Introduction
Lipolysis refers to the breakdown of lipids, and
involves the hydrolysis of triglycerides into glycerol and free
fatty acids (FFAs). To maintain glucose homeostasis, the secretion
of glucagon, epinephrine and norepinephrine increases, which
stimulates lipase secretion to promote lipolysis. In addition,
increases in levels of tumor necrosis factor (TNF)-α produced by
adipose cells promote lipolysis in metabolic diseases including
obesity. High rates of lipolysis in adipose tissues leads to the
excess production of FFAs, which may be important components of
insulin resistance and metabolic syndrome.
The majority of research on lipolysis has focused on
lipases. A previous study demonstrated that the protein perilipin-A
contributes to lipolysis by adjusting lipase activity. The primary
underlying mechanisms of the action of perilipin-A are not known;
however, a negative association between perilipin-A content and
lipase activity in human adipose cells has been identified
(1). TNF-α may inhibit perilipin-A
expression levels via the c-Jun N-terminal kinase (JNK) signaling
pathway, which promotes lipolysis and induces insulin resistance
(2). Ozcan et al (3) revealed that endoplasmic reticulum
(ER) stress (ERS) serves an important role in metabolic disease.
ERS often results in the unfolded protein response (UPR);
therefore, markers of UPR may be used to estimate the extent of
ERS. Such marker proteins include three types of ER transmembrane
protein: Inositol-requiring enzyme (IRE) 1, protein kinase RNA-like
ER kinase (PERK) and activating transcription factor 6 (ATF6). In
addition, sensitivity to ERS is associated with
immunoglobulin-binding protein (BiP). JNK in the IRE signaling
pathway is considered a key factor that regulates insulin;
therefore, activation of the JNK signaling pathway is involved in
the pathogenesis of obesity, type-2 diabetes mellitus (T2DM) and
insulin resistance in the periphery (4). The present study hypothesized that
TNF-α may inhibit perilipin-A expression levels via activation of
ERS in 3T3-L1 adipocytes. A previous study demonstrated that
molecular chaperones including tauroursodeoxycholic acid (TUDCA)
may affect ER function, which alleviates the increased ERS observed
in obesity, and reverses insulin resistance and T2DM (5).
TUDCA is a hydrophilic bile acid that is normally
produced endogenously in the liver, and is used as a bile acid
replacement therapy for the treatment of cholestasis and
hepatocellular necrosis. Furthermore, TUDCA may inhibit PERK and
eukaryotic translation initiation factor 2A (eIF2a) phosphorylation
to reduce ER stress by facilitating correct protein folding.
The aim of the present study was to investigate the
effects of TUDCA on the lipolytic action of TNF-α in 3T3-L1
adipocytes, and the underlying mechanisms of action.
Materials and methods
Reagents
The 3T3-L1 preadipocyte cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA).
Dulbecco's modified Eagle's medium (DMEM) and trypsin were obtained
from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Fetal bovine serum was purchased from PAA Laboratories; GE
Healthcare Life Sciences (Chalfont, UK). Oil Red O, dexamethasone
and 1-methyl-3-isobuthylxanthine were purchased from Sigma-Aldrich;
Merck Millipore (Darmstadt, Germany). Insulin was obtained from
Novo Nordisk (Bagsværd, Denmark, Germany). TNF-α was purchased from
PeproTech, Inc. (Rocky Hill, NJ, USA). TUDCA was obtained from
Sigma-Aldrich, Merck Millipore. A glycerol-3-phosphate
oxidase-peroxidase assay kit was obtained from Chaoyan
Biotechnology Co., Ltd. (Shanghai, China). A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay kit was purchased from Beyotime Institute of Biotechnology
(Haimen, China).
Antibodies
The primary antibodies used were rabbit anti-mouse
β-actin monoclonal antibody (catalog no. BA2305; BOSTER, Wuhan,
Hubei, China). Bovine anti-mouse Perilipin-A antibody (catalog no.
PB1061; BIOSS, Beijing, China). Rabbit anti-mouse phosphorylated
(p)-IRE polyclonal antibody (catalog no. ab48187; Abcam, Cambridge,
UK). Rabbit anti-mouse IRE polyclonal antibody (catalog no.
ab37037; Abcam). Rabbit anti-mouse BIP monoclonal antibody (catalog
no. 3183; Cell Signaling Technology Inc., Danvers, MA, USA). Rabbit
anti-mouse JNK polyclonal antibody (catalog no. 9252; Cell
Signaling Technology Inc.). Rabbit anti-mouse p-JNK monoclonal
antibodies (catalog no. 9251; Cell Signaling Technology Inc.). The
secondary antibodies used were horseradish peroxidase-conjugated
anti-rabbit IgG (catalog no. ZDR-5401; ZSGB-Bio, Beijing, China).
Horseradish peroxidase-conjugated anti-bovine IgG (catalog no.
ZDR-5105; ZSGB-Bio).
Cultivation and differentiation of
3T3-L1 preadipocytes
Cultivation and differentiation of 3T3-L1
preadipocytes was performed as previously described by Wang et
al (6).
