Introduction
Peritoneal dialysis (PD) is an efficient kidney
replacement therapy for patients with end-stage renal failure
(ESRF). Its lower infection rates and reduced costs significantly
improve quality of life and increase the survival rates of patients
(1). However, the peritoneal
membrane, which is directly and continuously exposed to the
peritoneal dialysis solution (PDS) exhibits structural and
functional alterations following long-term exposure to PD, and
causes a decline in peritoneal ultrafiltration capacity, eventually
resulting in ultrafiltration failure (UFF) (2). The potential factors responsible for
UFF are complex, predominantly including the peritonitis caused by
repeated microbial infection and the chronic aseptic inflammation
caused by non-physiological dialysis fluid (3). The physiological morphology of the
peritoneum eventually evolves showing loss of the mesothelial cells
layer, abnormal proliferation of connective tissue, angiogenesis,
obliterating vasculopathy, submesothelial fibrosis and
calcification, resulting in dysfunction of the exchange between the
blood and dialysate (4).
Peritoneal mesothelial cells (PMCs) are the most important cell
populations of the peritoneum, and are critical in the maintenance
of peritoneal homeostasis, immune surveillance, antigen
presentation, inflammation and wound healing, regulating the
structure and function of the peritoneum (5).
The expression of aquaporin-1 (AQP-1) and zonula
occluden-1 (ZO-1) on the PMCs modulates ultrafiltration during PD
through different mechanisms, and are important indicators for the
evaluation of peritoneal ultrafiltration function (6,7). As
water constitutes the most important component of all viable cells,
water uptake and discharge are two of the most basic activities in
life. It has been reported that there are two ways for water to
cross the cell membrane; one being through diffusion, which is
affected by temperature, and the other being via the
water-selective AQP-1 channel (6).
The water transport mediated by AQP-1 accounts for ~90% of the
overall ultrafiltration (8).
ZO-1-associated tight junction formation is considered to be
important for maintaining the osmotic pressure gradient between the
peritoneal capillaries and the dialysate (7). Therefore, abnormal expression of
AQP-1 and ZO-1 leads to degenerated function of the peritoneum.
Peroxisome proliferator-activated receptor-γ
(PPAR-γ) is a member of a nuclear hormone receptor family, which
regulates various metabolic pathways as a transcription factor. It
is crucial in the regulation of cellular inflammation, fibrosis and
endothelial function (9).
Thiazolidinedione, similar to rosiglitazone is a selective PPAR-γ
activator, which is used extensively in the treatment of type 2
diabetes mellitus (10). Previous
reports have demonstrated that pretreatment with rosiglitazone
results in decreased inflammation and peritoneal thickness in a
lipopolysaccharide-induced peritonitis rat model (11). Aramwit et al (12) found that, in patients receiving
continuous ambulatory peritoneal dialysis who were administered
with rosiglitazone (2 mg BID) for 12 weeks, the total body water
and extracellular fluid increased significantly (12).
The present study was designed to investigate the
potential protective effects of the PPAR-γ agonist, rosiglitazone,
on the peritoneal alterations in a PDS-induced RPMC model.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM)/nutrient
mixture F12 (1:1; DMEM/F12) medium and fetal bovine serum (FBS)
were obtained from Invitrogen; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). PDS (1.5, 2.5 and 4.25%) were from Baxter
Healthcare Co., Ltd. (Shanghai, China). Rosiglitazone was from
Molekula GmbH (Nienburg/Weser, Germany), and 15d-PGJ2 and GW-9662
were purchased from Cayman Chemical Company (Ann Arbor, MI, USA).
Monoclonal antibodies against Vimentin (Vim), CD45, GAPDH and AQP-1
were from Abcam (Cambridge, UK). Monoclonal antibody against Factor
VIII was from Bioss, Inc. (Woburn, MA, USA). Monoclonal antibodies
against ZO-1 and PPAR-γ, and secondary horseradish peroxidase
(HRP)-conjugated antibodies were from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA).
RPMC isolation and culture
The RPMCs were isolated and cultured, as described
previously (13). Briefly, male
Sprague-Dawley rats (150–250 g) were purchased from the Animal
Experimental Center of Sun Yat-Sen University, (Guangzhou, China).
The rats (n=40) were housed in plastic cages on bedding of chips.
All rats were given the distilled water to drink and allowed free
access to the water and pellet food. All rats were maintained at
25°C with 12-h light/12-h dark cycle. The mice were sacrificed with
pentobarbital natrium (30 mg/kg at day 7 after surgery. All
procedures were followed the rules of the Southern Medical
University (Guangzhou, China) Animal Experiment Committee.
Individual rats were injected intraperitoneally with 30 ml of 0.25%
trypsinase-0.02% EDTA-Na2, and 5 ml abdominal fluid of
individual rats was collected 2 h later, which was centrifuged at
820 × g for 10 min. The cells were washed in PBS and
suspended in DMEM/F12 medium with 10% (v/v) FBS, and were then
cultured in 25 cm2 tissue culture flasks at 37°C in a
humidified 5% CO2 atmosphere. The cells were passaged
every 3–5 days, and RPMCs from the second and third passages at 80%
confluence were used for the following experiments.
