Introduction
Rho GDP-dissociation inhibitors (RhoGDIs) are
regulators of the Rho family of proteins and function as molecular
switches in numerous cellular processes, including actin
cytoskeletal organization, microtubule dynamics, vesicle
trafficking, cell polarity and cell cycle progression (1). A total of three RhoGDIs
(RhoGDIα/RhoGDI1, RhoGDIβ/RhoGDI2/LyGDI/D4GDI and RhoGDIγ/RhoGDI3)
have been identified in mammals (2). RhoGDIβ is expressed abundantly in
hematopoietic cells (3,4) and is also expressed in
non-hematopoietic cells, including keratinocytes, fibroblasts and
amnion cells (5),
non-hematopoietic tumors (6–8) and
various cancer cells (9).
During apoptosis of hematopoietic cells, RhoGDIβ is
cleaved by caspase-3 at Asp19 (10–16),
and the cleaved form, Δ19-RhoGDIβ, translocates to the nucleus
(12,15–17),
suggesting the pro-apoptotic role of Δ19-RhoGDIβ. Therefore, the
cleavage of RhoGDIβ by caspase-3 has been implicated in the
apoptotic pathway, however, the precise role of Δ19-RhoGDIβ remains
to be elucidated. Caspase-3 is known to have apoptosis-independent
roles in the differentiation and proliferation of various cell
types, including hematopoietic cells (18). In the differentiation of monocytes
into macrophages, caspase-3 and caspase-9 are activated, and the
inhibition of these caspases prevents this differentiation
(19). Furthermore, phorbol
12-myristate 13-acetate (PMA)-stimulated THP-1 cells express
increased levels of caspase-3 (20). However, whether RhoGDIβ is cleaved
at Asp19 during the differentiation of these cells remains to be
elucidated.
In the present study, to clarify the role of
Δ19-RhoGDIβ in the differentiation of macrophages, the expression
of RhoGDIβ and Δ19-RhoGDIβ were examined during PMA-stimulated
differentiation of human THP-1 monocytic cells to macrophages. The
results confirmed that RhoGDIβ was cleaved at Asp19 and it was
shown that this cleaved form was expressed in non-apoptotic cells.
These results suggested the apoptosis-independent role of
Δ19-RhoGDIβ during the differentiation of THP-1 cells to
macrophages.
Materials and methods
Cell culture and induction of
differentiation
The THP-1 human myelomonocytic cell line was
provided by Dr Masaharu Wano, Department of Hematology and
Immunology, Kanazawa Medical University (Uchinada, Japan). The
cells were cultured in RPMI 1640 medium containing 2 mM L-glutamine
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) supplemented
with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), and were maintained at 37°C in a
humidified atmosphere of 5% CO2 in air. The
differentiation of THP-1 cells into macrophages was achieved using
the method described by Daigneault et al (21). Briefly, THP-1 cells
(~1.5×105/ml) were cultured with 200 nM PMA
(Sigma-Aldrich; Merck Millipore) for 3 days at 37°C, the
PMA-containing media was removed and the cells were incubated for a
further 5 days. For counting the total cell number, the culture
medium containing floating cells was removed and reserved, and the
attached cells were detached using 0.25% Trypsin/0.01% EDTA in
phosphate-buffered saline (PBS), following which they were
suspended with the previously reserved medium containing the
floating cells. The cell numbers were then counted using a
hemocytometer. The relative numbers of flattened cells on the dish
were observed under a phase-contrast microscope. The proportion of
flattened cells was estimated as it was difficult to distinguish
between flattened and unflattened cells precisely.
Antibodies
Anti-RhoGDIβ antibody (cat. no. sc-6047) raised
against amino acid residues 175–194 was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). This antibody recognizes
full-length and Δ19-RhoGDIβ. Anti-Δ19-RhoGDIβ antibody (clone
97A1015; cat. no. 14-6628-81) raised against the caspase-3 cleavage
site of human RhoGDIβ was purchased from eBioscience, Inc. (San
Diego, CA, USA). Anti-a-tubulin antibody (clone B-5-1-2; cat. no.
T6074) was purchased from Sigma-Aldrich; Merck Millipore.