Determination of cell viability by MTT
assay
Cells were counted and seeded into 96-well culture
plates at ~100 µl/well (~5×104 cells). The method previously
described by Wang et al (6)
was used to induce production of 3T3-L1 preadipocytes following
fusion. TNF-α (0.1, 1, 10, 50 or 100 ng/ml) and control (untreated)
was added to 3T3-L1 adipocytes for 24 h. Following this, 10 µl MTT
solution (5 mg/ml) was added to each well and the plates incubated
at 37°C for 4 h. Following incubation, the remaining MTT solution
was removed and dimethyl sulfoxide was added to each well to
dissolve the formazan crystals. Plates were agitated for 10 min on
a shaker to ensure adequate solubility. Absorbance readings for
each well on plates were performed at a wavelength of 490 nm. Cell
viability was subsequently calculated. All experiments were carried
out in triplicate. From the procedures described above, 50 ng/ml
was determined to be the optimal concentration of TNF-α; therefore,
3T3-L1 adipocytes were subsequently treated with TNF-α at this
concentration for 0, 2, 4 and 6 h.
Experimental groups
The present study used six well plates and seeded
3T3-L1 cells at 80% density.
For western blot analysis following TNF-α treatment,
3T3-L1 adipocytes were divided into four groups: A control group,
treated with serum-free DMEM media only, or experimental groups
treated with 50 ng/ml TNF-α for 2, 4 or 6 h.
To assess the impact of TUDCA pretreatment on the
effects of TNF-α treatment, cells were divided into four groups:
Control, treated with serum-free DMEM media only; TNF-α, treated
with TNF-α at 50 ng/ml for 4 h; TUDCA + TNF-α, cultured in
serum-free medium for 12 h after which 1 mmol/l TUDCA was added,
incubated for 3 h, and 50 ng/ml TNF-α was added for a further 4 h;
and TUDCA, cultured in serum-free medium for 12 h and 1 mmol/l
TUDCA was added for 3 h followed by culture for 4 h.
Lipolysis measurement
Lipolysis was evaluated in 3T3-L1 adipocytes from
different groups by measuring the amount of glycerin released into
the medium using the glycerol-3-phosphate oxidase-peroxidase assay,
following a 4 h incubation period. The enzyme label plate contained
five wells/group. The microplate spectrophotometer and 96-well
enzyme label plate were used to measure the absorbance. In order to
create a standard curve, five wells in every group were set in a
96-well enzyme label plate Samples (50 µl) 1×106/ml were incubated
with 150 µl operating fluid from the aforementioned glycerol assay
kit for 15 min at 37°C. The absorbance was measured at a wavelength
of 550 nm using an automatic microplate spectrophotometer (BioTek
Instruments, Inc., Winooski, VT, USA). A standard curve was used to
calculate the glycerin concentration.
Staining with Oil Red O
3T3-L1 adipocytes were washed three times with PBS
and fixed with paraformaldehyde. Each well in the 6-well culture
plates was stained with 1.5 ml diluted Oil Red O. A total of 0.05 g
Oil red powder was dissolved in 10 ml isopropanol as storage
