Introduction
Renal fibrosis is a common primary pathological
feature of progressive kidney disease. Characteristics include
hyperplasia of myofibroblasts and excessive deposition of the
extracellular matrix (ECM) (1,2). The
ECM is primarily derived from myofibroblasts in kidneys, and
excessive accumulation of ECM decreases the glomerular infiltration
rate and renal function, eventually resulting in renal failure
(3). Therefore, renal fibrosis is
considered to be an irreversible process leading to end-stage renal
failure. Previous studies have demonstrated that renal fibrosis is
involved in numerous renal diseases, including chronic allograft
nephropathy and membranous nephropathy (4,5). Few
strategies have been identified as effective for the prevention and
treatment of renal fibrosis. Thus, there is a requirement for the
development of novel therapies that prevent or ameliorate renal
fibrosis.
Accumulating evidence indicates that in the event of
renal injury, epithelial-mesenchymal transition (EMT) occurs,
during which tubular epithelial cells lose their phenotypic markers
[including neural (N)- and epithelial (E)-cadherin] and transform
into fibroblasts, myofibroblasts or mesenchymal cells, gaining
mesenchymal phenotypic markers, including vimentin and α-smooth
muscle actin (SMA) (6). Various
factors are involved in this process, including cytokines, adhesion
molecules and growth factors. Of these factors, transforming growth
factor-β1 (TGF-β1) is considered an important regulator of EMT, as
it causes renal scarring by activating the downstream mothers
against decapentaplegic (Smad) signaling pathway. It has been
demonstrated that the binding of TGF-β1 to its receptor triggers
the phosphorylation of Smad2 and 3, which combine with Smad4 to
form the Smad complex. The complex is subsequently translocated to
the nucleus to regulate target gene transcription, resulting in
fibrogenesis (3,7). Thus, inhibition of the production of
TGF-β1 or blockade of the TGF-β1 signaling pathway is a potential
strategy to prevent fibrogenesis.
Baicalin was originally isolated from the root of
Scutellaria baicalensis, and is widely used for treating
infectious and inflammatory diseases (8–10).
Our previous study reported that this traditional Chinese herb root
possesses protective effects against renal ischemia reperfusion
injury via inhibition of the production of proinflammatory
cytokines, including tumor necrosis factor (TNF)-α and interleukin
(IL)-1β (11). A previous study
demonstrated the antifibrotic effects of baicalin in rat livers via
a reduction of profibrotic cytokines including serum TGF-β1, TNF-α
and IL-6, and an increase of the antifibrotic cytokine IL-10
(12). In addition, Hu et
al (13) examined 21 compounds
isolated from plants and demonstrated potential antifibrotic
activities of baicalin in vitro; however, this requires
further investigation in vivo. The underlying mechanisms of
the antifibrotic activities of baicalin in vitro, and
whether TGF-β1 is involved in its effects on renal fibrosis, as has
been demonstrated for hepatic fibrosis, remain to be
determined.
The present study aimed to examine the antifibrotic
effects of baicalin in a unilateral ureteral obstruction
(UUO)-induced renal fibrosis rat model, and to investigate whether
its underlying mechanisms involve the TGF-β1/Smad signaling
pathway.
Materials and methods
Experimental animals
A total of 15 male Sprague-Dawley rats (weight,
200–250 g) were purchased from Shanghai SLAC Laboratory Animal Co.,
Ltd. (Shanghai, China). The rats were housed in specific
pathogen-free grade conditions under a 12-h light/dark cycle with
temperature and humidity ranging between 20–26°C and 40–70%,
respectively; free access to food and water was available for 1
week prior to the experiment. The present study was approved by the
Care and Use of Laboratory Animals of the Ethical Commission of
Fudan University (Shanghai, China) and performed under accepted
ethical guidelines. Briefly, animals were acclimatized to
laboratory conditions for 1 week prior to experimentation and
anesthetized to minimize pain or discomfort during operation.
Animals were sacrificed by pentobarbital overdose for tissue
collection.
Unilateral renal fibrosis model
Rats were randomly allocated to three groups: Sham,
UUO-induced fibrosis treated with normal saline and UUO-induced
fibrosis treated with baicalin (n=5/group). Baicalin (catalog no.
572667; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was
diluted in normal saline to 20 and 100 mg/kg was administered by
intraperitoneal injection every 2 days for 10 days. The UUO-induced
fibrosis model was established as previously described (14). Pentobarbital (40 mg/kg, Shanghai
USEN Biological Technology Co., Ltd., Shanghai, China) was used to
anesthetize the rats. Following this, the abdominal cavity was
exposed. The left ureter was identified, ligated at two points with
non-absorbable 5–0 silk and cut between the ligatures. Rats in the
sham group underwent ureter manipulation, but not ligation. Rats
were placed on a 37°C heat pad during surgery to maintain body
temperature. After 10 days, rats were anesthetized with
pentobarbital (40 mg/kg), and blood samples were collected from the
heart and centrifuged at 2,000 × g for 10 min at 20°C to obtain
serum samples. Following this, rats were sacrificed by overdose of
pentobarbital. The kidneys were transversally cut at the midline
and harvested.
Hematoxylin and eosin (H&E)
staining
Paraffin-embedded rat kidney tissue was cut into
5-mm sections and subsequently deparaffinized and rehydrated prior
to H&E staining. The severity of renal interstitial injury was
evaluated under light microscopy (magnification, ×200). Sections
from the sham, UUO and baicalin groups were evaluated according to
a previous study performed by Lin et al (11). Tubular dilation, interstitial
expansion and inflammatory cell infiltration were assessed using a
tubulointerstitial injury scoring system as follows: 0, normal; 1,
mild (≤25%); 2, moderate (>25 to 50%); 3, severe (>50%)
(11). A total of 10 fields per
specimen were randomly selected and assessed blindly by two
examiners. Mean values of these estimates were used in the
analyses.
Sirius red staining
Following deparaffinization and rehydration,
paraffin-embedded kidney sections were incubated with 0.1% Sirius
red solution at room temperature for 1 h. The slides were
subsequently washed twice in acidified water, dehydrated in
absolute alcohol and cleared in xylene. A total of 10 fields per
specimen were randomly selected and analyzed using Image-Pro Plus
software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
to evaluate the degree of renal fibrosis.
Immunohistochemical staining
Following deparaffinization and rehydration,
paraffin-embedded kidney sections were blocked with 3% hydrogen
peroxide and subsequently incubated for 1 h at 37°C with the
following rabbit primary antibodies: Anti-E-cadherin (1:25; catalog
no. ab15148; Abcam, Cambridge, UK), anti-N-cadherin (1:100; catalog
no. 13116; Cell Signaling Technology, Inc. Danvers, MA, USA),
anti-α-SMA (1:200; catalog no. ab32575; Abcam, Cambridge, UK) and
anti-vimentin (1:100; catalog no. 5741; Cell Signaling Technology,
Inc.). They were subsequently incubated with a horseradish
peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody
(1:200; catalog no. ab6721; Abcam) for 1 h at room temperature. The
reaction was visualized with a 3,3′-diaminobenzidine chromogen
solution (Gene Tech Co., Ltd., Shanghai, China) and counterstained
with hematoxylin. The slides were analyzed using Image-Pro Plus
software version 6.0.
