Introduction
Spinal cord injury (SCI) is an injury to the spinal
cord caused by trauma or disease, which may lead to alterations to
the normal motor, sensory or autonomic function of the spinal cord
(1). SCI is associated with high
morbidity and mortality rates, and the SCI annual incidence rate
ranges between 12.1–57.8 cases/million individuals (1,2). In
addition, the epidemiology of SCI is variable in different
countries, and there is currently no effective treatment (3,4).
Therefore, an effective therapy for the treatment of SCI is
required, and it has been suggested that genes associated with SCI
may be able to provide novel strategies for such a treatment.
Despite the lack of effective treatments, there have
been some notable findings associated with the molecular mechanism
of SCI. It has been observed that SCI results in secondary
degeneration involving apoptosis, with an increased expression of
genes associated with apoptosis and decreased expression of
anti-apoptotic genes (5). In
addition, a reduction in excessive M1 macrophage polarization and
an enhancement of M2 macrophage polarization produced by regulating
the levels of cytokines, including tumor necrosis factor a and
interleukin (IL)-1β in the SCI microenvironment, may be a desirable
treatment method (6). Tachibana
et al (7) observed that 3
genes, including heat shock 27 kDa protein, tissue inhibitor of
metalloproteinase-1 and epidermal fatty acid-binding protein, were
upregulated in SCI. A recent study indicated that deletion of the
IL-1α gene protected oligodendrocytes from SCI by overexpressing
TOX high mobility group box family member 3 (8). Furthermore, numerous genes associated
with inflammation, such as Arginase 1, are differentially expressed
in the ephrin type A-receptor 4 knockout mouse model of SCI
(9). In addition, it has been
demonstrated that a temporal blockade of the IL-6 signaling pathway
may modify the inflammatory response following SCI, and thus
promote regeneration of the spinal cord (10). However, there are various
additional important genes and pathways associated with SCI that
have yet to be explored completely. Thus, a greater understanding
of these genes and pathways is required as they may provide novel
targets for SCI therapy.
In the present study, GSE45550 microarray data was
obtained from the Gene Expression Omnibus (GEO) and used to
identify the differentially expressed genes (DEGs) associated with
SCI. Functional enrichment analyses were performed for DEGs.
Furthermore, functions of gene modules were analyzed. The aim of
the present study was to identify critical genes or significant
signaling pathways associated with SCI, and clarify the underlying
molecular mechanisms involved.
Materials and methods
Affymetrix microarray data
The microarray data from GSE45550 was downloaded
from the GEO database (http://www.ncbi.nlm.nih.gov/geo/) (11). The following 4 groups were applied:
6 control samples, 6 samples at 3 days post-SCI (SCI3d), 6 samples
at 8 days post-SCI (SCI8d) and 6 samples at 14 days post-SCI
(SCI14d). Data from the GPL1355 platform [(Rat230_2) Affymetrix Rat
Genome 230 2.0 Array; Affymetrix Inc., Santa Clara, CA, USA] were
used for subsequent analysis.
Data preprocessing
The microarray data was preprocessed using the
robust multi-array average algorithm with the Affy package
(12) in Bioconductor (version
1.46.1; http://www.bioconductor.org/).
Background correction, normalization and calculation of expression
were all included in the process of preprocessing. The probe of the
microarray data was transformed to gene symbols with Bioconductor
AnnotationData software packages. If several probes were mapped to
one gene symbol, then the mean value was set as the final
expression value of this gene. A total of 18,634 gene expression
matrixes were obtained from the above process.
DEGs analysis
The DEGs in the following three comparison groups:
SCI3d vs. Control, SCI8d vs. Control and SCI14d vs. Control were
analyzed using the limma package (13) in Bioconductor. The DEG P-values
were calculated using the unpaired Student's t-test (14) provided by the limma package, and
the P-values were adjusted to false discovery rate (FDR) values
using the Benjamini-Hochberg correction (15). Log2 fold-change (FC) ≥1
and FDR values <0.05 were used as cut-off criterion for DEGs.
Hierarchical clustering analysis of the DEGs was then performed and
visualized using g-plots (16) in
the R package.
Venn diagram analysis of DEGs
Venny is an interactive tool used to compare lists
with Venn diagrams (17). The
Kyoto Encyclopedia of Genes and Genomes (KEGG; www.genome.jp/kegg/) database is used to put
associated gene sets into their respective pathway (18). The Database for Annotation,
Visualization and Integrated Discovery (DAVID; http://david.ncifcrf.gov), used for analyzing gene
lists, is an integrated data-mining environment (19).
The intersections of upregulated and downregulated
genes in different sample groups were respectively analyzed using
Venny 2.0 (17) (http://bioinfogp.cnb.csic.es/tools/venny/index.html)
online tool. KEGG pathway enrichment analysis was performed for the
intersection of genes by DAVID. P≤0.05 and gene counts ≥2 were used
as threshold values.
Analysis of the correlation between
gene modules and phenotype
Weighted gene co-expression network analysis (WGCNA)
(20) is a tool used to identify
gene clusters or modules which are highly associated with the
phenotype of samples in expression profile data, and generalize
module characteristic genes among these gene clusters. Furthermore,
WGCNA provides correlation coefficients and significant thresholds
in every module.
