Introduction
Worldwide, lung cancer caused the mortality of 1.5
million people in 2010, with an overall 5-year survival of 16% in
the USA and <10% in the UK (1).
The primary types of lung cancer are small-cell lung cancer (SCLC)
and non-small cell lung cancer (NSCLC). The majority of lung
cancers (80%) are NSCLC (2); of
these patients, >65% were diagnosed with locally advanced or
metastatic disease (3). Therefore,
NSCLC is a malignancy with poor prognosis. Despite advances in
chemotherapy, further investigation is required to identify novel
therapeutic agents to reduce mortality.
Bile is a product of vertebrate liver cells. It
contains high levels of bile acids, including chenodeoxycholic
acid, ursodeoxycholic acid, cholic acid and deoxycholic acid
(4). Bile acids are the main
components of bile (50–70%) and have important physiological
functions in organisms. Additionally, bile acids act as a valuable
biosurfactant and may form several supramolecular self-assemblies,
including micelles and vesicles, which possess potential drug
delivery properties.
Animal bile as a natural product has been used as
traditional medicine without side effects for thousands of years in
China. Since the 1980s, bile and bile acids have received extensive
attention in the fields of chemistry and medicine (5). The medicinal value of animal bile,
such as bear and snake bile, have been recognized for their
immunity enhancing, anti-inflammatory, anti-convulsion and
analgesic effects (6). The
medicinal value of bile has recently investigated in cancer
research fields. It has been determined that animal bile has
anti-cancer activity (7).
The crocodile is an ancient vertebrate animal, which
has existed for >200 million years. Currently, systematic
research on the crocodile, particularly regarding aspects of
medicinal value, has indicated the crocodile may have various
unexplored uses. Previous research on the use of Siamese crocodile
bile (SCB) has been limited; therefore, the present study aimed to
identify its biological activity and particularly the anti-cancer
activity of SCB. The Siamese crocodile is a freshwater crocodile
primarily located in South East Asia. Siamese crocodile populations
have declined greatly due to commercial hunting for the leather
industry. The species was granted ‘Critically Endangered’ status by
the International Union for Conservation of Nature Red List in 1996
(8), conservation measures, such
as improved habitat management have been put in place in order to
stabilize the existing populations. As Siamese crocodile farming is
legal and widespread in the south of China any future use of SCB
should not affect the wild populations and allow for sustainable
sourcing of SCB. Previous studies determined that SCB has an
anti-cancer effect against cholangiocarcinoma and hepatocarcinoma
cells (9,10). However, the potential use of SCB
against human NSCLC cells and the underlying mechanism of its
action have not been fully elucidated. Therefore, the present study
evaluated the effects of SCB on the apoptosis of NCI-H1299 human
NSCLC cells. To the best of our knowledge, this is the first study
to demonstrate that SCB inhibited the proliferation of NCI-H1299
human NSCLC cells and induced apoptosis via a mitochondria-mediated
intrinsic pathway in vitro. Additionally, it was observed
that SCB suppressed the NSCLC xenograft tumorigenesis. These
results suggested that SCB may be a potential therapeutic agent for
the treatment of human NSCLC.
Materials and methods
Reagents
Rhodamine 123 (Rh123), 3-(4,5-dimethylthiazol-2-yl)
2,5-diphenyl tetrazolium bromide (MTT), z-VAD-fmk and 2′,
7′-dichlorofluorescin diacetate (DCF-DA) were purchased from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany).
Annexin-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
Apoptosis Assay kit and Caspase-3 Activity Apoptosis Assay kit were
purchased from KeyGen Biotech Co., Ltd. (Nanjing, China). Cell
Mitochondria Isolation kit was purchased from Takara Bio Inc.