Immunocytochemical staining
RPMCs at a density of 2×105/well were
cultured overnight on glass cover-slips in 3.5 cm-diameter tissue
culture plates. For immunostaining for DAPK-and RASSF1A, antigen
retrieval was performed using microwaves for 10 min. The slides
were exposed to Power Block (BioGenex Laboratories, San Ramon, CA,
USA) for 45 min. The cells were fixed in cold 4% paraformaldehyde
overnight, permeabilized with 0.5% Triton X-100 and then blocked
with 5% normal goat serum (BioGenex Laboratories). The cells were
then incubated with the following primary antibodies: Anti-Vim
(1:150; cat. no. MMS-464S), anti-CD45 (1:150; cat. no. PB9096) and
anti-Factor VIII (1:150; cat. no. PRO-318) in 1% BSA at 4°C
overnight. Following washing three times in PBS, the slips were
incubated with HRP-conjugated secondary antibodies for 2 h at 37°C.
The reaction was terminated with 3,3-diaminobenzidine. Images of
the results were captured (magnification, ×400) using a Nikon
ECLIPSE 80i microscope (Nikon, Tokyo, Japan). The optical densities
and numbers of DAPK- and RASSF1A-positive cells were counted in
five randomly selected fields per sample in spinal anterior horn,
and quantification was performed using Image-Pro Plus version 6.0
(Media Cybernetics, Inc., Rockville, MD, USA). Histology and
immunohistochemistry for each marker were performed simultaneously
in all samples and in negative controls without primary
antibodies.
Western blot analysis
Western blot analysis was performed according to
standard western blot procedures as previously described (9). The proteins were extracted using
Pro-Pprep protein extraction solution (Intron Biotechnology, Inc.,
Seongnam, South Korea) from the frozen kidney tissues. The protein
concentration was measured using Dc Protein Assay kit (Bio-Rad
Laboratories, Hercules, CA, USA). Proteins (30 g per lane) were
separated by 10% SDS-PAGE and then transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Following blocking in 5% nonfat milk, the membranes were incubated
with the following primary antibodies: AQP-1 (1:300; cat. no.
sc-32739), ZO-1 (1:300; cat. no. 33-9100), PPAR-γ (1:300; cat. no.
sc-7273) and GAPDH (1:1,000; cat. no. E12-052-1) overnight at 4°C,
followed by incubation with the anti-rabbit HRP-conjugated
secondary antibodies (cat. no. 1721011; Bio-Rad Laboratories) for 1
h at room temperature. Chemiluminescence was detected using ECL
Advance Western Blotting Detection reagents (GE Healthcare Life
Sciences, Chalfont, UK). The relative expression levels of AQP-1
and ZO-1 were quantified using ImageJ version 1.45.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent
(Ambion; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocols. The cDNAs used to examine the expression
of AQP-1 and ZO-1 were synthesized using a PrimeScript RT reagent
kit (Takara Bio, Inc., Otsu, Japan) according to manufacturer's
protocols. The expression of AQP-1 and ZO-1 were examined using
SYBR® Premix Ex Taq II (Takara Bio, Inc.), and GAPDH
served as an internal reference. PCR was performed using 1 µl cDNA
in a total volume of 25 µl in the presence of 12.5 µl 2xiQ Supermix
(Bio-Rad Laboratories), 200 nM AQP-1, or ZO-1 primer set. PCR
conditions were as follows: 40 cycles at 95°C for 30 sec, 60°C for
1 min using iCycler iQ qPCR detection system (Bio-Rad
Laboratories). All experiments were performed in duplicate and
repeated twice. The results are presented as the fold induction,
determined using the 2−ΔΔCq method (14). The primers for AQP-1 were: Sense
5′-GCACAATGGGCTCTATCTTC-3′ and antisense
5′-GACAGTCGTTC-TATGGTGGG−3′. The primers for ZO-1 were: Sense
5′-AAAAGTGAACCACGAG-ATGCT-3′ and antisense
5′-AAAGGTAAGGGACTGGAGATGA-3′. The primers for β-actin were: Sense
5′-GGCAAGTTCAATGGCACAGT-3′ and antisense
5′-AAGGTGGAGGAATGGGAGTT-3′.
ELISA
With the collected cell culture supernatant, the
concentrations of IL-6 and IL-8 were determined using commercial
ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA) according
to the manufacturer's protocol. Three independent assessments were
performed.
Statistical analysis
The results of the analyses are expressed as the
mean ± standard deviation. Statistical analaysis was performed
using Statview version 5.0 (SAS Intelligence, Cary, NC, USA)
Statistically significant differences between groups were
identified using one-way analysis of variance, followed by
Student's t-test (paired). P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of RPMCs
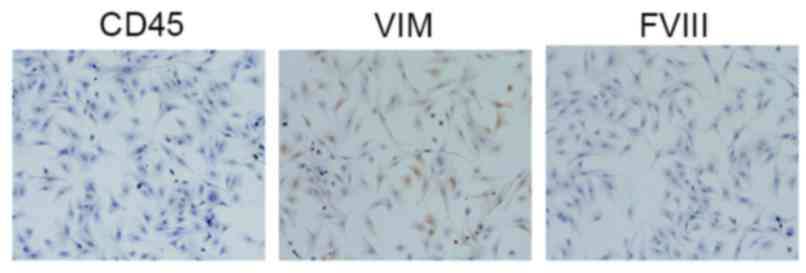
The identification of the cultured cells as RPMCs
was based on their typical markers, CD45, Vim, Desmin and Factor
VIII. Immunocytochemical staining of the cultured cells was
performed, and the cells were then examined under an optical
microscope. Of the characterized markers, it was found that the
cells were positively stained for CD45 and Vim, and negatively
stained for Desmin and Factor VIII (Fig. 1).