In the present study DEGs in the SCI3d, SCI8d and
SCI14d groups were combined, and the correlation between these DEGs
and SCI3d, SCI8d and SCI14d were analyzed, and gene sets with
higher correlation were dug. Modules enriched by DEGs were selected
by WGCNA in the R package, and modules significantly associated
with SCI were identified with cluster analysis. The higher the
absolute value of the correlation coefficient, the closer the
correlation was between gene expression levels in modules and
SCI.
Enrichment analysis of module
function
Gene Ontology (GO) is a tool used to generate gene
annotations by collecting defined, structured and controlled
vocabulary (21). GO annotation
and KEGG pathway analyses were performed for DEGs using DAVID.
P<0.05 and gene counts ≥2 were set as threshold values.
Results
Normalized analysis of sample
data
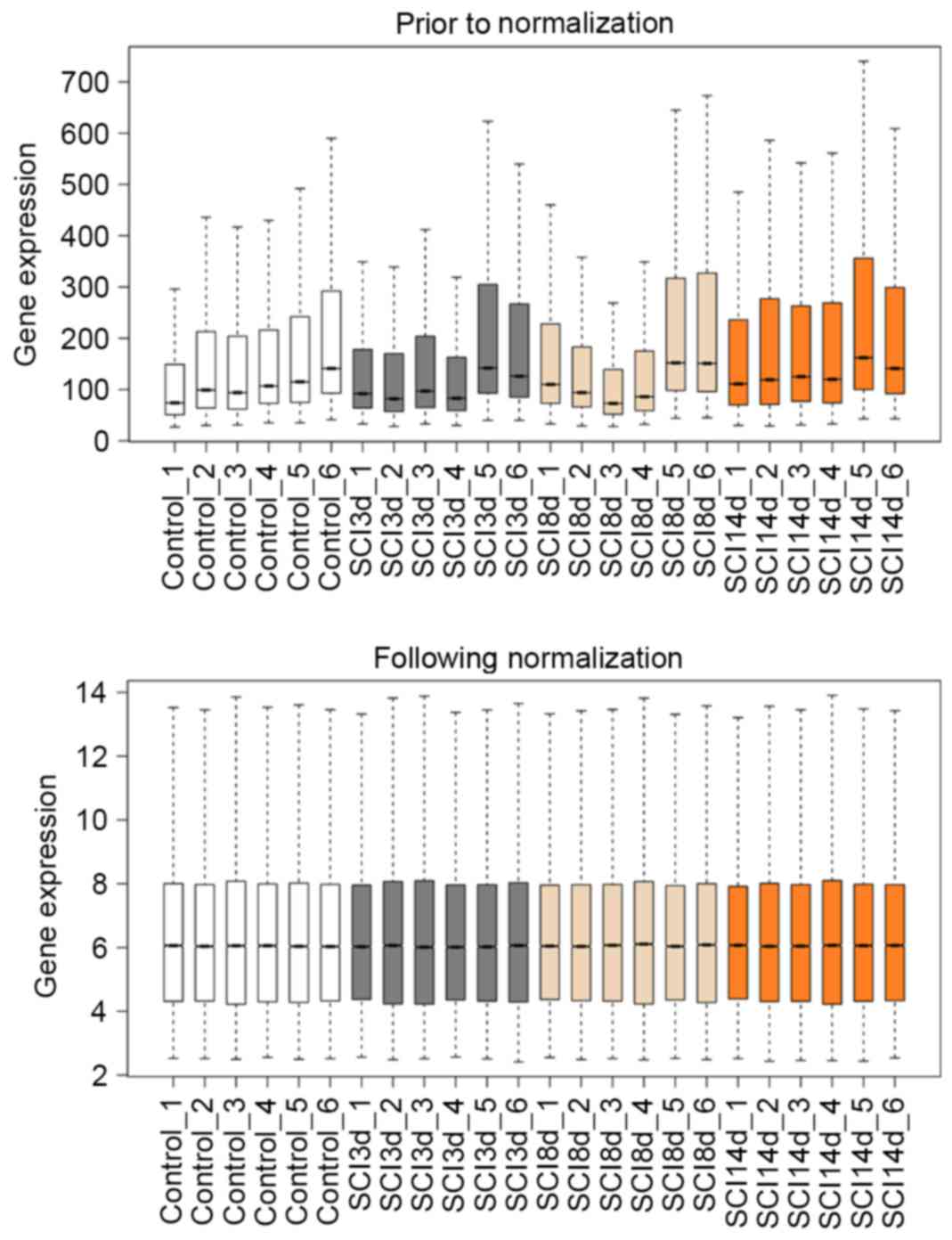
The boxplots of sample data prior to and following
normalization are depicted in Fig.
1. The median line of the boxplot was at the same level
following normalization, indicating that all data were successfully
normalized.