Western blot analysis
Renal tissue was homogenized in ice-cold
radioimmunoprecipitation assay buffer (Vazyme Biotech Co., Ltd,
Nanjing, China). Lysates were centrifuged at 12,000 × g at 4°C for
25 min, following which the supernatant was collected and total
proteins were quantified by bicinchoninic acid assay (Beyotime
Institute of Biotechnology, Haimen, China). Samples (20 µg) were
separated via 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto polyvinylidene
difluoride membranes at 310 mA for 110 min. Membranes were blocked
with 5% milk and subsequently incubated overnight at 4°C with the
following rabbit primary antibodies: Anti-E-cadherin (1:500;
Abcam), anti-N-cadherin (1:1,000), anti-α-SMA (1:1,000; catalog no.
14968; Cell Signaling Technology, Inc.), anti-vimentin (1:1,000),
anti-GAPDH (1:1,000; catalog no. 2118), anti-phosphorylated
(p)-Smad2 (1:1,000; catalog no. 3108), anti-p-Smad3 (catalog no.
9520) and anti-Smad2/3 (catalog no. 8685), all from Cell Signaling
Technology, Inc., with gentle agitation. Membranes were washed with
TBS-Tween-20 (1%) and incubated with a HRP-conjugated goat
anti-rabbit secondary antibody (1:5,000; catalog no. ab6721; Abcam)
for 1 h at room temperature. Proteins were visualized using an
Enhanced Chemiluminescence system (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Band intensities were analyzed using
Quantity-One 1-D Analysis software (version no. 4.62; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and values were normalized
against the value of GAPDH.
ELISA of serum TGF-β1 expression
levels
Serum TGF-β1 levels were measured using an ELISA kit
(catalog no. F16920; Shanghai Xitang Biotechnology Co., Ltd.,
Shanghai, China), according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from kidney tissue using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA (4 µg) was reverse-transcribed using RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) into
cDNA according to the manufacturer's protocol. qPCR was performed
using a Mastercycler® ep realplex system (Eppendorf,
Hamburg, Germany) in combination with the All-in-one™ qPCR
Mix(GeneCopoeia, Rockville, MD, USA). Thermocycling conditions were
as follows: Incubation for 2 min at 50°C and 10 min at 95°C. This
was followed by a 2-step PCR program of 95°C for 15 sec and 60°C
for 20 sec for 45 cycles. Expression levels of target genes were
normalized against GAPDH using the 2-ΔΔCq method
(15). Gene-specific primers for
rat TGF-β1, fibronectin, collagen I and GAPDH are presented in
Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| GAPDH | F:
AGGTCGGTGTGAACGGATTT |
|
| R:
GGGGTCGTTGATGGCAACA |
| Collagen I | F:
GAGAGAGCATGACCGATGGA |
|
| R:
CGTGCTGTAGGTGAATCGAC |
| TGF-β1 | F:
CTTTGTACAACAGCACCCGC |
|
| R:
CGGGTGACTTCTTTGGCGTA |
| Fibronectin | F:
TGGAGAGACAGGAGGAAATAGC |
|
| R:
CAGTGACAGCATACAGGGTGAT |
Cell culture and in vitro fibrosis
model
The NRK-52E rat renal tubular epithelial cell line
was purchased from the Shanghai Branch of the Chinese Academy of
Science (Shanghai, China) and maintained in Dulbecco's modified
Eagle's medium/F-12 nutrient mixture (DMEM/F-12; Shanghai BioSun
