Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer, accounting for ~5% of malignant cancers in males,
and 3% in females, according to a survey in 2013 in the USA
(1,2). The estimated statistics in the study
demonstrated that the incidence and the mortality rate of RCC were
among the ten leading types of cancer, with >65150 new cases
accounting for >13680 mortalities recorded (2). During the early stage of RCC,
patients have no characteristic clinical feature and 30% of the RCC
patients have an evidence of metastasis at diagnosis (3). Common metastatic sites of RCC are the
lung, bone, lymph node, liver, adrenal glands and brain (4,5).
Surgical treatment followed by radiotherapy and chemotherapy is the
predominant therapeutic strategy for RCC. However, high recurrence
rates of 20–40% patients are observed during the treatment
(6). The 5-year survival rate of
RCC is estimated to be ~55% and <10% in patients with metastatic
RCC (7). Thus, diagnosis of RCC at
an early stage may improve the prognosis of RCC patients, and it is
key to identify a novel biomarker to diagnose or offer a targeted
therapy for RCC.
Noncoding RNA (ncRNA) is considered to serve a
critical role in the regulation of multiple cellular processes.
microRNAs (miR) are a class of small ncRNAs at a length of ~20
nucleotides (8,9). In the majority of types of cancer,
miRNAs may modulate key processes by binding to mRNA to induce
translational repression or degradation (10,11).
miRs may act as oncogenes or tumor suppressor genes, according to
the mRNA that they target (12). A
number of studies have focused on aberrantly expressed miRs and
function in different diseases, particularly in tumors. miR-30b, a
member of the miR-30b family, has been identified as abnormally
expressed in different tumors, including those found in gastric
cancer, colorectal cancer and non-small cell lung cancer (NSCLC)
(13–15). A previous study demonstrated using
microarray that miR-30b was downregulated in RCC (16), whilst another indicated miR-30b was
upregulated in RCC tissues and serum (17). The expression of miR-30b has not
previously been verified by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) in RCC tissues. In the present
study, the expression of miR-30b was detected in RCC tissues and
cell lines, and, the function of miR-30b in RCC cell lines (ACHN
and 786-O) was investigated.
Materials and methods
Sample collection
A total of 31 paired tissues were collected from
Peking University Shenzhen Hospital (Shenzhen, China) between
December 2012 and December 2014. There were 19 males and 12 female
patients (age range, 25–75). Written informed consent was obtained
from all patients. Collection and usage of the samples were
reviewed and approved by the Ethics Committees of Peking University
Shenzhen Hospital. The tissues were immersed in RNAlater (Qiagen
GmbH, Hilden, Germany) for 30 min following dissection, prior to
storing the tissues at −80°C until further use. A pair of tissue
constitutes an affected RCC tissue and adjacent normal tissue,
which is 2 cm away from visible RCC lesions. The tissues collected
were reviewed and classified by hematoxylin and eosin staining. The
clinical and pathological characteristics of the patients are
presented in Table I.
 | Table I.Clinicopathological features of renal
cell carcinoma patients. |
Table I.
Clinicopathological features of renal
cell carcinoma patients.
| Characteristic | Number of
cases |
|---|
| Mean age/age range
(years) | 50/25–70 |
| Gender |
|
|
Male/female | 19/12 |
| Histological
type |
|
| Clear
cell/papillary | 26/5 |
| pT-stage |
|
|
T1/T2/T3+T4 | 16/11/4 |
| Fuhrman grade |
|
|
I/II/III/IV | 10/14/4/3 |
| AJCC clinical
stages |
|
|
I/II/III+IV | 16/12/3 |
Cell culture
The 293T human embryo kidney cell line was obtained
from the Type Culture Collection of the Chinese Academy of Medical
Sciences (Shanghai, China) and three RCC cell lines, 786-O, ACHN
and 769-P were purchased from the American Type Culture Collection
(Manassas, VA, USA) for use in the present study.
Cells were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo, Fisher
Scientific, Inc.), 1% antibiotics (100 µl/ml penicillin and 100
mg/ml streptomycin sulfates) and 1% glutamine in the humidified
incubator containing 5% CO2 at 37°C.
RNA extraction, cDNA synthesis and
qPCR
Total RNA was extracted from the tissues and cells
by TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
purified with the RNeasy Maxi kit (Qiagen GmbH) according to the
manufacturer's protocols. The concentration of RNA was measured
using a NanoDrop 2000/2000c (Thermo Fisher Scientific, Inc.). A
total of 1 µg total RNA of each sample was used for reverse
transcription by miScript Reverse Transcription kit (Qiagen)
according to the manufacturer's instructions to obtain cDNA.
RT-qPCR was performed to detect the expression level of miR-30b
using the miScript SYBR®green PCR Kit (Qiagen GmbH) on
the Roche Lightcycler 480 Real-Time PCR system (Roche Diagnostics,
Basel, Switzerland) according to the manufacturer's protocols. The
conditions of RT-qPCR were: 95°C for 1 min, then 95°C for 10 sec,
55°C for 30 sec, 70°C for 30 sec, for 40 cycles. U6 served as the
internal control. The sequences of the primers are presented in
Table II. The universal primer
was provided by the miScript SYBR®green PCR kit, which
was used as the reverse primer for miR-30b. The expression levels
of miR-30b were analyzed using the 2-ΔΔCq method
(18).
 | Table II.Sequences used in the present
study. |
Table II.
Sequences used in the present
study.
| Gene | Sequence |
|---|
| miR-30b mimic | Sense:
5′-UGUAAACAUCCUACACUCAGCU-3′ |
|
| Antisense:
5′-CUGAGUGUAGGAUGUUUACAUU-3′ |
| NC | Sense:
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
| Antisense:
5′-ACGUGACACGUUCGGAGAATT-3′ |
| miR-30b
inhibitor |
5′-AGCUGAGUGUAGGAUGUUUACA-3′ |
|
Inhibitor NC |
5′-CAGUACUUUUGUGUAGUACAA-3′ |
| miR-30b forward
primer |
5′-TGTAAACATCCTACACTCAGCT-3′ |
| U6
forward primer |
5′-CTCGCTTCGGCAGCACA-3′ |
| U6
reverse primer |
5′-ACGCTTCACGAATTTGCGT-3′ |
Cell transfection
For upregulation or downregulation of miR-30b,
synthesized miR-30b mimic or inhibitor (Shanghai GenePharma Co.,
Ltd., Shanghai, China) was transfected into cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
which was mixed in Opti-MEM® I Reduced Serum medium
(Gibco; Thermo Fisher Scientific, Inc.) following plating for 24 h.
Cells were transfected for 4 h at 37°C. Cells transfected with the
miR-30b mimic negative control (NC) or inhibitor NC formed the
control group. RT-qPCR was then performed, as before, to verify the
changes of miR-30b expression following transfection for 24 h. The
sequences are presented in Table
II.
Wound healing assay
The wound healing assay was performed to assess the
cell migration ability of 786-O and ACHN cells in vitro.
Approximately 3×105 cells were seeded in each well of the 12-well
plate, and the cells were transfected with 40 pmol of miR-30b
mimics, inhibitors, negative control or inhibitor negative control
after 24 h. A vertical horizontal line was scratched with a sterile
200 µl pipette tip 6 h after transfection. The cells were rinsed
with phosphate-buffered saline (PBS) to remove the floating cells.
A digital camera system was used to acquire the images of the
scratches at 0 and 24 h following the scratch. The experiments were
performed in triplicate and repeated at least three times.
Transwell assay
A Transwell assay was performed to assess the cell
migration and invasion ability of 786-O and ACHN cells in
vitro. Transwell chamber inserts (BD Biosciences, Franklin
Lakes, NJ, USA) with (for invasion) or without Matrigel (BD
Biosciences) were used in the assay according to the manufacturer's
protocols. The transfected cells were seeded in the upper chamber
of the insert at a density of 1×104 cells in 200 µl serum-free
medium. The bottom of the inserts was incubated in the medium
supplemented with 10% FBS. The cells were allowed to migrate for 40
h and invade for 60 h. The cells that had migrated or invaded to
the bottom of the inserts were stained with crystal violet and
counted using a light microscope.