DEG analysis
The DEG count of the three SCI groups compared with
the control group are summarized in Table I with |log2FC| values ≥1
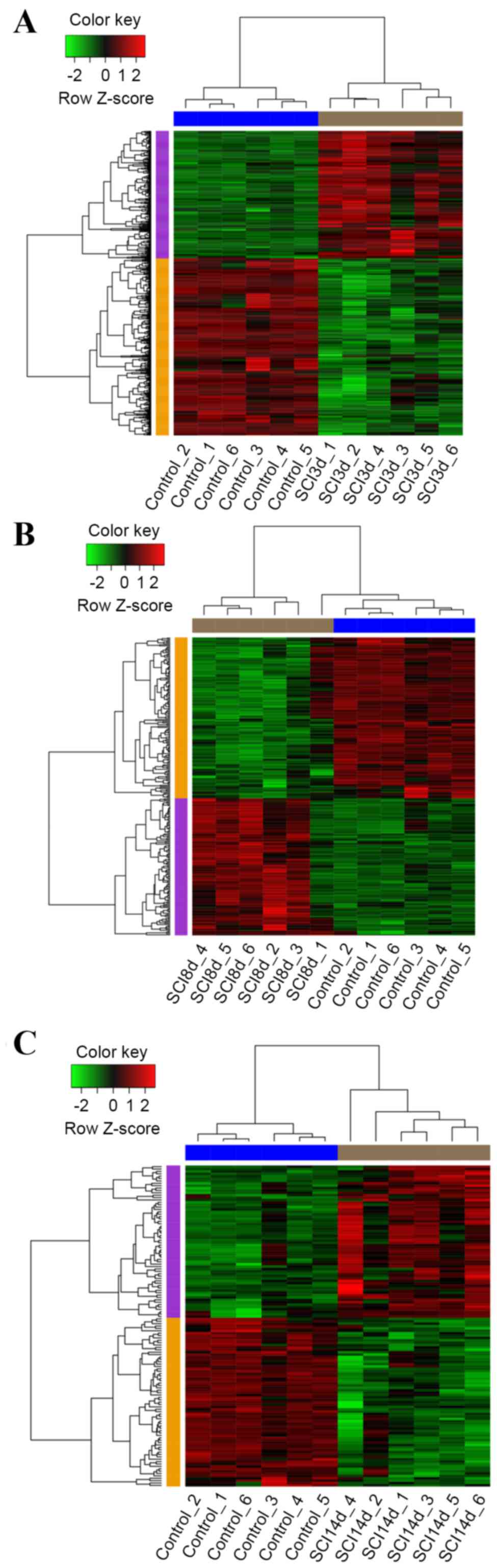
and FDR values <0.05. The heat maps of the DEGs are depicted in
Fig. 2.
 | Table I.Differentially expressed genes count
relative to the control group. |
Table I.
Differentially expressed genes count
relative to the control group.
| Group | Upregulated genes
count | Downregulated genes
count | Total |
|---|
| SCI3d | 232 | 322 | 554 |
| SCI8d | 121 | 142 | 263 |
| SCI14d | 64 | 71 | 135 |
Analysis of overlapping DEGs among
groups
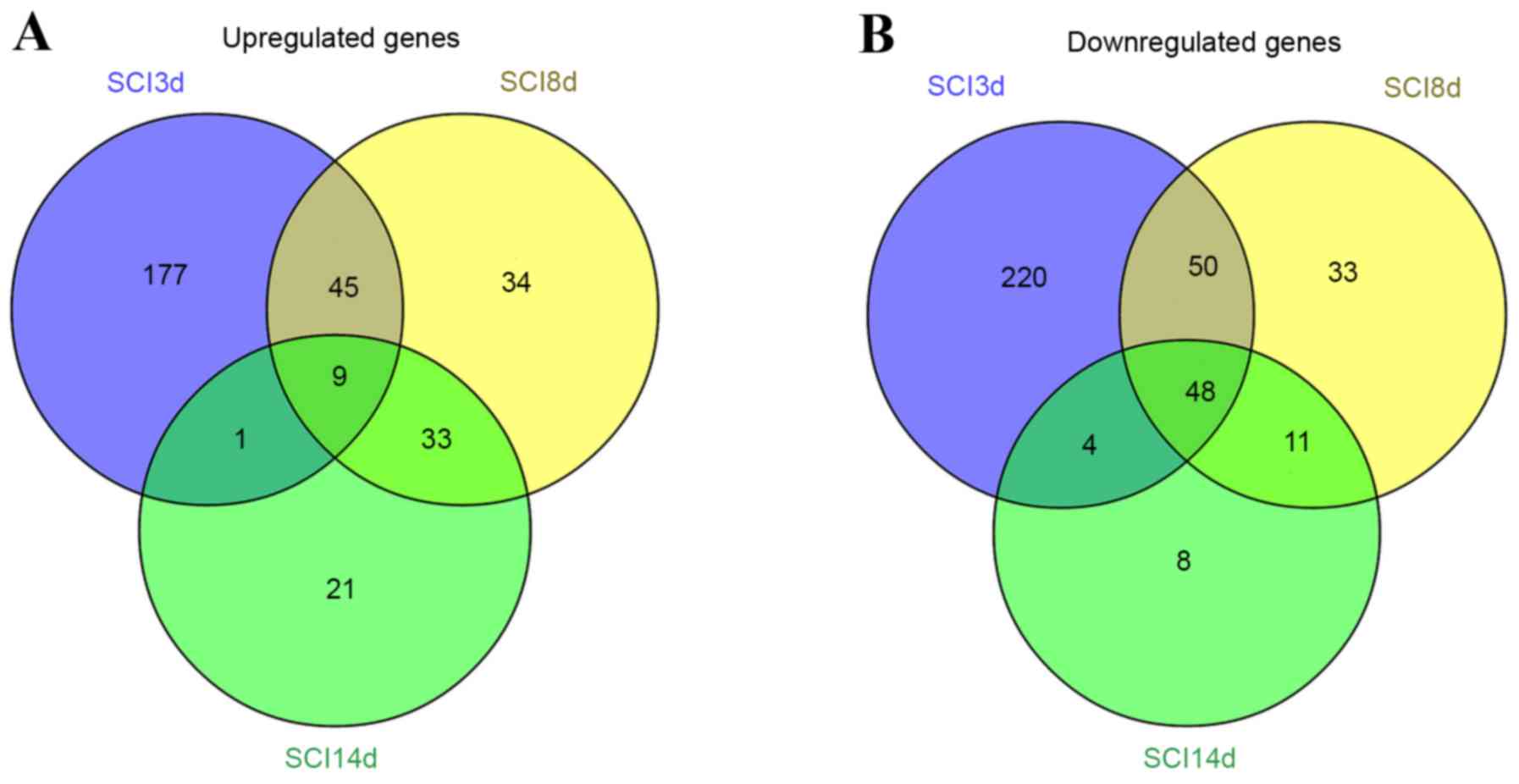
Venn diagram analyses for the upregulated and
downregulated genes in the SCI3d, SCI8d and SCI14d groups were
performed, and overlapping DEGs among groups is depicted in
Fig. 3. A total of 9 genes were
upregulated at all 3 time points and 48 genes were downregulated at
all 3 time points.
