Introduction
Thyrotoxicosis is a condition with multiple
etiologies, manifestations and potential therapies. The term
‘thyrotoxicosis’ refers to a clinical state that results from
increased thyroid hormone levels in tissues. The most common
manifestation is termed Graves' disease (GD). In China, the
prevalence of GD is approximately 1.1–1.4% (1). GD is an organ-specific autoimmune
thyroid disease with a genetic predisposition. Typical features of
GD include high metabolic syndrome, goiter and Graves' orbitopathy
(GO). Patients with GD should be treated with any of the following
modalities: Anti Thyroid Drugs (ATDs), radioactive iodine (RAI)
therapy, or thyroidectomy. In China, ATD therapy has been the most
preferred by physicians, however recent developments have suggested
an increase in the use of RAI therapy to reduce the use of ATDs. In
GD, the generation of an autoantibody, thyrotrophin receptor
antibody (TRAb), against the thyroid-stimulating hormone (TSH)
receptor (TSHR) causes continuous stimulation of the thyroid gland
and hyperthyroidism (2,3). The precise immunological mechanism of
TRAb production, however, remains elusive. Previous studies have
indicated that TRAb is detected in the serum of most patients with
GD (4,5). TRAb is an important independent risk
factor for GD (6). The titer of
TRAb influences the duration of ATD therapy and the dose of RAI
therapy (7). The establishment of
a GD animal model allows the study of its etiology and potential
therapeutic targets. In recent years, various animal models of GD
have been developed that have demonstrated an autoimmune response
to TSHR. Two attempts were made to immunize mice using human TSHR:
one via the injection of TSHR-transfected cells simultaneously
expressing MHC class II (8), and
the other by genetic immunization using TSHR cDNA (9). These immunized mice demonstrated
typical phenotypes associated with hyperthyroidism, however the
response was temporary and TRAb was produced against non-self TSHR.
The process of autoantibody production in these mice presumably
lacks the persistent abrogation of self-tolerance. The present
study investigated an improved method for inducing GD in BALB/c
mice by injecting the recombinant plasmid pcDNA3.1/TSHR268 and
performing electroporation (EP). This novel method has numerous
advantages, including a short model cycle, high success rates,
stability and reproducibility. This model may not precisely mimic
human disease; however, the present study forms a theoretical basis
for further study on the etiology and pathogenesis of GD, and for
the development of novel therapeutic strategies.
Materials and methods
Materials
A total of 90 female BALB/c mice (age, 6–8 weeks;
weight, 18–20 g) were obtained from the Institute of Laboratory
Animal Sciences, Chinese Academy of Medical Sciences (Beijing,
China). All the animals were housed in individual cages with a
constant temperature (18–20°C) and humidity (65–69%) at 12/12 h
light/dark cycle with free access to food and water. The
experimental procedures were approved by the animal ethics
committee of Tianjin Medical University. EP was performed using an
ECM®830 electroporater (BTX; Harvard Apparatus,
Holliston, MA, USA). Pertechnetate (99mTcO4-) was
produced by a molybdenum technetium generator (China Institute of
Atomic Energy, Beijing, China). The mice were imaged using a
single-photon emission computer tomography (SPECT) machine
(Discovery NM-670; GE Healthcare Life Sciences, Shanghai,
China).
Preparation of recombinant plasmid
pcDNA3.1/TSHR268
Total RNA was extracted from healthy human thyroid
tissue and reverse transcribed. The tissue was obtained from male
and female adult patients, during the resection of benign thyroid
nodules. The Total RNA extraction kit (Protein and RNA Extraction
Kit) and reverse transcription kit (PrimeScript 1st Strand cDNA
Synthesis kit) were supplied by Takara Biotechnology Co., Ltd.
(Dalian, China). The experiments were performed following the
manufacturer's protocol. The experimental procedures and materials
were approved by the ethics committee of Tianjin Medical University
General Hospital. Human TSHR 164–967 bp (TSHR268) was amplified by
polymerase chain reaction (PCR) using the following primer
sequences: Forward, 5′-ACGCGAATTCGCCACCATGGGGTGTTCGTCTCC-3 and
reverse, 5′-AGGCGGATCCCTGATTCTTAAAAGCACAGCAGT-3. The PCR reaction
mixture contained: 0.25 µl Pyrobest™ DNA polymerase (Takara
Biotechnology Co., Ltd.), 5 µl 10X Pyrobest buffer, 4 µl
2′-deoxynucleoside 5′-triphosphates mix (2.5 mmol/l), 1 µl upstream
primer (20 µmol/l), 1 µl downstream primer (20 µmol/l), 2.5 ng
template cDNA and deionized water to a final volume of 50 µl. The
cycling conditions were as follows: Pre-denaturation at 94°C for 4
min, followed by 30 cycles of denaturation at 94°C for 45 sec,
annealing at 56°C for 30 sec and extension at 72°C for 1 min, and a
final extension step at 72°C for 5 min. The PCR product was cloned
into the pcDNA3.1 vector by pcDNA3.1/V5-His TOPO TA Expression Kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
experimental procedure was conducted according to the
manufacturer's protocol.
Experimental groups
The BALB/c mice were randomly divided into
experimental (n=50), control (n=20) and blank (n=20) groups. Mice
were injected with the recombinant plasmid pcDNA3.1/TSHR268
(experimental group) or an equal volume of saline (control and
blank groups). Experimental and control group mice were subjected
to EP.
