Introduction
Proliferative vitreoretinopathy (PVR), a retinal
damage repair process, is a common cause of severe visual
impairment or blindness (1).
Retinal pigment epithelial (RPE) cells are a primary component of
PVR membranes, and abnormal RPE cell migration and proliferation
contributes to PVR (2). The
pathological basis for PVR is the destruction of the blood-retinal
barrier, which causes the subretinal RPE cells to directly contact
the vitreous. RPE cells lose their connection to the RPE
extracellular matrix by an unknown underlying mechanism. The cells
subsequently undergo epithelial-mesenchymal transition, localize to
the vitreous, proliferate and organize into an epiretinal membrane.
Contraction of the membrane may cause retinal detachment (3,4).
During PVR, components of the extracellular matrix, including
fibronectin (FN), collagen and laminin, appear in the epiretinal
membrane; however, the importance of this is unclear. FN is a
glycoprotein that is crucial for promoting cell growth and
adhesion, maintaining cell structure, and wound repair and healing
(5,6). Subretinal and epiretinal membranes
exhibit high levels of FN (7,8).
Chloride channels (ClCs) are widely distributed
throughout mammalian organs and serve important roles in
maintaining cell volume and regulating cell activity,
proliferation, differentiation and division (9). ClC activity determines whether cells
enter the S or G0 phase (10–12).
In addition, ClC-2 and −3 are involved in morphological
alterations, which are closely associated with cell migration and
invasion. Numerous ClCs have been cloned, including ClC-1 to −7,
ClC-ka and ClC-kb. It has been demonstrated that ClCs are expressed
in RPE cells (13,14). ClC blockers include
5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) and tamoxifen
(TAM), which may prevent the proliferation and migration of glioma
cells (15–17). Little is known about the
association between ClCs, the proliferation and migration of RPE
cells, and PVR.
ARPE-19 is a well-established adult human RPE cell
line that has been widely used as a model for in vitro RPE
cell research (18,19). The present study cultured ARPE-19
cells with ClC blockers in vitro to investigate the effects
of ClCs on the proliferation and cell cycle of human RPE cells.
Additionally, the present study established an RPE cell model of
phagocytosis, using FN-coated latex beads, to examine the effect of
ClCs on RPE cell migration. These results may provide a basis for
novel studies aimed at the prevention and treatment of PVR.
Materials and methods
RPE cell culture
The ARPE-19 human adult RPE cell line (American Type
Culture Collection, Manassas, VA, USA) was cultured in Dulbecco's
modified Eagle's medium/Nutrient Mixture F-12 (DMEM/F-12; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml
streptomycin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany).
The cells were maintained in a humidified incubator at 37°C and 5%
CO2.
Dose-dependence of RPE cell
viability
NPPB and TAM (Sigma-Aldrich; Merck Millipore) stock
solutions were prepared in dimethyl sulfoxide and serially diluted
with serum-free DMEM/F12 medium. ARPE-19 cells were cultured in the
medium, in the presence or absence of 10, 50 or 100 µM NPPB or 5,
10 or 50 µM TAM, for 48 h and subsequently suspended in
phosphate-buffered saline (PBS) with 0.1% trypan blue dye
(Sigma-Aldrich; Merck Millipore) for 10 min. Staining was measured
by flow cytometry (Coulter EPICS® XL™; Beckman Coulter,
Inc., Brea, CA, USA) and cell viability was calculated under an
inverted light microscope (CH20-BIM; Olympus Corporation, Tokyo,
Japan).
Cell proliferation assay
ARPE-19 cells were seeded into 24 Petri dishes at a
density of 4×104 cells/ml, and incubated for 48 h with NPPB or TAM
as described in the previous section. The cells were digested with
0.25% parenzyme (Gibco; Thermo Fisher Scientific, Inc.). Trypan
blue (1%) was added to 0.9 ml of each cell suspension. Cells were
counted by a blinded observer using a hemocytometer. Mean values
were calculated from three replicates for each group.
Cell cycle determination
ARPE-19 cells were seeded into six Petri dishes at a
density of 4×104 cells/ml in media containing 100 µM NPPB or 50 µM
TAM. After 48 h, the cells were digested with 0.25% parenzyme,
washed three times with PBS, and resuspended in 0.5 ml PBS. Triton
X-100 (0.15%) and RNase (5 mg/ml; Sigma-Aldrich; Merck Millipore)
were added to each suspension. Following incubation for 10 min at
room temperature, DNA was stained with 25 µg/ml propidium iodide
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 10 min at
room temperature. The stained cells were filtered through a nylon
filter membrane (Sigma-Aldrich; Merck Millipore) and DNA was
quantified by flow cytometry at an excitation wavelength of 488 nm.
MULTICYCLE® software version 5.0 (Beckman Coulter, Inc.)
was used to analyze the cell cycle in 10,000 cells per sample.
Establishment of a model for RPE cell
phagocytosis
ARPE-19 cells were cultured for 24 h in 6 Petri
dishes at a density of 2.5×105 cells/ml, to a confluence of 70–95%.
Fluorescent-labeled latex beads (emission wavelength, 515 nm;
diameter, 1.0 µm; Thermo Fisher Scientific, Inc.) were diluted in
PBS to a density of 5×107 latex beads/ml and mixed with FN
(Sigma-Aldrich; Merck Millipore) to a final concentration of 1.0
µg/ml FN. This mixture was incubated at 37°C for 10 min. FN-coated
or uncoated latex beads were added to the cell culture medium in
each well at a density of 5×106, to a final volume of 100 µl. The
cells were incubated at 37°C for 0, 1, 3, 6 or 12 h. Following
this, they were digested and dispersed with 0.25% parenzyme. The
digests were washed three times with PBS to remove the non-adherent
latex beads and were subsequently resuspended in PBS.