MTT assay
An MTT assay was performed to assess the cell
proliferation ability of 786-O and ACHN cells in vitro. In
each well of the 96-well plate, ~5,000 cells were seeded and then
transfected with 5 pmol miR-30b mimics, inhibitors, mimic NC or
inhibitor NC. MTT (20 µl, 5 mg/ml; Sigma-Aldrich; Merck-Millipore,
Darmstadt, Germany) was added into the wells to be detected at 0,
24, 48 and 72 h post-transfection. Following this, the 96-well
plate was incubated at 37°C for 4 h. The mixed medium was then
replaced by 150 µl dimethyl sulfoxide (Sigma-Aldrich, Merck
Millipore) and then agitated for 15 min at room temperature.
Subsequently, the optical density (OD) of each well was measured by
an ELISA microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) at a wavelength of 490 nm.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation of 786-O and ACHN cells was also
detected using the Cell Counting Kit-8 (Beyotime Institute of
Biotechnology, Haimen, China) following the manufacturer's
protocols. In each well of 96-well plate ~5,000 cells were seeded
and 24 h later the cells were be transfected with 5 pmol miR-30b
mimics, inhibitors, mimic NC or inhibitor NC. At 0, 24, 48 and 72 h
after transfection, 15 µl CCK-8 was added to the wells and OD of
each well was measured after 1.5 h by an ELISA microplate reader
(Bio-Rad Laboratories, Inc.) at a wavelength of 490 nm.
Flow cytometry assay for
apoptosis
The apoptosis rates of 786-O and ACHN cells in
vitro were measured by performing a flow cytometry assay. A
total of ~3×105 cells were seeded in each well of a 6-well plate
and then transfected with 200 pmol miR-30b mimics, inhibitors,
mimic NC or inhibitor NC. At 48 h post-transfection, all cells were
harvested and washed twice with cold PBS. Subsequently, the cells
were resuspended in 100 µl 1X binding buffer, and 5 µl Annexin
V-fluorescein isothiocyanate (Invitrogen; Thermo Fisher Scientific,
Inc.) and 5 µl propidium iodide (Invitrogen; Thermo Fisher
Scientific, Inc.) were added into each cell suspension. After 15
min, 400 µl binding buffer was added to each tube and flow
cytometry (EPICS XL 4; Beckman Coulter, Inc., Brea, CA, USA) was
used to quantify and analyze the apoptosis rate. FlowJo software
(version, X) fl (FlowJo LLC, Ashland, OR) was used.
Hoechst 33342 staining assay
Cell apoptosis was observed using a Hoechst 33342
staining assay. After 48 h transfection, ~1×105 cells were cultured
in a six-well plates and were washed with PBS and stained with
Hoechst 33342 (5 µg/ml; Thermo Fisher Scientific, Inc.) for 10 min.
Following washing with PBS twice, images of the cells were acquired
with a fluorescence microscope.
Statistical analysis
A paired Student's t-test was used to compare the
expression levels of miR-30b in matching tissues. Student's t-test
was used to analyze assays for characterizing phenotypes of cells.
The association between the expression level of miR-30b and
clinical clinicopathological parameters was analyzed by Fisher's
exact test or χ2 test. All statistical analysis was conducted by
SPSS software (version, 19.0; IBM SPSS. Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
miR-30b was upregulated in RCC tissues
and cell lines
To determine the expression of miR-30b in RCC
tissues and cell lines, RT-qPCR was performed in RCC cell lines and
31 paired tissues. The ratios of miR-30b in 31 paired tissues
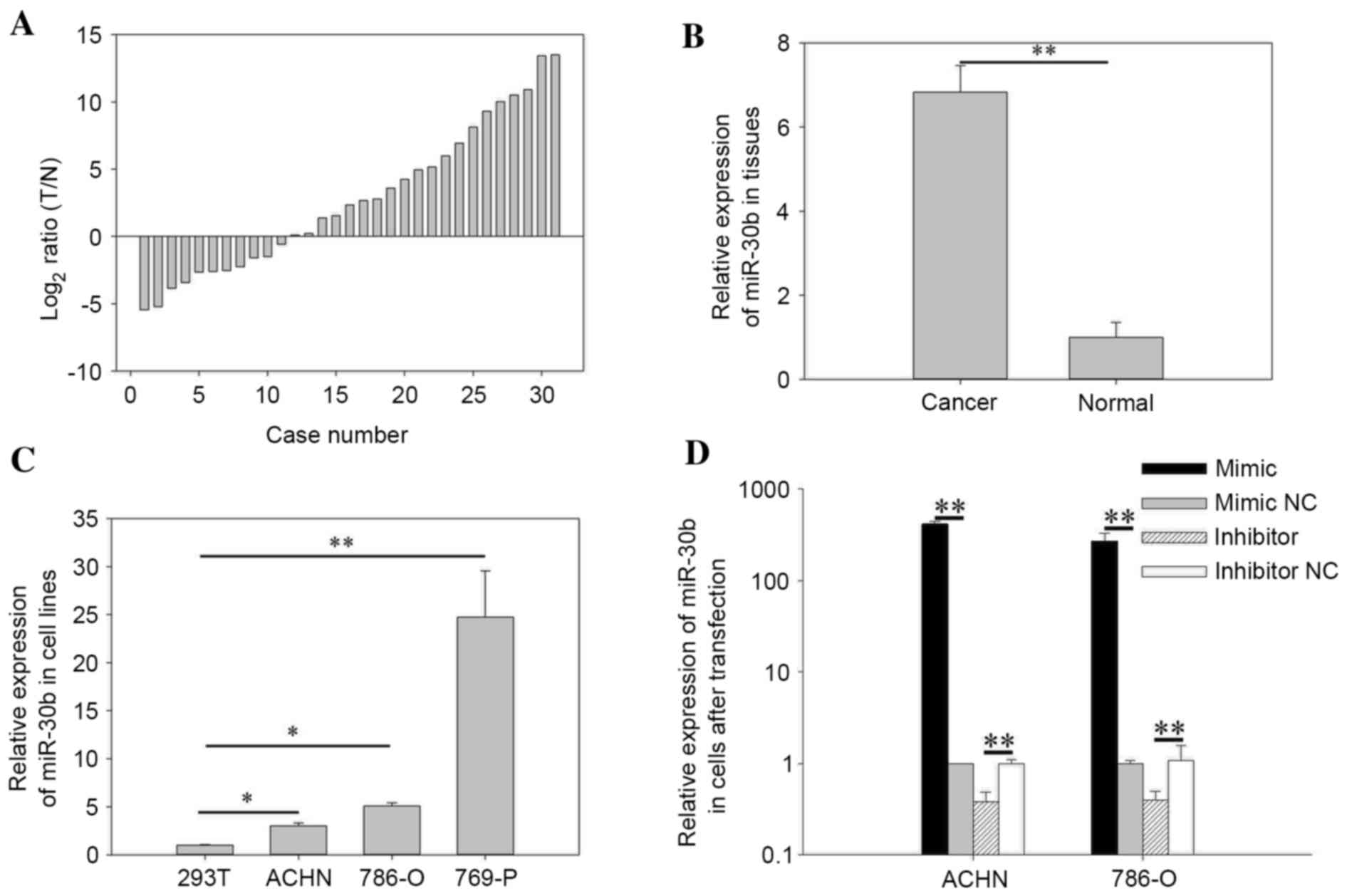
[log2 ratio (T/N)] are presented in Fig. 1A, in which miR-30b was upregulated
in 20 RCC tissues. The results demonstrated that the expression of
miR-30b in RCC tissues (mean relative expression=6.83) was
significantly higher than normal tissues (P=0.009). Relative
expression of miR-30b in paired tissues is presented in Fig. 1B. The expression levels of miR-30b
in RCC cell lines (786-O, ACHN and 769-P) were significantly higher
when compared with the 293T human embryo kidney cell line (786-O,
P=0.032; ACHN, P=0.012; 769-P, P=0.006), as indicated in Fig. 1C. The results suggest that miR-30b
may function as an oncogene in RCC.