Sci&Tech Co., Ltd., Shanghai, China). A total of nine Petri
dishes were seeded with NRK-52E cells at a density of 1×106 cells
per dish. Cells were cultured with 10 µl DMEM/F-12 containing 100
µl PBS (control group), 100 µl TGF-β1 (3 ng/ml; Peprotech, Inc.,
Rocky Hill, NJ, USA; TGF-β1 group) or 100 µl TGF-β1 + 40 µl
baicalin (100 µmol/l; baicalin group; n=3/group). After 3 days,
cells were collected for western blot analysis.
Statistical analysis
SPSS software version 19.0 (IBM SPSS, Armonk, NY,
USA) was used for statistical analysis of the data by one-way
analysis of variance, followed by a Bonferroni correction. Data are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
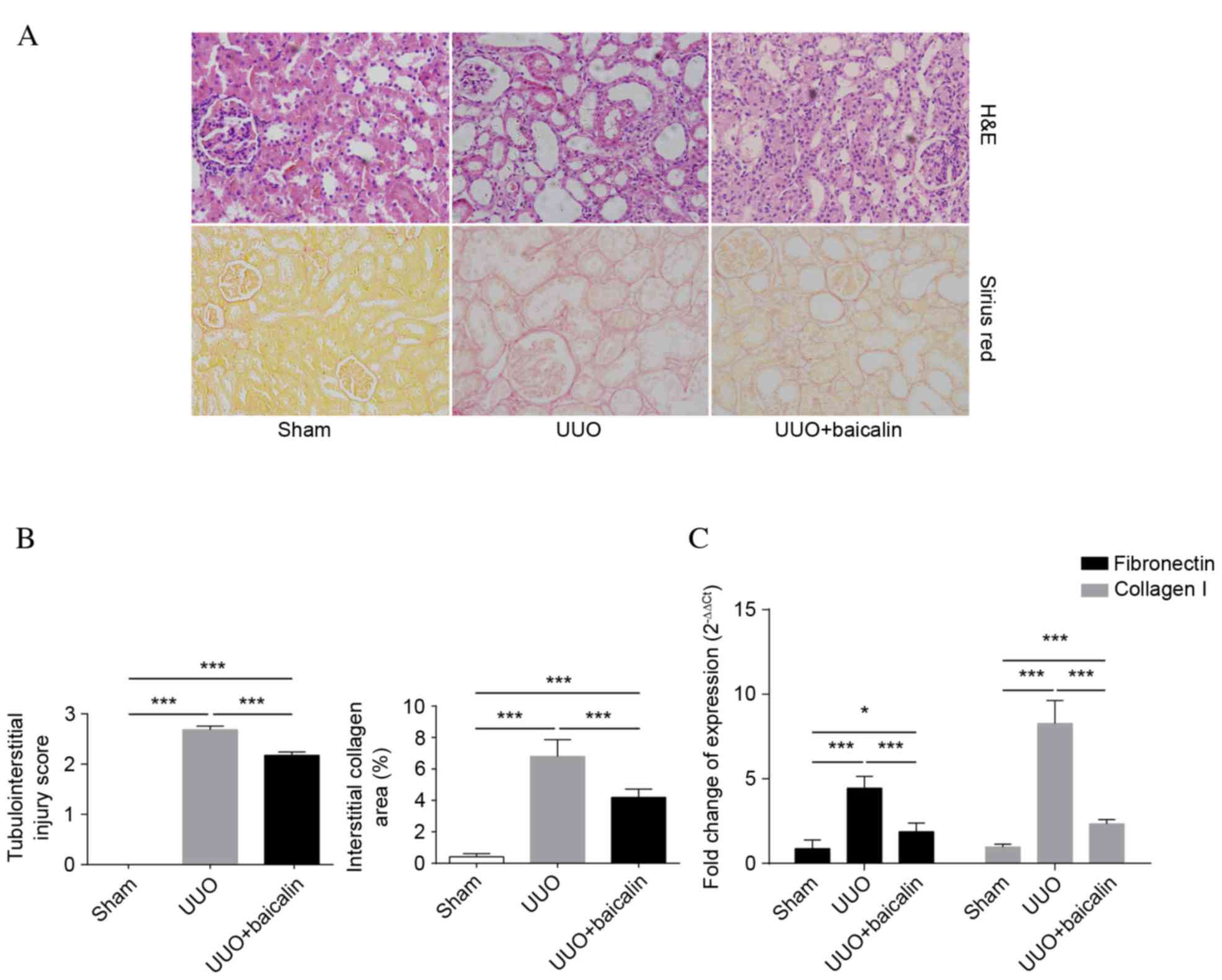
Baicalin attenuates UUO-induced renal
fibrosis and injury
A total of 10 days following UUO, H&E staining
of obstructed kidneys was performed in all groups to assess the
degree of renal injury and fibrosis. As presented in Fig. 1A, tubular structures had a healthy
morphology and architecture in the sham group, whereas severe
tubular dilation, interstitial expansion and inflammatory cell
infiltration were observed in the UUO group. By contrast, baicalin
treatment ameliorated tubulointerstitial lesions compared with the
UUO group (P<0.001). Collagen accumulation was determined by
Sirius red staining. Interstitial collagen accumulation in kidney
tissue markedly increased following UUO (P<0.001). However,
compared with the UUO group, baicalin significantly reduced the
area of interstitial collagen deposition (P<0.001), although not
to the level of the sham group (P<0.001). These results were
further confirmed by tubular injury scores and the relative area of
tubulointerstitial collagen deposition, as presented in Fig. 1B. Fibronectin and collagen I,
components of ECM, were additionally analyzed to evaluate the
degree of renal fibrosis. As presented in Fig. 1C, mRNA expression levels of the
previously mentioned indicators were significantly elevated in
kidney tissue following UUO compared with the sham group
(P<0.001), whereas baicalin treatment markedly inhibited this
effect (P<0.001), suggesting that baicalin inhibits the degree
of renal injury and fibrosis in a UUO-induced fibrosis model.
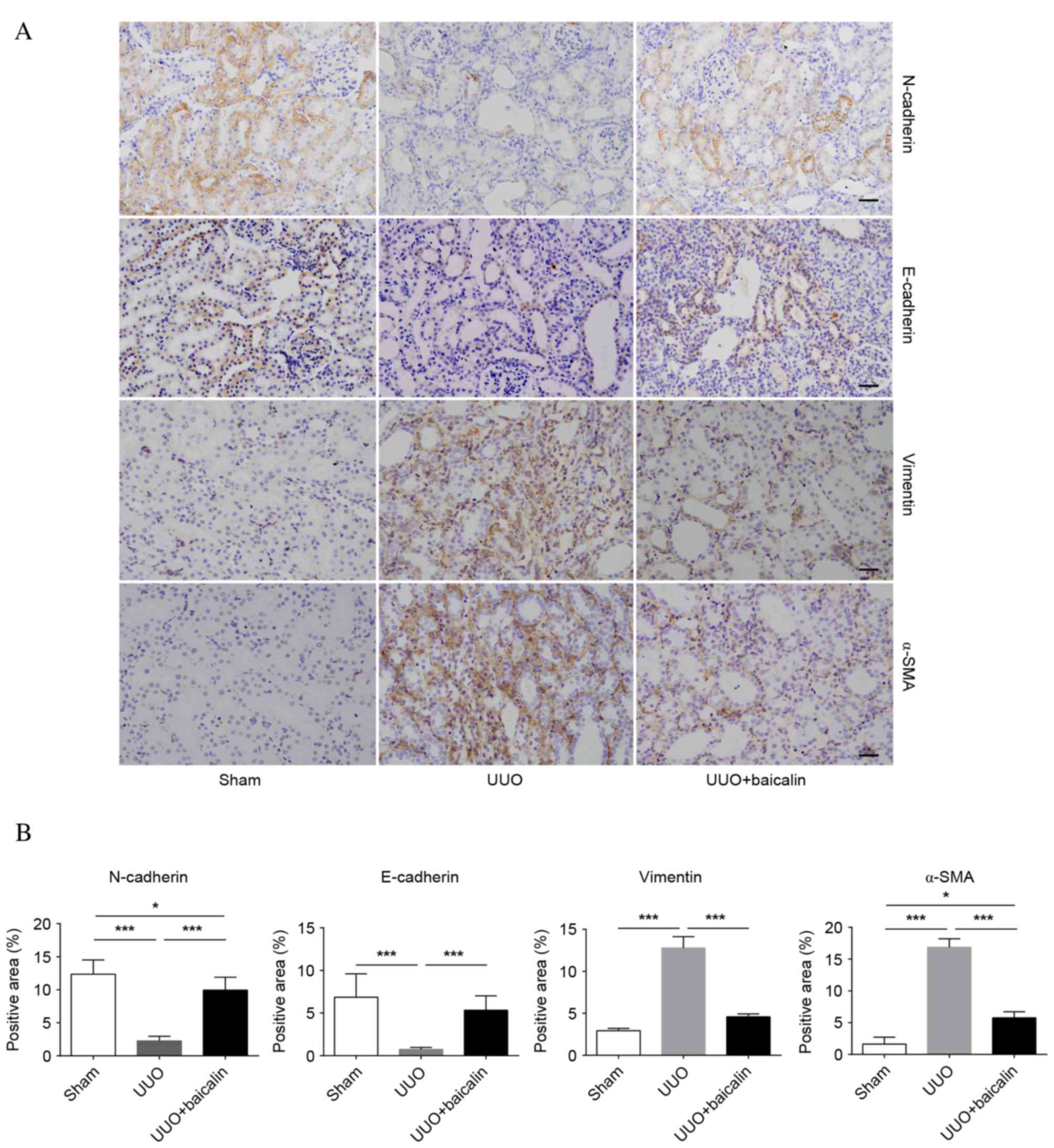
Baicalin inhibits EMT in kidney
tissue
EMT is a core process in the progression of renal
fibrosis. The epithelial markers N- and E-cadherin and the
mesenchymal markers α-SMA and vimentin were examined to assess EMT
(Fig. 2A). Immunohistochemical
analysis revealed low baseline expression levels of vimentin and
α-SMA in the sham group, whereas expression levels of these
proteins increased 10 days following UUO in the UUO group
(P<0.001); by contrast, enhanced expression levels of N- and
E-cadherin were observed in the sham group, which markedly
decreased following UUO (P<0.001), indicating that renal
fibrosis had occurred. However, compared with the UUO group,
baicalin treatment significantly decreased the expression levels of
α-SMA and vimentin (P<0.001), and reversed the increased
expression levels of N- and E-cadherin (P<0.001); however, not
to the levels of the sham group (Fig.