liquid. Following this, 6 ml storage liquid was diluted into 10 ml
with distilled water on standby prior to use, for 40 min and rinsed
twice with 60% isopropanol. Following a further three washes with
PBS, the forms of intracellular lipid droplets were observed under
a microscope.
Western blotting
Cells were lysed with radiommunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology) for 20 min on
ice. Cell lysates were centrifuged at 4°C, 12,000 × g for 20 min
and supernatants were saved for analysis. Protein samples (2 mg/ml)
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis. Following separation, proteins were transferred
onto polyvinylidene difluoride membranes. Membranes were blocked
with 5% skimmed milk powder at room temperature, incubated
overnight at 4°C with different primary antibodies: Perilipin A
(1:1,000), IRE (1:500), p-IRE (1:500), Bip (1:1,000), JNK
(1:1,000), p-JNK (1:1,000), β-actin (1:1,000) and membranes washed
three times with TBS containing Tween-20 (TBST). The membranes were
subsequently incubated with horseradish peroxidase-conjugated
secondary anti-bovine IgG antibody (Perilipin, 1:2,000) and
anti-rabbit IgG antibody (IRE, 1:500; p-IRE, 1:500; Bip, 1:1,000;
JNK, 1:1,000; p-JNK, 1:1,000; β-actin, 1:1,000) for 1 h at room
temperature. Membranes were washed three times with TBST,
visualized by Enhanced Chemiluminescence (EMD Millipore, Billerica,
MA, USA) and quantified using Bandscan analysis software version
5.0 (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
Statistical analyses were performed using SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Significance was
assessed by one-way analysis of variance and the least-significant
difference test, as the post hoc test for multiple group
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
TNF-α influences the survival of
3T3-L1 adipocytes
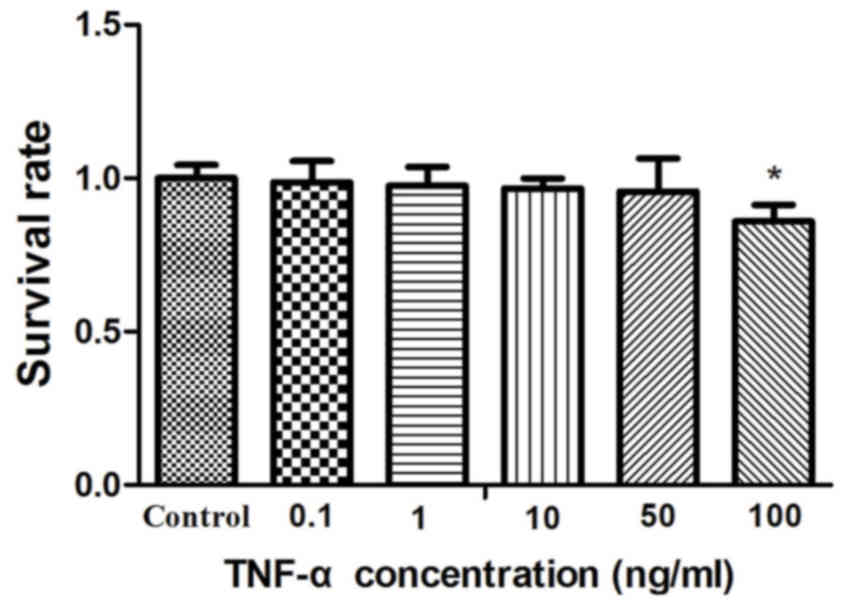
Following treatment with 0.1, 1, 10 or 50 ng/ml
TNF-α for 24 h, cell survival decreased as TNF-α concentration
increased (0.9871±0.069, P=0.6662; 0.9753±0.062, P=0.3740;
0.9669±0.032, P=0.0523 and 0.9568±0.108, P=0.3722 respectively;) in
3T3-L1 adipocytes, compared with the control group (the value of
control group was 1.0000±0.043). Cell survival was significantly
decreased following treatment with 100 ng/ml TNF-α for 24 h
(0.8598±0.053; P<0.01) compared with the control group (Fig. 1). Thus, the concentration of 50
ng/ml was selected for further experiments.
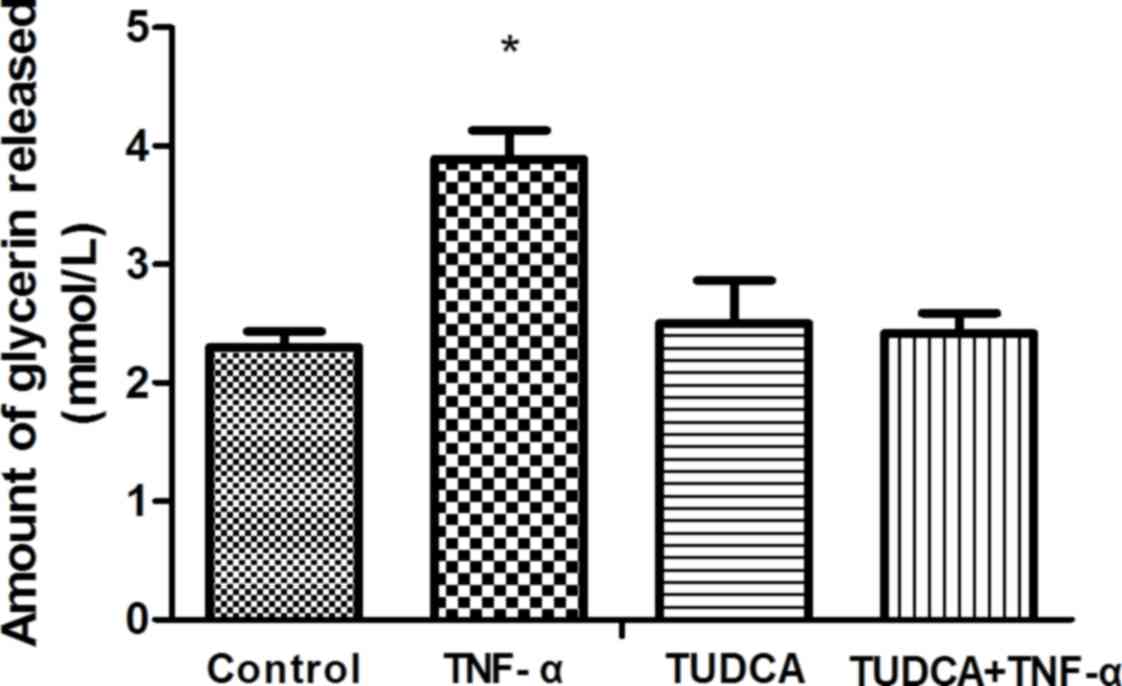
TUDCA inhibits TNF-α-induced lipolysis
in 3T3-L1 adipocytes
Lipolysis was evaluated by measuring the quantity of
glycerin released into the medium. Glycerin content in the TNF-α
group (3.890±0.240) was significantly increased compared with the
control group (2.303±0.131; P<0.0001). Glycerin content in the
TUDCA+TNF-α group (2.417±0.174) was significantly reduced compared
with the TNF-α group (P<0.0001). No significant differences were
observed in glycerin content between the control and TUDCA+TNF-α
groups (P=0.2755; Fig. 2).