Association between the expression
level of miR-30b and clinicopathological characteristics in RCC
patients
Patients were divided into two groups (high
expression and low expression groups) according to the mean
expression level of miR-30b. Fisher's exact test or χ2 test was
performed to investigate the association between the expression and
clinicopathological characteristics of patients. No association was
observed between the expression of miR-30b and the age, gender, T
stage, histological type, Fuhrman grade and American Joint
Committee on Cancer clinical stages (all P>0.05). However, the
validity of the results must be questioned due to the low number of
experimental cases; the results should be verified in future
studies (Data not shown).
Validation of cell transfection
efficiency
RT-qPCR was performed to quantify the transfection
efficiency of miR-30b mimics or inhibitors compared with mimic NC
or inhibitor NC. The results indicated that the expression levels
of miR-30b in the miR-30b mimic group were 271.58-fold higher
(786-O) and 417.68-fold higher (ACHN) when compared with the
negative control group (P=0.005 786-O, P=0.008, ACHN), and
expression in the inhibitor group was 0.38 times (786-O) and 0.40
times of the inhibitor NC group (P=0.002 786-O, P=0.008 ACHN,
Fig. 1D).
miR-30b promotes cell
proliferation
Cell proliferation was measured using MTT and CCK-8
assays in vitro. The results of MTT and CCK-8 assay
suggested that upregulation of miR-30b promoted cell proliferation,
whereas downregulation of miR-30b attenuated cell proliferation. As
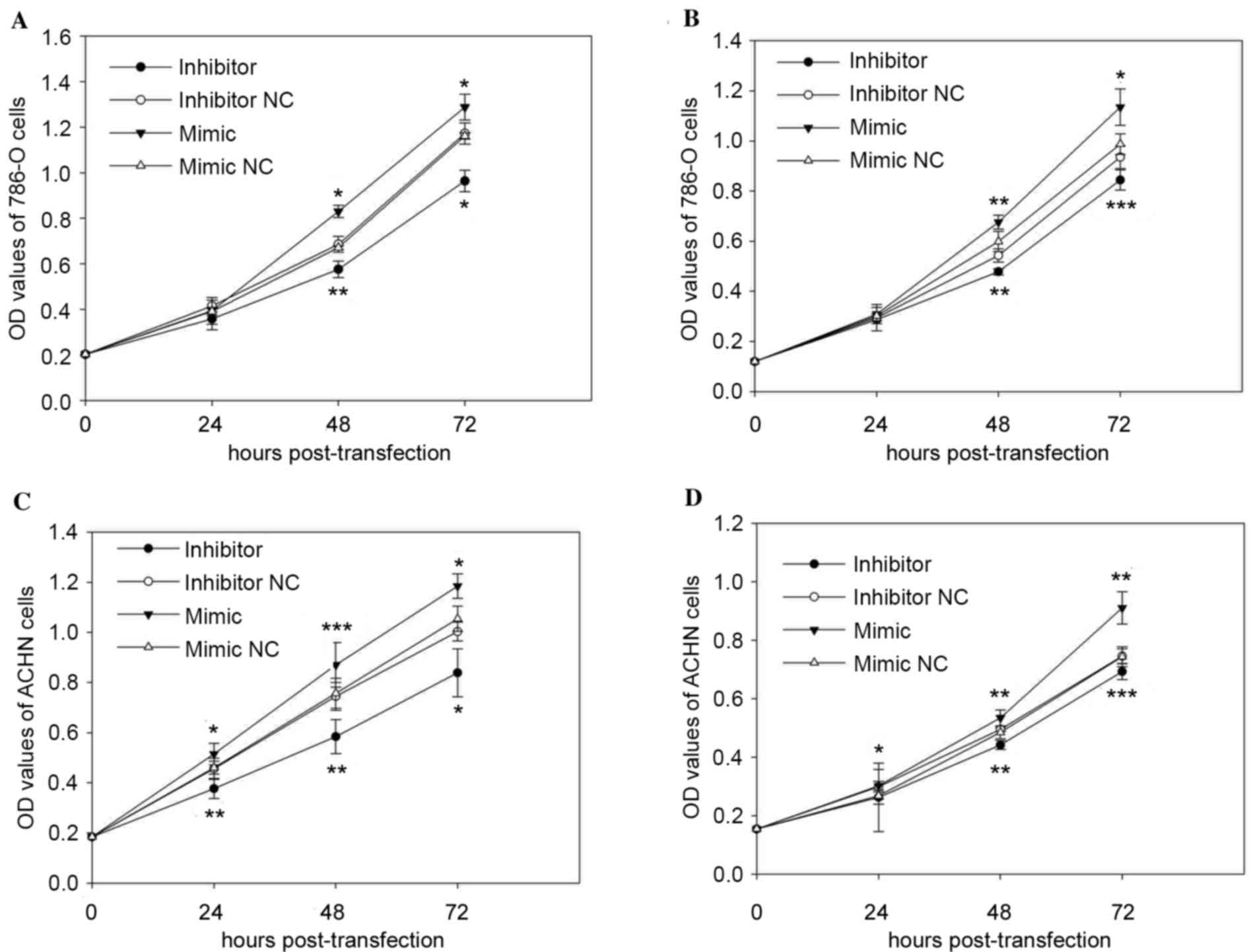
demonstrated in Fig. 2A, the CCK-8
assay reduced cell proliferation of 786-O cells in the inhibitor
group by 13.76% (P>0.05), 16.01% (P=0.002), 17.78% (P=0.048),
while the mimic group was promoted by 0.64% (P>0.05), 23.75%
(P=0.013), 10.90% (P=0.047), after transfection at 0, 24, 48 and 72
h, respectively, compared with those transfected with the
respective inhibitor NC or mimic NC. Similarly, the MTT assay
(Fig. 2B) demonstrated that cell
proliferation of 786-O cells in the inhibitor group was reduced by
2.70% (P>0.05), 11.94% (P=0.009), 9.78% (P=0.0002), with the
mimic group promoted by 2.31% (P>0.05), 12.86% (P=0.006), 14.98%
(P=0.015) compared with the inhibitor NC or mimic NC group at 24,
48 and 72 h post-transfection, respectively.