2B). Similar results were obtained from western blot analysis
(Fig. 3). These findings suggested
that obstructive nephropathy following UUO was accompanied by the
downregulation of N- and E-cadherin, and the upregulation of α-SMA
and vimentin. Furthermore, baicalin may partially reverse the
alterations in the levels of these proteins, indicating that
baicalin inhibits EMT in kidney tissue.
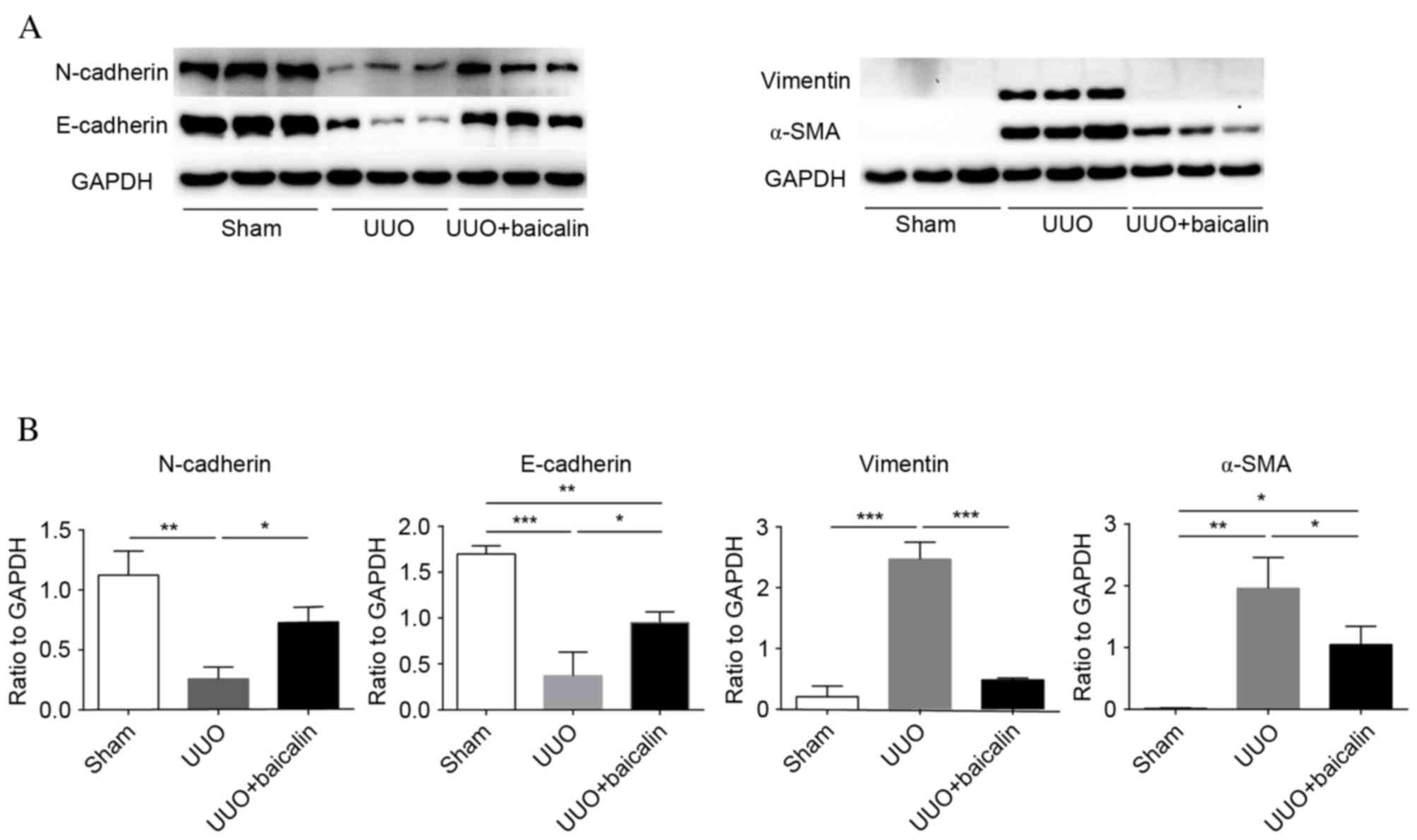 | Figure 3.Baicalin treatment inhibits
epithelial-mesenchymal transition in kidney tissue, as assessed by
western blotting. (A) Representative western blot images
demonstrating the expression of N- and E-cadherin, vimentin, and
α-SMA in kidney tissue from the sham, UUO and UUO + baicalin
groups. (B) The ratio of N- and E-cadherin, vimentin, and α-SMA
expression levels to GAPDH. Data are expressed as the mean ±
standard deviation. *P<0.05; **P<0.01; ***P<0.001. UUO,
unilateral ureteral obstruction; α-SMA, α-smooth muscle actin; N,
neural; E, epithelial. |
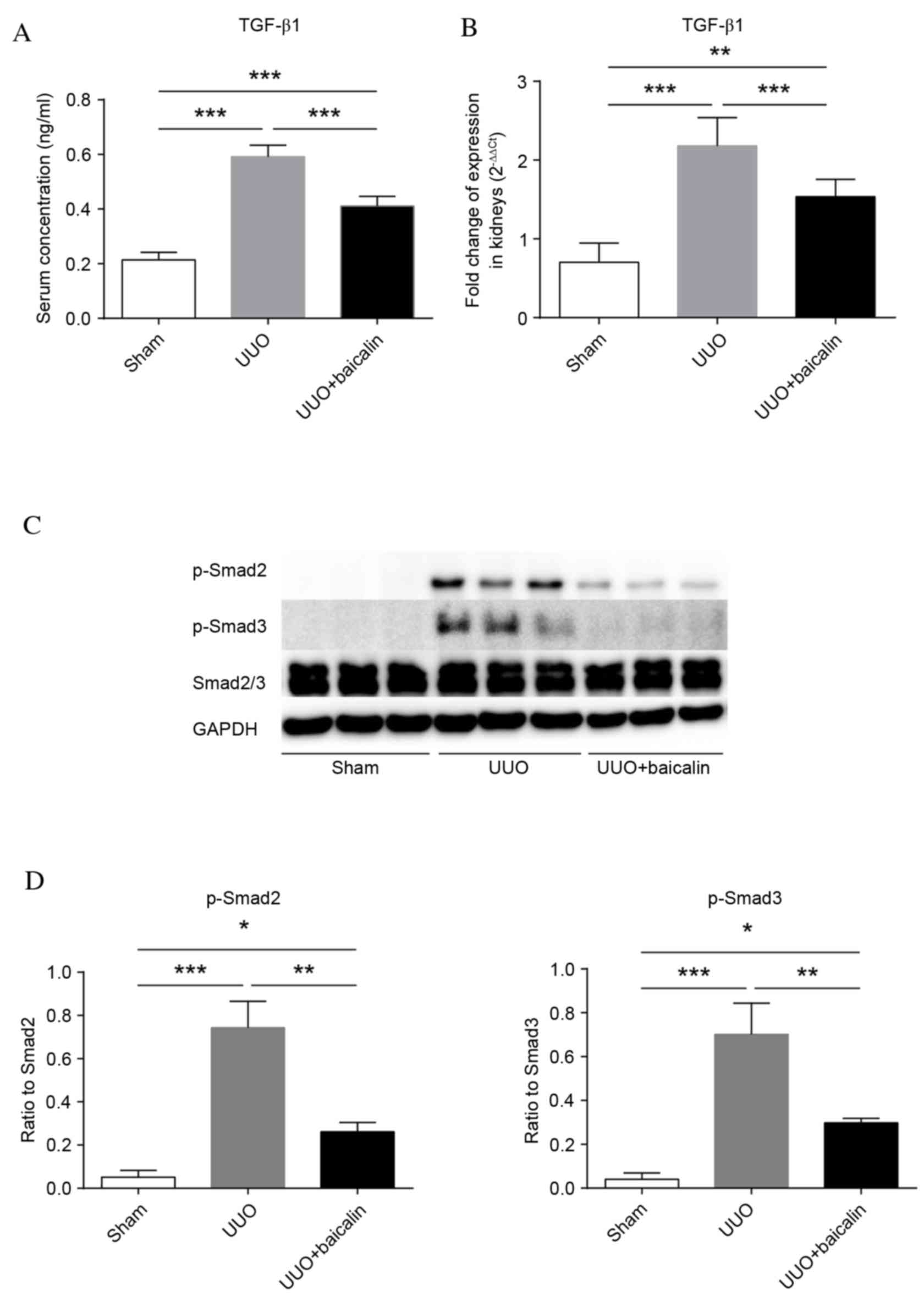
Baicalin treatment inhibits TGF-β1
synthesis and phosphorylation of Smad2/3 in vivo
TGF-β1 serves important roles in fibrogenesis. To
identify whether TGF-β1 is involved in the baicalin-mediated
amelioration of renal fibrosis, TGF-β1 levels were examined in
serum and kidney tissue. As presented in Fig. 4A, and Table II, the levels of TGF-β1 in serum
following UUO (0.592±0.042 ng/ml) were significantly elevated
compared with the sham group (0.214±0.027 ng/ml; P<0.001),
whereas baicalin treatment markedly decreased these levels
(0.411±0.036 ng/ml; P<0.001), although they remained increased
compared with the sham group (P<0.001). Similarly, kidney TGF-β1
mRNA expression levels were significantly increased in the UUO
group compared with the sham group (P<0.001) (Fig. 4B). However, TGF-β1 mRNA expression
levels in the baicalin-treated group were significantly reduced
compared with the UUO group (P<0.001). Therefore, baicalin
treatment may reduce the production of TGF-β1 in UUO-induced
fibrogenesis.
 | Table II.Serum transforming growth factor-β1
expression levels. |
Table II.
Serum transforming growth factor-β1
expression levels.
| Group | Value | Mean | Standard
deviation | P-value |
|---|
| Sham | 0.233 | 0.214 | 0.027 | <0.001 (vs.
UUO) |
|
| 0.229 |
|
| <0.001 (vs. UUO
+ baicalin) |
|
| 0.212 |
|
|
|
|
| 0.206 |
|
|
|
|
| 0.251 |
|
|
|
|
| 0.257 |
|
|
|
|
| 0.183 |
|
|
|
|
| 0.181 |
|
|
|
|
| 0.194 |
|
|
|
|
| 0.197 |
|
|
|
| UUO | 0.567 | 0.592 | 0.042 | <0.001 (vs.
sham) |