Where P<0.05, there was no significant enrichment
observed among the 9 intersecting upregulated genes using KEGG
pathway analysis. However, the 48 intersecting downregulated genes
were markedly enriched in the peroxisome proliferator-activated
receptor (PPAR) signaling pathway (P=8.01×10−4; enriched
genes including lipoprotein lipase, fatty acid binding protein 3,
aquaporin 7 and adiponectin, C1Q and collagen domain containing)
and in the synthesis and degradation of ketone bodies signaling
pathway (P=2.39×10−2; enriched genes including 3-oxoacid
CoA transferase 1, 3-hydroxybutyrate dehydrogenase, type 1) (data
not shown).
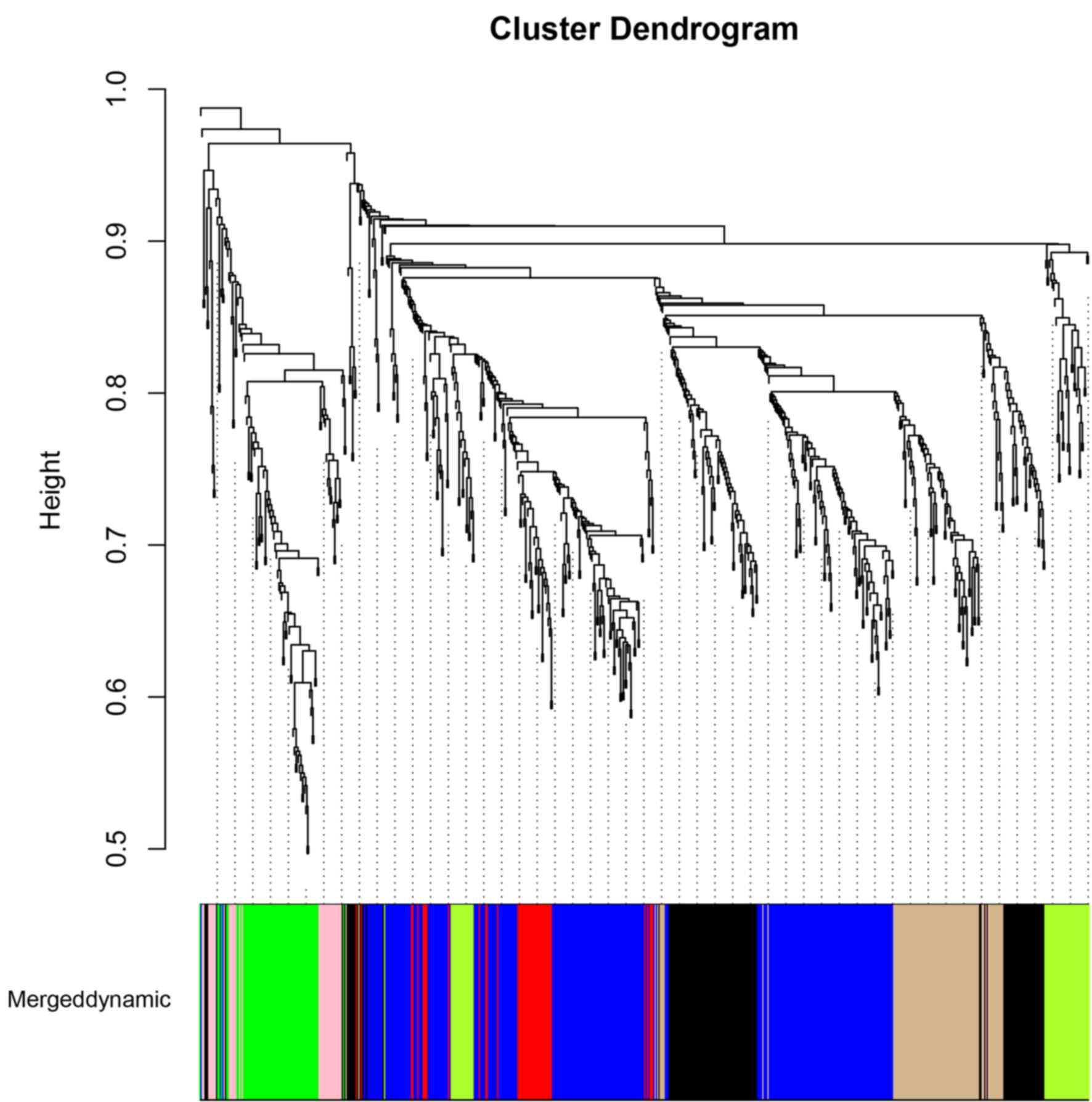
WGCNA module analyses of DEGs
A total of 693 genes were obtained by combining the
DEGs of the SCI3d, SCI8d and SCI14d groups. Cluster analyses using
WGCNA were performed using the expression data of these genes, and
the cluster dendrogram is presented in Fig. 4. The DEGs were divided into 7
modules (pink module, green/yellow module, black module, blue
module, green module, red module and tan module). The correlation
coefficient and P-value between gene counts of every module and SCI
are summarized in Table II. The
data indicated that the pink module and green module with smaller
P-values demonstrated a higher correlation with SCI. The functional
enrichment results of pink and green modules are summarized in
Table III. It was demonstrated
that the PPAR signaling pathway that cluster of differentiation 36
(CD36) was significantly enriched in, was one of the
significant pathways in the pink module. In addition, the p53
signaling pathway, that caspase-3 (Casp3) was significantly
enriched in, was one of the significant pathways in the green
module (Table III).
 | Table II.Results of module analysis. |
Table II.
Results of module analysis.
| Factor | Pink module | Green/yellow
module | Black module | Blue module | Green module | Red module | Tan module |
|---|
| Gene count | 37 | 53 | 125 | 273 | 69 | 46 | 90 |
| Correlation | −7.30E-01 | −1.00E-01 | −5.80E-01 | −9.00E-02 | 7.50E-01 | −7.80E-02 | 2.80E-01 |
| P-value |
5.29E-05 |
6.39E-01 |
3.08E-03 |
6.77E-01 | 2.28E-05 |
7.18E-01 | 1.88E-01 |
 | Table III.Functional enrichment results of pink
and green modules. |
Table III.
Functional enrichment results of pink
and green modules.
| A, Pink module |
|---|
|
|---|
| GO/KEGG | Term | Description | P-value | Gene |
|---|
| GO-BP | GO:0006631 | Fatty acid
metabolic process | 8.00E-04 | Cd36, Mlycd, Acot2,
Fabp4, Decr1 |
|