Peroxidase-conjugated anti-mouse (cat. no; K4001) and anti-rabbit
IgG antibodies (cat. no. K4002) were purchased from DakoCytomation
(Glostrup, Denmark). Peroxidase-conjugated anti-goat IgG antibody
(cat. no; 414351) was purchased from the Nichirei Corporation
(Tokyo, Japan). Alexa Fluor 594-conjugated goat anti-mouse IgG
(H+L; cat. no. A-11032) was purchased from Invitrogen; Thermo
Fisher Scientific, Inc.
Immunoblotting
The cells not exposed to PMA (untreated cells) were
found not to attach to the culture dish, whereas >95% of the
PMA-stimulated cells attached. When the cell lysates of the
PMA-stimulated cells were prepared, floating cells and cell debris
were removed to avoid contamination by the dead cells. The cells
were lysed using Laemmli buffer containing 4% sodium dodecyl
sulfate (SDS), 20% glycerol, 10% 2-mercaptoethanol, 0.004%
bromophenol blue and 0.0125 M Tris-HCl (pH 6.8), and the protein
concentrations of the lysate were measured using a Bradford Ultra
kit (Novexin, Ltd., Cambridge, UK). The proteins (10 µg) were
resolved by SDS-polyacrylamide gel electrophoresis and transferred
onto Immobilon-P membranes (EMD Millipore, Billerica, MA, USA). The
membranes were then probed with a primary antibody (sc-6047,
1:10,000 dilution; clone 97A1015, 1:10,000 dilution; clone B-5-1-2,
1:100,000 dilution) overnight at 4°C, followed by incubation with a
peroxidase-conjugated secondary antibody (1:500 dilution) for 90
min at room temperature. The immunoreactive proteins were
visualized using ECL Prime reagents (GE Healthcare Life Sciences,
Ltd., Little Chalfont, UK). The same quantity of protein was
applied in all immunoblot experiments.
Annexin V and immunofluorescence
staining
The cells were grown in 35-mm culture dishes. To
remove dead and apoptotic cells, the dishes were washed twice with
PBS at 4°C. The cells were stained with the Annexin V-FITC
Apoptosis Detection kit I (BD Biosciences, San Jose, CA, USA)
according to the manufacturer's protocol, and fixed with freshly
prepared 3.7% paraformaldehyde in Annexin V binding buffer
containing 140 mM NaCl, 2.5 mM CaCl2 and 10 mM HEPES (pH
7.5) for 30 min at room temperature. The cells were then
permeabilized with 0.5% Triton X-100 for 5 min at room temperature.
Following washing with PBS, the cells were incubated with 0.5%
bovine serum albumin (BSA) in PBS for 60 min at room temperature,
and then incubated overnight at 4°C with anti-Δ19-RhoGDIβ antibody
(clone 97A1015) diluted 1:400 in PBS containing 0.5% BSA. Following
three washes with PBS, the cells were incubated for 60 min at room
temperature with a secondary antibody (A-11032), diluted 1:400 in
PBS containing 0.5% BSA and 0.1 µg/ml
4′,6-diamidino-2-phenylindole. Following three washes with PBS, the
cells were mounted with ProLong Gold (Invitrogen; Thermo Fisher
Scientific, Inc.). Images were captured using an Axiovert 200
inverted fluorescence microscope (Plan Neofluar 40X/0.75 NA
objective lens) with AxioVision 4.4 software (Carl Zeiss AG, Jena,
Germany). Images of the green, red and blue channels were captured
using a 38HE bandpass filter (excitation, 450–490 nm; emission,
500–550 nm), a 43HE bandpass filter (excitation, 537–563 nm;
emission 570–640 nm) and a 49 bandpass filter (excitation, G 365
nm; Emission, 420–470 nm), respectively.