In ACHN cells, the results demonstrated that cell
proliferation of cells transfected with the miR-30b inhibitor was
reduced by 17.43% (P=0.002), 21.59% (P=0.004), 16.41% (P=0.011) in
CCK-8 assay (Fig. 2C) and 12.12%
(P>0.05), 10.76% (P=0.009), 7.11% (P=0.0003) in MTT (Fig. 2D) after transfection at 24, 48 and
72 h compared with cells transfected with inhibitor NC. Inversely,
the proliferation of ACHN cells transfected with miR-30b mimic was
promoted by 11.86% (P=0.018), 14.97% (P=0.0007), 12.36% (P=0.037)
in CCK-8 assay (Fig. 2C), with
12.59 (P=0.031), 10.16% (P=0.005), 22.45% (P=0.001) in MTT assay
(Fig. 2D) at 24, 48 and 72 h,
respectively, compared with cells transfected with mimic NC.
miR-30b promoted RCC cell
mobility
In order to investigate the effect of miR-30b on
cell mobility in RCC cell lines (786-O and ACHN), Transwell
invasion, migration and wound scratch assays were performed. As
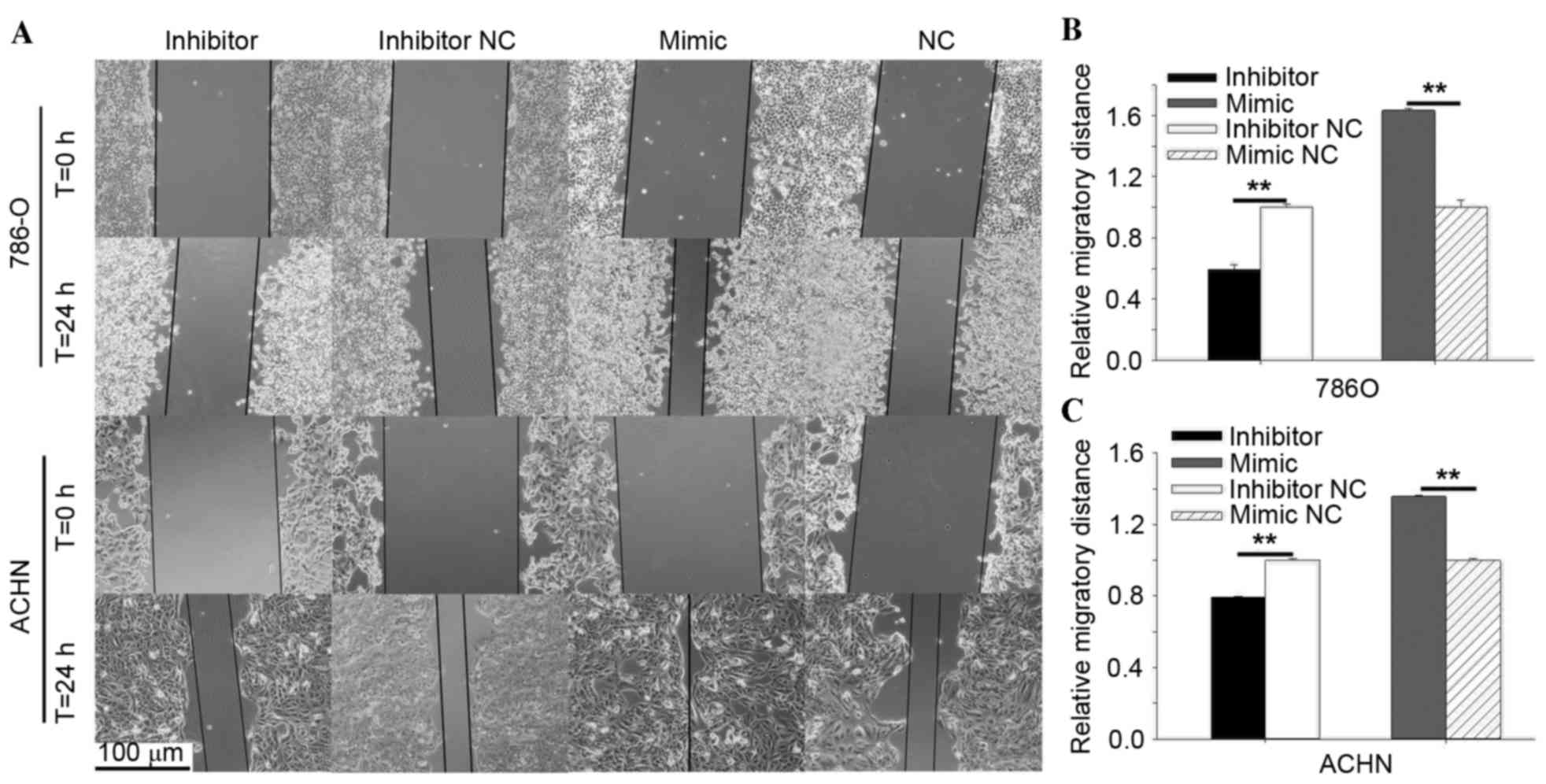
demonstrated in Fig. 3A and B, the
results of the wound scratch assay of 786-O indicated that the
migratory distance of cells transfected with miR-30b inhibitor was
reduced significantly by 40.58% (P=0.001) at 24 h post-transfection
compared with cells transfected with inhibitor NC. In contrast,
upregulation of miR-30b by transfecting the miR-30b mimic promoted
migratory distances by 63.45% (P=0.003) in 786-O at 24 h
post-transfection compared with cells transfected with mimic NC. In
ACHN cells, the downregulation of miR-30b reduced the migratory
distance by 20.68% (P=0.001) at 24 h post-transfection (Fig. 3A and C). By contrast, upregulation
of miR-30b promoted migratory distances by 35.64% (P=0.008) in ACHN
cells at 24 h post-transfection (Fig.
3A and C).
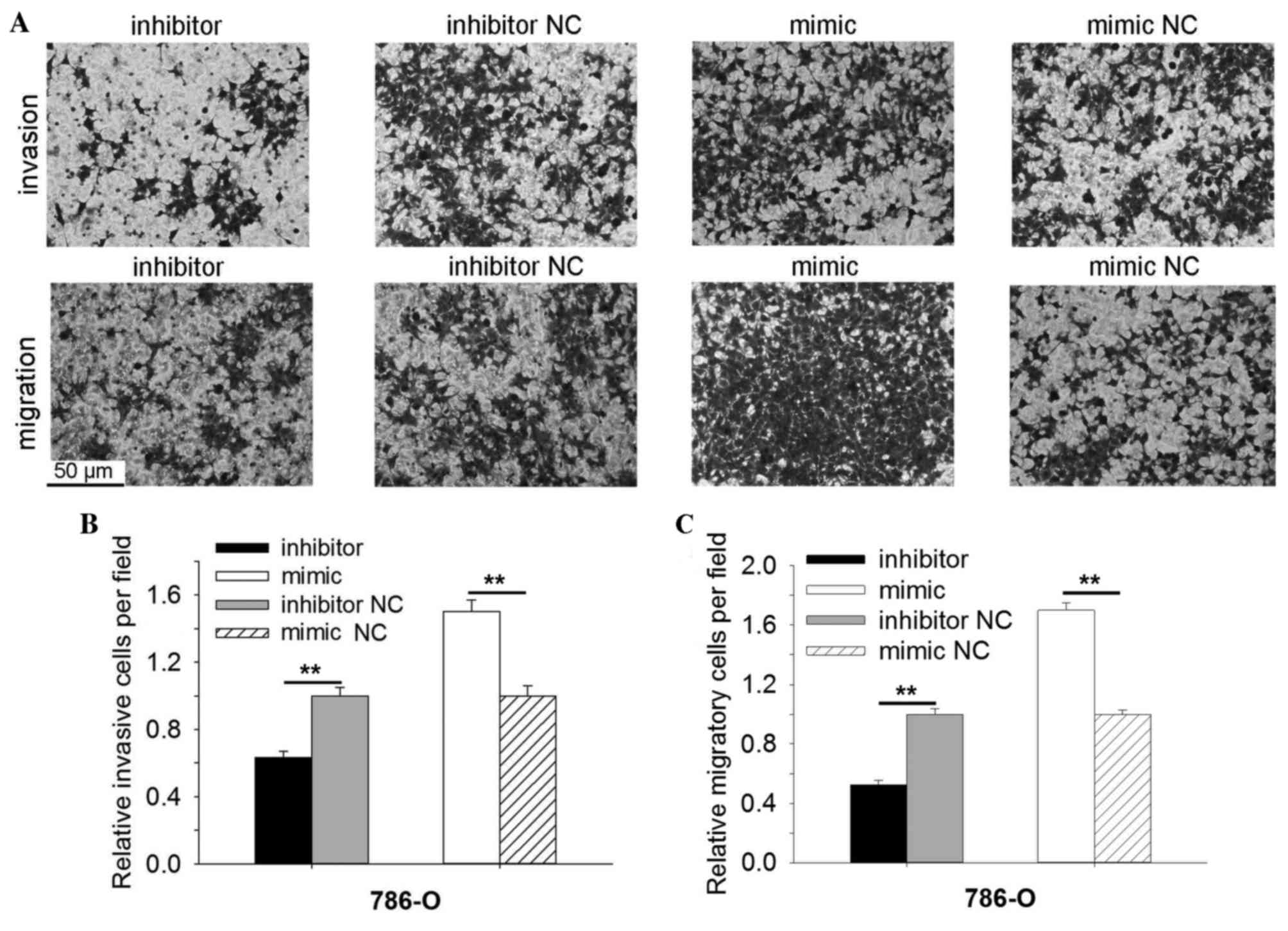
As indicated in Fig.