mRNA and protein levels of AQP-1 and
ZO-1 in RPMCs treated with 4.25% PDS for different durations
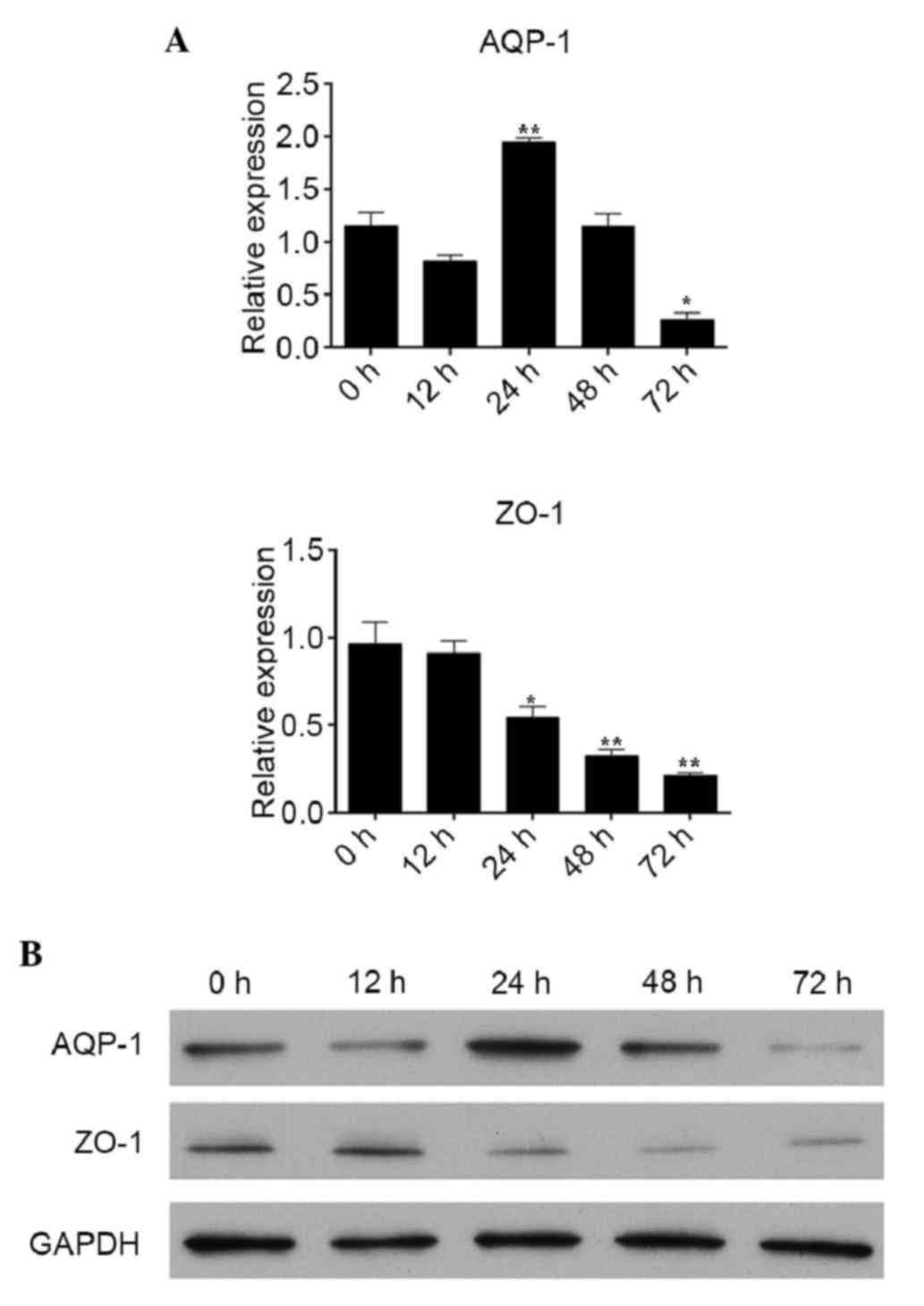
To observe the effects of PDS on the levels of AQP-1
and ZO-1 in the RPMCs, the RPMCs were cultured with 4.25% PDS for
0, 12, 24, 48 and 72 h. Compared with the expression at 0 h, the
gene expression of AQP-1 was significantly increased at 24 h,
however, a decreasing trend was observed at 48 and 72 h, and this
decrease was statistically significantly at 72 h. The mRNA levels
of ZO-1 were significantly reduced 24, 48 and 72 h following
treatment with 4.25% PDS, compared with the level at 0 h (Fig. 2A). As shown in Fig. 2B, compared with the levels at 0 h,
the protein levels of AQP-1 were significantly decreased at 72 h,
and the protein expression levels of ZO-1 were significantly
decreased at 24, 48 and 72 h.