Results
Induction of THP-1 cell
differentiation into macrophages
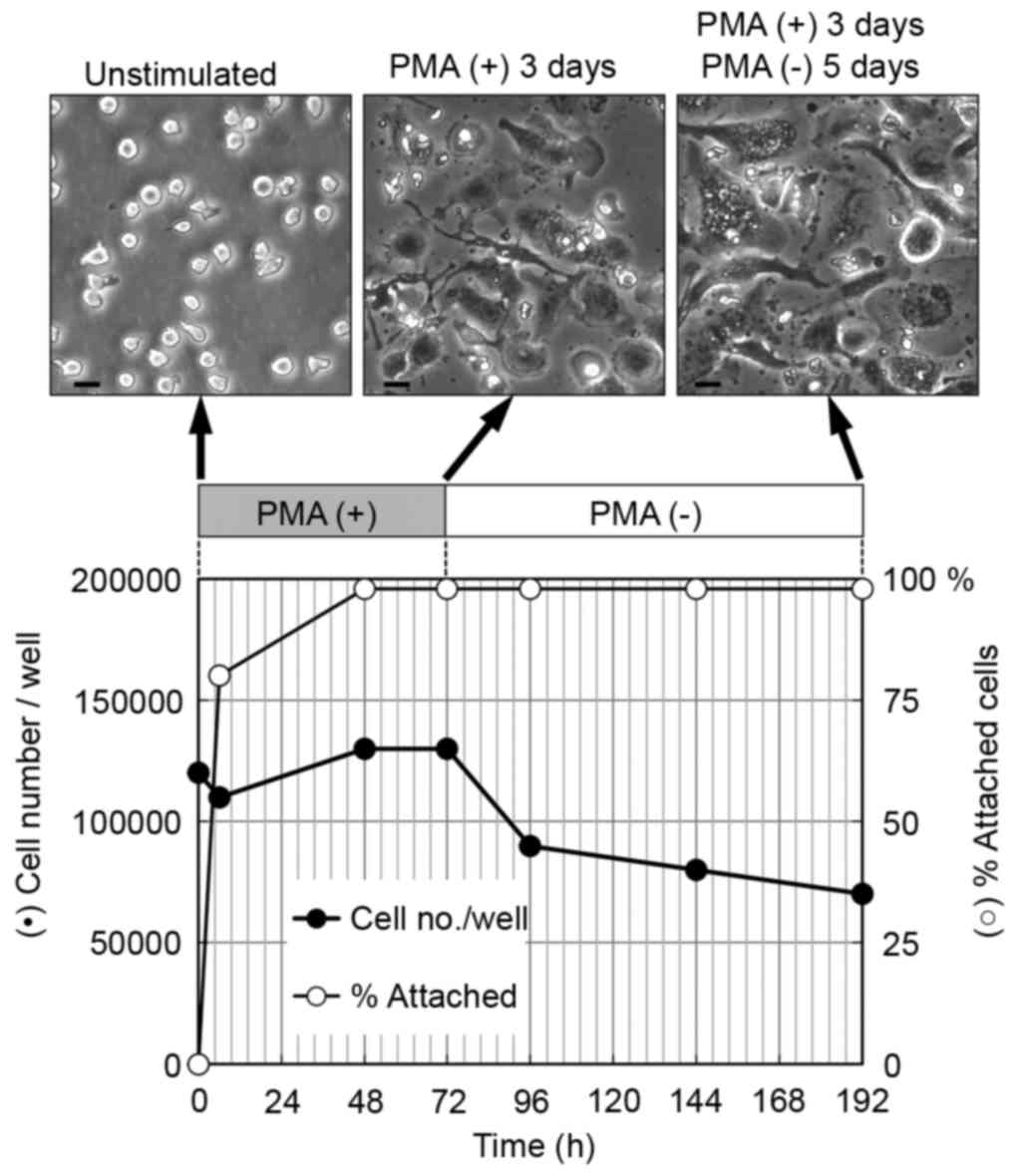
The THP-1 cells were cultured with 200 nM PMA for 3
days, following which the PMA-containing media was removed and the
cells were then incubated for a further 5 days (Fig. 1). Cell proliferation was suppressed
and the proportion of attached cells increased considerably within
2 days. In addition >90% of the attached cells were flattened 2
days following treatment with PMA. The differentiation of THP-1
cells to macrophages is known to be accompanied by a loss of
proliferation (22); furthermore,
cell adhesion and spreading are used as functional indicators of
macrophage differentiation (21,23).
The results obtained indicated that the majority of THP-1 cells
were induced to differentiate into macrophages.
The cells cultured for 5 days following PMA removal
showed a higher degree of flattened morphology, compared with the
cells cultured for 3 days with PMA (Fig. 1, top panel). It has been reported
that cultures rested for 5 days in PMA-free media adopt a
phenotype, which more closely resembles human monocyte-derived
macrophages, compared with differentiated THP-1 cells cultured for
only 3 days with PMA (21). The
flattened morphology observed in the present study of the cells
cultured 5 days following PMA removal is likely to reflect the
increased degree of differentiation of these cells.