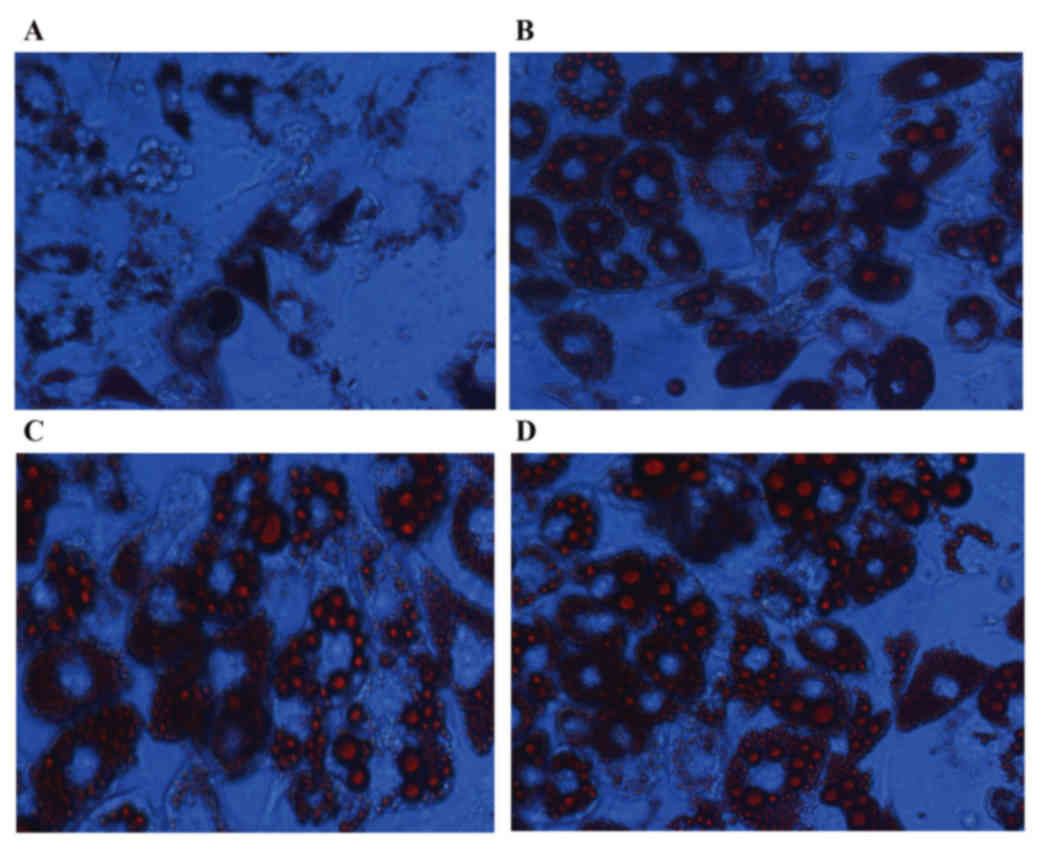
Forms of intracellular lipid droplets
in 3T3-L1 adipocytes
Lipid droplets were dispersed in the TNF-α group
(Fig. 3A) following treatment with
TNF-α for 24 h, compared with the control group. However, this
degree of dispersion was not observed in the TUDCA (Fig. 3B) or TUDCA+TNF-α (Fig. 3C) groups, compared with the control
group (Fig. 3D).
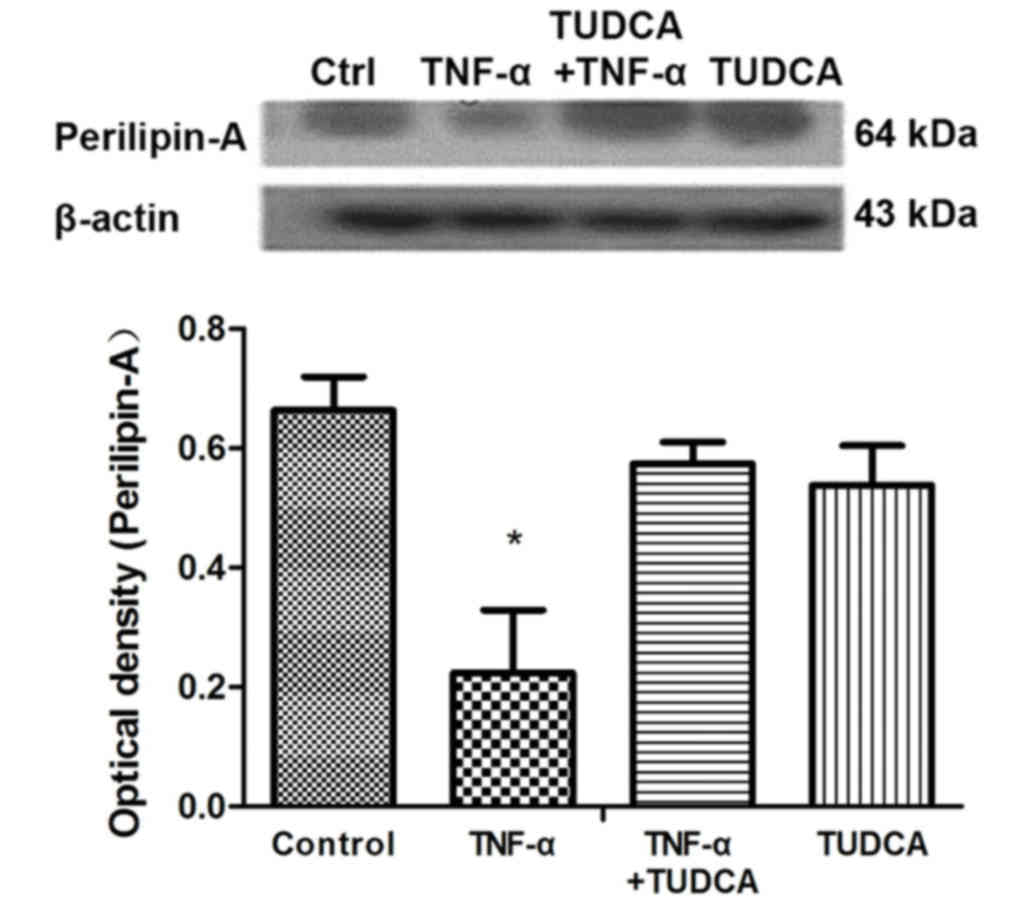
TUDCA inhibits TNF-α-induced
expression of perilipin-A in 3T3-L1 adipocytes
Western blotting revealed that perilipin-A protein
expression levels in 3T3-L1 adipocytes were significantly reduced
in the TNF-α group compared with the control group (P=0.0031);
however, they were not altered in the TUDCA+TNF-α group compared
with the control group (P=0.0792; P=0.0055 compared with the TNF-α
group; Fig. 4).