| GO:0006936 | Muscle
contraction | 9.00E-04 | Tnnt3, Tnnc2,
Mylpf, Actn3 |
|
| GO:0003012 | Muscle system
process | 1.30E-03 | Tnnt3, Tnnc2,
Mylpf, Actn3 |
|
| GO:0034440 | Lipid
oxidation | 3.70E-03 | Cd36, Mlycd,
Decr1 |
|
| GO:0019395 | Fatty acid
oxidation | 3.70E-03 | Cd36, Mlycd,
Decr1 |
| GO-CC | GO:0015629 | Actin
cytoskeleton | 9.80E-03 | Tnnt3, Tnnc2,
Mylpf, Myoz1 |
|
| GO:0005861 | Troponin
complex | 1.70E-02 | Tnnt3, Tnnc2 |
|
| GO:0030016 | Myofibril | 1.83 E-02 | Tnnt3, Tnnc2,
Mylpf |
|
| GO:0005865 | Striated muscle
thin filament | 1.91E-02 | Tnnt3, Tnnc2 |
|
| GO:0043292 | Contractile
fiber | 2.12E-02 | Tnnt3, Tnnc2,
Mylpf |
| GO-MF | GO:0048037 | Cofactor
binding | 1.70E-03 | Ldhb, Nox1, Actn3,
Decr1, Nqo1 |
|
| GO:0008092 | Cytoskeletal
protein binding | 4.30E-03 | Tnnt3, Tnnc2,
Mylpf, Myoz1, Actn3 |
|
| GO:0050662 | Coenzyme
binding | 6.80E-03 | Ldhb, Nox1, Decr1,
Nqo1 |
|
| GO:0003779 | Actin binding | 7.50E-03 | Tnnt3, Tnnc2,
Mylpf, Actn3 |
| KEGG | rno04510 | Focal adhesion | 1.67E-02 | Col6A3, Mylpf,
Actn3, Col5A3 |
|
| rno03320 | PPAR signaling
pathway | 1.70E-02 | Cd36, Fabp4,
Aqp7 |
|
| rno04512 | ECM-receptor
interaction | 2.18 E-02 | Cd36, Col6A3,
Col5A3 |
|
| rno04670 | Leukocyte
transendothelial migration | 4.17E-02 | Nox1, Mylpf,
Actn3 |
|
| B, Green
module |
|
| GO/KEGG | Term | Description | P-value | Gene |
|
| GO-BP | GO:0030199 | Collagen fibril
organization | 1.00E-04 | Col3A1, Lox,
Col5A2, Col5A1 |
|
| GO:0030198 | Extracellular
matrix organization | 4.00E-04 | Col3A1, Ccdc80,
Lox, Col5A2, Col5A1 |
|
| GO:0042060 | Wound healing | 6.00E-04 | Ccnb1, Casp3,
Col3A1, Lox, Tfpi2, Col5A1 |
|
| GO:0030155 | Regulation of cell
adhesion | 1.30E-03 | Tnc, Ccdc80, Jak2,
Mmp14, Col8A1 |
|
| GO:0007049 | Cell cycle | 2.10E-03 | Ccnb1, Ccnb2,
Mki67, Cks2, Pttg1, Cdkn3, Ube2C, Racgap1 |
| GO-CC | GO:0031012 | Extracellular
matrix | 6.06E-14 | Matn2, Aspn,
Cthrc1, Tnc, Col3A1, Ccdc80, Mmp14, Col5A2, Col5A1, Col6A2, Col6A1,
Lox, Loxl1, Thbs4, Adamts4, Spon1 |
|
| GO:0005578 | Proteinaceous
extracellular matrix | 4.22E-12 | Matn2, Aspn,
Cthrc1, Tnc, Col3A1, Ccdc80, Lox, Mmp14, Col5A2, Loxl1, Col5A1,
Adamts4, Spon1, Thbs4 |
|
| GO:0044421 | Extracellular
region part | 1.36E-09 | Matn2, Aspn,
Cthrc1, Tnc, Col3A1, Ccdc80, Mmp14, Col5A2, Col5A1, Ctsk, Cpxm1,
Col6A2, Col6A1, Lox, Loxl1, Thbs4, Adamts4, Spon1 |
|
| GO:0005576 | Extracellular
region | 1.18E-08 | Matn2, Aspn,
Cthrc1, Aebp1, Tnc, Col3A1, Ccdc80, Mmp14, Col5A2, Col5A1, Ctsk,
Penk, Cpxm1, Sfrp4, Col6A2, Col6A1, Lox, Loxl1, Tfpi2, Thbs4,