Expression of Δ19-RhoGDI in THP-1
cells differentiated into macrophages
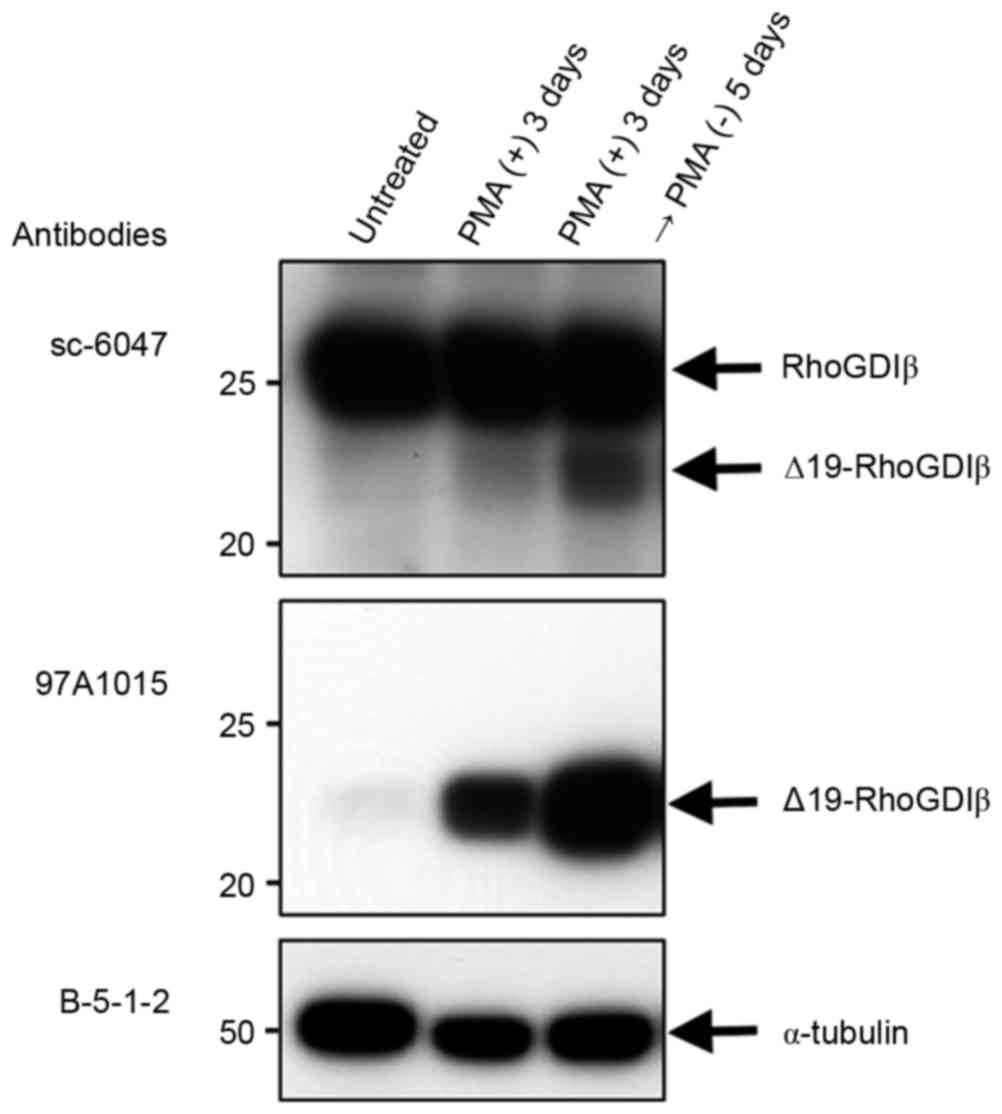
The present study used immunoblotting to examine the
expression of RhoGDIβ and its cleaved form at Asp19 during the
PMA-stimulated differentiation of THP-1 cells to macrophages.
Following treatment with PMA, the expression of full-length RhoGDIβ
remained unchanged, whereas the expression of Δ19-RhoGDIβ increased
and was correlated with the differentiation of the cells into
macrophages, although only a small fraction of full-length RhoGDIβ
was cleaved at Asp19 (Fig. 2, top
panel). The increased expression of Δ19-RhoGDIβ was confirmed using
anti-Δ19-RhoGDIβ antibody (97A1015; Fig. 2, middle panel). The expression
level of Δ19-RhoGDIβ was greater in cells cultured 5 days following
PMA removal, compared with cells cultured for 3 days only with PMA.
