerning ERK activation, there was no significant
change in the expression of total-ERK following formalin addition
(Fig. 4). However, formalin
induced the activation of pERK, which was significantly reduced
when subsequently treated with alfentanil (P=0.02) or propofol
(P=0.03) alone, or combination treatment (P=0.01), compared with
the formalin only group (FA). Furthermore, formalin-induced
activation was significantly reduced by combination treatment (Pro
+ Alf) compared with those treated with propofol (P=0.01) or
alfentanil (P=0.03) only groups (Fig.
4). The current study subsequently investigated whether
propofol and alfentanil had any effect on basal levels of c-fos
expression. As illustrated in Fig.
5, there was a clear increase in the expression of c-fos
following injection of formalin, highlighted in the FA group. This
increased the level of c-fos expression was then significantly
reduced when treated with alfentanil (P=0.02), propofol (P=0.02) or
combination treatment (P=0.03) compared with the FA group. Notably,
the Alf+Pro group also exhibited significantly decreased c-fos
expression when compared with treatment with alfentanil (P=0.01) or
propofol (P=0.02) alone (Fig.
5).
Discussion
The major finding of the current study was that the
combination of alfentanil and propofol led to synergistic
antinociceptive effects in the formalin test. Subcutaneous hind paw
injection of formalin triggers biphasic nociceptive responses.
While phase I is considered to reflect acute nociceptive pain due
to direct stimulation of the nerve by formalin, phase II is
attributed to the combination of ongoing inflammatory-associated
afferent input from peripheral tissue and functional changes in the
spinal dorsal horn (central sensitization) (24). Accordingly, the present study
investigated the inhibitory effects of alfentanil or propofol on
the number of flinches in a given time following formalin
injection, flinches were considered to indicate a nociceptive
response. Injection of formalin led to persistent inflammatory pain
throughout the test. The current study observed that nociceptive
behavior during phase I, but not phase II, was reduced when treated
with alfentanil, propofol or both. Furthermore, it was established
that combined treatment with alfentanil and propofol led to
synergistic antinociceptive effects. The present study calculated
the ED50 mix of the alfentanil-propofol combination in
phase I of the formalin test. The ED50 for treatment
with alfentanil or propofol alone were calculated as 25.3±2.21
µg/kg and 8.7±1.344 mg/kg, respectively. The ED50
mix was significantly less than their corresponding
ED50 add, and the calculated interaction
index (γ) was <1. The results of this study initially
demonstrated the synergistic interaction between alfentanil and
propofol in the formalin test. Notably, the ED50 of
propofol in the formalin test was less than its ED50 for
clinical application, suggesting that the synergistic
antinociceptive effect may potentially be beneficial for its use in
clinic for pain treatment.
A previous study reported that propofol at
sub-hypnotic dosage (0.25 mg/kg) reduced acute pain induced by
argon laser stimulation in humans (24). Furthermore, a study that involved
healthy volunteers suggested that propofol delivered intravenously,
at 0.25 mg/kg followed by 25 µg/kg/min or more, led to a reduction
in pain intensity (25).
Additionally, another study demonstrated that intravenously
administered propofol (0.25–0.5 mg/kg) depressed pain induced by
tibial pressure algesimetry in patients who had been through
gynecologic surgery (26).
However, one study concluded that propofol (0.5 mg/kg) did not
affect thermal pain detection thresholds (27). In the current study, the
ED50 of propofol was calculated to be 8.7±1.344 mg/kg in
a formalin test performed on rats. Propofol is a commonly used
intravenous general anesthetic that acts on GABAAR and
enhances the action of GABA. It is indistinct from the analgesic
actions of propofol despite its profound anesthetic effects. Goto
et al (28) concluded that
propofol had no effect on phase II nociceptive behavioral responses
induced by formalin injection in the hind paw of rats. However,
Gilron et al (6), reported
that propofol reduced hind paw formalin-induced expression of
fos-like immunoreactivity in spinal neurons. Their results
indicated the analgesic effect of propofol. Antinociceptive effects
of propofol have previously been reported in humans (24). It is suggested, based on in
vivo studies, that propofol reduced pain in rats via spinal
GABAARs (29,30). Alfentanil is an analgesic that acts
as an agonist of µ opioid receptors and it is used to relieve acute
pain or the severe, chronic and disabling pain associated with
terminal conditions, including cancer and degenerative conditions,
which include rheumatoid arthritis. The analgesic effects of
alfentanil and propofol are regulated through different receptors
at the level of the spinal cord. However, the synergistic
antinociceptive effects of their combined treatment and the
underlying molecular mechanisms of alfentanil and propofol in acute
nociceptive pain remain unclear. The current study demonstrated
that the synergistic antinociceptive action of alfentanil and
propofol in the formalin test is regulated by ERK1/2 and c-fos, as
described above (Figs. 4 and
5) (9,10).