TNF-α induces ERS in a time-dependent
manner in 3T3-L1 adipocytes
Following treatment with 50 ng/ml TNF-α for 0, 2, 4
and 6 h, western blotting revealed that the expression of marker
proteins of ERS (BiP, p-IRE and p-JNK) was induced by TNF-α, and
that their expression levels peaked at 4 h (BiP at 4 h, P=0.0026
P=0.0099, P=0.0073 compared with 0, 2 and 6 h groups; p-IRE at 4 h,
P=0.0003, P=0.0003, P=0.0009 compared with 0, 2 and 6 h groups;
p-JNK at 4 h, P<0.0001, P=0.0001, P=0.0045 compared with 0, 2
and 6 h groups) and began to decline at 6 h (BiP at 6 h, P=0.0073
compared with 4 h groups; p-IRE at 6 h, P=0.0009 compared with 4 h
groups; p-JNK at 6 h, P=0.0045 compared with 4 h groups; Fig. 5). Therefore, TNF-α treatment for 4
h was selected to assess the effect of TUDCA on the expression
levels of these proteins.
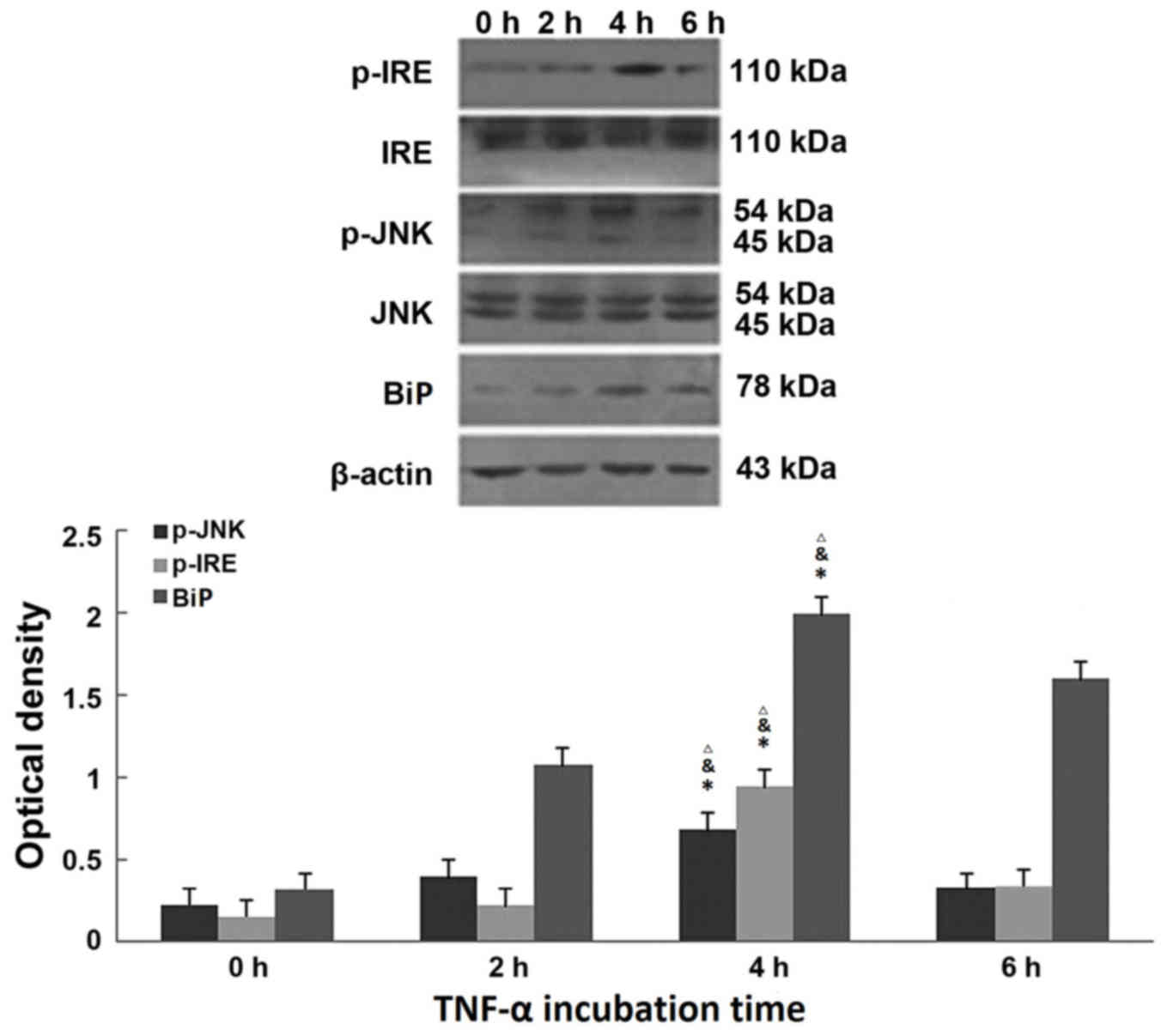 | Figure 5.TNF-α influences expression of the
endoplasmic reticulum stress marker proteins BiP, p-IRE and p-JNK.