Therefore, the expression level of Δ19-RhoGDIβ appeared to
correlate with the degree of differentiation into macrophages. To
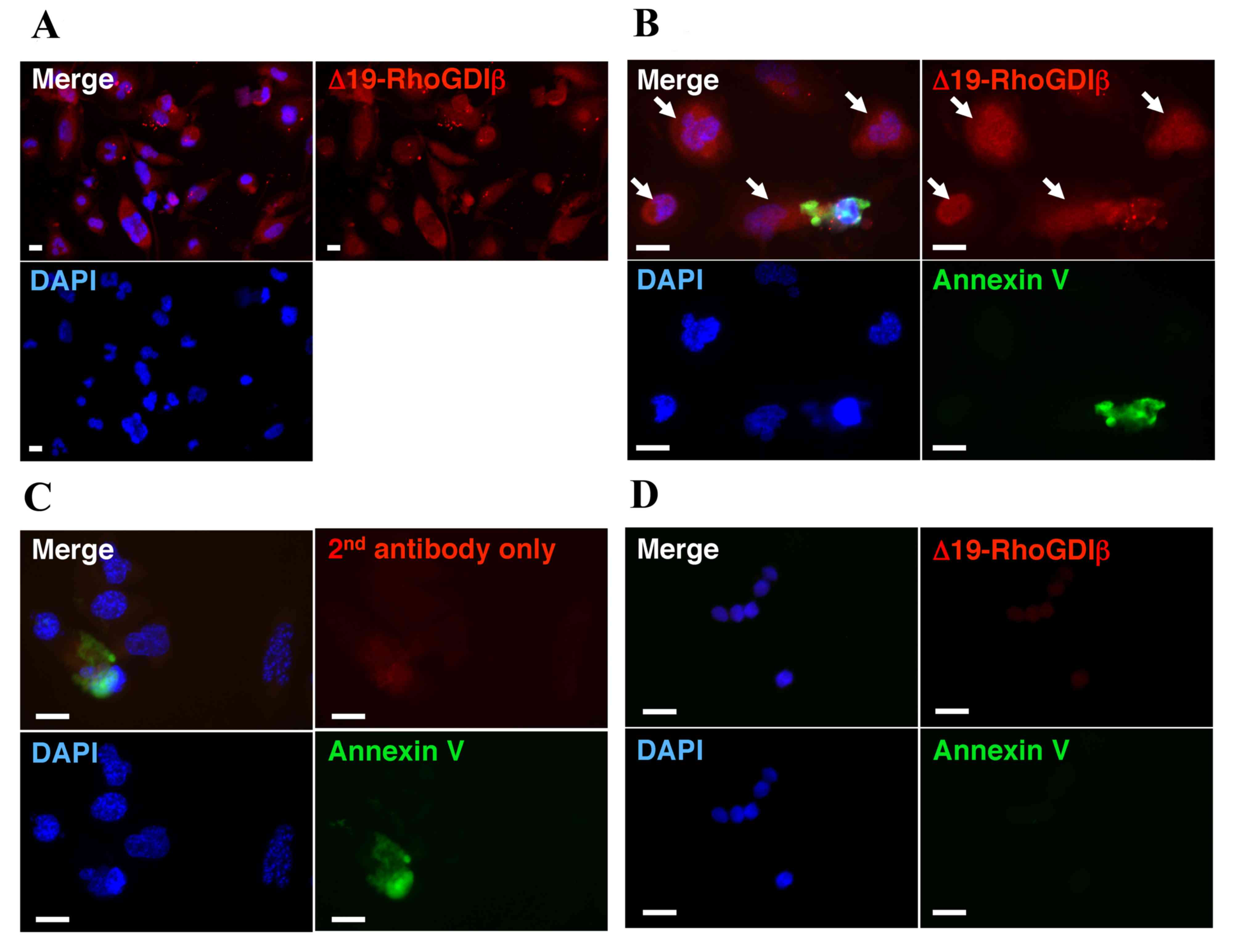
confirm the expression of Δ19-RhoGDIβ in macrophage-differentiated
THP-1 cells, the differentiated cells were stained with
anti-Δ19-RhoGDIβ antibody. Δ19-RhoGDIβ was detected in the
macrophage-differentiated THP-1 cells (Fig. 3A).