Flow cytometry (excitation wavelength, 488 nm;
emission wavelength, 530±15 nm) was used to calculate the
phagocytic activity of 10,000 ARPE-19 cells following treatment
with latex beads. The percentage of cells containing phagocytosed
latex beads and the amount of latex beads that were phagocytosed
was defined as the phagocytic index.
Effect of ClC blockers on RPE cell
phagocytosis of FN
ARPE-19 cells were treated with 10, 50 or 100 µM
NPPB, or 5, 10 or 50 µM TAM for 1 h. These concentrations do not
cause cell death (20–22). Following this, FN-coated or
uncoated latex beads were added into the culture media and
incubated at 37°C for 3 h. The cells were subsequently digested and
dispersed, and the phagocytic index was measured using flow
cytometry.
Measurement of intracellular calcium
concentration
Fura-2-acetoxymethyl ester (AM) was used to measure
intracellular calcium (Ca2+) levels. ARPE-19 cells were incubated
with 10 µmol/l membrane-permeant Fura-2AM in hypotonic saline
solution for 1 h in the dark at room temperature. NPPB (50 or 100
µM) or TAM (50 or 100 µM) was added to the hypotonic solutions to
the final concentration (reported in the Results section) and
fluorescence emission was measured using a RF-5301 PC
Spectrofluorophotometer (Shimadzu Corporation, Kyoto, Japan) at a
wavelength of 510 nm using excitation wavelengths of 340 and 380
nm.
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± standard deviation. One-way
analysis of variance was used to analyze significance, and
comparisons between the groups was made by analyzing data using
one-way analysis of variance and the Student-Newman-Keuls method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of ClC blockers NPPB and TAM on
ARPE-19 cell viability and proliferation
An initial screening assessment was used to
determine the optimal concentrations of NPPB and TAM that were not
cytotoxic to ARPE-19 cells during a 48-h treatment period. In all
groups, the cells demonstrated >95% viability, and no
significant differences were observed between the control and
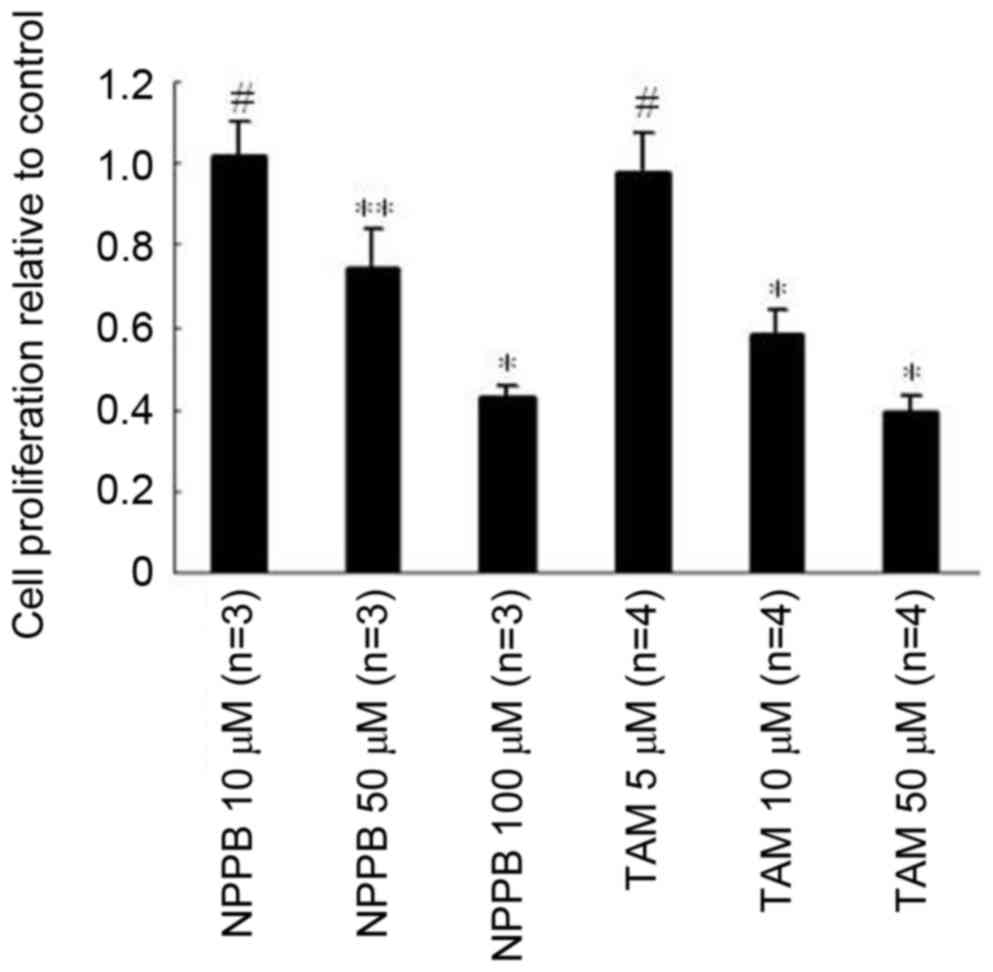
inhibitor groups. The effects of NPPB and TAM on the proliferation
of ARPE-19 cells are presented in Fig.