Establishment of the GD mouse
model
Mice were anesthetized with an intraperitoneal
injection of 50 mg/kg sodium pentobarbital (Shanghai Haling
Biotechnology Co., Ltd., Shanghai, China). At weeks 1, 4, 7 and 10,
recombinant plasmid pcDNA3.1/TSHR268 (50 µl, 1 g/l) was injected
into the bilateral gastrocnemius of experimental group mice. An
equal volume of saline was injected into the control and blank
group mice at the same time. The experimental and control group
mice were subjected to EP at the same time and location to enhance
immunization. For EP, a pair of electrode needles was injected into
the muscle across the site at which the recombinant plasmid was
injected. Electric pulses were delivered using an electroporator. A
total of three 50 V pulses and three additional pulses of the
opposite polarity were administered at each injection site at a
rate of one pulse per 200 msec, a frequency of 5 pulses/sec and a
duration of 50 msec (10).
T4 and TSH
measurements
Blood from the angular vein was collected to measure
the levels of total serum T4 and serum TSH using
radioimmunoassay and immunoradiometric kits, respectively (Beijing
North Institute of Biotechnology, Beijing, China) 7 to 10 days
after each immunization and every three weeks until week 21.
ELISA for measuring TRAb N-terminal
(N) and C-terminal (C)
Blood from the angular vein was collected to measure
the levels of TRAb N and C by ELISA at the same time points as
above. Thyroid-stimulating antibody (TSAb) was used in the N method
and thyroid stimulation blocking antibody (TSBAb) was used in the C
method, as respective antigens. A total of two ELISA methods for
the detection of TSAb and TSBAb have been established and evaluated
by our laboratory (11). The
absorbance value A450 served as the quantitative
parameter to reflect the levels of TRAb. TRAb C levels were
measured in arbitrary units per milliliter (AU/ml) (12).
99mTcO4− radioactive isotope
imaging
Whole body 99mTcO4- imaging was performed
at weeks 0, 12 and 21. A total of three mice from each group were
randomly selected and anesthetized with pentobarbital.
99mTcO4- was intraperitoneally injected into each mouse
(37 MBq/0.1 ml). Scintigraphy was performed 10 min later using a
SPECT machine. A low-energy universal collimator was used for
imaging. The imaging conditions were as follows: Energy peak, 140
keV; matrix, 128×256; magnification, 3.0; and counts, 3×105.