Further studies are required to investigate the mechanism in
further detail.
In summary, the present research suggests synergism
between alfentanil and propofol. These results provide evidence for
the potential benefits that the development of synergistic drug
combinations of opioid analgesics with intravenous anesthetics may
have if applied clinically. The combination of alfentanil with
propofol may prove beneficial for the treatment of pain, including
neuropathic or anti-inflammatory disease. Furthermore, the
combination of alfentanil and propofol treatments may produce
synergistically antinociceptive effects through inhibition of
pERK1/2 and decreased expression of c-fos in the spinal cord.
Acknowledgements
This study was financially supported by grants from
the National Nature Science Foundation of China (grant no.
81403159) and the National New Drug ‘R&D’ project (grant no.
2011ZXJ09302).
References
|
1
|
Hendrickx JF, EI II Eger, Sonner JM and
Shafer SL: Is synergy the rule? A review of anesthetic interactions
producing hypnosis and immobility. Anesth Analg. 107:494–506. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kieffer BL and Evans CJ: Opioid receptors:
From binding sites to visible molecules in vivo. Neuropharmacology.
56 Suppl 1:S205–S212. 2009. View Article : Google Scholar
|
|
3
|
Hug CC, Chaffman M, Camu F and Spierdijk
J: Alfentanil: Pharmacology and uses in anaesthesia. Adis.
1984.
|
|
4
|
Taylor BK, Peterson MA, Roderick RE, Tate
J, Green PG, Levine JO and Basbaum AI: Opioid inhibition of
formalin-induced changes in plasma extravasation and local blood
flow in rats. Pain. 84:263–270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith I, White PF, Nathanson M and
Gouldson R: Propofol. An update on its clinical use.
Anesthesiology. 81:1005–1043. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gilron I, Quirion R and Coderre TJ: Pre-
versus postinjury effects of intravenous GABAergic anesthetics on
formalin-induced Fos immunoreactivity in the rat spinal cord.
Anesth Analg. 88:414–420. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Y, Jia N, Zhao C, Li Y, Shi XP, Li YW,
Wang C, Li RL, Wang JW and Wen AD: Synergistic antinociception of
propofol-alfentanil combination in mice. Pharmacol Biochem Behav.
116:25–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun YY, Li KC and Chen J: Evidence for
peripherally antinociceptive action of propofol in rats: Behavioral
and spinal neuronal responses to subcutaneous bee venom. Brain Res.
1043:231–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji RR, Befort K, Brenner GJ and Woolf CJ:
ERK MAP kinase activation in superficial spinal cord neurons
induces prodynorphin and NK-1 upregulation and contributes to
persistent inflammatory pain hypersensitivity. J Neurosci.
22:478–485. 2002.PubMed/NCBI
|
|
10
|
Hunt SP, Pini A and Evan G: Induction of
c-fos-like protein in spinal cord neurons following sensory
stimulation. Nature. 328:632–634. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morgan JI and Curran T:
Stimulus-transcription coupling in neurons: Role of cellular
immediate-early genes. Trends Neurosci. 12:459–462. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vuyk J, Engbers FH, Burm AG, Vletter AA,
Griever GE, Olofsen E and Bovill JG: Pharmacodynamic interaction
between propofol and alfentanil when given for induction of
anesthesia. Anesthesiology. 84:288–299. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vogel HG, et al: Guidelines for the care
and use of laboratory animalsDrug Discovery and Evaluation.