1. No significant differences were observed in cell
proliferation in the 10 µM NPPB or 5 µm TAM-treated groups,
compared with the control. Treatment with 50 µM NPPB or 10 µM TAM
inhibited cell proliferation by ~20 and 40%, respectively, and
treatment with 100 µM NPPB or 50 µM TAM inhibited proliferation by
~50 or 60%, respectively.
Effect of NPPB and TAM on the cell
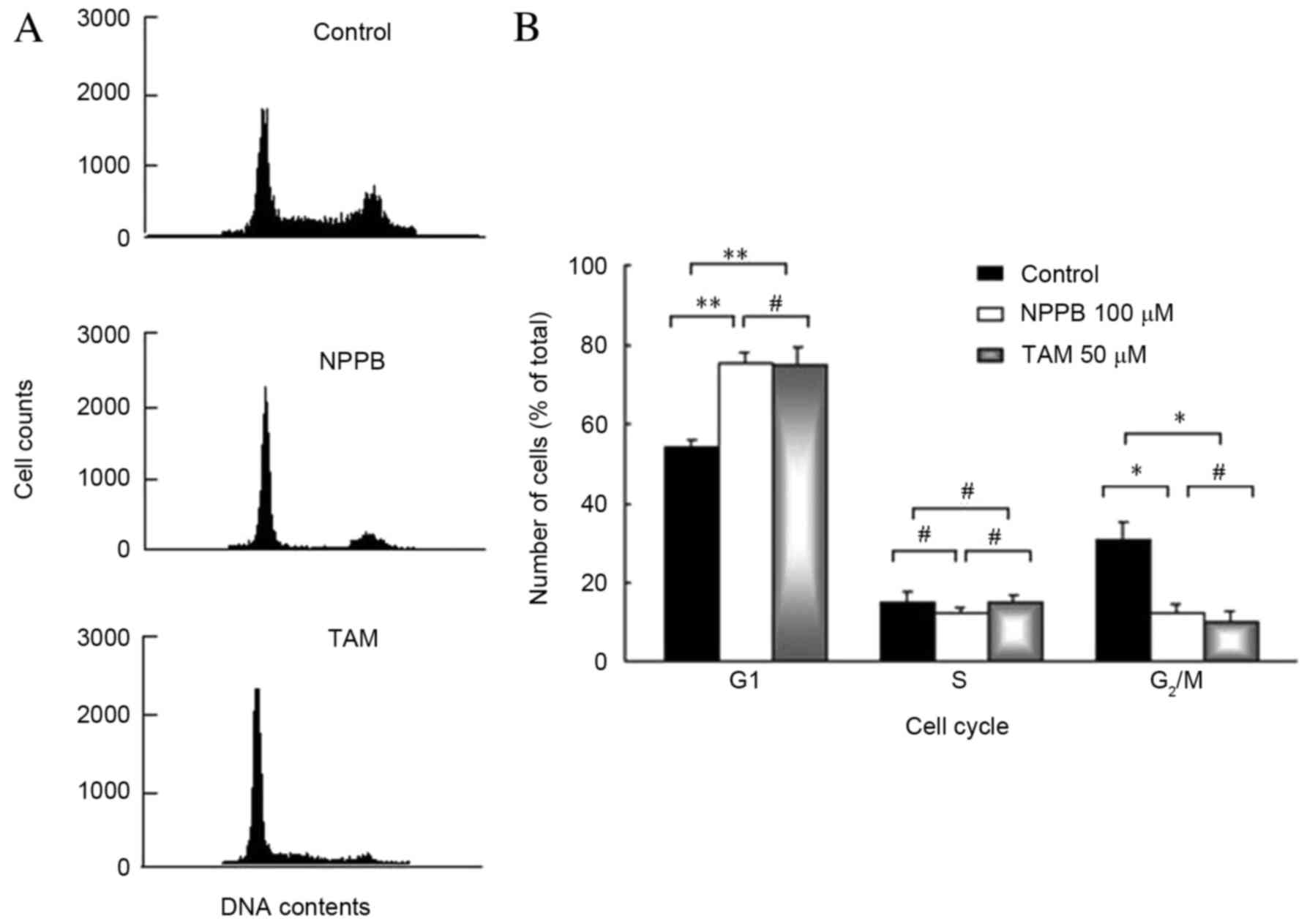
cycle
The effect of ClC blockers on the cell cycle is
presented in Fig. 2. ARPE-19 cells
were treated with 100 µM NPPB or 50 µM TAM for 48 h. The first peak
was generated by cells in the G1 phase. The second peak
was generated by cells in the G2/M phase, and S phase
cells made up the area between the two peaks. The number of cells
in the G1 phase was significantly increased in cells
treated with 100 µM NPPB or 50 µM TAM, compared with untreated
cells (P<0.05). The number of cells in the G2/M phase
was significantly reduced in cells treated with 100 µM NPPB or 50
µM TAM, compared with untreated cells (P<0.05). No significant
differences were observed in the number of cells in the S phase
between the groups. Therefore, ClC blockers significantly inhibited
cells from entering the DNA synthesis phase.