Weight, thyroid morphology and
pathology analysis
All mice were weighed 7 to 10 days following each
immunization and every three weeks until week 21. They were
subsequently sacrificed by cervical vertebra dislocation at the end
of week 21. The thyroid glands were removed and fixed with 10%
formalin. After one week, the tissue was embedded in paraffin
blocks, sectioned and stained with hematoxylin and eosin for
analysis (13).
Statistical analysis
Data were analyzed using an independent samples
t-test or one-way analysis of variance followed by a least
significant difference post hoc test. SPSS software version 19.0
(IBM SPSS, Armonk, NY, USA) was used to analyze the data. Data are
expressed as the mean ± standard deviation or number (%). P<0.05
was considered to indicate a statistically significant
difference.
Results
Induction of GD in mice
During the course of the experiment, two mice in the
experimental group and one in the blank group died. Prior to the
experiment, T4, and TRAb N and C levels of all mice in
the experimental group were within the same range as those in the
control and blank groups. At the end of the immunization protocol
(week 12), 38 of the 48 mice in the experimental group had
increased levels of T4, and TRAb N and C. All 38 mice
demonstrated reduced levels of TSH. The rate of disease induction
was therefore 79.17%.
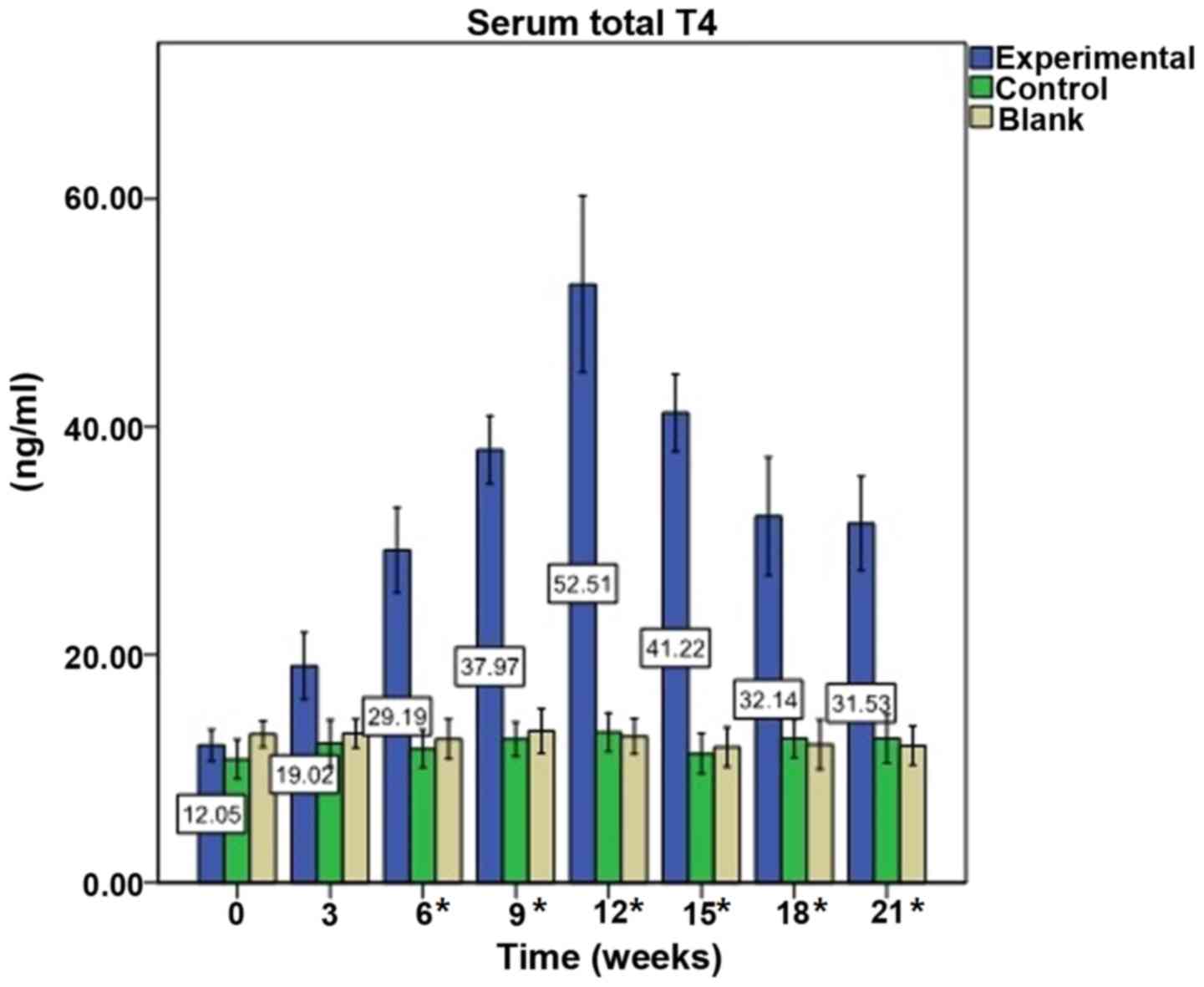
Immunization elevates serum
T4 levels
Prior to immunization (week 0), baseline mean levels
of T4 in the experimental mice were 12.05±4.23 ng/ml.
Following the first immunization, the mean levels of T4
increased slowly and peaked following the fourth immunization at
week 12, where T4 was 52.51±23.58 ng/ml (P<0.0001).
T4 levels decreased from weeks 15 to 21; however, they
remained significantly increased compared with pre-immunization
levels (all P=0.000). T4 levels in the control group
were 13.21±3.73 ng/ml at week 12 vs. 10.84±3.79 ng/ml at week 0
(P=0.593), and in the blank group were 12.84±3.36 ng/ml at week 12
vs. 13.03±2.47 ng/ml at week 0 (P=0.892). There were no significant
alterations in T4 levels in the control and blank groups
during the experiment (Fig.
1).
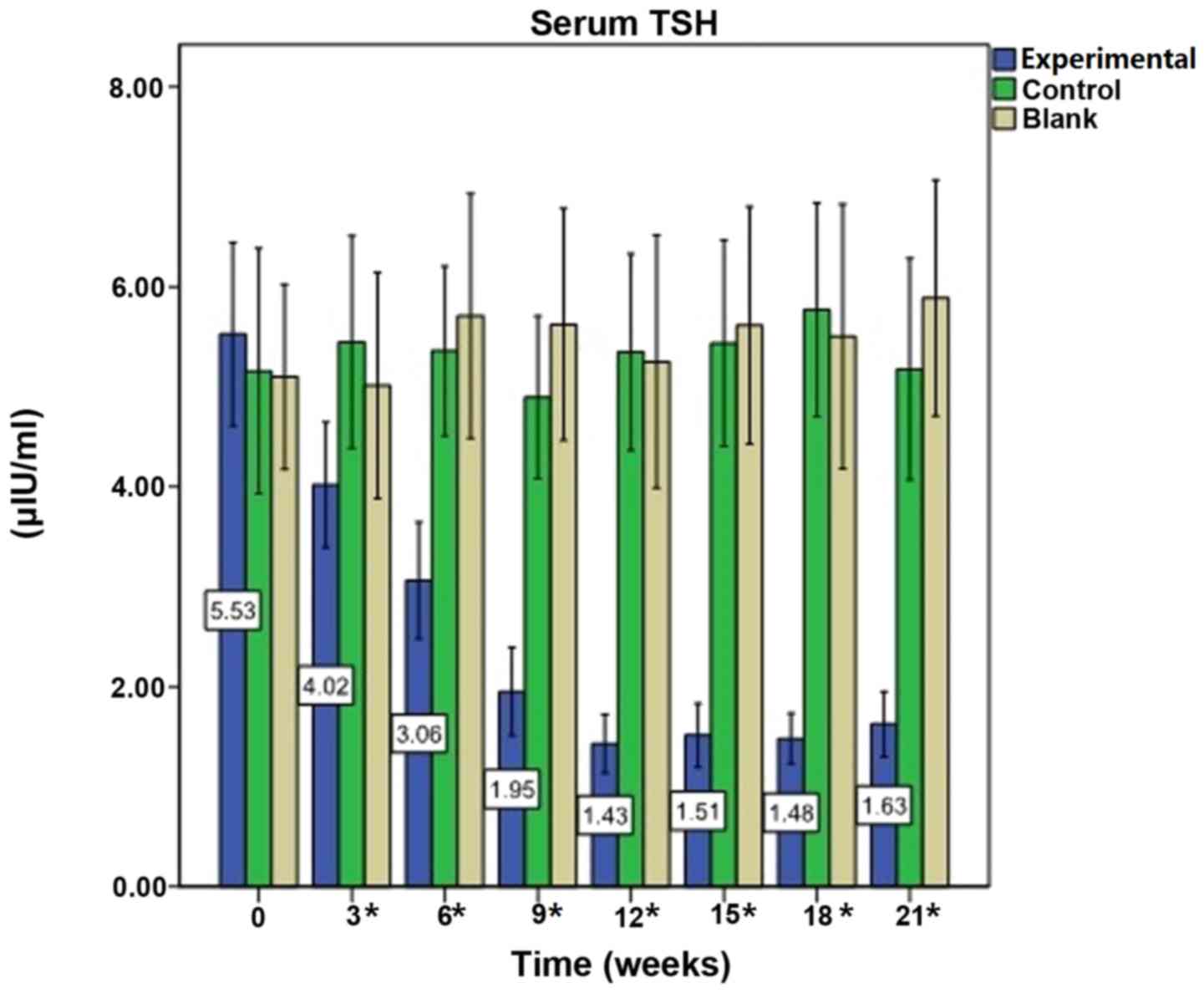
Immunization reduces serum TSH
levels
Prior to immunization (week 0), baseline mean levels
of serum TSH in the experimental mice were 5.53±2.78 µIU/ml.