Representative western blot images and quantification of p-IRE,
IRE, p-JNK, JNK and BiP protein expression levels in 3T3-L1
adipocytes, following treatment with 50 ng/ml TNF-α for 0, 2, 4 or
6 h. β-actin served as an internal control. Data are presented as
the mean ± standard deviation. *P<0.01. vs. 0 h treated group;
&P<0.01 vs. 2 h treated group;
∆P<0.01 vs. 6 h treated group. p, phosphorylated;
JNK, c-Jun N-terminal kinase; BiP, immunoglobulin-binding protein;
IRE, inositol-requiring enzyme; TNF-α, transforming growth
factor-α. |
TUDCA inhibits TNF-α-induced ERS in
3T3-L1 adipocytes
Western blotting revealed that the greatest
expression levels of marker proteins of ERS (BiP, p-IRE and p-JNK)
were present in the TNF-α group (P=0.0003, P=0.0027, P=0.0017
compared with the control group). The expression levels of these
three proteins were reduced in the TUDCA+TNF-α group compared with
the TNF-α group (P=0.0003, P=0.0005, P=0.0011; P>0.05 compared
with the control group; Fig.
6).
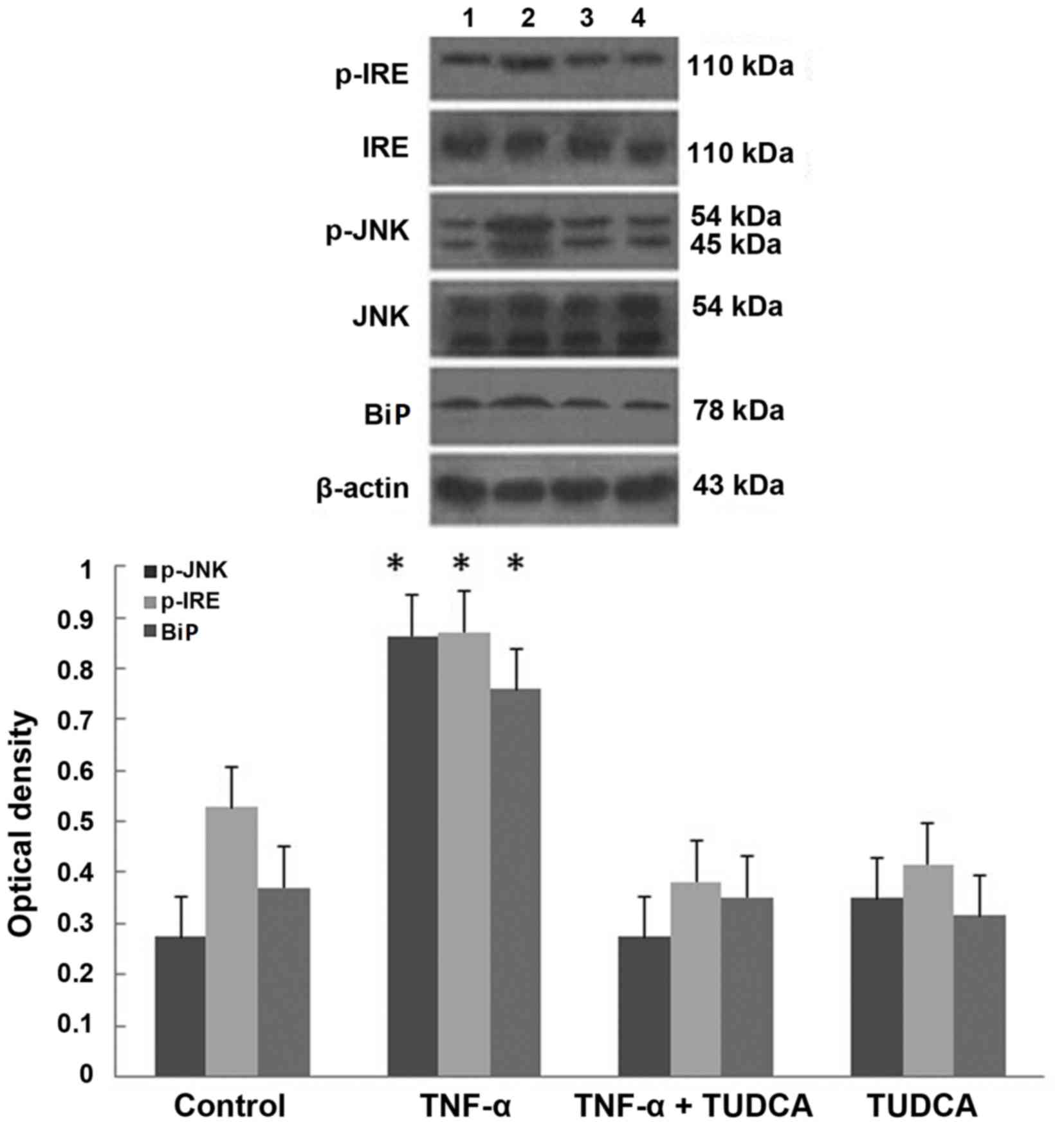 | Figure 6.TUDCA inhibits TNF-α-induced
endoplasmic reticulum stress in 3T3-L1 adipocytes. Representative
western blot images and quantification of p-IRE, IRE, p-JNK, JNK
and BiP protein expression levels in 3T3-L1 adipocytes treated with
TNF-α, TNF-α+TUDCA, or TUDCA. Data are presented as the mean ±
standard deviation. *P<0.01 vs. control and TUDCA +TNF-α groups.
TUDCA, tauroursodeoxycholic acid; p, phosphorylated; JNK, c-Jun
N-terminal kinase; BiP, immunoglobulin-binding protein; IRE,
inositol-requiring enzyme; TNF-α, transforming growth factor-α. |
Discussion
Obesity and T2DM are primary global health concerns,
associated with disorders of lipid metabolism and accompanied by
increased levels of FFAs. Prolonged exposure to FFAs causes reduced
glucose-stimulated insulin secretion, and apoptosis of beta cells
in the pancreas (7). An increase
in lipolysis causes levels of FFAs to increase.
The present study demonstrated that increased levels
of TNF-α promote lipolysis in terms of intracellular lipid droplets
and glycerin content. TNF-α may increase lipolysis via the nuclear
factor-κB (NF-κB)-JNK and p44/42 signaling pathways (8). Perilipin-A has been the primary
target of various mechanistic studies involving lipolysis (9); perilipin-A expression has previously
been demonstrated to be blocked by inhibitors of NF-κB-JNK and
p44/42 signaling pathways (10).
Perilipin-A surrounds lipid droplets in adipocytes and restricts
the access of lipases to the triglyceride core within the droplet,
thus inhibiting lipolysis. However, perilipin-A additionally
regulates lipase phosphorylation, thereby enhancing lipolytic