|
| 0.571 |
|
| <0.001 (vs. UUO
+ baicalin) |
|
| 0.621 |
|
|
|
|
| 0.616 |
|
|
|
|
| 0.575 |
|
|
|
|
| 0.573 |
|
|
|
|
| 0.657 |
|
|
|
|
| 0.653 |
|
|
|
|
| 0.544 |
|
|
|
|
| 0.541 |
|
|
|
| UUO + baicalin | 0.435 | 0.411 | 0.036 | <0.001 (vs.
sham) |
|
| 0.441 |
|
| <0.001 (vs.
UUO) |
|
| 0.419 |
|
|
|
|
| 0.426 |
|
|
|
|
| 0.373 |
|
|
|
|
| 0.378 |
|
|
|
|
| 0.453 |
|
|
|
|
| 0.448 |
|
|
|
|
| 0.362 |
|
|
|
|
| 0.372 |
|
|
|
To investigate if the TGF-β1 signaling pathway was
attenuated by baicalin treatment, phosphorylation of Smad2 and 3
was examined by western blot analysis (Fig. 4C). Phosphorylation of Smad2 and 3
was almost undetectable in the sham group, and significantly
increased 10 days following UUO (P<0.001). Notably, although
increased compared with the sham group (P<0.05), phosphorylation
of these proteins in the baicalin-treated group was significantly
reduced compared with the UUO group (P<0.01) (Fig. 4D). These results indicated that 10
days of ureteral obstruction triggered the phosphorylation of Smad2
and 3, which was markedly attenuated by baicalin.
Baicalin inhibits TGF-β1 signaling in
an in vitro TGF-β1-induced fibrosis model
To investigate whether the phosphorylation of
Smad2/3 may be decreased by baicalin in the presence of TGF-β1, the
phosphorylation of Smad2/3 was examined by western blot analysis in
an NRK-52E rat renal tubular epithelial cell line (Fig. 5A). Similar to the results in
vivo, the phosphorylation of Smad2/3 was significantly
increased in the TGF-β1 group compared with the control group
(P<0.001 and P<0.01, respectively; Fig. 5B). Notably, baicalin treatment was
demonstrated to significantly decrease Smad2/3 phosphorylation
(P<0.01 and P<0.05, respectively), although the levels of
phosphorylation remained increased compared with the control group,
further demonstrating that baicalin treatment may ameliorate renal
fibrosis. To demonstrate the effect of the inhibition of
phosphorylation of Smad2/3, protein expression levels of EMT
markers in NRK-52E cells were additionally assessed by western blot
analysis (Fig. 5C). Similar to the
results in vivo, baicalin treatment inhibited the
TGF-β1-induced decrease of N- and E-cadherin expression levels
(P<0.01 and P<0.001, respectively), and inhibited the
increase of vimentin and α-SMA induced by TGF-β1 (P<0.001 and
P<0.05, respectively; Fig. 5D).
This indicated that baicalin treatment decreases the
phosphorylation of Smad2/3 in NRK-52E cells even in the presence of
TGF-β1, thereby inhibiting the process of EMT.