Following the first immunization, the mean levels of TSH declined
(all P<0.0001). During the course of the experiment, TSH levels
fluctuated; however, they remained reduced compared with
pre-immunization levels (all P<0.0001). TSH levels in the
control group were 5.35±2.17 µIU/ml at week 12 vs. 5.16±2.70 µIU/ml
at week 0 (P=0.969), and in the blank group were 5.25±2.72 µIU/ml
at week 12 vs. 5.10±1.99 µIU/ml at week 0 (P=0.960). There were no
significant alterations in serum TSH levels in the control and
blank groups (Fig. 2).
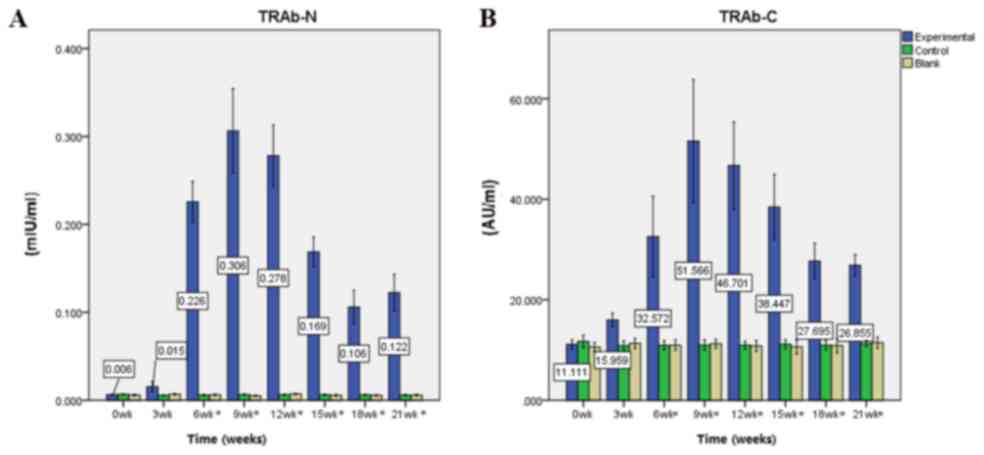
Immunization elevates TRAb N and C
levels
Prior to immunization (week 0), baseline mean levels
of serum TRAb N and C in experimental mice were 0.006±0.002 mIU/ml
and 11.111±2.808 AU/ml, respectively. Following the first
immunization, the mean levels of TRAb N and C increased steadily.
Following the third immunization at week nine, TRAb N and C levels
peaked at 0.306±0.146 mIU/ml and 51.566±37.357 AU/ml, respectively
(all P<0.0001). From the fourth immunization (week 12) to the
end of the experiment (week 21), the mean levels of TRAb N and C
decreased; however, they remained significantly increased compared
with pre-immunization levels (TRAb C at week 21, P=0.001, the rest
P<0.0001). TRAb N levels in the control group were 0.006±0.002
mIU/ml at week 9 vs. 0.007±0.002 mIU/ml at week 0 (P=0.520), and in
the blank group were 0.005±0.002 mIU/ml at week 9 vs. 0.006±0.002
mIU/ml at week 0 (P=0.885). TRAb C levels in the control group were
10.97±2.35 AU/ml at week 9 vs. 11.69±2.78 AU/ml at week 0
(P=0.933), and in the blank group were 11.25±1.91 AU/ml at week 9
vs. 10.59±1.99 AU/ml at week 0 (P=0.939). There were no significant
alterations in TRAb N and C levels in the control and blank groups
(Fig. 3).