(Otsu, Japan). RPMI-1640 medium and fetal bovine serum (FBS) were
obtained from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). The primary antibodies for human B cell leukemia/lymphoma
(Bcl-2; cat. no. sc-7382; 1:1,000), Bcl2 associated X (Bax; cat.
no. sc-23959; 1:1,000), cytochrome c (cat. no. sc-13156;
1:1,000), apoptotic peptidase activating factor 1 (Apaf-1; cat. no.
sc-65890; 1:1,000), survivin, cytochrome c oxidase subunit 4
(COX IV; cat. no. sc-69359; 1:1,000), β-actin (cat. no. sc-8432;
1:1,000) and proliferating cell nuclear antigen (PCNA; cat. no.
sc-56; 1:1,000), vascular endothelial growth factor (VEGF; cat. no.
sc-7269; 1:1,000) used for immunohistochemistry (IHC) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
The antibodies for cleaved caspase-3 (cat. no. ab136812; 1:250;
Abcam, Cambridge, UK) and −9 (cat. no. 9501; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), cyclin D1 (cat. no.
ab134175; 1:5,000; Abcam), cyclin E1 (cat. no. 4129; 1:1,000; Cell
Signaling Technology, Inc.) and cyclin-dependent kinase 2 (Cdk2;
cat. no. 2546; 1:1,000; Cell Signaling Technology, Inc.).
Polyvinylidene difluoride (PVDF) membranes obtained from Merck
Millipore. Goat anti-rabbit and anti-mouse secondary antibodies
conjugated to horse-radish peroxidase (HRP) or FITC were purchased
from Tiangen Biotech Co., Ltd. (Beijing, China). Enhanced HRP-DAB
Chromogenic Substrate kit and Ultrasensitive SAP kit were purchased
from MaiXin Bio (Fuzhou, China). All remaining chemicals were
purchased from Sigma-Aldrich.
SCB preparation
Siamese crocodile gallbladders were supplied by
Sriracha Tiger Zoo Co., Ltd., (Sriracha, Thailand). The
gallbladders were sliced to obtain the fresh bile juice. The bile
juice was subsequently centrifuged at 10,000 × g for 30 min
at 4°C. The supernatant was pooled and vacuum dried into a powder.
The SCB powder was stored in aliquots at 4°C. Concentrations (w/v
in medium or normal saline) of SCB were used for the in
vitro and in vivo experiments.
Cell culture
NCI-H1299 human NSCLC cells were obtained from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI 1640
supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin
(100 µg/ml). The cells were incubated at 37°C in a humidified
atmosphere with 5% CO2.
Cell viability assay
Cell viability was determined using an MTT assay.
Briefly, cells were seeded in 96-well plates at a density of
5.0×103 cells/well. Following an overnight culture, the
cells were treated with increasing concentrations of SCB (6.25,
12.5, 25, 50, 75 and 100 µg/ml), the same volume medium was used
for the control. The treatment was applied for 12, 24 and 48 h.
Following treatment, 20 µl MTT (5 mg/ml) was added to each well and
the cells were incubated for another 4 h at 37°C. The medium was
subsequently removed and 150 ml DMSO was added to each well. The
absorbance of each well was recorded at 490 nm using a microplate
spectophotometer. All experiments were repeated at least three
times.
Cell colony formation assay
Cells were seeded at densities of 500, 1,000, 2,000
cells in 100 mm plates and divided into two groups. One group was
treated with normal medium as the control and the other group was
treated with 40 µg/ml SCB. After 2 weeks, the adherent cell
colonies were fixed with methanol for 15 min at room temperature
and then stained with Giemsa at a dilution of 1:10 for 10 min and
washed with PBS three times. Finally, the cell colony numbers were
counted.
Cell cycle analysis
NCI-H1299 cells were treated with different
concentrations of SCB (20, 40, 60 µg/ml) for 12, 24 and 48 h.
Following treatment, cells were harvested and washed with PBS. The
cells were centrifuged at 400 × g for 5 min at 10°C and the
supernatant was removed. The pellet was fixed in cold 70% ethanol
on ice for 30 min. The cells were washed twice and centrifuged
again at 400 × g for 5 min at 10°C. The pellet was
re-suspended in binding buffer. Subsequently, the cells were
treated with 50 µl RNase (stock 100 mg/ml) and 200 µl PI (stock
solution 50 µg/ml) and incubated at 37°C for 30 min without light.