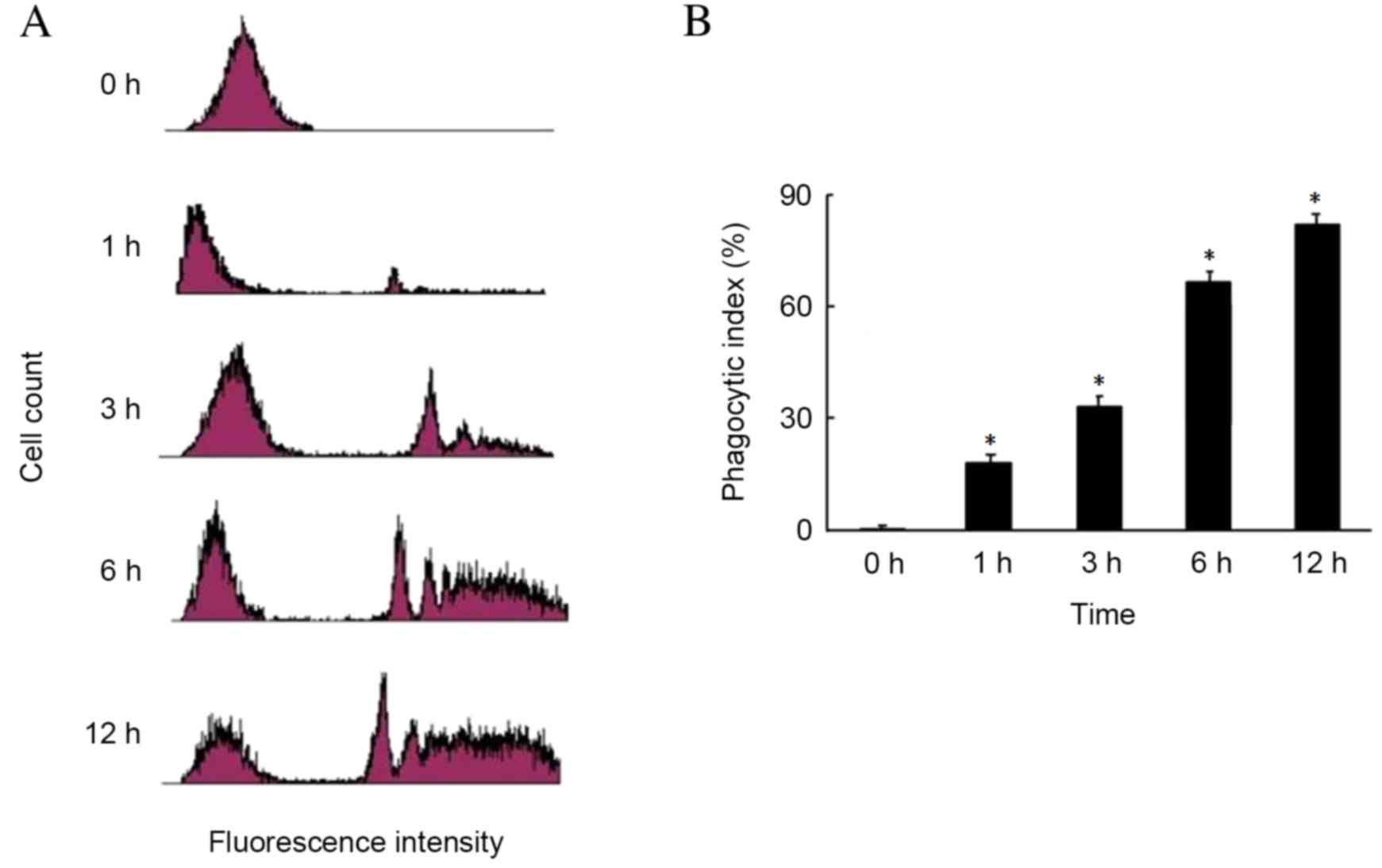
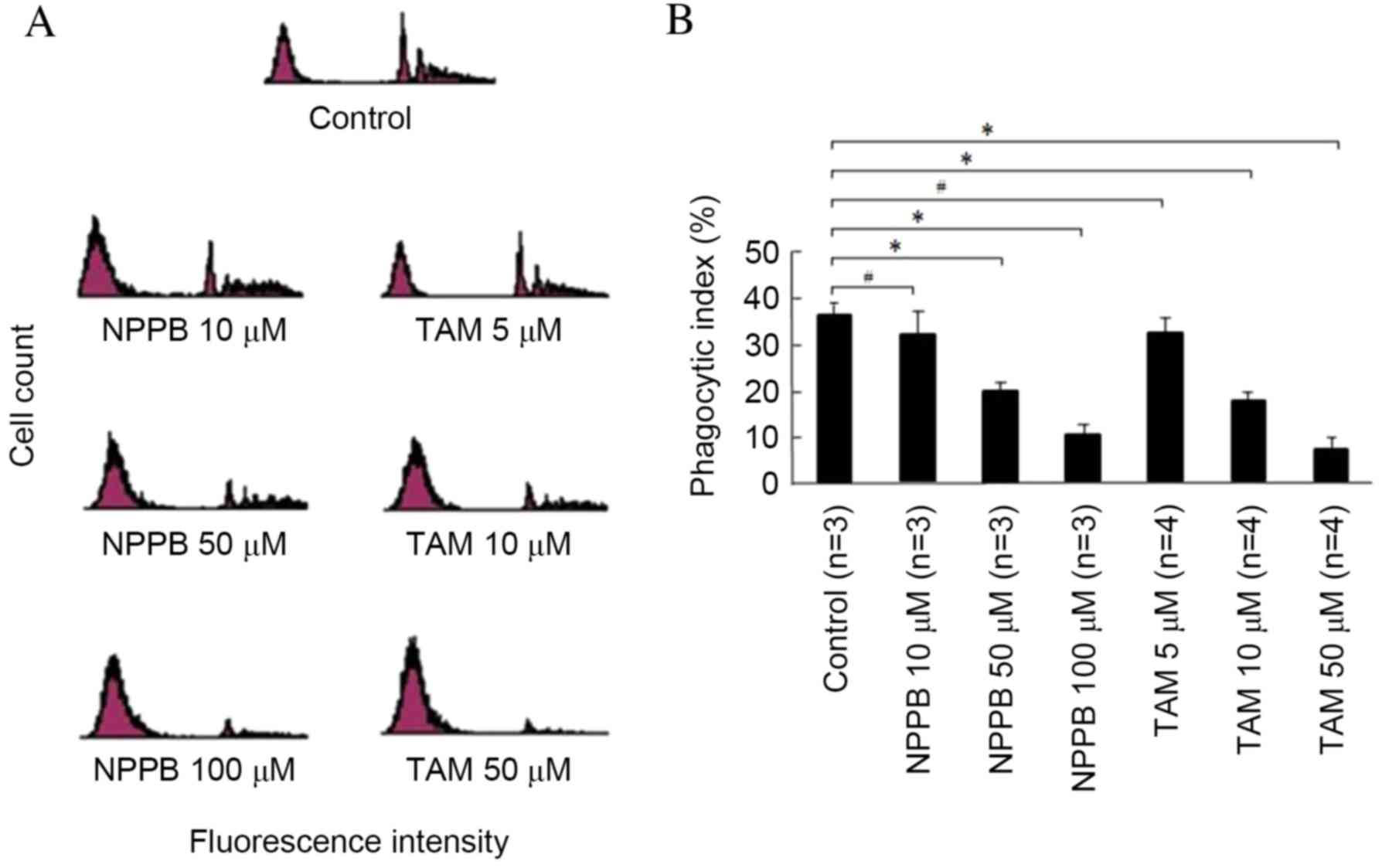
Phagocytosis of FN by RPE cells
The phagocytosis of FN by RPE cells is presented in
Fig. 3. The first peak represents
RPE cells that have not phagocytosed FN. The latter peaks represent
RPE cells that have phagoctosed FN. The phagocytic index of RPE
cells increased with incubation time in a time-dependent manner,
being 15% at 1 h, 35% at 3 h and 70% at 6 h, peaking at 80% after
12 h. The phagocytic index was reduced in the uncoated latex bead