reactions. A previous study revealed that perilipin-A-null mice
exhibit reduced adipose mass, increased basal lipolysis in adipose
tissues, and increased expression and activity of hormone-sensitive
and adipose triglyceride lipases (11). Increased lipolytic activity may
accelerate the efflux of FFAs from adipose tissues to the
bloodstream, and therefore insulin resistance in perilipin-A null
mice (11).
Obesity and T2DM are additionally closely associated
with ERS. ERS is the accumulation of un- or misfolded proteins
resulting from various causes in ER lumina. Signal transduction of
ERS is mediated by IRE1, PERK and ATF6, which combine with BiP to
remain inactive under non-pathologic conditions. In the presence of
ERS, these proteins may transduce ERS signals from the membrane to
the nucleus by oligomerization and phosphorylation. IRE (a type-1
transmembrane protein that contains silk threonine kinase and
ribonuclease domains) increases JNK phosphorylation upon
activation.
Low-level inflammation is present in obesity and
T2DM; inflammatory factors are produced primarily in adipose
tissue. TNF-α may induce ERS in L929 murine fibrosarcoma cells
after 4 h to increase levels of the ERS marker proteins BiP, ATF6
and PERK (12). To ascertain if
ERS is involved in TNF-α-stimulated lipolysis in 3T3-L1 adipocytes,
the present study assessed the survival of 3T3-L1 adipocytes
treated with different concentrations of TNF-α for 24 h. The
concentration of 50 ng/ml TNF-α was selected for use in the present
study. Following treatment, expression levels of ERS marker
proteins were measured at various time points. The present study
revealed significant differences after 2-h treatment, compared with
the control group, and the expression levels of the ERS marker
proteins BiP, IRE and JNK increased in a time-dependent manner,
peaking at 4 h and declining at 6 h. Thus, treatment with 50 ng/ml
TNF-α for 4 h was selected for TUDCA intervention experiments.
Ozcan et al (5) were the first to propose that TUDCA
may alter ER function, alleviating the increased ERS observed in
obesity, and reversing insulin resistance and T2DM. Jiao et
al (13) demonstrated that
TUDCA may inhibit FFA-induced ERS and inflammation to improve
insulin sensitivity. TUDCA has previously been demonstrated to
reverse obesity-associated metabolism alterations in C57BL/6 mice,
including glucose intolerance, systemic and hepatic insulin
resistance, hyperinsulinemia, and increases in blood pressure
(14). Based on this information,
the present study selected to use TUDCA to further investigate the
effects of ERS on FFA-induced lipolysis.