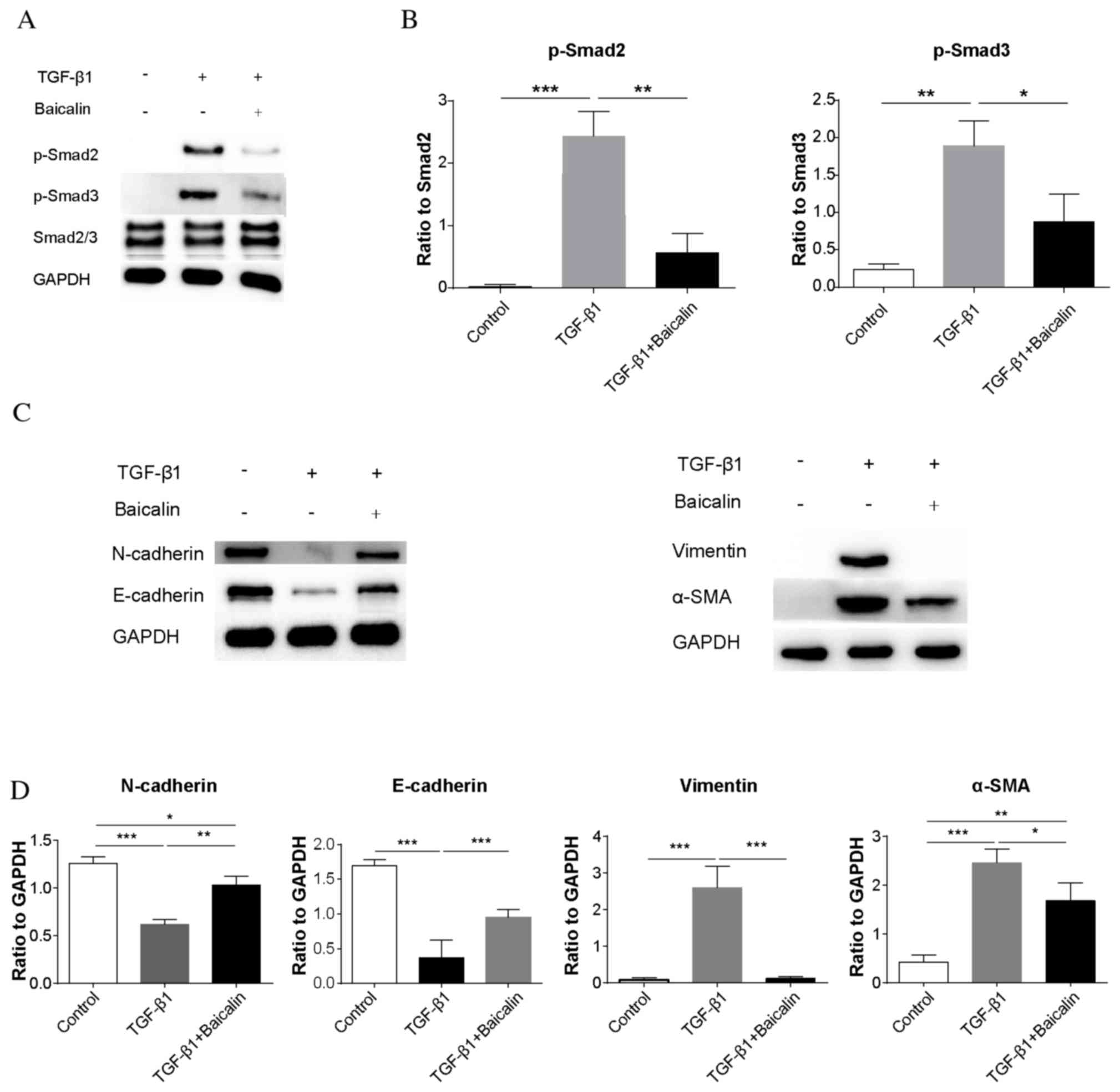 | Figure 5.Baicalin treatment inhibits TGF-β1
signaling in a NRK-52E rat renal tubular epithelial cell line in an
in vitro TGF-β1-induced fibrosis model. (A) Representative
western blot images of p-Smad2, p-Smad3 and Smad2/3 expression in
control, TGF-β1 and TGF-β1 + baicalin groups. GAPDH served as an
internal control. (B) The ratio of p-Smad2/3 to Smad2/3 expression
levels. (C) Representative western blot images of N- and
E-cadherin, vimentin and α-SMA expression. (D) Quantification of
the ratio of N and E-cadherin, vimentin, and α-SMA expression
levels to GAPDH. Data are expressed as the mean ± standard
deviation. *P<0.05; **P<0.01; ***P<0.001. α-SMA, α-smooth
muscle actin; N, neural; E, epithelial; p, phosphorylated; Smad,
mothers against decapentaplegic; α-SMA, α smooth muscle actin;
TGF-β1, transforming growth factor-β1. |
Discussion
Various treatments and therapeutic targets with the
aim of ameliorating renal fibrosis have previously been evaluated,
including drugs, endocrine hormones, the complement system and
microRNAs (2,5,16–18).
However, inactivation of a specific hormone may affect the
secretion of other hormones, inhibition of complement may impair
immune function and antagonists of microRNAs are not as stable as
compounds and only function for a short period of time, which
limits the clinical application of these methods. Therefore, safe
and efficient strategies to prevent renal fibrosis are required. A
previous study demonstrated the low cytotoxicity of baicalin, which
indicates the safety of baicalin (13). Additionally, another previous study
reported that baicalin treatment may ameliorate the effects of
fibrosis within various organs. Jia et al (19) demonstrated that baicalin attenuated
bleomycin-induced lung fibrosis via downregulation of connective
tissue growth factor. Liu et al (20) revealed that baicalin inhibited the
synthesis of collagen I in pulmonary arteries, and attenuated
pulmonary vascular remodeling via upregulation of a disintegrin and
metalloproteinase with thrombospondin motifs 1 expression. Qiao
et al (21) demonstrated
that the combination of mesenchymal stem cells and baicalin
exhibited a more effective therapeutic effect in hepatic fibrosis
compared with treatment with mesenchymal stem cells alone. Yang
et al (22) demonstrated
that the active components of Yang-Gan-Wan, rosmarinic acid and
baicalin had an antifibrotic effect on liver fibrosis via
inhibition of peroxisome proliferator-activated receptor-γ. In the
present study, baicalin was systematically demonstrated to have a
protective effect against renal fibrosis by reducing interstitial
collagen accumulation, decreasing mRNA expression levels of
fibronectin and collagen I, upregulating N- and E-cadherin
expression levels, and downregulating α-SMA and vimentin expression
levels. In addition, baicalin was identified to ameliorate renal
fibrosis via inhibition of TGF-β1 production and its downstream
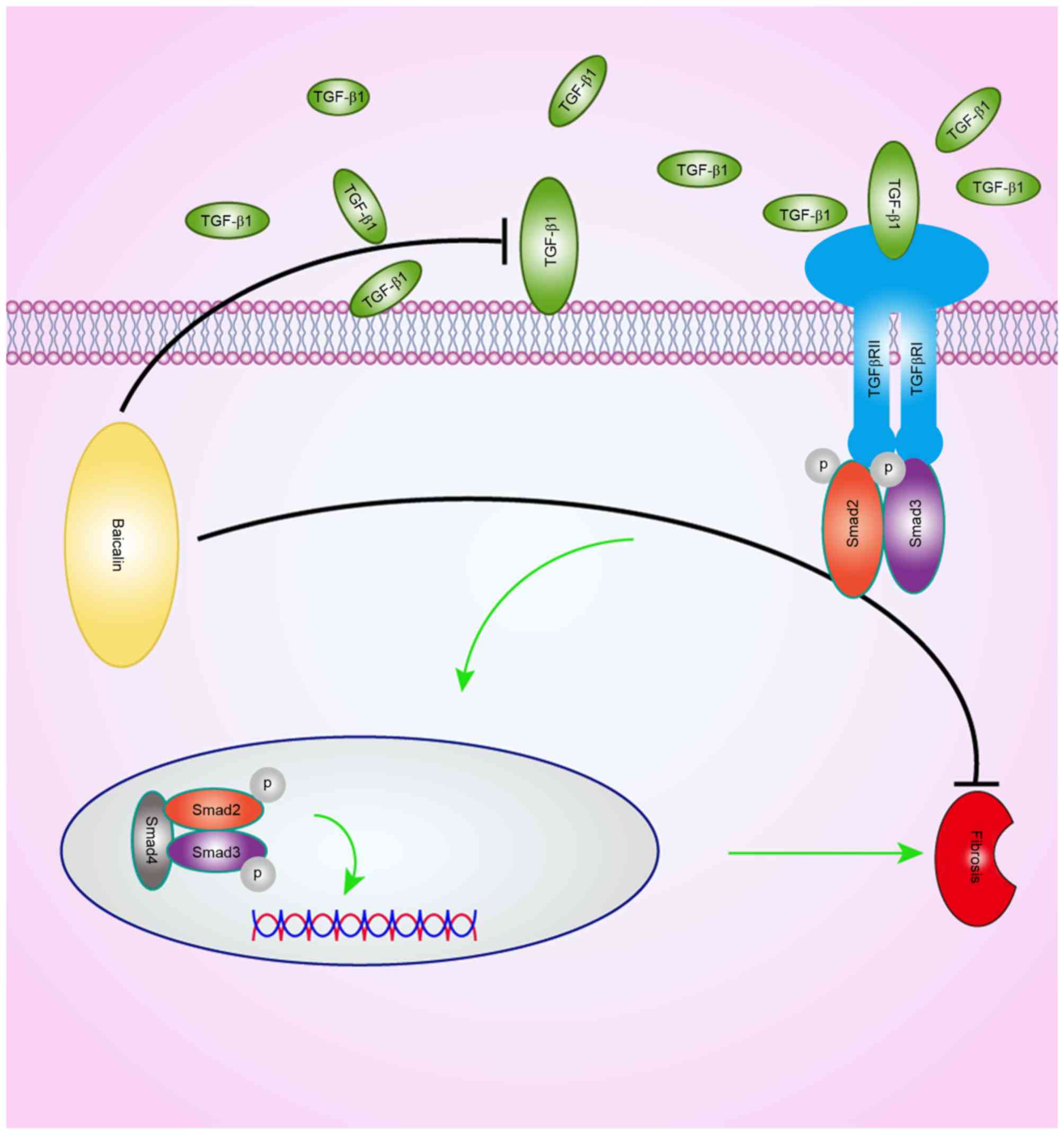
signal transduction. A schematic of the potential effects of
baicalin on the TGF-β1 signaling pathway is presented in Fig. 6. Even in the presence of TGF-β1,
baicalin reduced the phosphorylation of Smad2/3 in
vitro.
The majority of chronic kidney diseases (CKDs) are
characterized by excessive ECM accumulation in the interstitium and
glomerulus, disrupting healthy kidney structure, decreasing the
glomerular infiltration rate and eventually resulting in renal
failure (3). The UUO-induced renal
fibrosis model has been frequently used to mimic the pathological
alterations of chronic obstructive nephropathy that are commonly
observed in patients with CKD (23). The present study demonstrated that