group, compared with the FN-coated latex bead group (data not
shown).
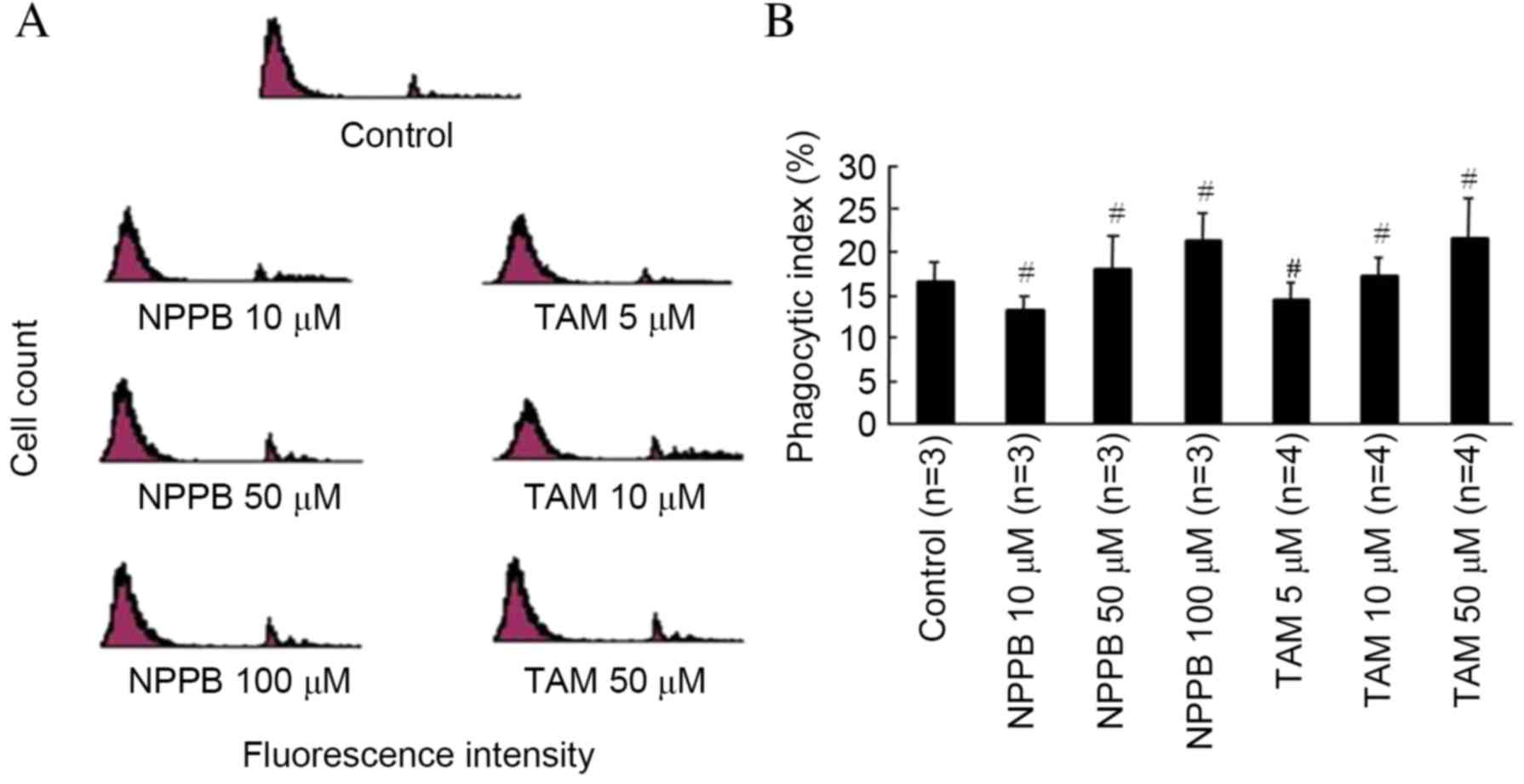
Effects of NPPB and TAM on RPE cell
phagocytosis of uncoated latex beads
The effects of NPPB and TAM on RPE cell phagocytosis
of uncoated latex beads are presented in Fig. 4. The phagocytic index was ~17% in
the control group following a 3-h incubation with uncoated latex
beads. No concentrations of NPPB or TAM had significant effects on
phagocytosis of uncoated latex beads.