4, the results demonstrated that the invasive ability of 786-O
cells transfected with miR-30b inhibitors was reduced significantly
by 36.80% (P=0.003) when compared with cells transfected with
inhibitor NC, and promoted by 50.09% in the miR-30b mimic group
(P=0.002) when compared with the mimic NC group (Fig. 4A and B). The migratory ability of
786-O cells transfected with miR-30b inhibitors was reduced by
47.54% (P=0.004) and promoted by 60.95% (P=0.003) in cells
transfected with miR-30b mimic, when compared with the inhibitor NC
or mimic NC group (Fig. 4A and C).
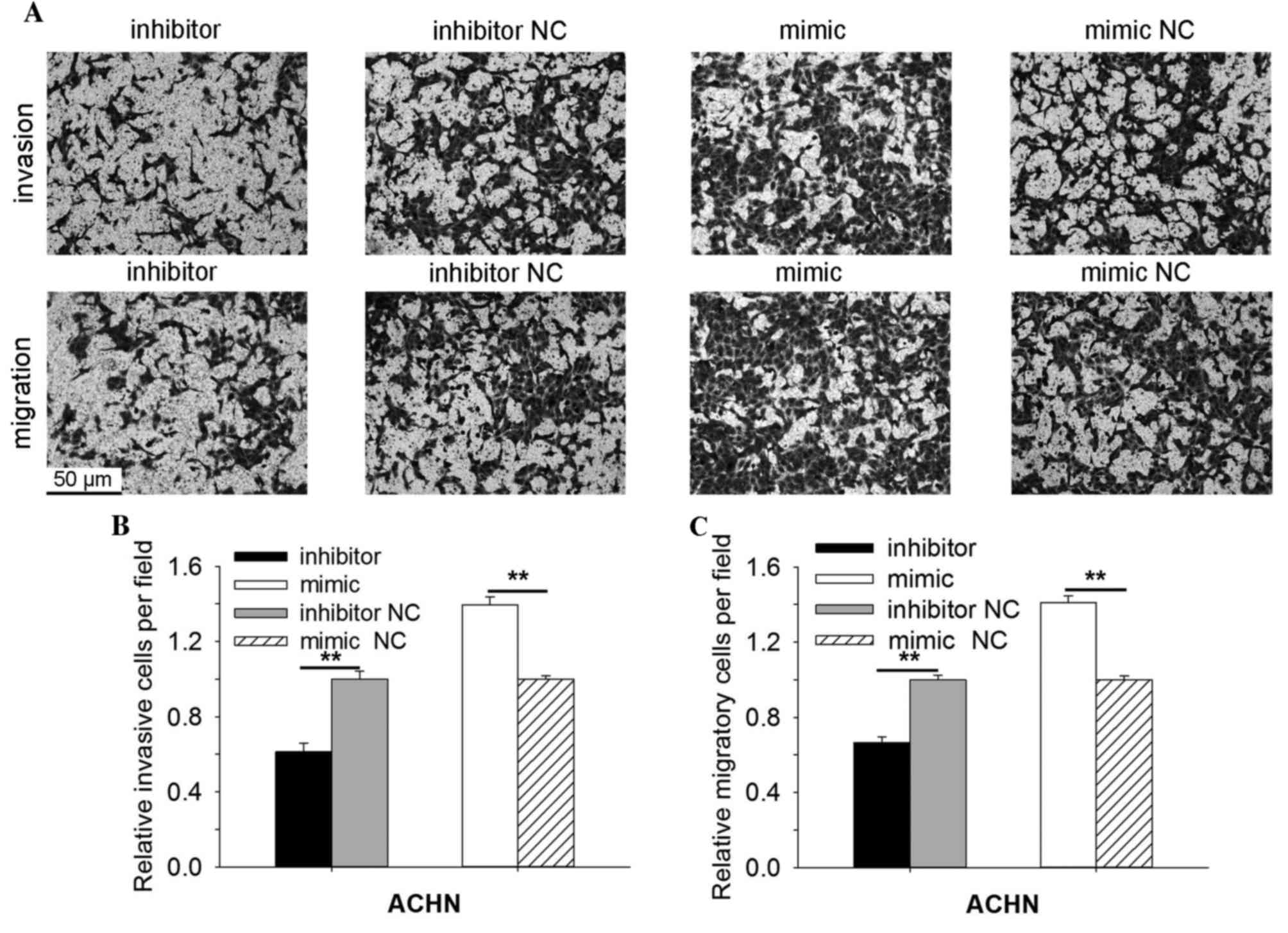
In ACHN cells, the Transwell invasion assay demonstrated that the
invasive ability of cells transfected with miR-30b mimic was
increased by 39.60% (P=0.004) and reduced by 38.78% (P=0.004) in
cells transfected with miR-30b inhibitor compared with the mimic NC
or inhibitor NC group (Fig. 5A and
B). As indicated in Fig. 5A and
C, the migratory ability of cells transfected with miR-30b
mimic was increased by 40.93% (P=0.003) and reduced by 33.54%
(P=0.006) in cells transfected with miR-30b inhibitor, compared
with cells transfected with mimic NC or inhibitor NC. The results
of the Transwell invasion, migration and wound scratch assays
indicated that miR-30b promoted the ability of RCC cell
mobility.