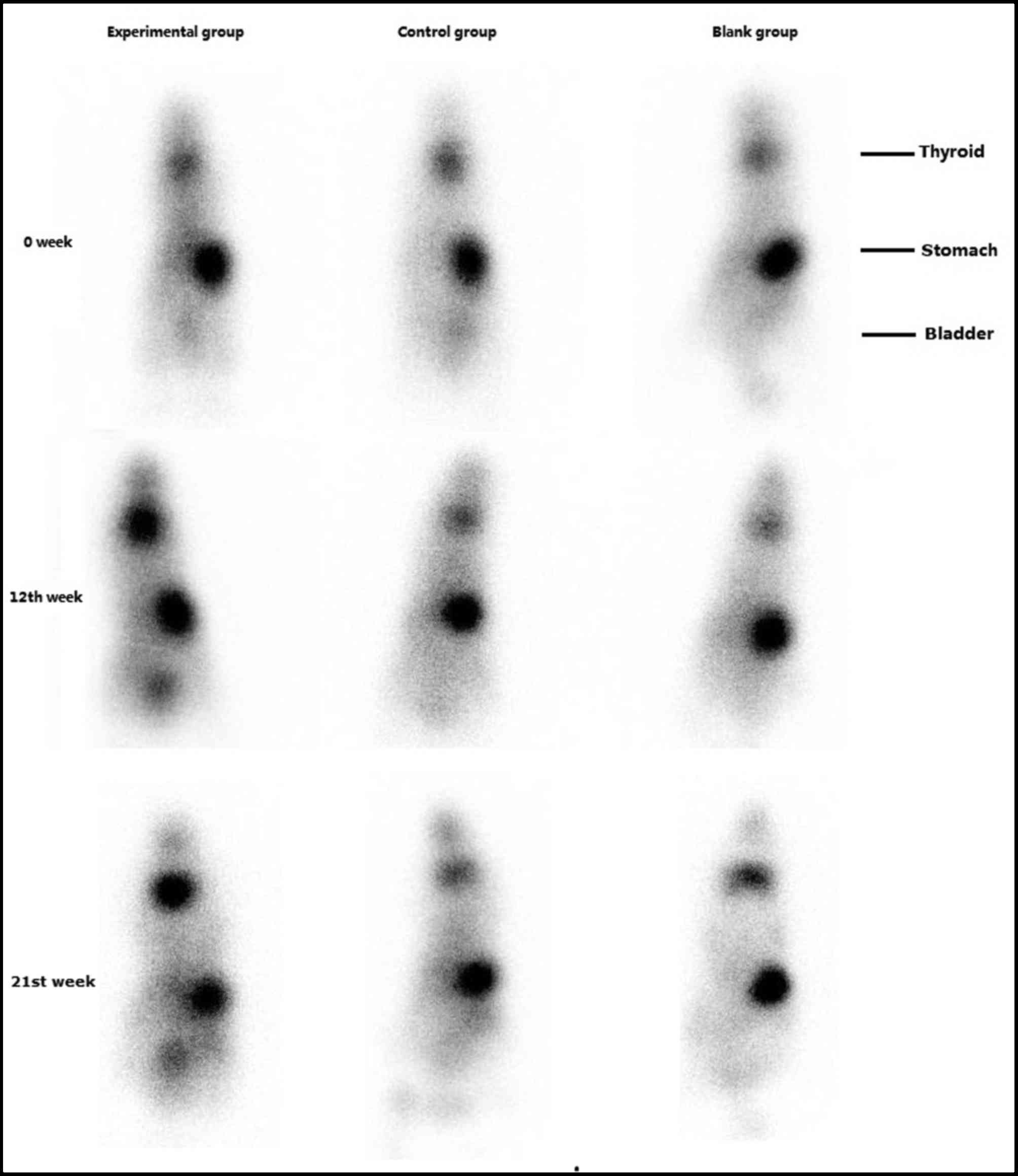
99mTcO4− radioactive isotope
imaging
There were no significant differences in thyroid
99mTcO4- uptake between the three groups prior to
immunization. Following the fourth immunization (week 12), the
uptake of 99mTcO4- by the thyroid was increased in the
experimental group. At week 21, this remained increased compared
with pre-immunization levels. There were no significant alterations
in 99mTcO4- thyroid uptake in the control and blank
groups (Fig. 4).
Weight, thyroid morphology and
pathology analysis
At week 0, the mean weights of mice in the three
groups were similar. The weight of mice increased steadily during
the experiment in all groups. At week 21, the mean weight of the
experimental mice was significantly reduced compared with the blank
and control groups (Table I).
 | Table I.Weight analysis. |
Table I.
Weight analysis.