Effects of NPPB and TAM on RPE cell
phagocytosis of FN-coated latex beads
The effects of NPPB and TAM on RPE phagocytosis of
FN-coated latex beads are presented in Fig. 5. Compared with the control group,
treatment with 10 µM NPPB or 5 µM TAM had no significant effect.
Phagocytosis was significantly reduced in cells treated with ≥50 µM
NPPB or ≥10 µM TAM, in a dose-dependent manner (P<0.05).
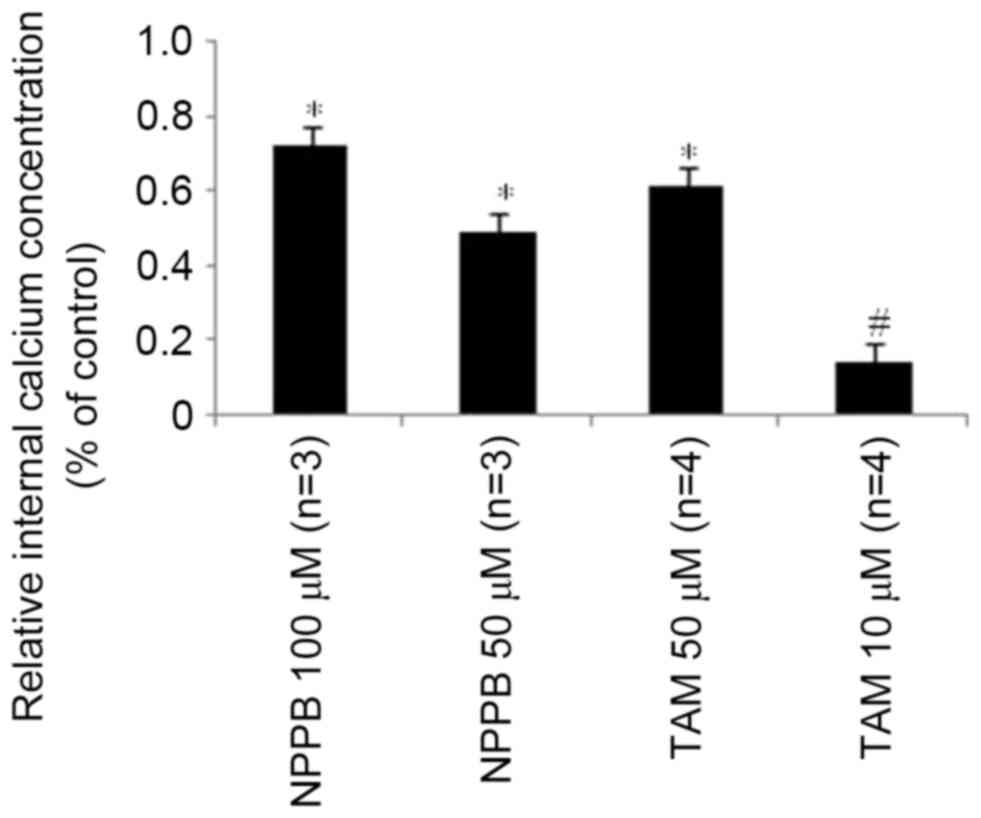
Effects of NPPB and TAM on
Ca2+ concentration
To further investigate the underlying mechanisms of
the influence of NPPB and TAM on RPE cell-directional migration
prior to phagocytosis of FN, intracellular Ca2+ levels were
examined. Cells were incubated in a hypotonic environment, which
induced a transient marked increase of intracellular Ca2+.
Treatment with NPPB or TAM significantly reduced intracellular Ca2+
levels, in a dose-dependent manner (P<0.05, Fig. 6).
Discussion
Abnormal proliferation and migration of RPE cells
are the primary processes that mediate PVR formation. The present
study demonstrated that NPPB or TAM treatment inhibited the
proliferation of ARPE-19 human RPE cells in a dose-dependent
manner, and blocked cells from entering S phase. Furthermore, the
presence of FN enhanced phagocytosis. Following the development of
PVR, FN predominantly enters in plasma via the damaged
blood-retinal barrier, and is generated by displaced RPE cells
(8). A previous study demonstrated
that the composition of PVR membranes varies over time (23). The FN content of PVR membranes is
significantly elevated in the first four months of PVR development
(23), as in the earlier stages of
wound healing, FN serves an important role in cellular adhesion.
However, following scar formation, FN disappears (24). This suggests that as PVR
progresses, FN may be degraded. Based on the results of the present
study, this transient presence of FN may be due to RPE cells, and
phagocytosis may be critical for the abnormal migration of RPE
cells into the vitreous.