miR-30b suppressed apoptosis
Apoptosis rate was qualified by flow cytometry and
Hoechst 33342 staining assay. The results of the flow cytometry
indicated that miR-30b had a negative effect on apoptosis, as the
upregulation of miR-30b suppressed apoptosis while downregulation
of miR-30b invoked apoptosis in RCC cells (Figs. 6 and 7). After a 48 h transfection of the
miR-30b mimic, inhibitor, mimic NC or inhibitor NC, all cells were
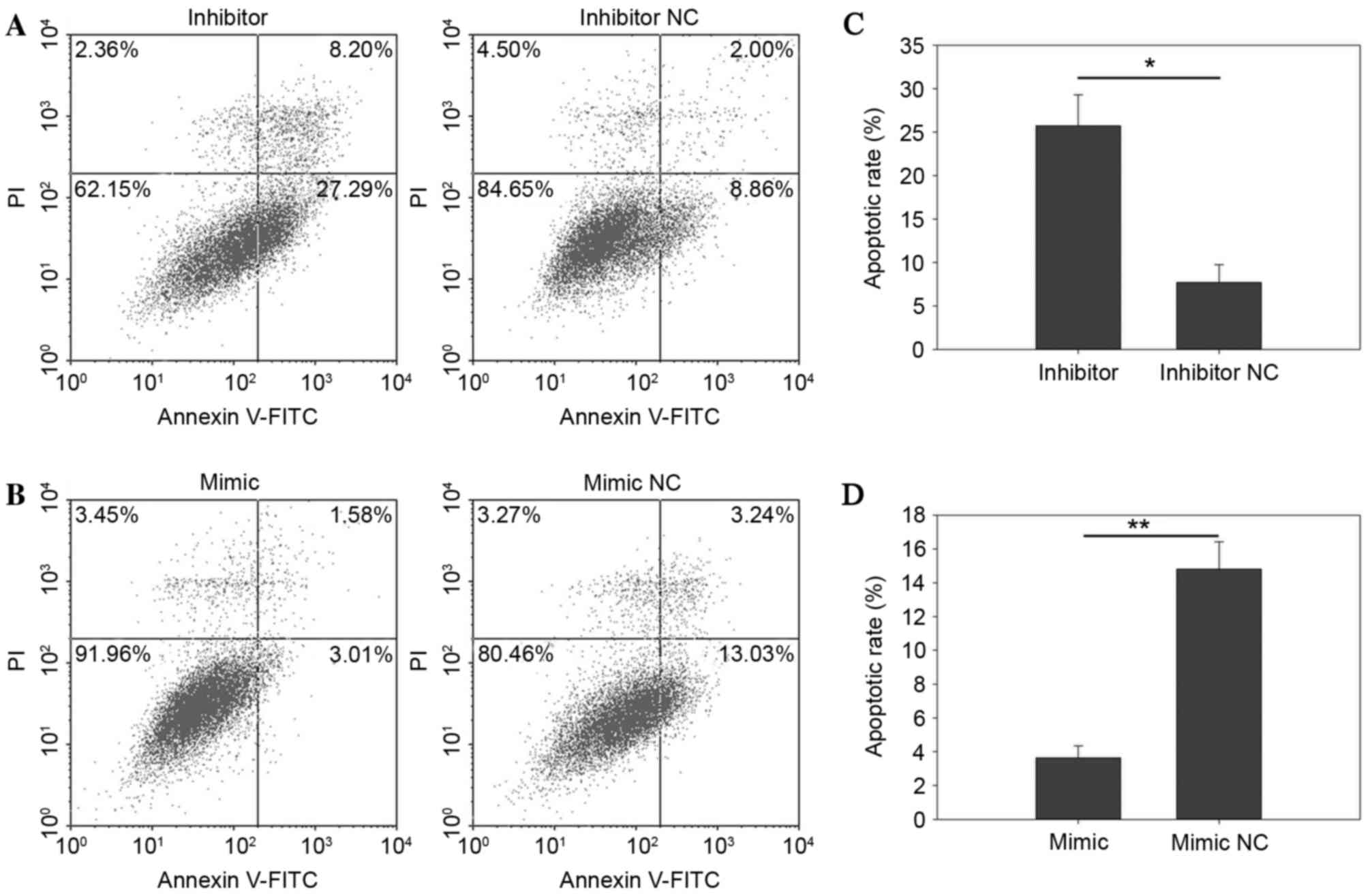
collected for measurement. As presented in Fig. 6C, the results demonstrated that the
early apoptosis rate of cells transfected with miR-30b inhibitor or
inhibitor NC was 25.74±3.55 vs. 7.71±2.04 (P=0.025) in 786-O cells.
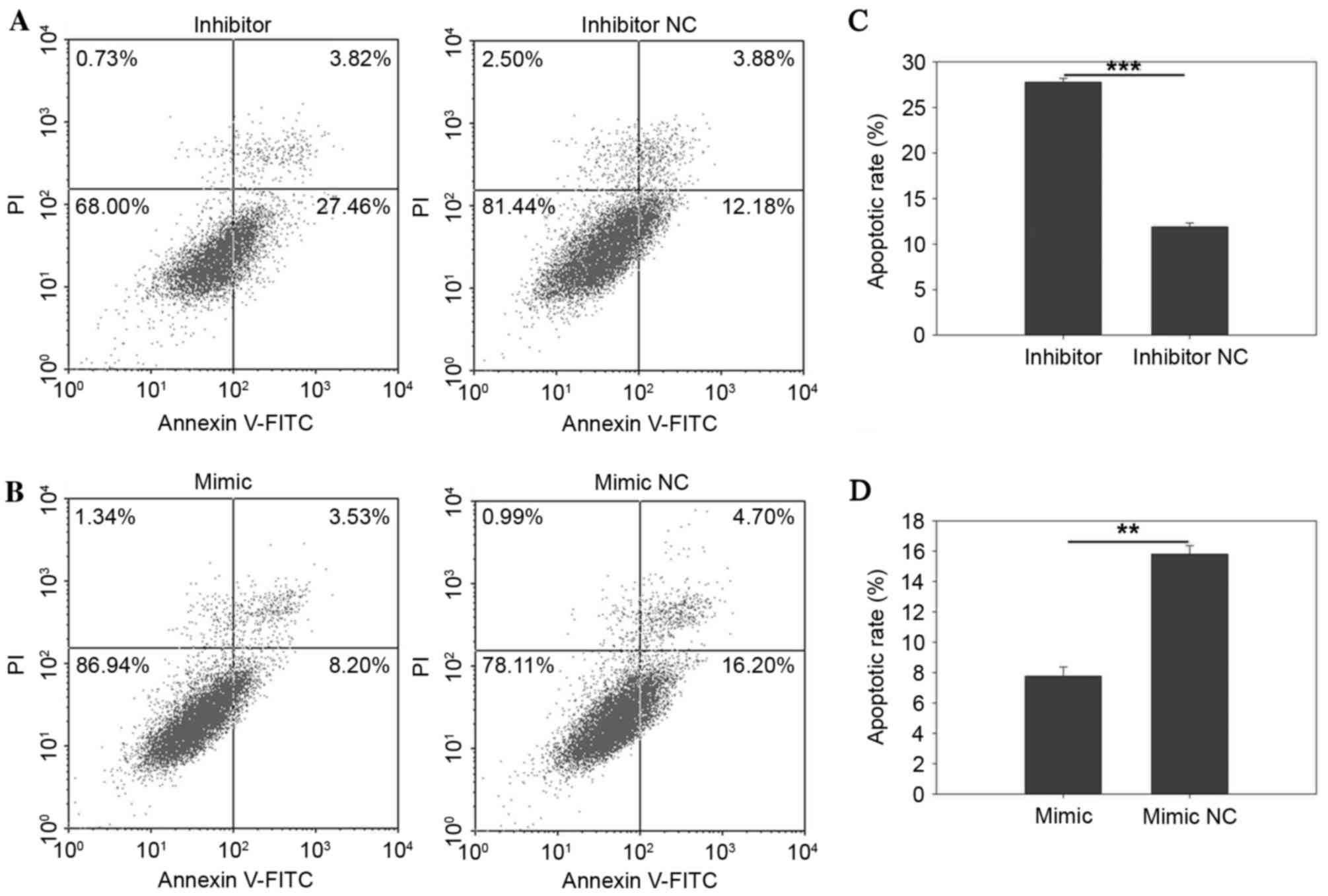
Fig. 7C demonstrated that the
apoptotic rate of ACHN cells was 27.76±0.42 vs. 11.88±0.42%
(P=0.0002), while upregulation of miR-30b by transfection the
miR-30b mimic reduced the apoptotic rate from 14.81±1.62 to
3.64±0.71% (P=0.003) in 786O cells (Fig. 6D) and 15.80±0.57 to 7.78±0.60%
(P=0.002) in ACHN cells (Fig. 7D),
respectively. The results of the present study demonstrated that
upregulation of miR-30b may inhibit cell apoptosis, while
downregulation of miR-30b may induce apoptosis in RCC cells.