|
|
| Weight of mice
(g) |
|---|
|
|
|
|
|---|
| Group | Number of mice | Week 0 | Week 3 | Week 6 | Week 9 | Week 12 | Week 15 | Week 18 | Week 21 |
|---|
| Experimental | 38 | 20.20±1.48 | 20.74±1.38 |
21.45±1.70b |
20.83±1.87a,b |
20.78±1.29a,b |
21.13±2.61a,b |
21.92±1.57a,b | 21.84±2.28
a,b |
| Control | 20 | 20.73±1.31 | 20.23±1.51 | 21.96±1.13 | 22.34±1.92 | 24.45±1.27 | 24.76±1.86 | 25.90±1.65 | 25.46±1.45 |
| Blank | 19 | 20.89±1.36 | 20.31±1.88 | 22.04±1.62 | 22.88±1.95 | 24.23±1.59 | 24.27±1.40 | 25.40±1.64 | 25.36±1.90 |
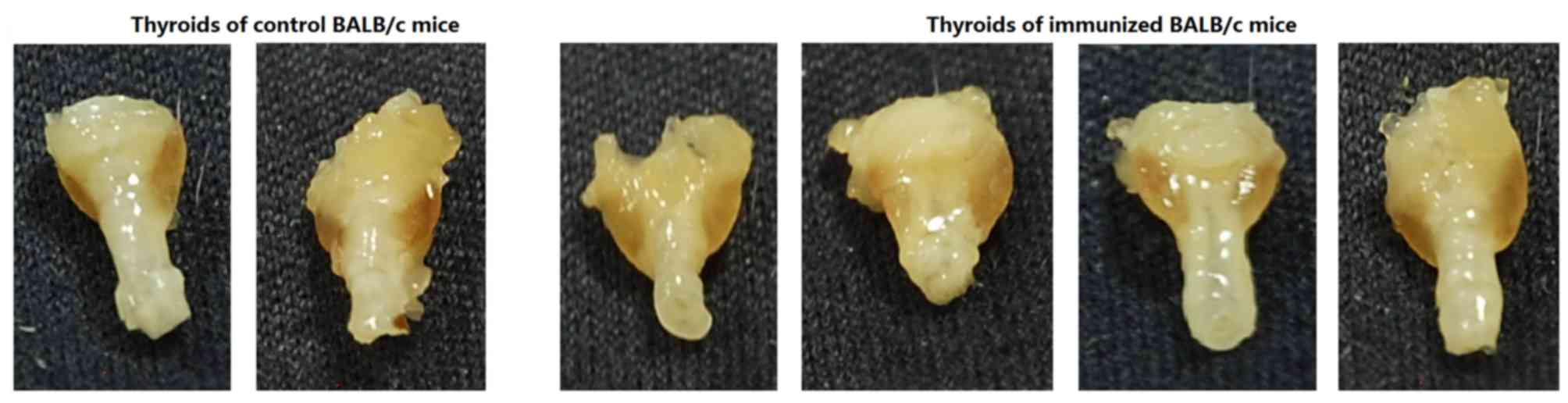
The thyroid glands from experimental group mice
exhibited diffuse enlargement with hypertrophy, unlike the blank
and control mice (Fig. 5).
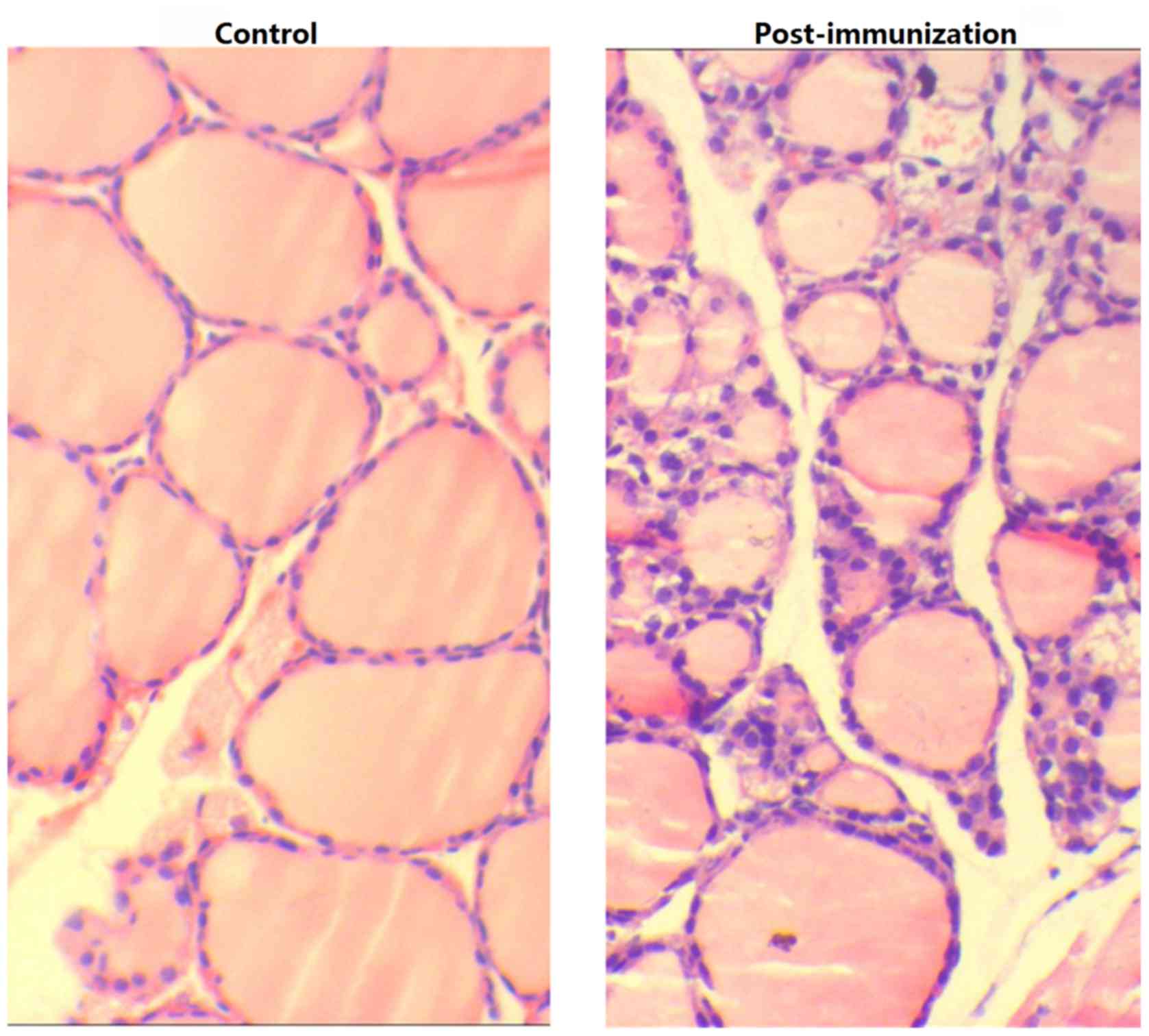
Examination of the thyroid tissue via microscopy revealed
lymphocyte infiltration, fewer colloid nodules and an increase in
the height of epithelial cells in experimental mice compared with
blank and control group mice (Fig.