baicalin markedly protected against renal damage, reduced the area
of interstitial collagen deposition, and suppressed the
accumulation of the ECM components fibronectin and collagen I,
demonstrating its protective effect against renal fibrosis.
EMT, defined by the loss of epithelial
characteristics and the acquisition of a mesenchymal phenotype, has
been hypothesized to be a primary underlying mechanism of ECM and
fibrogenesis (24). Myofibroblasts
are regarded as the effector cells of renal fibrosis (3). During EMT, myofibroblast derivation
occurs within the epithelium. The transformation of renal tubular
epithelial cells to myofibroblasts results in the excessive
production and accumulation of ECM and eventually renal
fibrogenesis (25,26). A previous study demonstrated that
in the absence of EMT, the accumulation of myofibroblasts was
reduced and reversed (27),
revealing the crucial role of EMT in fibrogenesis. In the present
study, following UUO, baicalin was demonstrated to exert a
protective effect against the process of EMT, as evidenced by
upregulation of the epithelial markers N- and E-cadherin, and
downregulation of the mesenchymal markers α-SMA and vimentin.
TGF-β1, the most abundant subtype of the TGF-β
family, has been extensively studied as a mediator of fibrogenesis
(28). Accumulating evidence has
demonstrated that TGF-β1 serves a central role in the pathogenesis
of renal fibrosis in experimental models and human kidney diseases
(3). Various underlying mechanisms
have been reported, including induction of ECM production via
Smad-dependent canonical and non-canonical signaling pathways,
inhibition of ECM degradation via suppression/inhibition of matrix
metalloproteinases, and induction of the transdifferentiation of
renal tubular cells to myofibroblasts by EMT (3). Furthermore, Ding and Choi (29) reported that autophagy, which is
additionally regulated by TGF-β, was involved in renal fibrosis. In
the present study, the reduction of serum and kidney tissue TGF-β1
expression levels by baicalin treatment implied that baicalin
affects the expression levels of this pro-fibrotic cytokine.
The TGF-β1/Smad signaling pathway is regarded as a
crucial mediator of fibrogenesis (3). In the present study, the
significantly enhanced phosphorylation of Smad2/3 in the UUO group
compared with the sham group, and the decreased phosphorylation of
Smad2/3 in the baicalin group compared with the UUO group in
vivo, revealed the potential participation of the TGF-β1/Smad
signaling pathway in the amelioration of renal fibrosis by
baicalin. To further investigate whether the phosphorylation of
Smad2/3 may be decreased by baicalin in the presence of TGF-β1, the
effect of baicalin on the phosphorylation of Smad2/3 in NRK-52E
cells was examined under controlled concentrations of TGF-β1 in
vitro. In comparison with the TGF-β1 group, baicalin inhibited
the phosphorylation of Smad2/3. Furthermore, phosphorylation of
Smad2/3 reversed the process of EMT even in the presence of TGF-β1,
demonstrating that baicalin may ameliorate renal fibrosis even when
TGF-β1 is expressed. However, whether baicalin ameliorates renal
fibrosis by decreasing the binding of TGF-β1 to its receptors, or
by directly inhibiting the phosphorylation of Smad2/3 in
vitro, remains unknown and requires further investigation. In
conclusion, the present study demonstrated that baicalin may
ameliorate renal fibrosis in vivo, via reduction of TGF-β1
and its downstream signal transduction, and in the presence of
TGF-β1 in vitro. This suggested that baicalin may be a
potential novel therapeutic agent for the clinical prevention of
renal fibrosis.