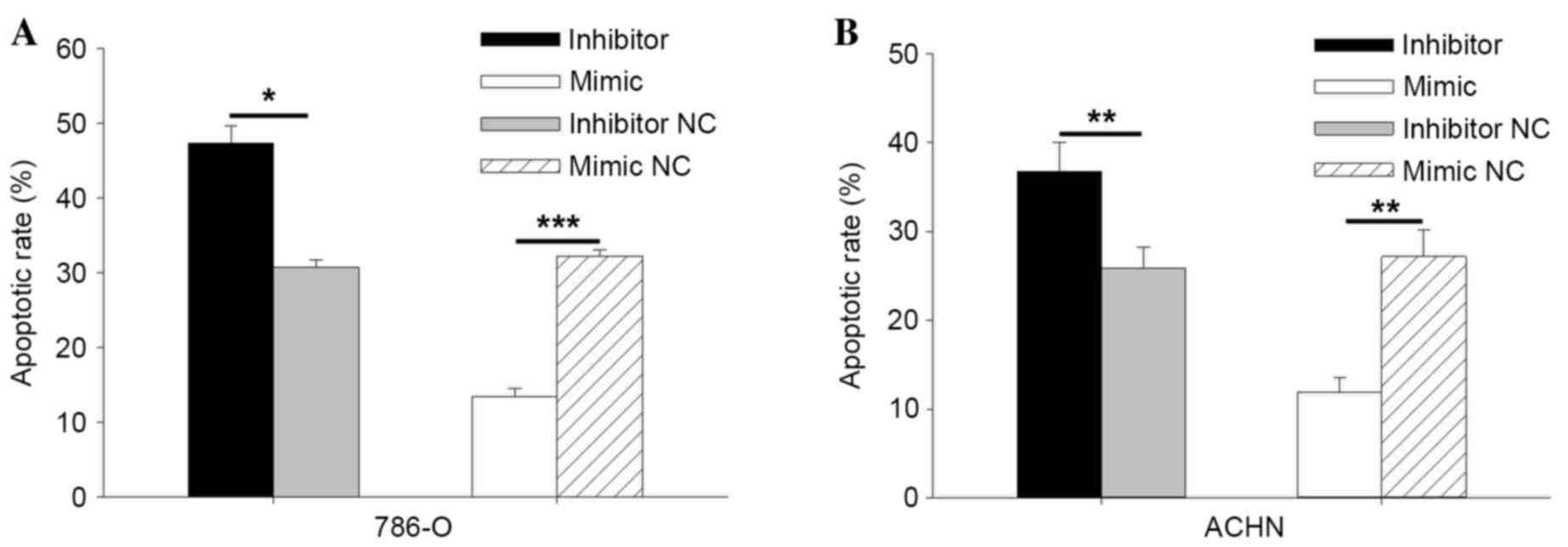
The results of Hoechst 33342 staining assay are
consistent with results of the flow cytometry. As indicated in
Fig. 8A, the apoptotic rate of
786-O cells was 47.33% in the inhibitor group and 30.78% in the
inhibitor NC group (P=0.011). The apoptotic rate in cells
transfected with the miR-30b mimic was 13.39%, and 32.27% in cells
transfected with the miR-30b mimic NC (P=0.0001). The results
indicated that miR-30b may inhibit cell apoptosis. In ACHN cells
transfected with the miR-30b inhibitor, the apoptotic rate was
36.80, and 25.89% in cells transfected with inhibitor NC (P=0.003).
The apoptotic rate of cells transfected with the miR-30b mimic and
mimic NC was 11.93 and 27.17%, respectively (P=0.008; Fig. 8B). The results demonstrated that
miR-30b may regulate RCC cell apoptosis.
Discussion
The identification of biomarkers for the diagnosis
of cancer may lead to more efficient treatment and lower mortality
rates (19). However, there are
currently no reliable biomarkers for patients with RCC. MicroRNAs
have been identified as a potential source of cancer biomarkers,
due to their abnormal expression in numerous types of cancers.
Tumorigenesis is associated with activation of
oncogenes and dysfunction of anti-oncogenes. MicroRNAs may serve an
important role as either as an oncogene or tumor suppressor gene
via regulating the expression of oncogenes or anti-oncogenes at
post-transcription level (20).
Upregulated microRNAs could act as oncogenes, the downregulated
microRNAs as tumor suppressors.
miR-30b has been previously described as a tumor
suppressor in certain types of cancer, including NSCLC, gastric
cancer and colorectal cancer (CRC) (14,21,22).
Conversely, miR-30b has been described as an oncogene in bladder
cancer (23), oral squamous cell
cancer (10) and medulloblastoma
(24). However, the function of
miR-30b in RCC remains to be elucidated. Previous microarray
studies have indicated different levels of expression of miR-30b in
RCC, which may result from the high sensitivity and limited number
of sample tissues (16,17). Another previous study revealed that
the deregulation of miR-30 family (miR-30a, miR-30b and miR-30c)
was correlated with distant metastasis (25). Thus, in the present study, RT-qPCR
was performed to detect the expression level of miR-30b in RCC
tissues and cell lines. The association between expression of
miR-30b and clinicopathological characteristic was also
investigated in the current study. The function of miR-30b in RCC
cell lines was investigated by performing MTT, CCK-8, Transwell,
wound scratch, Hoechst 33342 staining and flow cytometry assays.
The results revealed that miR-30b was upregulated in RCC tissues
and cell lines (ACHN, 786-O and 769-P) when compared with adjacent
normal tissues and 293T cells. In addition, miR-30b may regulate
RCC cell proliferation, migration, invasion and apoptosis. All the
results suggested miR-30b acts as an oncogene in RCC. No
association was identified between the expression of miR-30b and
the clinicopathological characteristics observed in patients. The
present study is, to the best of our knowledge, the first to
describe miR-30b as an oncogene in RCC.
In certain types of cancer, the mechanism of miR-30b
is understood. Tian et al (22) demonstrated that miR-30b regulated
gastric cancer cell processes by targeting eukaryotic translation
initiation factor 5A2. In gastric cancer, Zhu et al
(15) indicated that miR-30b could
promote cell apoptosis and suppress tumor growth in vivo by
targeting plasminogen activator inhibitor-1. In addition, it has
been reported that DNA (cytosine-5)-methyltransferase 1 may
suppress miR-30b expression partly via methylation of its promoter
(12). In NSCLC, miR-30b may
target the collagen triple helix repeat containing 1 protein to
inhibit cell invasion and migration (13). Gu et al (26) identified that patients with low
miR-30b expression in NSCLC had a lower overall survival rate,
which indicated that miR-30b could be selected as a biomarker for
NSCLC. Furthermore, it was reported that miR-30b could regulate
migration and invasion of CRC by targeting SIX homeobox 1 (14). In CRC, miR-30b was also reported to
serve a role as a tumor suppressor by targeting the KRAS
proto-oncogene GTPase, phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit δ and B-cell lymphoma 2 genes (27).
A previous study identified that the miR-30 family
may modulate endothelial cell behavior during angiogenesis by
targeting delta-like 4 (28).
Another previous study reported that the expression of miR-30
family is inversely correlated with MYB proto-oncogene like 2 in
acute myeloid leukemia patients (29). Furthermore, miR-30b has been
demonstrated to be associated with cancer therapy. It has been
reported that overexpression miR-30b may improve p53 gene therapy
for laryngeal carcinoma (30). In
breast cancer cells, trastuzumab produces therapeutic actions by
upregulating miR-30b and miR-26a (31). These findings suggest that miR-30b
is closely associated with tumorigenesis, where it acts as an
oncogene or anti-oncogene.
The underlying mechanism of miR-30b in RCC requires
further elucidation. The present study demonstrated its role as an
oncogene in RCC. The overexpression may promote RCC cell
proliferation, migration, invasion and inhibit cell apoptosis.
Whilst the downregulation of miR-30b could inhibit RCC cell
proliferation, migration, invasion and promote cell apoptosis.
Thus, miR-30b has the potential to be an effective biomarker of
RCC.
In conclusion, the current study is the first to
describe miR-30b as an oncogene within RCC, in addition to
identifying that miR-30b could regulate cell proliferation,
migration, invasion and apoptosis. Further research should focus on
the underlying mechanism of miR-30b in RCC, and on investigating
the possible use of miR-30 as a biomarker for RCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101922), the
Science and Technology Development Fund Project of Shenzhen (grant
nos. JCYJ20130402114702124 and JCYJ20150403091443329) and the Fund
of Guangdong Key Medical Subject.