mRNA and protein levels of AQP-1 and
ZO-1 in RPMCs treated with different concentrations of PDS for 72
h
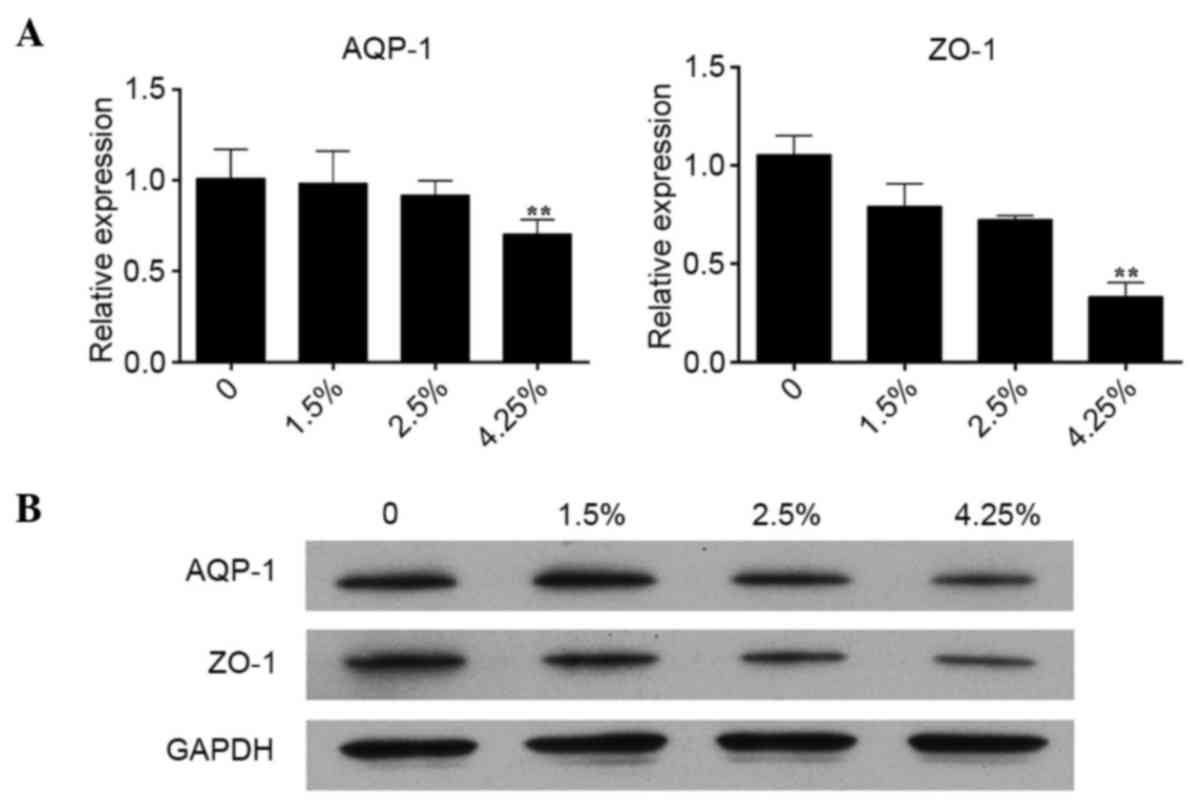
To evaluate the effect of PDS on the levels of AQP-1
and ZO-1 levels in RPMCs, the RPMCs were cultured with different
PDS concentrations of 0, 1.5, 2.5 and 4.25% for 72 h. Compared with
the controls (PDS 0%), the gene expression levels of AQP-1 and ZO-1
showed a decreased trend, and these decreases were statistically
significantly in the 4.25% group (Fig.
3A). Consistent with the results of the RT-qPCR analysis, the
protein expression levels of AQP-1 and ZO-1 were significantly
reduced in the 4.25% group, as demonstrated by the results of the
western blot analysis (Fig.
3B).
Effects of rosiglitazone on the mRNA
and protein expression of 4.25% PDS-induced AQP-1 and ZO-1
To evaluate the effect of rosiglitazone in the
PDS-induced RPMC model, the RPMCs were pre-treated with 15 µmol/l
rosiglitazone or with 5 µmol/l 15-PGJ2, another PPAR-γ agonist, for
2 h, or were pre-treated with 15 µmol/l rosiglitazone for 2 h
following pre-treatment with 3 µmol/l GW9662, a PPAR-γ inhibitor,
for 1 h. As shown in Fig. 4A, the
gene expression levels of AQP-1 and ZO-1 in the 4.25% PDS-induced
RPMCs were significantly enhanced by the PPAR-γ agoinst,
rosiglitazone, compared the with model group (DMSO+4.25% PDS). The
effects of the other PPAR-γ activator, 15-PGJ2, were consistent
with that of rosiglitazone. By contrast, the mRNA levels of AQP-1
and ZO-1 in the RPMCs pre-treated with rosiglitazone and the PPAR-γ
inhibitor were reduced significantly, compared with the RPMCs
pre-treated with rosiglitazone only. As shown in Fig. 4B, further examination using western
blot analysis revealed that the protein levels of AQP-1 and ZO-1
were significantly increased in the rosiglitazone group and 15-PGJ2
group, compared with the model group. The use of GW-9662 appeared
to completely prevent the effects of rosiglitazone.