Acknowledgements
The present study was supported by the Shanghai
Health and Family Planning Commission (grant no. 2014JQ008A) and
the National Natural Science Foundation of China (grant no.
81500568).
References
|
1
|
Wynn TA: Common and unique mechanisms
regulate fibrosis in various fibroproliferative diseases. J Clin
Invest. 117:524–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo Y, Li Z, Ding R, Li H, Zhang L, Yuan W
and Wang Y: Parathyroid hormone induces epithelial-to-mesenchymal
transition via the Wnt/β-catenin signaling pathway in human renal
proximal tubular cells. Int J Clin Exp Pathol. 7:5978–5987.
2014.PubMed/NCBI
|
|
3
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Becker LE, Weritz B, Yi X, Gross-Weissmann
ML, Waldherr R, Zeier M and Sommerer C: Evolution of allograft
fibrosis and function in kidney transplant recipients: A
retrospective analysis of stable patients under CNI and mTORi.
Transpl Int. 28:553–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li TT, Zhang XH, Jing JF, Li X, Yang XQ,
Zhu FH, Tang W and Zuo JP: Artemisinin analogue SM934 ameliorates
the proteinuria and renal fibrosis in rat experimental membranous
nephropathy. Acta Pharmacol Sin. 36:188–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lan HY: Tubular epithelial-myofibroblast
transdifferentiation mechanisms in proximal tubule cells. Curr Opin
Nephrol Hypertens. 12:25–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakao A, Imamura T, Souchelnytskyi S,
Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH,
Miyazono K and ten Dijke P: TGF-beta receptor-mediated signalling
through Smad2, Smad3 and Smad4. EMBO J. 16:5353–5362. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang
X, Min D, Sun H, Xie N and Cai J: Baicalin attenuates global
cerebral ischemia/reperfusion injury in gerbils via anti-oxidative
and anti-apoptotic pathways. Brain Res Bull. 85:396–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang YJ, Zhou FW, Luo ZQ, Li XZ, Yan HM,
Wang MJ, Huang FR and Yue SJ: Multiple therapeutic effects of
adjunctive baicalin therapy in experimental bacterial meningitis.
Inflammation. 33:180–188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu J, Wang J, Sheng Y, Zou Y, Bo L, Wang
F, Lou J, Fan X, Bao R, Wu Y, et al: Baicalin improves survival in
a murine model of polymicrobial sepsis via suppressing inflammatory
response and lymphocyte apoptosis. PLoS One. 7:e355232012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R
and Zhu T: The protective effect of baicalin against renal
ischemia-reperfusion injury through inhibition of inflammation and
apoptosis. BMC Complement Altern Med. 14:192014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng XD, Dai LL, Huang CQ, He CM and Chen
LJ: Correlation between anti-fibrotic effect of baicalin and serum
cytokines in rat hepatic fibrosis. World J Gastroenterol.
15:4720–4725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Q, Noor M, Wong YF, Hylands PJ,
Simmonds MS and Xu Q, Jiang D, Hendry BM and Xu Q: In vitro
anti-fibrotic activities of herbal compounds and herbs. Nephrol
Dial Transplant. 24:3033–3041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Satoh M, Kashihara N, Yamasaki Y, Maruyama
K, Okamoto K, Maeshima Y, Sugiyama H, Sugaya T, Murakami K and
Makino H: Renal interstitial fibrosis is reduced in angiotensin II
type 1a receptor-deficient mice. J Am Soc Nephrol. 12:317–325.
2001.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang DT, Huang RH, Cheng X, Zhang ZH, Yang
YJ and Lin X: Tanshinone IIA attenuates renal fibrosis and
inflammation via altering expression of TGF-β/Smad and NF-κB
signaling pathway in 5/6 nephrectomized rats. Int Immunopharmacol.
26:4–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Chen L, Zang J, Tang X, Liu Y, Zhang
J, Bai L, Yin Q, Lu Y, Cheng J, et al: C3a and C5a receptor
antagonists ameliorate endothelial-myofibroblast transition via the
Wnt/β-catenin signaling pathway in diabetic kidney disease.
Metabolism. 64:597–610. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Denby L, Ramdas V, Lu R, Conway BR, Grant
JS, Dickinson B, Aurora AB, McClure JD, Kipgen D, Delles C, et al:
MicroRNA-214 antagonism protects against renal fibrosis. J Am Soc
Nephrol. 25:65–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jia H, Chen XL, Chen C, Hu YY and Yun XJ:
Baicalin prevents the up-regulation of connective tissue growth
factor in fibrotic lungs of rats. Sheng Li Xue Bao. 62:535–540.
2010.(In Chinese). PubMed/NCBI
|
|
20
|
Liu P, Yan S, Chen M, Chen A, Yao D, Xu X,
Cai X, Wang L and Huang X: Effects of baicalin on collagen I and
collagen III expression in pulmonary arteries of rats with hypoxic
pulmonary hypertension. Int J Mol Med. 35:901–908. 2015.PubMed/NCBI
|
|
21
|
Qiao H, Tong Y, Han H, Xu W, Ren Z, Ouyang
J and Chen Y: A novel therapeutic regimen for hepatic fibrosis
using the combination of mesenchymal stem cells and baicalin.
Pharmazie. 66:37–43. 2011.PubMed/NCBI
|
|
22
|
Yang MD, Chiang YM, Higashiyama R, Asahina
K, Mann DA, Mann J, Wang CC and Tsukamoto H: Rosmarinic acid and
baicalin epigenetically derepress peroxisomal
proliferator-activated receptor γ in hepatic stellate cells for
their antifibrotic effect. Hepatology. 55:1271–1281. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W, Zhou PH, Xu CG, Zhou XJ, Hu W and
Zhang J: Baicalein attenuates renal fibrosis by inhibiting
inflammation via down-regulating NF-κB and MAPK signal pathways. J
Mol Histol. 46:283–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masszi A and Kapus A: Smaddening
complexity: The role of Smad3 in epithelial-myofibroblast
transition. Cells Tissues Organs. 193:41–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Connor JW and Gomez EW: Cell adhesion
and shape regulate TGF-beta1-induced epithelial-myofibroblast
transition via MRTF-A signaling. PLoS One. 8:e831882013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeisberg M and Kalluri R: The role of
epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med
(Berl). 82:175–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Shultz RW, Mars WM, Wegner RE, Li
Y, Dai C, Nejak K and Liu Y: Disruption of tissue-type plasminogen
activator gene in mice reduces renal interstitial fibrosis in
obstructive nephropathy. J Clin Invest. 110:1525–1538. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lebrin F, Deckers M, Bertolino P and Ten
DP: TGF-beta receptor function in the endothelium. Cardiovasc Res.
65:599–608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding Y and Choi ME: Regulation of
autophagy by TGF-β: Emerging role in kidney fibrosis. Semin
Nephrol. 34:62–71. 2014. View Article : Google Scholar : PubMed/NCBI
|