During the differentiation of THP-1 cells to
macrophages, the total cell number gradually decreased (Fig. 1). As low levels of apoptotic cell
death are reported to occur in macrophage-differentiated THP-1
cells in the absence of apoptotic stimuli (24), the decrease in cell number observed
in the present study was likely due to apoptotic cell death. To
exclude the possibility that the detected levels of Δ19-RhoGDIβ in
the differentiated cells were derived from apoptotic cells, the
differentiated cells attached to the culture dish were stained with
Annexin V and anti-Δ19-RhoGDIβ antibody (Fig. 3B). The unattached dead and
apoptotic cells were almost completely removed by washing with PBS
prior to staining. The proportion of cells stained with Annexin V
was <1%. The observed Annexin V-positive cells are shown in
Fig. 3B and C. Δ19-RhoGDIβ was
detected in the apoptotic cells and macrophage-differentiated THP-1
cells, and the majority of the expression of Δ19-RhoGDIβ was
observed in the non-apoptotic cells, which differentiated into
macrophages.
Discussion
During the apoptosis of hematopoietic cells, RhoGDIβ
is cleaved by caspase-3 at Asp19 (10–16),
and the cleaved form, Δ19-RhoGDIβ, has been suggested to have a
pro-apoptotic function in K562 leukemia cells (17). In the present study, it was shown
that, during the PMA-stimulated differentiation of THP-1 cells to
macrophages, RhoGDIβ was also cleaved at Asp19 in non-apoptotic
differentiating cells. Although it is unknown whether Δ19-RhoGDIβ
is involved in the differentiation process or the differentiation
phenotype, these results suggested that Δ19-RhoGDIβ is involved in
cellular processes other than apoptosis, at least in THP-1 cells.
Δ19-RhoGDIβ may have different roles depending on the cellular
context.
RhoGDIβ is implicated in cancer progression;
however, the correlation between malignancy and the expression
level of RhoGDIβ is contradictory (25). It has been previously reported that
RhoGDIβ may have a positive (7,26,27)
and negative (28) role in cancer
progression. Caspase-3 is considered to be involved in cancer
susceptibility in squamous-cell carcinomas of the head and neck
(29), endometrial cancer
(30) and lung cancer (31), therefore, RhoGDIβ may be cleaved by
casapse-3 in various cancer cells. The inconsistent correlation of
RhoGDIβ with malignancy may be a reflection of the different
functions of Δ19-RhoGDIβ in different types of cancer.
The function of Δ19-RhoGDIβ remains to be
elucidated, and cleavage of RhoGDIβ at Asp19 is unlikely to result
in the inactivation of RhoGDIβ. Δ22-RhoGDIα (32,33)
and Δ20-RhoGDIα (34) have been
reported to be almost fully functional for extracting GTPases from
the membrane, although the inhibition of GTPase activity by
Δ20-RhoGDIα is less effective, compared with that by wild-type
RhoGDIα (34). The amino acid
sequence and protein structure are similar between RhoGDIα and
RhoGDIβ; therefore, Δ19-RhoGDIβ is expected to retain certain
regulatory functions of full-length RhoGDIβ. Our previous study
reported that RhoGDIs may act as a positive and negative regulator
of Rho GTPases, depending on their expression level, and their
affinity for guanine nucleotide exchange factors (GEFs) and
GTPase-activating proteins (GAPs) (35). The cleavage of RhoGDIβ at Asp19 may
modify the regulatory function of RhoGDIβ towards Rho GTPases by
altering the affinity of RhoGDIβ to GEFs and GAPs.
In conclusion, the present study showed that
Δ19-RhoGDIβ was expressed in non-apoptotic, differentiating cells,
thereby suggesting an apoptosis-independent role for this protein.
The identification of this novel function of Δ19-RhoGDIβ may assist
in further elucidating the regulatory system of Rho GTPases, which
is involved in the regulation of numerous cellular processes.
Acknowledgements
Mr. Mamoru Fujiwara and Dr Masaaki Tatsuka
(Department of Life Sciences, Life and Environmental Sciences,
Prefectural University of Hiroshima, Shoubara, Japan) were
supported by grants from the Prefectural University of Hiroshima
Important Research Project; Interdisciplinary/Priority Research
(grant nos. (S) H-26 and (S) H-27).