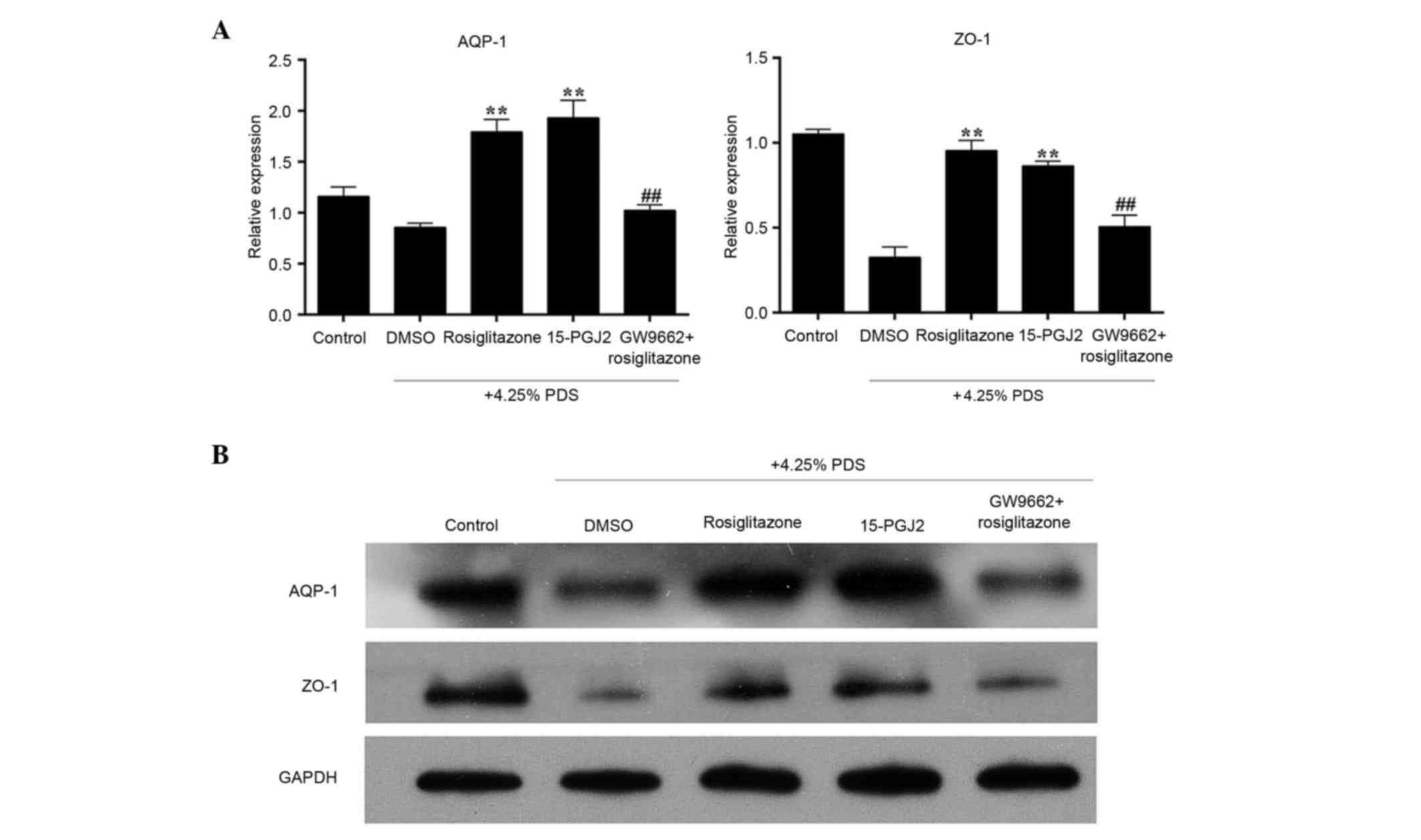 | Figure 4.Effects of rosiglitazone on the mRNA
and protein expression of 4.25% PDS-induced AQP-1 and ZO-1. The
five groups assessed were: Control group, RPMCs cultured with
normal medium only; DMSO group, RPMCs cultured with 0.1% DMSO and
4.25% PDS for 72 h; Rosiglitazone group, RPMCs pre-treated with 15
µmol/l rosiglitazone dissolved in 0.1% DMSO for 2 h, then with
4.25% PDS for 72 h; 15-PGJ2 group, RPMCs pre-treated with 5 µmol/l
15-PGJ2 (PPAR-γ activator) dissolved in 0.1% DMSO for 2 h, then
with 4.25% PDS for 72 h; GW9662 + rosiglitazone group, RPMCs
pre-treated with 3 µmol/l GW9662 (PPAR-γ inhibitor) for 1 h, then
with 15 µmol/l rosiglitazone for 2 h, followied by 4.25% PDS for 72
h. (A) mRNA levels of AQP-1 and ZO-1 in RPMCs were determined using
reverse transcription-quantitative polymerase chain reaction
analysis. (B) Protein expression of AQP-1 and ZO-1 in RPMCs,
determined using western blot analysis. Values are presented as the
mean + standard deviation (n=3). **P<0.01, vs. DMSO group;
##P<0.01, vs. rosiglitazone group. AQP-1,
aquaporin-1; ZO-1, zonula occluden-1; RPMCs, rat peritoneal
mesothelial cells; PDS, peritoneal dialysis solution; PPAR-γ,
peroxisome proliferator-activated receptor-γ. |
Effects of rosiglitazone on the
protein levels of PPAR-γ and the secretion of IL-6 and IL-8
Previous studies have suggested that PPAR-γ is a
target of rosiglitazone. In the present study, it was found that
rosiglitazone was important in the injury induced by PDS in the
RPMCs. The present study then investigated whether PPAR-γ is
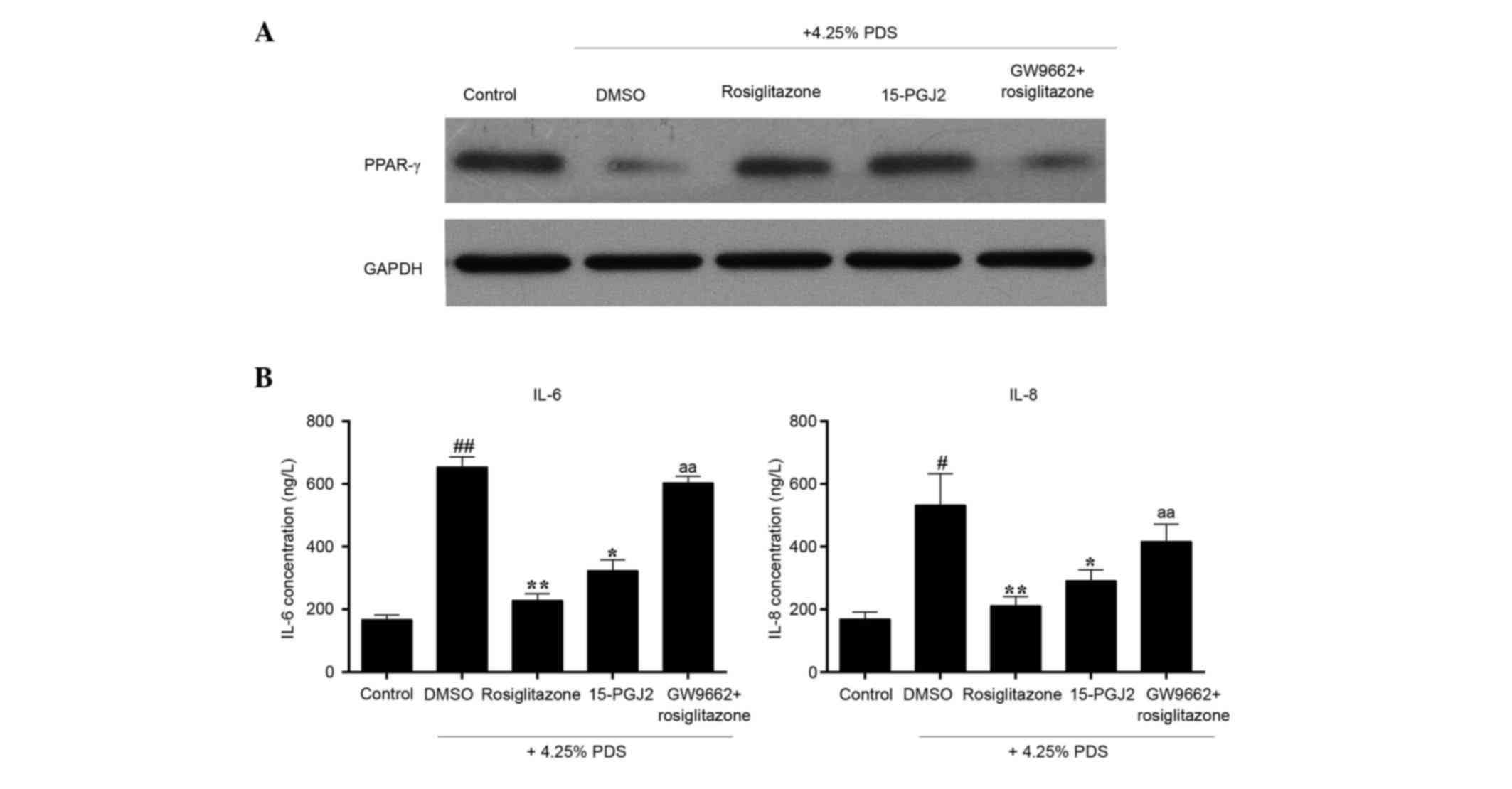
involved in the PDS-induced RPMC model. As shown in Fig. 5A, compared with the control group,
the protein levels of PPAR-γ were reduced by 4.25% PDS. The
expression of PPAR-γ was significantly elevated in the
rosiglitazone group and the 15-PGJ2 group, compared with the model
group (DMSO+4.25% PDS). The use of GW-9662 did not completely
prevent the effects of rosiglitazone. The present study further
investigated whether rosiglitazone has effects on the 4.25%
PDS-induced secretion of the IL-6 and IL-8 inflammatory cytokines
in the RPMCs. As shown in Fig. 5B,
the levels of IL-6 and IL-8 in the cellular supernatant of the
model group were significantly increased, compared with those in
the control group. However, pre-treatment of the cells with
rosiglitazone or 15-PGJ2 reduced these two inflammatory cytokines,