The cell cycle stages were immediately analyzed by flow cytometry
using FlowJo version 9.0 (Tree Star, Inc., Ashland, OR, USA). For
each measurement, at least 20,000 cells were counted.
Transmission electron microscopy
(TEM)
NCI-H1299 cells with and without SCB treatment (20,
40 and 60 µg/ml for 48 h) were fixed with 2.5% glutaraldehyde in
0.1 M PBS (pH 7.4) overnight at 4°C and post-fixed in 1% osmium
tetraoxide for 30 min. Following washing with PBS, the cells were
progressively dehydrated in a 10% graded series of 50–100% ethanol
and propylene oxide and embedded in Epon 812 resin. The blocks were
cut into ultrathin sections (5 µm) using a microtome and the
sections were stained with saturated uranyl acetate and lead
citrate. The ultrastructure of cells and mitochondria were then
observed under a transmission electron microscope (JEM-2100HC;
Jeol, Ltd., Tokyo, Japan).
Cell apoptotic assay
NCI-H1299 cells were treated with different
concentrations of SCB (20, 40, 60 µg/ml) for up to 24 h. Following
the treatment, the cells were harvested, washed in PBS and
re-suspended in 100 µl Annexin-binding buffer. The suspension was
incubated with 5 µl Annexin V-FITC and 10 µl PI (working solution
in the aforementioned Annexin-FITC/PI Apoptosis Assay kit) for 15
min at room temperature in the dark. Following staining, 400 µl
Annexin-binding buffer was added and the cells were immediately
analyzed by flow cytometry using FlowJo version 9.0. For each
measurement, at least 20,000 cells were counted.
Quantification of reactive oxygen
species (ROS)
DCF-DA is a fluorogenic freely permeable tracer
specific for ROS. NCI-H1299 cells were plated in a 6-well plate at
a density of 1×106 cells/well. The cells were treated with
different concentrations of SCB (20, 40, 60 µg/ml) for 24 and 48 h.
Following treatment, the cells were incubated with 10 mM DCF-DA at
37°C for 30 min in the dark. Subsequently, the cells were harvested
and washed in PBS. ROS generation was expressed as mean
fluorescence intensity, which was detected by flow cytometry using
FlowJo version 9.0.
Quantification of mitochondrial
membrane potential (ΔΨm)
Rh123 was used to detect changes in the ΔΨm of
NCI-H1299 cells. NCI-H1299 cells were plated in 6-well plates at a
density of 2×105 cells/well. Following an overnight culture, the
cells were treated with 40 µg/ml SCB for up to 48 h. The cells
washed with PBS three times and then stained with Rh123 staining
solution at room temperature for 20 min in the dark and observed
under an ordinary inverted phase-contrast microscope (Olympus
Corporation, Tokyo, Japan).
The ΔΨm was quantified by flow cytometry. Following
SCB treatment (20, 40, 60 µg/ml) for 24 h, NCI-H1299 cells were
harvested, washed in PBS, and incubated with Rh123 (1 mg/ml) at
37°C in a 5% CO2 incubator for 20 min. The cells were
re-suspended in PBS. Subsequently, the ΔΨm was analyzed by flow
cytometry using FlowJo version 9.0 at an excitation wavelength of
488 nm and an emission wavelength of 530 nm.
Cytochrome c release assay
Mitochondria were isolated from the cells using the
aforementioned Cell Mitochondria Isolation kit according to the
manufacturer's protocol. Briefly, the cells were treated with SCB
(40 µg/ml) for different time periods (0, 12, 24, 48 h), harvested
and re-suspended in hypotonic buffer. Following the lysis of cells,
mitochondrial fractions were isolated by differential
centrifugation: Centrifuged at 700 × g for 10 min at 4°C,
the supernatant was collected and centrifuged at 12,000 × g
for 15 min at 4°C. Subsequently, the supernatant was removed, the
pellet washed and centrifuged at 12,000 × g for 5 min at 4°C.