6).
Discussion
The immunological processes involved in autoimmune
thyroid disease, including GD, are hypothesized to be mediated
primarily by antibodies, similarly to other autoimmune diseases
(14). Hyperactivity of the
thyroid gland is the result of thyroid-stimulating antibodies,
which are now considered to recognize and activate the TSHR. One of
the unique characteristics of GD is the presence of TRAb, which is
detectable in the serum of the majority of patients with the
disease, and leads to hyperplasia and hyperfunctioning of the
thyroid gland due to activation of the thyrotropin receptor
(4,5,15).
Our previous study demonstrated that, as one of the most important
risk factors for GD, TRAb influenced prognosis and treatment
efficacy, particularly in female patients (16). A total of three varieties of TRAb
are present in most GD patients: TSAb, TSBAb and a neutral
antibody. TSAb and TSBAb bind to the N- and C-terminal regions of
the TSHR extracellular domain, respectively. It is hypothesized
that TSAb serves a central role in the pathogenesis of GD, by
stimulating TSH-mediated activation of thyroid function. However,
TSBAb may act as a weak TSH agonist to induce hypothyroidism by
preventing TSH from binding to the receptor. The neutral TSHR
antibody does not block TSH binding or action (17). Previous studies have indicated that
TSBAb may be the precursor form of TSAb (18).
The establishment of an animal model of GD
contributes to the investigation of its etiology, pathogenesis and
potential therapeutic targets. Following decades of developments,
animal models of GD have greatly improved. However, limitations of
GD mouse models include the complex model preparation process, low
success rate and poor reproducibility. An ideal GD mouse model
should be spontaneous, reproducible and have a high disease
incidence in a variety of mouse strains (19). Thus far, the common features of all
successful GD models include TSHR expression, immune system
stimulation via repeated injections.
In the present study, following four immunizations,
38 of 48 BALB/c mice (79.17%) developed hyperthyroidism, a
percentage that is consistent with or greater than previous studies
(20–23). All hyperthyroid mice demonstrated
increased serum T4, TRAb N and C levels, and reduced TSH
and weight levels. It was observed that TRAb N levels markedly
increased following the second immunization at week 6, and peaked
at week 9. TRAb C levels demonstrated a similar trend; however, the
increase was more gradual. Under the stimulation of TRAb, serum
T4 levels increased gradually and peaked following the
fourth immunization at week 12. Over the same period, serum TSH
levels declined gradually. Following the fourth immunization, serum
TSH levels stabilized. Following cessation of immunization, serum
T4 and TRAb levels decreased slightly; however, at the
end of the experiment, they remained significantly increased
compared with pre-immunization levels. The uptake of
99mTcO4− by the thyroid increased
significantly in immunized mice. Compared with the control and
blank groups, mice in the experimental group were emaciated and
exhibited enlarged thyroid glands. Examination of the thyroid
tissue via microscopy revealed lymphocyte infiltration, fewer
colloid nodules and an increase in the height of epithelial cells
in the experimental mice compared with the blank and control
groups. All of the above are characteristic of GD, thus indicating
the establishment of a successful of model of the disease.
Furthermore, the increased levels of TRAb C support the hypothesis
that TSBAb may be the precursor form of TSAb.
It is widely accepted that the study of animal
models of disease provides an improved understanding of the
underlying pathogenesis, and may be used for evaluating novel
therapeutic strategies. An appropriate animal model of GD would
include the following features: i) Elevated T4 and/or
reduced TSH levels, ii) TSHR-associated biologically active
antibodies, iii) alterations in thyroid architecture, iv)
lymphocytic thyroiditis, v) clinical signs of hyperthyroidism
including weight loss, vi) orbital alterations similar to those in
thyroid eye disease, and vii) lymphocytic infiltration in the
pretibial skin (24). With the
exception of pathological alterations of eyes and pretibial skin,
the additional five clinical characteristics were observed in the
model established in the present study.
In conclusion, the present study demonstrated an
improved animal model of GD in which repeated intramuscular
injection of recombinant plasmid pcDNA3.1/TSHR268 and EP in
vivo efficiently induced TRAb with thyroid stimulating activity
and hyperthyroidism. This novel method has the advantages of a
short modeling cycle, high induction rate, and good stability and
reproducibility. This model may not precisely mimic human disease,
and therefore requires further examination; however, based on these
findings, it may be possible to further investigate the etiology
and pathogenesis of GD, and develop novel ideas for the clinical
treatment of this disease.
Acknowledgements
This paper is supported by the National Natural
Science Foundation of China (grant no. 81601523 and 81501510).