Glossary
Abbreviations
Abbreviations:
|
BSA
|
bovine serum albumin
|
|
GAP
|
GTPase-activating protein
|
|
GEF
|
guanine nucleotide exchange factor
|
|
PBS
|
phosphate-buffered saline
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
|
RhoGDI
|
Rho GDP-dissociation inhibitor
|
|
SDS
|
sodium dodecyl sulphate
|
References
|
1
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dovas A and Couchman JR: RhoGDI: Multiple
functions in the regulation of Rho family GTPase activities.
Biochem J. 390:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lelias JM, Adra CN, Wulf GM, Guillemot JC,
Khagad M, Caput D and Lim B: cDNA cloning of a human mRNA
preferentially expressed in hematopoietic cells and with homology
to a GDP-dissociation inhibitor for the rho GTP- binding proteins.
Proc Natl Acad Sci USA. 90:1479–1483. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scherle P, Behrens T and Staudt LM:
Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding
protein, is expressed preferentially in lymphocytes. Proc Natl Acad
Sci USA. 90:7568–7572. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leffers H, Nielsen MS, Andersen AH, Honoré
B, Madsen P, Vandekerckhove J and Celis JE: Identification of two
human Rho GDP dissociation inhibitor proteins whose overexpression
leads to disruption of the actin cytoskeleton. Exp Cell Res.
209:165–174. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gildea JJ, Seraj MJ, Oxford G, Harding MA,
Hampton GM, Moskaluk CA, Frierson HF, Conaway MR and Theodorescu D:
RhoGDI2 is an invasion and metastasis suppressor gene in human
cancer. Cancer Res. 62:6418–6423. 2002.PubMed/NCBI
|
|
7
|
Ota T, Maeda M, Suto S and Tatsuka M:
LyGDI functions in cancer metastasis by anchoring Rho proteins to
the cell membrane. Mol Carcinog. 39:206–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seraj MJ, Harding MA, Gildea JJ, Welch DR
and Theodorescu D: The relationship of BRMS1 and RhoGDI2 gene
expression to metastatic potential in lineage related human bladder
cancer cell lines. Clin Exp Metastasis. 18:519–525. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harding MA and Theodorescu D: RhoGDI
signaling provides targets for cancer therapy. Eur J Cancer.
46:1525–1559. 2010. View Article : Google Scholar
|
|
10
|
Essmann F, Wieder T, Otto A, Müller EC,
Dörken B and Daniel PT: GDP dissociation inhibitor D4-GDI (Rho-GDI
2), but not the homologous rho-GDI 1, is cleaved by caspase-3
during drug-induced apoptosis. Biochem J 346 Pt. 3:777–783. 2000.
View Article : Google Scholar
|
|
11
|
Kettritz R, Xu YX, Faass B, Klein JB,
Müller EC, Otto A, Busjahn A, Luft FC and Haller H:
TNF-alpha-mediated neutrophil apoptosis involves Ly-GDI, a Rho
GTPase regulator. J Leukoc Biol. 68:277–283. 2000.PubMed/NCBI
|
|
12
|
Krieser RJ and Eastman A: Cleavage and
nuclear translocation of the caspase 3 substrate Rho
GDP-dissociation inhibitor, D4-GDI, during apoptosis. Cell Death
Differ. 6:412–419. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Na S, Chuang TH, Cunningham A, Turi TG,
Hanke JH, Bokoch GM and Danley DE: D4-GDI, a substrate of CPP32, is
proteolyzed during Fas-induced apoptosis. J Biol Chem.
271:11209–11213. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rickers A, Brockstedt E, Mapara MY, Otto
A, Dörken B and Bommert K: Inhibition of CPP32 blocks surface
IgM-mediated apoptosis and D4-GDI cleavage in human BL60 Burkitt
lymphoma cells. Eur J Immunol. 28:296–304. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiede B, Siejak F, Dimmler C and Rudel T:
Prediction of translocation and cleavage of heterogeneous
ribonuclear proteins and Rho guanine nucleotide dissociation
inhibitor 2 during apoptosis by subcellular proteome analysis.
Proteomics. 2:996–1006. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Suto S, Ota T and Tatsuka M:
Nuclear Translocation of Cleaved LyGDI dissociated from Rho and Rac
during Trp53-dependent ionizing radiation-induced apoptosis of
thymus cells in vitro. Radiat Res. 162:287–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi MR, Groot M and Drexler HC:
Functional implications of caspase-mediated RhoGDI2 processing
during apoptosis of HL60 and K562 leukemia cells. Apoptosis.