Adamts4, Spon1 |
|
| GO:0005581 | Collagen | 9.18E-05 | Col3A1, Lox,
Col5A2, Col5A1 |
| GO-MF | GO:0016641 | Oxidoreductase
activity, acting on the CH-NH2 group of donors, oxygen as
acceptor | 1.50E-03 | Lox, Loxl2,
Loxl1 |
|
| GO:0016638 | Oxidoreductase
activity, acting on the CH-NH2 group of donors | 2.20E-03 | Lox, Loxl2,
Loxl1 |
|
| GO:0005201 | Extracellular
matrix structural constituent | 5.20E-03 | Col3A1, Col5A2,
Col5A1 |
|
| GO:0016702 | Oxidoreductase
activity, acting on single donors with incorporation of molecular
oxygen, incorporation of two atoms of oxygen | 6.40E-03 | Plod2, P4Ha3,
Hpd |
|
| GO:0016701 | Oxidoreductase
activity, acting on single donors with incorporation of molecular
oxygen | 6.80E-03 | Plod2, P4Ha3,
Hpd |
| KEGG | rno04512 | ECM-receptor
interaction | 6.37E-07 | Tnc, Col3A1,
Col6A2, Col6A1, Col5A2, Col5A1, Thbs4 |
|
| rno04510 | Focal adhesion | 1.03E-04 | Tnc, Col3A1,
Col6A2, Col6A1, Col5A2, Col5A1, Thbs4 |
|
| rno04115 | p53 signaling
pathway | 2.96E-02 | Ccnb1, Casp3,
Ccnb2 |
Discussion
In the present study, a total of 693 genes were
identified by combining the DEGs of the SCI3d, SCI8d and SCI14d
groups. The results obtained demonstrated that the PPAR signaling
pathway, in which CD36 was significantly enriched, was one
of the significant pathways in the pink module, while the p53
signaling pathway that Casp3 was significantly enriched in,
was one of the significant pathways in the green module.
PPAR, which includes 3 isoforms (PPAR-α PPAR-γ
PPAR-β/δ), is involved in the inflammation process (22). A previous study demonstrated that
PPAR-γ and PPAR-δ are involved in the protective effects of
palmitoylethanolamide (PEA) in SCI, indicating that PPAR-γ and
PPAR-δ may contribute to the anti-inflammatory effects of PEA in
SCI (23). It has been reported
that PPAR participates in the pathogenesis of diseases, such as
diabetes and SCI (24,25). In addition, it has been observed
that the decrease of PPAR-δ expression in the spinal cord of
streptozotocin (STZ)-diabetic rats may explain the higher mortality
rate observed following SCI in STZ-diabetic rats (26). Thus, activation of PPAR-δ may
reduce the severity of SCI, and PPAR-δ may be a target for therapy
in SCI patients (27). In
addition, van Neerven and Mey (28) indicated that endogenous PPAR
ligands may contribute by preventing the expansion of the initial
damage via modulating inflammation post-SCI, and concluded that the
PPAR signaling pathway may be a target for treatment of SCI.