compared with the model group. Compared with the rosiglitazone
group, the decreases in the levels of IL-6 and IL-8 induced by
rosiglitazone were reversed by GW-9662.
Discussion
PD is an important treatment modality for patients
with ESRF, however, a decrease in ultrafiltration function and UFF
are common complications in patients receiving long-term PD, and
are a leading cause of PD dropout (4). The impaired ultrafiltration leads to
the retention of body water and sodium, increases the risk of
cardiovascular disease and results in poor prognosis, particularly
in patients with loss of residual renal function (15).
AQPs are a group of water-selective channel
proteins. They are important in maintaining fluid balance in an
organism. Until now, 14 isoforms have been identified. AQP-l was
the first member to be identified in this family (16). AQP-l has been reported to be
responsible for osmotically driven water movement across the
peritoneal membrane (16). It is
reported that high glucose dialysate upregulates the expression of
AQP-l in uremic rats, however, it is not accompanied by an increase
in ultrafiltration. Ni et al (17) investigated the effects of the
deletion of AQP-1 on the structure of the mouse peritoneum, and
found that, compared with AQP-l(+/+) litter mates, AQP-l(−/−) mice
had no sodium sieving. The initial, solute-free ultrafiltration
decreased by ~70% and cumulative ultrafiltration decreased by
almost 50%. Endothelial cells and tight junctions form the basic
structures. The structure comprises a complex of a set of protein
molecular elements, including transmembrane proteins, predominantly
occludins, claudins, in the junction-associated molecules in the
protein family composition (18,19).
The membrane components of tight junction molecules interact with
each other and their neighbors in tight junctions membrane protein
polymerization to form stable tight junctions between cells. ZO-1
is the first component to have been confirmed to be closely
connected to the protein and belongs to the membrane-associated
guanylic acid kinase protein family (20). ZO-1 is predominantly composed of
the PDZ domain, Src homologous 3 domain and guanylate kinase
domain. ZO-1 functions to maintain epithelial cell polarity, is
indirectly involved in the formation of the cytoskeleton, and is
one of the important proteins present in cell tight junctions
(20,21). In addition, ZO-1 is a signal
transducer in the barrier function and metastasis of cancer cells.