Based on previous literature, and a previous
experiment (data not shown), the present study selected to treat
cells with 1 mmol/l TUDCA for 3 h in vitro to ensure cell
survival (15). The present study
demonstrated that glycerin content in the TUDCA+TNF-α group was
reduced significantly, and that dispersal of lipid droplets in the
TUDCA+TNF-α group was reduced, compared with the TNF-α group.
Therefore, TUDCA may inhibit the downregulation of perilipin-A
expression. Additionally, the present study revealed that
expression levels of the ERS marker proteins BiP, p-IRE and p-JNK
in 3T3-L1 adipocytes reduced significantly following pretreatment
with TUDCA. Thus, TUDCA may alleviate TNF-α-induced ERS.
In conclusion, TUDCA may inhibit TNF-α-induced
lipolysis in 3T3-L1 adipocytes. The underlying mechanism of action
is potentially associated with the inhibition of the IRE-JNK
signaling pathway, which affects perilipin-A expression levels. The
underlying mechanisms of action of TNF-α-induced lipolysis merit
further studies to identify potential targets for therapy against
obesity, dyslipidemia, insulin resistance and DM. TUDCA may reduce
insulin resistance and improve insulin sensitivity. Furthermore, it
may alleviate insulin ER stress and protect microvessels.
Therefore, TUDCA may act as a potential therapeutic agent for the
treatment of obesity and T2DM in the future.
Acknowledgements
The present study was supported by the National and
Fujian Province's Key Clinical Specialty Discipline Construction
Programs (grant no. 2015-SLN-11).
References
|
1
|
Zhang HH, Halbleib M, Ahmad F, Manganiello
VC and Greenberg AS: Tumor necrosis factor-alpha stimulates
lipolysis in differentiated human adipocytes through activation of
extracellular signal-related kinase and elevation of intracellular
cAMP. Diabetes. 51:2929–2935. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hickenbottom SJ, Kimmel AR, Londos C and
Hurley JH: Structure of a lipid droplet protein; the PAT family
member TIP47. Structure. 12:1199–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaneto H, Katakami N, Kawamori D,
Miyatsuka T, Sakamoto K, Matsuoka TA, Matsuhisa M and Yamasaki Y:
Involvement of oxidative stress in the pathogenesis of diabetes.
Antioxid Redox Signal. 9:355–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chaperones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Zhang W, Liu X, Huang P and Liu L:
The Development of Dexamethasone Induced Insulin Resistance in
3T3-L1 Adipocyte. J Fujian Med Univ. 41:282–284. 2007.
|
|
7
|
Lupi R, Dotta F, Marselli L, Del Guerra S,
Masini M, Santangelo C, Patané G, Boggi U, Piro S, Anello M, et al:
Prolonged exposure to free fatty acids has cytostatic and
pro-apoptotic effects on human pancreatic islets: Evidence that
beta-cell death is caspase mediated, partially dependent on
ceramide pathway, and Bcl-2 regulated. Diabetes. 51:1437–1442.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bézaire V, Mairal A, Anesia R, Lefort C
and Langin D: Chronic TNFalpha and cAMP pre-treatment of human
adipocytes alter HSL, ATGL and perilipin to regulate basal and
stimulated lipolysis. FEBS Lett. 583:3045–3049. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Xun K, Chen L and Wang Y:
TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct.
27:407–416. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rydén M, Arvidsson E, Blomqvist L, Perbeck
L, Dicker A and Arner P: Targets for TNF-alpha-induced lipolysis in
human adipocytes. Biochem Biophys Res Commun. 318:168–175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhai W, Xu C, Ling Y, Liu S, Deng J, Qi Y,
Londos C and Xu G: Increased lipolysis in adipose tissues is
associated with elevation of systemic free fatty acids and insulin
resistance in perilipin null mice. Horm Metab Res. 42:247–253.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue X, Piao JH, Nakajima A, Sakon-Komazawa
S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H and Nakano H:
Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein
response (UPR) in a reactive oxygen species (ROS)-dependent fashion
and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem.
280:33917–33925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiao P, Ma J, Feng B, Zhang H, Diehl JA,
Chin YE, Yan W and Xu H: FFA-induced adipocyte inflammation and
insulin resistance: Involvement of ER stress and IKKβ pathways.
Obesity (Silver Spring). 19:483–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Purkayastha S, Zhang H, Zhang G, Ahmed Z,
Wang Y and Cai D: Neural dysregulation of peripheral insulin action
and blood pressure by brain endoplasmic reticulum stress. Proc Natl
Acad Sci USA. 108:2939–2944. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malo A, Krüger B, Seyhun E, Schäfer C,
Hoffmann RT, Göke B and Kubisch CH: Tauroursodeoxycholic acid
reduces endoplasmic reticulum stress, trypsin activation, and
acinar cell apoptosis while increasing secretion in rat pancreatic
acini. Am J Physiol Gastrointest Liver Physiol. 299:G877–G886.
2010. View Article : Google Scholar : PubMed/NCBI
|