Therefore, the results of the present study are in agreement with
the results of previous studies and, as such, the PPAR signaling
pathway may be closely associated with the pathogenesis of SCI. In
addition, the results demonstrated that CD36 was
significantly enriched in the PPAR signaling pathway. It has been
indicated that the upregulation of CD36, an integral
membrane protein, may resolve inflammation via the
5-lipoxygenase-dependent and PPAR-γ signaling pathways (29). The wnt-1 proto-oncogene protein
promotes CD36 expression via β-catenin and PPAR-γ signaling
pathways (30). CD36 is
involved in the PPAR-γ signaling pathway, and activation of PPAR-γ
leads to upregulation of CD36 in the PPAR signaling pathway
(31). Previous studies have
indicated that CD36 is involved in the PPAR-γ signaling
pathway (22,32). Although the roles of CD36 in
SCI have not been extensively studied, when considering the
association between the PPAR signaling pathway and SCI, it is
possible that CD36 may be involved in the progression of SCI
via the PPAR signaling pathway.
The p53 signaling pathway was observed to be one of
the significant KEGG pathways in the green module in the present
study. A previous study observed DNA damage-induced p53-mediated
mitochondrial apoptosis in SCI (5). Nerve injury is a significant
consequence of SCI, and p53 is involved in glial cell death and
survival in SCI (33). p53, a key
molecular regulator of apoptotic cell death, is known to promote
apoptosis by increasing the transcription of target genes (34,35).
In addition, a number of apoptosis-associated molecules are
expressed via p53, and apoptosis induction via the p53 pathway is a
complex process (33).
Furthermore, minocycline has been shown to reduce apoptosis in
models of SCI (36). Therefore,
the results of the present study are consistent with previous
studies, and therefore indicate that the p53 signaling pathway may
be significant in the progression of SCI. In the present study, it
was demonstrated that Casp3 was significantly enriched in
the p53 signaling pathway. It has been observed that Casp3
and the p53 signaling pathway may regulate nitric oxide-induced
extracellular signal-regulated protein kinase and p38 kinase
(37). In addition, p53 prevents
the α6β4 integrin-mediated activation of serine/threonine-specific
protein kinase B by promoting Casp3 dependent cleavage
(38). It has been suggested that
Casp3 is a critical mediator of p53-induced apoptosis
(39). Zhang et al
(40) investigated the apoptosis
process in SCI, and demonstrated that the Casp3 apoptotic
pathway components are activated following SCI in rats. Ultimately,
it is thought that Casp3 may be involved in the progression
of SCI via the p53 signaling pathway.
In conclusion, the PPAR and p53 signaling pathways
may be important pathways associated with SCI. In addition,
CD36 and Casp3 may be involved in the progression of
SCI via the PPAR and p53 signaling pathways, respectively. However,
the results of the present study are limited by the small sample
size, and therefore further studies are required to evaluate the
molecular mechanisms underlying SCI progression.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472120) and the
Science and Technology Commission of Shanghai Municipality (grant
no. 14140903900).
Glossary
Abbreviations
Abbreviations:
|
Casp3
|
Caspase-3
|
|
DEGs
|
differentially expressed genes
|
|
CD36
|
cluster of differentiation 36
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
PPAR
|
peroxisome proliferator-activated
receptor
|
|
SCI
|
spinal cord injury
|
References
|
1
|
Shah RR and Tisherman SA: Spinal Cord
Injury. Springer; London: 2014
|
|
2
|
Me VDB, Castellote JM, Mahillo-Fernandez I
and De P-CJ: Incidence of spinal cord injury worldwide: A
systematic review. Neuroepidemiology. 34:184–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sekhon LH and Fehlings MG: Epidemiology,
demographics, and pathophysiology of acute spinal cord injury.
Spine (Phila Pa 1976). 26(24 Suppl): S2–S12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wyndaele M and Wyndaele JJ: Incidence,
prevalence and epidemiology of spinal cord injury: What learns a
worldwide literature survey? Spinal Cord. 44:523–529. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kotipatruni RR, Dasari VR, Veeravalli KK,
Dinh DH, Fassett D and Rao JS: p53- and bax-mediated apoptosis in
injured rat spinal cord. Neurochem Res. 36:2063–2074. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wanner IB, Anderson MA, Song B, Levine J,
Fernandez A, Gray-Thompson Z, Ao Y and Sofroniew MV: Glial scar
borders are formed by newly proliferated, elongated astrocytes that
interact to corral inflammatory and fibrotic cells via
STAT3-dependent mechanisms after spinal cord injury. J Neurosci.
33:12870–12886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tachibana T, Noguchi K and Ruda MA:
Analysis of gene expression following spinal cord injury in rat
using complementary DNA microarray. Neurosci Lett. 327:133–137.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bastien D, Landete V Bellver, Lessard M,
Vallières N, Champagne M, Takashima A, Tremblay MÈ, Doyon Y and
Lacroix S: IL-1α gene deletion protects oligodendrocytes after
spinal cord injury through upregulation of the survival factor
Tox3. J Neurosci. 35:10715–10730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Munro KM, Perreau VM and Turnley AM:
Differential gene expression in the EphA4 knockout spinal cord and
analysis of the inflammatory response following spinal cord injury.