The results of the present study suggested that
during the pathological process, retinal tears lead to a breach in
the blood-eye barrier. This causes the release of FN to facilitate
repair of the breach. RPE cells may migrate through the retinal
tear to clear the FN; therefore, inhibiting FN-induced RPE cell
migration may have a positive effect in the prevention of PVR.
To further investigate the potential effects of ClCs
on RPE cell migration, intracellular Ca2+ concentration
was examined. Ca2+ is an intracellular secondary
messenger, which serves a central role in signal transduction,
resulting in numerous cellular responses (25). It is involved in the specific
phagocytosis by RPE cells of rod outer segments, and serves an
important role in cell migration (26–28).
Ca2+ may facilitate loosening of cell-matrix connections
(29), and the rise of
intracellular Ca2+ level in the cell cytoplasm is more
prominent than in the lamellipodium (30), where it may alter the structure of
the cortical actomyosin gel, causing contraction (31). The present study demonstrated that
the ClC blockers NPPB and TAM reduced intracellular Ca2+
levels, suggesting that ClCs may regulate intracellular
Ca2+ levels in RPE cells, and subsequently contribute to
the regulation of the cell migration prior to phagocytosis of
FN.
These results revealed that ClCs serve important
roles in human RPE cell proliferation and phagocytosis of FN, which
cause RPE cell migration to the vitreous during PVR. To inhibit the
formation of PVR, the molecular processes underlying RPE cell
proliferation and migration have been widely investigated. A series
of agents have been reported to have inhibitory effects on these
processes, including mammalian target of rapamycin kinase
inhibitior, protein tyrosine phosphatase 1B and insulin-like growth
factor binding protein-6 (32–34).
Numerous signaling pathways are involved in RPE cell proliferation
and migration; primarily the phosphoinositide 3-kinase/protein
kinase B and mitogen activated protein kinase kinase/extracellular
signal-regulated kinase-associated signaling pathways (32,35).
Previous studies regarding the pathogenesis of PVR have focused on
the study of cytokines and proteases (36–39).
The results of the present study offered novel insight into the
pathogenesis of PVR by examining the nonselective ClC blockers,
NPPB and TAM; however, further research is required to determine
which ClC isoforms may be involved. In conclusion, ClCs may be
important for the proliferation and migration of RPE cells.
Targeting ClCs may provide a novel way to inhibit PVR
formation.
References
|
1
|
Ho PC and McMeel JW: Retinal detachment
with proliferative vitreoretinopathy: Surgical results with scleral
buckling, closed vitrectomy, and intravitreous air injection. Br J
Ophthalmol. 69:584–587. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Machemer R: Proliferative
vitreoretinopathy (PVR): A personal account of its pathogenesis and
treatment. Proctor lecture. Invest Ophthalmol Vis Sci.
29:1771–1783. 1988.PubMed/NCBI
|
|
3
|
Rowen SL and Glaser BM: Retinal pigment
epithelial cells release a chemoattractant for astrocytes. Arch
Ophthalmol. 103:704–707. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glaser BM, Cardin A and Biscoe B:
Proliferative vitreoretinopathy. The mechanism of development of
vitreoretinal traction. Ophthalmology. 94:327–332. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan CM, Huang JH, Chiang HS, Wu WB, Lin
HH, Hong JY and Hung CF: Effects of (−)-epigallocatechin gallate on
RPE cell migration and adhesion. Mol Vis. 16:586–595.
2010.PubMed/NCBI
|
|
6
|
Proctor RA: Fibronectin: A brief overview
of its structure, function, and physiology. Rev Infect Dis. 9 Suppl
4:S317–S321. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma M, Tiwari A, Sharma S, Bhoria P,
Gupta V, Gupta A and Luthra-Guptasarma M: Fibrotic remodeling of
the extracellular matrix through a novel (engineered,
dual-function) antibody reactive to a cryptic epitope on the
N-terminal 30 kDa fragment of fibronectin. PLoS One. 8:e693432013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hiscott P, Waller HA, Grierson I, Butler
MG and Scott D: Local production of fibronectin by ectopic human
retinal cells. Cell Tissue Res. 267:185–192. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nilius B and Droogmans G: Amazing chloride
channels: An overview. Acta Physiol Scand. 177:119–147. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng YJ, Furukawa T, Tajimi K and Inagaki
N: Cl-channel blockers inhibit transition of quiescent (G0)
fibroblasts into the cell cycle. J Cell Physiol. 194:376–383. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng YJ, Furukawa T, Ogura T, Tajimi K
and Inagaki N: M phase-specific expression and
phosphorylation-dependent ubiquitination of the ClC-2 channel. J
Biol Chem. 277:32268–32273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Furukawa T, Ogura T, Zheng YJ, Tsuchiya H,
Nakaya H, Katayama Y and Inagaki N: Phosphorylation and functional
regulation of ClC-2 chloride channels expressed in Xenopus oocytes
by M cyclin-dependent protein kinase. J Physiol. 540:883–893. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wills NK, Weng T, Mo L, Hellmich HL, Yu A,
Wang T, Buchheit S and Godley BF: Chloride channel expression in
cultured human fetal RPE cells: Response to oxidative stress.