The present study focused on the regulation of expression and the
relevant functions of AQP-1 and ZO-1 in RPMCs, which are key
proteins in the peritoneum during PD and are important indicators
for the evaluation of peritoneal ultrafiltration function. In the
present study, RPMCs were cultured with 4.25% PDS for 0, 12, 24, 48
and 72 h. It was found that the expression of AQP-1 was increased
at 24 h, which was possibly due to the adaptive response of the
cells to 4.5% PDS pre-treatment. However, the expression of AQP-1
showed a significant decrease at 72 h, and the expression of ZO-1
was significantly decreased at 24, 48 and 72 h. A previous study
showed that the expression of AQP-1 in human peritoneal mesothelial
cells (HPMCs) was enhanced by glucose and other osmotically active
agents, and the potential mechanism involved the hypertonic
characteristics of PDS stablizing the AQP-1 protein and extending
its biological half-life (22,23).
However, Fusshoeller (4) found
that the expression of AQP-1 in peritoneal mesothelial cells
gradually decreased with prolonged dialysis duration (4). The results of the present study
confirmed this finding, therefore; 72 h was selected as the
treatment duration for the PDS-induced damage model. Subsequently,
the RPMCs were cultured with 0, 1.5, 2.5 and 4.25% of PDS for 72 h.
It was found that the expression levels of AQP-1 and ZO-1 were
reduced significantly in the 4.25% group. These results indicated
that RPMCs cultured with 4.5% PDS for 72 h were damaged by
assessment of the expression levels of AQP-1 and ZO-1.
PPARs belong to the nuclear hormone receptor super
family, which regulates transcription by binding to retinoid X
receptor, which is in turn bound to DNA in various cell types
(9). PPARs have three phenotypes
and PPAR-γ is one of these. It has been reported that the decreased
expression of PPAR-γ may be important in activating the
pathogenesis of UFF, and the ligands of PPAR-γ can inhibit or
reverse the development of UFF (24). Rosiglitazone is a synthetic ligand
of PPAR-γ, which can have effects on inflammation, fibrosis and
angiogenesis. Sandoval et al (25) revealed that PPAR-γ agonist,
rosiglitazone, ameliorated peritoneal membrane damage in a mouse
model of PD, the underlying mechanism being that rosiglitazone
reduced the accumulation of advanced glycation end-products,
preserved the mesothelial monolayer, decreased the number of
invading mesothelial cells, and reduced fibrosis and angiogenesis.
Furthermore, rosiglitazone treatment augments the levels of the
anti-inflammatory cytokine, IL-10, and increases the recruitment of
CD4+CD25+FoxP3+ cells. Sauter
et al (26) showed that the
activation of PPAR-γ by glitazones reduces the expression and
release of tumor necrosis factor α-stimulated monocyte
chemoattractant protein-1 in HPMCs (26). Yao et al (27) found that high concentrations of
glucose and glucose degradation products in PDS stimulate an
inflammatory response in HPMCs, and that rosiglitazone decreases
fibrosis by inhibiting the inflammatory factors, IL-6 and IL-8, and
regulating the transforming growth factor/small mothers against
decapentaplegic signaling pathway (27). In the present study, it was found
that rosiglitazone and another PPAR-γ agonist, 15-PGJ2, attenuated
the damage induced by 4.25% PDS by increasing the expression levels
of AQP-1 and ZO-1, however, these effects were reversed by the
PPAR-γ inhibitor, GW9662. Furthermore, the present study examined
the expression of PPAR-γ and inflammatory cytokines in RPMCs
cultured with 4.25% PDS for 72 h, and found a decrease in the
levels of PPAR-γ and increase in the levels of IL-6 and IL-8.
However, when the cells were pre-treated with rosiglitazone and
15-PGJ2, the expression of PPAR-γ was increased, and the levels of
IL-6 and IL-8 were reduced. These effects were also reversed by
GW9662.
In conclusion, rosiglitazone was found to directly
and specifically potentiate AQP-1-mediated water transport and
ZO-1-mediated tight junctions, through elevating the levels of
PPAR-γ and attenuating inflammation in the RPMCs treated with 4.25%
PDS. This suggests the requirement for further investigation for
the complications associated with long-term PDS.
Acknowledgements
This study was funded by the Guangzhou Medical Key
Subject Construction Project (grant no. 2013–2015) and the Program
of Huadu District Science and Technology, Guangzhou, China (grant
no. 13-HDWS2003).
References
|
1
|
Ditsawanon P and Aramwit P: Preserving the
peritoneal membrane in long-term peritoneal dialysis patients. J
Clin Pharm Ther. Aug 17–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuvaraj A, Koshy PJ, Rohit A, Nagarajan P,
Nair S, Revathi L and Abraham G: Diagnostic dilemma of
ultrafiltration failure in a continuous ambulatory peritoneal
dialysis patient. Perit Dial Int. 35:233–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teitelbaum I: Ultrafiltration failure in
peritoneal dialysis: A pathophysiologic approach. Blood Purif.