PLoS One. 7:e376352011. View Article : Google Scholar
|
|
10
|
Guerrero AR, Uchida K, Nakajima H,
Watanabe S, Nakamura M, Johnson WE and Baba H: Blockade of
interleukin-6 signaling inhibits the classic pathway and promotes
an alternative pathway of macrophage activation after spinal cord
injury in mice. J Neuroinflammation. 9:402012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41(Database issue):
D991–D995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: Affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smyth GK: Linear models and empirical
bayes methods for assessing differential expression in microarray
experiments. Stat Appl Genet Mol Biol. 3:Article32004.PubMed/NCBI
|
|
15
|
Ferreira JA: The Benjamini-Hochberg method
in the case of discrete test statistics. Int J Biostat. 3:Article
112007.PubMed/NCBI
|
|
16
|
Warnes GR, Bolker B, Bonebakker L,
Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A,
Moeller S, et al: gplots: Various R programming tools for plotting
data. R package version. 2:2005.
|
|
17
|
Oliveros JC: VENNY. An interactive tool
for comparing lists with Venn Diagrams. 2007.
|
|
18
|
Altermann E and Klaenhammer TR:
PathwayVoyager: Pathway mapping using the kyoto encyclopedia of
genes and genomes (KEGG) database. BMC Genom. 6:203–213. 2005.
View Article : Google Scholar
|
|
19
|
Huang DW, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nature Protoc. 4:44–57. 2008. View Article : Google Scholar
|
|
20
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene Ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamanaka M, Ishikawa T, Griep A, Axt D,
Kummer MP and Heneka MT: PPARγ/RXRα-induced and CD36-mediated
microglial amyloid-β phagocytosis results in cognitive improvement
in amyloid precursor protein/presenilin 1 mice. J Neurosci.
32:17321–17331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paterniti I, Impellizzeri D, Crupi R,
Morabito R, Campolo M, Esposito E and Cuzzocrea S: Molecular
evidence for the involvement of PPAR-δ and PPAR-γ in
anti-inflammatory and neuroprotective activities of
palmitoylethanolamide after spinal cord trauma. J
Neuroinflammation. 10:202013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy GJ and Holder JC: PPAR-γ agonists:
Therapeutic role in diabetes, inflammation and cancer. Trends
Pharmacol Sci. 21:469–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McTigue DM: Potential therapeutic targets
for PPAR gamma after Spinal Cord Injury. PPAR Res. 2008:5171622008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai CC, Lee KS, Chen SH, Chen LJ, Liu KF
and Cheng JT: Decrease of PPARδ in type-1-like diabetic rat for
higher mortality after spinal cord injury. PPAR Res.
2014:4563862014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esposito E and Cuzzocrea S: Targeting the
peroxisome proliferator-activated receptors (PPARs) in spinal cord
injury. Expert Opin Ther Targets. 15:943–959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Neerven S and Mey J: RAR/RXR and
PPAR/RXR signaling in spinal cord injury. PPAR Res. 2007:292752007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ballesteros I, Cuartero MI, Pradillo JM,
de la Parra J, Pérez-Ruiz A, Corbí A, Ricote M, Hamilton JA,
Sobrado M, Vivancos J, et al: Rosiglitazone-induced CD36
up-regulation resolves inflammation by PPARγ and 5-LO-dependent
pathways. J Leukoc Biol. 95:587–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan H, Wang S, Chen T and Zhu J: oxLDL
decreases wnt1 which promotes CD36 through b-catenin and PPAR-r
signaling pathway in macrophage. European heart journal Oxford Univ
Press; Great Clarendon St, Oxford OX2 6DP, England: pp. 1123.
2014
|
|
31
|
Nagaraj S, Raghavan AV, Rao SN and
Manjappara UV: Obestatin and Nt8U influence glycerolipid metabolism
and PPAR gamma signaling in mice. Int J Biochem Cell Biol.
53:414–422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lourenco MV and Ledo JH: Targeting
Alzheimer's pathology through PPARγ signaling: Modulation of
microglial function. J Neurosci. 33:5083–5084. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saito N, Yamamoto T, Watanabe T, Abe Y and
Kumagai T: Implications of p53 protein expression in experimental
spinal cord injury. J Neurotrauma. 17:173–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fridman JS and Lowe SW: Control of
apoptosis by p53. Oncogene. 22:9030–9040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Slee EA, O'Connor DJ and Lu X: To die or
not to die: How does p53 decide? Oncogene. 23:2809–2818. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Teng YD, Choi H, Onario RC, Zhu S,
Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME and
Friedlander RM: Minocycline inhibits contusion-triggered
mitochondrial cytochrome c release and mitigates functional
deficits after spinal cord injury. Proc Natl Acad Sci USA.
101:3071–3076. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK,
Kim JH, Yoo YJ, Bang OS, Kang SS and Chun JS: ERK-1/2 and p38
kinase oppositely regulate nitric oxide-induced apoptosis of
chondrocytes in association with p53, caspase-3, and
differentiation status. J Biol Chem. 277:1332–1339. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bachelder RE, Ribick MJ, Marchetti A,
Falcioni R, Soddu S, Davis KR and Mercurio AM: p53 inhibits alpha 6
beta 4 integrin survival signaling by promoting the caspase
3-dependent cleavage of AKT/PKB. J Cell Biol. 147:1063–1072. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Communal C, Sumandea M, De Tombe P, Narula
J, Solaro RJ and Hajjar RJ: Functional consequences of caspase
activation in cardiac myocytes. Proc Natl Acad Sci USA.
99:6252–6256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang N, Yin Y, Xu SJ, Wu YP and Chen WS:
Inflammation & apoptosis in spinal cord injury. Indian J Med
Res. 135:287–296. 2012.PubMed/NCBI
|