References
|
1
|
Laitinen M, Parry M, Ratasvuori M, Wedin
R, Albergo JI, Jeys L, Abudu A, Carter S, Gaston L, Tillman R and
Grimer R: Survival and complications of skeletal reconstructions
after surgical treatment of bony metastatic renal cell carcinoma.
EJSO the Journal Cancer Surgery. 41:886–892. 2015.
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rasmussen F: Metastatic renal cell cancer.
Cancer Imaging. 13:374–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rouvière O, Bouvier R, Négrier S, Badet L
and Lyonnet D: Nonmetastatic renal-cell carcinoma: Is it really
possible to define rational guidelines for post-treatment
follow-up? Nat Clin Pract Oncol. 3:200–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bianchi M, Sun M, Jeldres C, Shariat SF,
Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P,
et al: Distribution of metastatic sites in renal cell carcinoma: A
population-based analysis. Ann Oncol. 23:973–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, Zhang W, Yang J, Liu YL, Yan ZX,
Guo ZJ, Li YJ and Bian XW: High ERα36 Expression level and membrane
location predict poor prognosis in renal cell carcinoma. Medicine
(Baltimore). 94:e10482015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang HM, Yang FQ, Chen SJ, Che J and
Zheng JH: Upregulation of long non-coding RNA MALAT1 correlates
with tumor progression and poor prognosis in clear cell renal cell
carcinoma. Tumour Biol. 36:2947–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schaefer A, Jung M, Kristiansen G, Lein M,
Schrader M, Miller K, Stephan C and Jung K: MicroRNAs and cancer:
Current state and future perspectives in urologic oncology. Urol
Oncol. 28:4–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mu YP, Tang S, Sun WJ, Gao WM, Wang M and
Su XL: Association of miR-193b down-regulation and mir-196a
up-regulation with clinicopathological features and prognosis in
gastric Cancer. Asian Pac J Cancer Prev. 15:8893–9000. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao C, Yu Y, Yu L, Pei Y, Feng Q, Chu F,
Fang Z and Zhou Y: Amplification and up-regulation of microRNA-30b
in oral squamous cell cancers. Arch Oral Biol. 57:1012–1017. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu YC, Chang JT, Huang YC, Huang CC, Chen
WH, Lee LY, Huang BS, Chen YJ, Li HF and Cheng AJ: Combined
determination of circulating miR-196a and miR-196b levels produces
high sensitivity and specificity for early detection of oral
cancer. Clin Biochem. 48:115–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiao F, Zhang K, Gong P, Wang L, Hu J, Lu
S and Fan H: Decreased miR-30b-5p expression by DNMT1 methylation
regulation involved in gastric cancer metastasis. Mol Biol Rep.
41:5693–5700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Li P, Yang R, Cheng R, Zhang F,
Wang Y, Chen X, Sun Q, Zang W, Du Y, et al: microRNA-30b inhibits
cell invasion and migration through targeting collagen triple helix
repeat containing 1 in non-small cell lung cancer. Cancer Cell Int.
15:852015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao H, Xu Z, Qin H, Gao Z and Gao L:
miR-30b regulates migration and invasion of human colorectal cancer
via SIX1. Biochem J. 460:117–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu ED, Li N, Li BS, Li W, Zhang WJ, Mao
XH, Guo G, Zou QM and Xiao B: miR-30b, down-regulated in gastric
cancer, promotes apoptosis and suppresses tumor growth by targeting
plasminogen activator inhibitor-1. PLoS One. 9:e1060492014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ge YZ, Wu R, Xin H, Zhu M, Lu TZ, Liu H,
Xu Z, Yu P, Zhao YC, Li MH, et al: A tumor-specific microRNA
signature predicts survival in clear cell renal cell carcinoma. J
Cancer Res Clin Oncol. 141:1291–1299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wulfken LM, Moritz R, Ohlmann C,
Holdenrieder S, Jung V, Becker F, Herrmann E, Walgenbach-Brünagel
G, von Ruecker A, Müller SC and Ellinger J: MicroRNAs in renal cell
carcinoma: Diagnostic implications of serum miR-1233 levels. PLoS
One. 6:e257872011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang ZX, Li JL, Cao JX, Liu YS, Li D,
Zhang XY, Wang M, Wu M, Xu BL, Liu JL, et al: Cytokine-induced
killer cells in the treatment of patients with renal cell
carcinoma: A pooled meta-analysis. Immunotherapy. 6:787–795. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M, Tang Q, Qiu M, Lang N, Li M, Zheng
Y and Bi F: miR-21 targets the tumor suppressor RhoB and regulates
proliferation, invasion and apoptosis in colorectal cancer cells.
FEBS Lett. 585:2998–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhong K, Chen K, Han L and Li B:
MicroRNA-30b/c inhibits non-small cell lung cancer cell
proliferation by targeting Rab18. BMC Cancer. 14:7032014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian SB, Yu JC, Liu YQ, Kang WM, Ma ZQ, Ye
X and Yan C: MiR-30b suppresses tumor migration and invasion by
targeting EIF5A2 in gastric cancer. World J Gastroenterol.
21:9337–9347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahdavinezhad A, Mousavi-Bahar SH,
Poorolajal J, Yadegarazari R, Jafari M, Shabab N and Saidijam M:
Evaluation of miR-141, miR-200c, miR-30b Expression and
Clinicopathological Features of Bladder Cancer. Int J Mol Cell Med.
4:32–39. 2015.PubMed/NCBI
|
|
24
|
Lu Y, Ryan SL, Elliott DJ, Bignell GR,
Futreal PA, Ellison DW, Bailey S and Clifford SC: Amplification and
overexpression of Hsa-miR-30b, Hsa-miR-30d and KHDRBS3 at
8q24.22-q24.23 in medulloblastoma. PLoS One. 4:e61592009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heinzelmann J, Henning B, Sanjmyatav J,
Posorski N, Steiner T, Wunderlich H, Gajda MR and Junker K:
Specific miRNA signatures are associated with metastasis and poor
prognosis in clear cell renal cell carcinoma. World J Urol.
29:367–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu YF, Zhang H, Su D, Mo ML, Song P, Zhang
F and Zhang SC: miR-30b and miR-30c expression predicted response
to tyrosine kinase inhibitors as first line treatment in non-small
cell lung cancer. Chin Med J (Engl). 126:4435–4439. 2013.PubMed/NCBI
|
|
27
|
Liao WT, Ye YP, Zhang NJ, Li TT, Wang SY,
Cui YM, Qi L, Wu P, Jiao HL, Xie YJ, et al: MicroRNA-30b functions
as a tumour suppressor in human colorectal cancer by targeting
KRAS, PIK3CD and BCL2. J Pathol. 232:415–427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bridge G, Monteiro R, Henderson S, Emuss
V, Lagos D, Georgopoulou D, Patient R and Boshoff C: The
microRNA-30 family targets DLL4 to modulate endothelial cell
behavior during angiogenesis. Blood. 120:5063–5072. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fuster O, Llop M, Dolz S, García P, Such
E, Ibáñez M, Luna I, Gómez I, López M, Cervera J, et al: Adverse
prognostic value of MYBL2 overexpression and association with
microRNA-30 family in acute myeloid leukemia patients. Leuk Res.
37:1690–1696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L and Wang B: Overexpression of
microRNA-30b improves adenovirus-mediated p53 cancer gene therapy
for laryngeal carcinoma. Int J Mol Sci. 15:19729–19740. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ichikawa T, Sato F, Terasawa K, Tsuchiya
S, Toi M, Tsujimoto G and Shimizu K: Trastuzumab produces
therapeutic actions by upregulating miR-26a and miR-30b in breast
cancer cells. PLoS One. 7:e314222012. View Article : Google Scholar : PubMed/NCBI
|