39:70–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fusshoeller A: Histomorphological and
functional changes of the peritoneal membrane during long-term
peritoneal dialysis. Pediatr Nephrol. 23:19–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ranzinger J, Rustom A and Schwenger V:
Membrane nanotubes between peritoneal mesothelial cells: Functional
connectivity and crucial participation during inflammatory
reactions. Front Physiol. 5:4122014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morelle J and Devuyst O: Water and solute
transport across the peritoneal membrane. Curr Opin Nephrol
Hypertens. 24:434–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaneda K, Miyamoto K, Nomura S and
Horiuchi T: Intercellular localization of occludins and ZO-1 as a
solute transport barrier of the mesothelial monolayer. J Artif
Organs. 9:241–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Devuyst O and Rippe B: Water transport
across the peritoneal membrane. Kidney Int. 85:750–758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han JY, Kim YJ, Kim L, Choi SJ, Park IS,
Kim JM, Chu YC and Cha DR: PPARgamma agonist and angiotensin II
receptor antagonist ameliorate renal tubulointerstitial fibrosis. J
Korean Med Sci. 25:35–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma L, Shao Z, Wang R, Zhao Z, Dong W and
Zhang J, Zhang X, Sheng S, Ji Z and Zhang J: Rosiglitazone improves
learning and memory ability in rats with type 2 diabetes through
the insulin signaling pathway. Am J Med Sci. 350:121–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YF, Zou XL, Wu J, Yu XQ and Yang X:
Rosiglitazone, a peroxisome proliferator-activated receptor
(PPAR)-γ agonist, attenuates inflammation via NF-kB inhibition in
lipopolysaccharide-induced peritonitis. Inflammation. 38:2105–2115.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aramwit P, Bunmee P and Supasyndh O:
Effectiveness and tolerability of rosiglitazone on insulin
resistance and body composition in nondiabetic Thai patients
undergoing continuous ambulatory peritoneal dialysis: A 12-week
pilot study. Curr Ther Res Clin Exp. 70:377–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nie J, Dou X, Hao W, Wang X, Peng W, Jia
Z, Chen W, Li X, Luo N, Lan HY and Yu XQ: Smad7 gene transfer
inhibits peritoneal fibrosis. Kidney Int. 72:1336–1344. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo J, Xiao J, Gao H, Jin Y and Zhao Z,
Jiao W, Liu Z and Zhao Z: Cyclooxygenase-2 and vascular endothelial
growth factor expressions are involved in ultrafiltration failure.
J Surg Res. 188:527–536.e2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Devuyst O and Yool AJ: Aquaporin-1: New
developments and perspectives for peritoneal dialysis. Perit Dial
Int. 30:135–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ni J, Verbavatz JM, Rippe A, Boisdé I,
Moulin P, Rippe B, Verkman AS and Devuyst O: Aquaporin-1 plays an
essential role in water permeability and ultrafiltration during
peritoneal dialysis. Kidney Int. 69:1518–1525. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu L, Chen C, Ding G, Zhou Y and Zhang M:
The effects of electromagnetic pulse on the protein levels of tight
junction associated-proteins in the cerebral cortex, hippocampus,
heart, lung, and testis of rats. Biomed Environ Sci. 24:438–444.
2011.PubMed/NCBI
|
|
19
|
Hawkins BT and Davis TP: The blood-brain
barrier/neurovascular unit in health and disease. Pharmacol Rev.
57:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ito T, Yorioka N, Kyuden Y, Asakimori Y,
Kiribayashi K, Ogawa T and Kohno N: Effect of glucose polymer on
the intercellular junctions of cultured human peritoneal
mesothelial cells. Nephron Clin Pract. 93:c97–c105. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leung JC, Chan LY, Li FF, Tang SC, Chan
KW, Chan TM, Lam MF, Wieslander A and Lai KN: Glucose degradation
products downregulate ZO-1 expression in human peritoneal
mesothelial cells: The role of VEGF. Nephrol Dial Transplant.
20:1336–1349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leitch V, Agre P and King LS: Altered
ubiquitination and stability of aquaporin-1 in hypertonic stress.
Proc Natl Acad Sci USA. 98:2894–2898. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakayama M, Kawaguchi Y, Yamada K,
Hasegawa T, Takazoe K, Katoh N, Hayakawa H, Osaka N, Yamamoto H,
Ogawa A, et al: Immunohistochemical detection of advanced
glycosylation end-products in the peritoneum and its possible
pathophysiological role in CAPD. Kidney Int. 51:182–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Hooland S, Boey O, Van der Niepen P,
Van den Branden C and Verbeelen D: Effect of short-term
rosiglitazone therapy in peritoneal dialysis patients. Perit Dial
Int. 29:108–111. 2009.PubMed/NCBI
|
|
25
|
Sandoval P, Loureiro J, González-Mateo G,
Pérez-Lozano ML, Maldonado-Rodríguez A, Sánchez-Tomero JA, Mendoza
L, Santamaría B, Ortiz A, Ruíz-Ortega M, et al: PPAR-γ agonist
rosiglitazone protects peritoneal membrane from dialysis
fluid-induced damage. Lab Invest. 90:1517–1532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sauter M, Kastenmüller K, Belling F,
Wörnle M, Ladurner R, Mussack T and Sitter T: Activation of
peroxisome proliferator-activated receptor-gamma by glitazones
reduces the expression and release of monocyte chemoattractant
protein-1 in human mesothelial cells. Mediators Inflamm.
2012:2176962012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao Q, Ayala ER, Qian JQ, Stenvinkel P,
Axelsson J and Lindholm B: A combination of a PPAR-gamma agonist
and an angiotensin II receptor blocker attenuates proinflammatory
signaling and stimulates expression of Smad7 in human peritoneal
mesothelial cells. Clin Nephrol. 68:295–301. 2007.PubMed/NCBI
|