Invest Ophthalmol Vis Sci. 41:4247–4255. 2000.PubMed/NCBI
|
|
14
|
Weng TX, Godley BF, Jin GF, Mangini NJ,
Kennedy BG, Yu AS, Yu AS and Wills NK: Oxidant and antioxidant
modulation of chloride channels expressed in human retinal pigment
epithelium. Am J Physiol Cell Physiol. 283:C839–C849. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ransom CB, O'Neal JT and Sontheimer H:
Volume-activated chloride currents contribute to the resting
conductance and invasive migration of human glioma cells. J
Neurosci. 21:7674–7683. 2001.PubMed/NCBI
|
|
16
|
Olsen ML, Schade S, Lyons SA, Amaral MD
and Sontheimer H: Expression of voltage-gated chloride channels in
human glioma cells. J Neurosci. 23:5572–5582. 2003.PubMed/NCBI
|
|
17
|
Mastronardi L, Puzzilli F and Ruggeri A:
Tamoxifen as a potential treatment of glioma. Anti-cancer drugs.
9:581–586. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dunn KC, Aotaki-Keen AE, Putkey FR and
Hjelmeland LM: ARPE-19, a human retinal pigment epithelial cell
line with differentiated properties. Exp Eye Res. 62:155–169. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou Q, Tang J, Wang Z, Wang C, Chen X, Hou
L, Dong XD and Tu L: Inhibitory effect of microRNA-34a on retinal
pigment epithelial cell proliferation and migration. Invest
Ophthalmol Vis Sci. 54:6481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei Y, Lin N, Zuo W, Luo H, Li Y, Liu S,
Meng L, Fan A, Zhu L, Jacob TJ, et al: Ethanol promotes cell
migration via activation of chloride channels in nasopharyngeal
carcinoma cells. Alcohol Clin Exp Res. 39:1341–1351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Z, Zhang ZX, Li S and Gao J: Effect of
a chloride channel inhibitor,
5-nitro-2-(3-phenylpropylamino)-benzoate, on ovarian cancer cell
migration. Clin Lab. 57:543–550. 2011.PubMed/NCBI
|
|
22
|
Li M, Wang B and Lin W: Cl-channel
blockers inhibit cell proliferation and arrest the cell cycle of
human ovarian cancer cells. Eur J Gynaecol Oncol. 29:267–271.
2008.PubMed/NCBI
|
|
23
|
Morino I, Hiscott P, McKechnie N and
Grierson I: Variation in epiretinal membrane components with
clinical duration of the proliferative tissue. Br J Ophthalmol.
74:393–399. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurkinen M, Vaheri A, Roberts PJ and
Stenman S: Sequential appearance of fibronectin and collagen in
experimental granulation tissue. Lab Invest. 43:47–51.
1980.PubMed/NCBI
|
|
25
|
Li M, Wang Q, Lin W and Wang B: Regulation
of ovarian cancer cell adhesion and invasion by chloride channels.
Int J Gynecol Cancer. 19:526–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mergler S, Steinhausen K, Wiederholt M and
Strauss O: Altered regulation of L-type channels by protein kinase
C and protein tyrosine kinases as a pathophysiologic effect in
retinal degeneration. FASEB J. 12:1125–1134. 1998.PubMed/NCBI
|
|
27
|
Feng W, Yasumura D, Matthes MT, LaVail MM
and Vollrath D: Mertk triggers uptake of photoreceptor outer
segments during phagocytosis by cultured retinal pigment epithelial
cells. J Biol Chem. 277:17016–17022. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei C, Wang X, Chen M, Ouyang K, Song LS
and Cheng H: Calcium flickers steer cell migration. Nature.
457:901–905. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lawson MA and Maxfield FR: Ca(2+)- and
calcineurin-dependent recycling of an integrin to the front of
migrating neutrophils. Nature. 377:75–79. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwab JC, Beckers CJ and Joiner KA: The
parasitophorous vacuole membrane surrounding intracellular
Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad
Sci USA. 91:509–513. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lauffenburger DA and Horwitz AF: Cell
migration: A physically integrated molecular process. Cell.
84:359–369. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du ZD, Hu LT, Zhao GQ, Wang Q, Xu Q, Jiang
N and Lin J: Protein tyrosine phosphatase 1B regulates migration of
ARPE-19 cells through EGFR/ERK signaling pathway. Int J Ophthalmol.
8:891–897. 2015.PubMed/NCBI
|
|
33
|
Zhao HM, Sheng MJ and Yu J: Expression of
IGFBP-6 in a proliferative vitreoretinopathy rat model and its
effects on retinal pigment epithelial cell proliferation and
migration. Int J Ophthalmol. 7:27–33. 2014.PubMed/NCBI
|
|
34
|
Calton MA and Vollrath D: The mTOR kinase
inhibitor INK128 blunts migration of cultured retinal pigment
epithelial cells. Adv Exp Med Biol. 854:709–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin D, Zheng XX and Jiang YR: Apelin-13
induces proliferation, migration, and collagen I mRNA expression in
human RPE cells via PI3K/Akt and MEK/Erk signaling pathways. Mol
Vis. 19:2227–2236. 2013.PubMed/NCBI
|
|
36
|
Gao Q and Ge J: The inhibition of CA2+
influx induced by hypericin in cultured human retinal pigment
epithelial cells analyzed by confocal imaging. Ophthalmic Res.
37:128–135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saika S: TGFbeta pathobiology in the eye.
Lab Invest. 86:106–115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao MW, Jin ML, He S, Spee C, Ryan SJ and
Hinton DR: A distinct integrin-mediated phagocytic pathway for
extracellular matrix remodeling by RPE cells. Invest Ophthalmol Vis
Sci. 40:2713–2723. 1999.PubMed/NCBI
|
|
39
|
Tanihara H, Yoshida M, Matsumoto M and
Yoshimura N: Identification of transforming growth factor-beta
expressed in cultured human retinal pigment epithelial cells.
Invest Ophthalmol Vis Sci. 34:413–419. 1993.PubMed/NCBI
|