References
|
1
|
Teng W, Shan Z, Teng X, Guan H, Li Y, Teng
D, Jin Y, Yu X, Fan C, Chong W, et al: Effect of iodine intake on
thyroid diseases in China. N Engl J Med. 354:2783–2793. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith B Rees, McLachlan SM and Furmaniak
J: Autoantibodies to the thyrotropin receptor. Endocr Rev.
9:106–121. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rapoport B, Chazenbalk GD, Jaume JC and
McLachlan SM: The thyrotropin (TSH) receptor: Interaction with TSH
and autoantibodies. Endocr Rev. 19:673–716. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andrade VA, Gross JL and Maia AL: Serum
thyrotropin-receptor autoantibodies levels after I therapy in
Graves' patients: Effect of pretreatment with methimazole evaluated
by a prospective, randomized study. Eur J Endocrinol. 151:467–474.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takasu N, Kamijo K, Sato Y, Yoshimura H,
Nagata A and Ochi Y: Sensitive thyroid-stimulating antibody assay
with high concentrations of polyethylene glycol for the diagnosis
of Graves' disease. Clin Exp Pharmacol Physiol. 31:314–319. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McKenna TJ: Graves' disease. Lancet.
357:1793–1796. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross DS, Burch HB, Cooper DS, Greenlee MC,
Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN and
Walter MA: 2016 American thyroid association guidelines for
diagnosis and management of hyperthyroidism and other causes of
thyrotoxicosis. Thyroid. 26:1343–1421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimojo N, Kohno Y, Yamaguchi K, Kikuoka
S, Hoshioka A, Niimi H, Hirai A, Tamura Y, Saito Y, Kohn LD and
Tahara K: Induction of Graves-like disease in mice by immunization
with fibroblasts transfected with the thyrotropin receptor and a
class II molecule. Proc Natl Acad Sci USA. 93:11074–11079. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Costagliola S, Many MC, Denef JF, Pohlenz
J, Refetoff S and Vassart G: Genetic immunization of outbred mice
with thyrotropin receptor cDNA provides a model of Graves' disease.
J Clin Invest. 105:803–811. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vicat JM, Boisseau S, Jourdes P, Lainé M,
Wion D, Bouali-Benazzouz R, Benabid AL and Berger F: Muscle
transfection by electroporation with high-voltage and short-pulse
currents provides high-level and long-lasting gene expression. Hum
Gene Ther. 11:909–916. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li N, Fang P, Zhang Y and Li S: A novel
human TSHR antibody ELISA using recombinant extracellular domain
fragments of human TSH receptor as antigen and initial clinical
evaluation. Chin J Nucl Med. 29:348–351. 2009.
|
|
12
|
Olesen H: Properties and units in the
clinical laboratory sciences. I. Syntax and semantic rules
(recommendation 1995). International union of pure and applied
chemistry (IUPAC) and international federation of clinical
chemistry (IFCC). Eur J Clin Chem Clin Biochem. 33:627–636.
1995.PubMed/NCBI
|
|
13
|
Hine IF: Block staining of mammalian
tissues with hematoxylin and eosin. Stain Technol. 56:119–123.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morshed SA, Latif R and Davies TF:
Delineating the autoimmune mechanisms in Graves' disease. Immunol
Res. 54:191–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marino M, Chiovato L and Pinchera A:
Graves' diseaseDe Groot LJ and Jameson JL: Endocrinology eds. 5th
edition. Philadelphia: Elsevier Saunders. 1979–1994. 2006,
View Article : Google Scholar
|
|
16
|
Zheng W, Tan J, Zhang G, Meng Z and Wang
R: Analysis of 131I therapy and correlation factors of Graves'
disease patients: A 4-year retrospective study. Nucl Med Commun.
33:97–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morshed SA, Latif R and Davies TF:
Characterization of thyrotropin receptor antibody-induced signaling
cascades. Endocrinology. 150:519–529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ochi Y, Kajita Y, Hachiya T and Hamaoki M:
A novel hypothesis for the etiology of Graves' disease: TSAb may be
thyroid stimulating animal IgG-like hormone and TBAb may be the
precursor of TSAb. Med Hypotheses. 78:781–786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagayama Y: Animal models of Graves'
hyperthyroidism. Endocr J. 52:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye F, Shi B, Wu X, Hou P, Gao L, Ma X, Xu
L and Wu L: Experience with lentivirus-mediated CD40 gene silencing
in a mouse model of Graves' disease. J Endocrinol. 208:285–291.
2011.PubMed/NCBI
|
|
21
|
Kaneda T, Honda A, Hakozaki A, Fuse T,
Muto A and Yoshida T: An improved Graves' disease model established
by using in vivo electroporation exhibited long-term immunity to
hyperthyroidism in BALB/c mice. Endocrinology. 148:2335–2344. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagayama Y, Kita-Furuyama M, Ando T, Nakao
K, Mizuguchi H, Hayakawa T, Eguchi K and Niwa M: A novel murine
model of Graves' hyperthyroidism with intramuscular injection of
adenovirus expressing the thyrotropin receptor. J Immunol.
168:2789–2794. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu LP, Shi BY, Xun LR, Guo LY, Yang J and
Xu L: An exploration of induction methodology and experimental
duration of Graves disease animal model. Zhonghua Nei Ke Za Zhi.
51:793–797. 2012.(In Chinese). PubMed/NCBI
|
|
24
|
Ludgate M: Animal models of Graves'
disease. Eur J Endocrinol. 142:1–8. 2000. View Article : Google Scholar : PubMed/NCBI
|