Springer; Berlin Heidelberg: pp. 2023–2037. 2007, View Article : Google Scholar
|
|
14
|
Wood MM and Cousins MJ: Iatrogenic
neurotoxicity in cancer patients. Pain. 39:1–3. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho IH, Chung YM, Park CK, Park SH, Lee H,
Kim D, Piao ZG, Choi SY, Lee SJ, Park K, et al: Systemic
administration of minocycline inhibits formalin-induced
inflammatory pain in rat. Brain Res. 1072:208–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tjølsen A, Berge OG, Hunskaar S, Rosland
JH and Hole K: The formalin test: An evaluation of the method.
Pain. 51:5–17. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guindon J, LoVerme J, Piomelli D and
Beaulieu P: The antinociceptive effects of local injections of
propofol in rats are mediated in part by cannabinoid CB1 and CB2
receptors. Anesth Analg. 104:1563–1569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia N, Zhao C, Wang L, Li Y, Cui J, Cao S,
Li R, Wang C, Wu Y and Wen A: The effects of a propofol/alfentanil
admixture on total intravenous anaesthesia in dogs undergoing
splenectomy. Veterinární Medicína. 60:194–201. 2015. View Article : Google Scholar
|
|
19
|
Tallarida RJ, Stone DJ Jr and Raffa RB:
Efficient designs for studying synergistic drug combinations. Life
Sci. 61:PL417–PL425. 1997. View Article : Google Scholar
|
|
20
|
Tomić MA, Vucković SM, Stepanović-Petrović
RM, Ugresić ND, Prostran MS and Bosković B: Synergistic
interactions between paracetamol and oxcarbazepine in somatic and
visceral pain models in rodents. Anesth Analg. 110:1198–1205.
2010.PubMed/NCBI
|
|
21
|
Tallarida RJ: Drug synergism: Its
detection and applications. J Pharmacol Exp Ther. 298:865–872.
2001.PubMed/NCBI
|
|
22
|
Tallarida RJ: The interaction index: A
measure of drug synergism. Pain. 98:163–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fürst S: Transmitters involved in
antinociception in the spinal cord. Brain Res Bull. 48:129–141.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anker-Møller E, Spangsberg N,
Arendt-Nielsen L, Schultz P, Kristensen MS and Bjerring P:
Subhypnotic doses of thiopentone and propofol cause analgesia to
experimentally induced acute pain. Br J Anaesth. 66:185–188. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zacny JP, Coalson DW, Young CJ, Klafta JM,
Lichtor JL, Rupani G, Thapar P and Apfelbaum JL: Propofol at
conscious sedation doses produces mild analgesia to cold
pressor-induced pain in healthy volunteers. J Clin Anesth.
8:469–474. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Briggs LP, Dundee JW, Bahar M and Clarke
RS: Comparison of the effect of diisopropyl phenol (ICI 35, 868)
and thiopentone on response to somatic pain. Br J Anaesth.
54:307–311. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wilder-Smith OH, Kolletzki M and
Wilder-Smith CH: Sedation with intravenous infusions of propofol or
thiopentone Effects on pain perception. Anaesthesia. 50:218–222.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goto T, Marota JJ and Crosby G:
Pentobarbitone, but not propofol, produces pre-emptive analgesia in
the rat formalin model. Br J Anaesth. 72:662–667. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nadeson R and Goodchild CS:
Antinociceptive properties of propofol: Involvement of spinal cord
gamma-aminobutyric acid(A) receptors. J Pharmacol Exp Ther.
282:1181–1186. 1997.PubMed/NCBI
|
|
30
|
Merrill AW, Barter LS, Rudolph U, EI II
Eger, Antognini JF, Carstens MI and Carstens E: Propofol's effects
on nociceptive behavior and spinal C-Fos expression after
intraplantar formalin injection in mice with a mutation in the
gamma-aminobutyric acid-type(A) receptor beta3 subunit. Anesth
Analg. 103:478–483. 2006. View Article : Google Scholar : PubMed/NCBI
|