12:2025–2035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sordet O, Rébè C, Plenchette S, Zermati Y,
Hermine O, Vainchenker W, Garrido C, Solary E and Dubrez-Daloz L:
Specific involvement of caspases in the differentiation of
monocytes into macrophages. Blood. 100:4446–4453. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao HS, Matsumoto A, Itakura H, Pittman
T, Kodama T and Geng YJ: De novo expression of the class-A
macrophage scavenger receptor conferring resistance to apoptosis in
differentiated human THP-1 monocytic cells. Cell Death Differ.
6:245–255. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daigneault M, Preston JA, Marriott HM,
Whyte MK and Dockrell DH: The Identification of Markers of
Macrophage differentiation in PMA-stimulated THP-1 cells and
monocyte-derived macrophages. PLoS One. 5:e86682010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwende H, Fitzke E, Ambs P and Dieter P:
Differences in the state of differentiation of THP-1 cells induced
by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol.
59:555–561. 1996.PubMed/NCBI
|
|
23
|
Auwerx J: The human leukemia cell line,
THP-1: A multifacetted model for the study of monocyte-macrophage
differentiation. Experientia. 47:22–31. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vicca S, Hennequin C, Nguyen-Khoa T, Massy
ZA, Descamps-Latscha B, Drueke TB and Lacour B: Caspase-dependent
apoptosis in THP-1 cells exposed to oxidized low-density
lipoproteins. Biochem Biophys Res Commun. 273:948–954. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Griner EM and Theodorescu D: The faces and
friends of RhoGDI2. Cancer Metastasis Rev. 31:519–528. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang YS, Maeda M, Okamoto M, Fujii M,
Fukutomi R, Hori M, Tatsuka M and Ota T: Centrosomal localization
of RhoGDIβ and its relevance to mitotic processes in cancer cells.
Int J Oncol. 42:460–468. 2013.PubMed/NCBI
|
|
27
|
Ota T, Maeda M, Murakami M, Takegami T,
Suto S and Tatsuka M: Activation of Rac1 by Rho-guanine nucleotide
dissociation inhibitor-beta with defective isoprenyl-binding
pocket. Cell Biol Int. 31:92–96. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ota T, Maeda M, Sakita-Suto S, Zhou X,
Murakami M, Takegami T and Tatsuka M: RhoGDIbeta lacking the
N-terminal regulatory domain suppresses metastasis by promoting
anoikis in v-src-transformed cells. Clin Exp Metastasis.
23:323–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen K, Zhao H, Hu Z, Wang LE, Zhang W,
Sturgis EM and Wei Q: CASP3 polymorphisms and risk of squamous cell
carcinoma of the head and neck. Clin Cancer Res. 14:6343–6349.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu HL, Xu WH, Cai Q, Feng M, Long J, Zheng
W, Xiang YB and Shu XO: Polymorphisms and haplotypes in the
caspase-3, caspase-7, and caspase-8 genes and risk for endometrial
cancer: A population-based, case-control study in a Chinese
population. Cancer Epidemiol Biomarkers Prev. 18:2114–2122. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jang JS, Kim KM, Choi JE, Cha SI, Kim CH,
Lee WK, Kam S, Jung TH and Park JY: Identification of polymorphisms
in the Caspase-3 gene and their association with lung cancer risk.
Mol Carcinog. 47:383–390. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gosser YQ, Nomanbhoy TK, Aghazadeh B,
Manor D, Combs C, Cerione RA and Rosen MK: C-terminal binding
domain of Rho GDP-dissociation inhibitor directs N-terminal
inhibitory peptide to GTPases. Nature. 387:814–819. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Platko JV, Leonard DA, Adra CN, Shaw RJ,
Cerione RA and Lim B: A single residue can modify target-binding
affinity and activity of the functional domain of the Rho-subfamily
GDP dissociation inhibitors. Proc Natl Acad Sci USA. 92:2974–2978.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Golovanov AP, Chuang TH, DerMardirossian
C, Barsukov I, Hawkins D, Badii R, Bokoch GM, Lian LY and Roberts
GC: Structure-activity relationships in flexible protein domains:
Regulation of rho GTPases by RhoGDI and D4 GDI. J Mol Biol.
305:121–135. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ota T, Maeda M, Okamoto M and Tatsuka M:
Positive regulation of Rho GTPase activity by RhoGDIs as a result
of their direct interaction with GAPs. BMC Syst Biol. 9:32015.
View Article : Google Scholar : PubMed/NCBI
|