Protein from the cytosolic and mitochondrial fractions of each
sample was analyzed by western blotting using an anti-cytochrome c
antibody.
Caspase-3 activation assay
A Caspase-3 Colorimetric Assay kit was used
according to the manufacturer's protocol to investigate the
caspase-3 activation following SCB treatment. Briefly, NCI-H299
cells were treated with SCB (20, 40, 60 µg/ml) for up to 48 h.
Following the treatment, the cells were harvested and lysed with a
RIPA lysis buffer on ice for 1 h. Cells were centrifuged at 10,000
× g for 1 min to obtain the lysate. The total protein
concentration was determined using the Coomassie brilliant blue
method. Enzymatic reactions were performed in a 96-well plate and
the same protein quantity of cell lysate was incubated with the
substrate for 4 h at 37°C. The absorbance was measured at 405
nm.
Western blot analysis
Western blot analysis was performed as previously
described (10). Protein (20 µg)
underwent SDS-PAGE and then transferred onto PVDF membranes. The
membranes were blocked by non-fat milk for 1 h and then incubated
with primary and subsequently secondary antibodies. The enhanced
chemiluminescence system was used to quantify protein
expression.
Xenograft tumor mouse model
All the protocols used were approved by the Xiamen
University Laboratory Animal Center (Xiamen, China). A total of 12
female athymic (BALB/c, nu/nu; 6-weeks old, weight, 20±5 g) nude
mice were used in the present study, purchased from Chinese Academy
of Sciences, Shanghai Institute for Animals (Shanghai, China). The
mice were maintained at 21°C, humidity 45% and light/dark cycle of
12 h. NCI-H1299 cells were harvested, washed in PBS, the cells were
then counted and suspended in fresh medium. Cells were diluted so
that 200 µl contained the required number of cells per injection.
Around 2×106 cells per mouse were injected subcutaneously into the
flank of BALB/c nude mice. When the tumor volume reached ~60 mm3,
the mice were divided into two groups randomly (6 mice per group).
One group received SCB (100 mg/kg) and the other group was used as
the control and received normal saline by intraperitoneal injection
daily for 5 days/week and 100 µl volume for each mouse. The dose of
SCB was selected based on our previous study about acute toxicity
and chronic toxicity (10). Mice
bearing xenograft tumors were monitored every day. The tumor volume
was measured once every 4 days using calipers. The tumor volume was
estimated according to the following formula: Tumor volume
(mm3)=L × W2/2; where L is the length and W
the width. Body weight was recorded once every 4 days; however, it
was monitored more frequently during the first 2 weeks in order to
identify potential drug-associated toxicity. Following 4 weeks of
treatment, the mice were sacrificed by CO2 (flow rate,
<2 L/min). The tumors were carefully removed, measured and
weighed individually.
Hematoxylin and eosin (H&E)
staining and IHC analysis
Tumors and internal organs (lung and liver) were
fixed in formalin and processed for H&E staining and IHC. The
samples were processed as previously described (11). The percentage of PCNA- and
VEGF-positive cells was calculated by counting the number of
positive-stained cells (crimson or brown color) and the total
number of cells in 5 randomly selected fields from each tumor at
×400 magnification.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis. Student's t test or one-way analysis of
variance were used to determine the significant differences between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
SCB inhibits cell proliferation and
colony-forming ability of NCI-H1299 human NSCLC cells
The cytotoxic activity of SCB against human NSCLC
NCI-H1299 cells was analyzed by MTT assay in vitro. The
cells were treated with indicated concentrations of SCB (6.25,
12.5, 25, 50, 75 and 100 µg/ml) for 12, 24 and 48 h. A clear
time-and dose-dependent cytotoxic inhibition was induced by SCB in
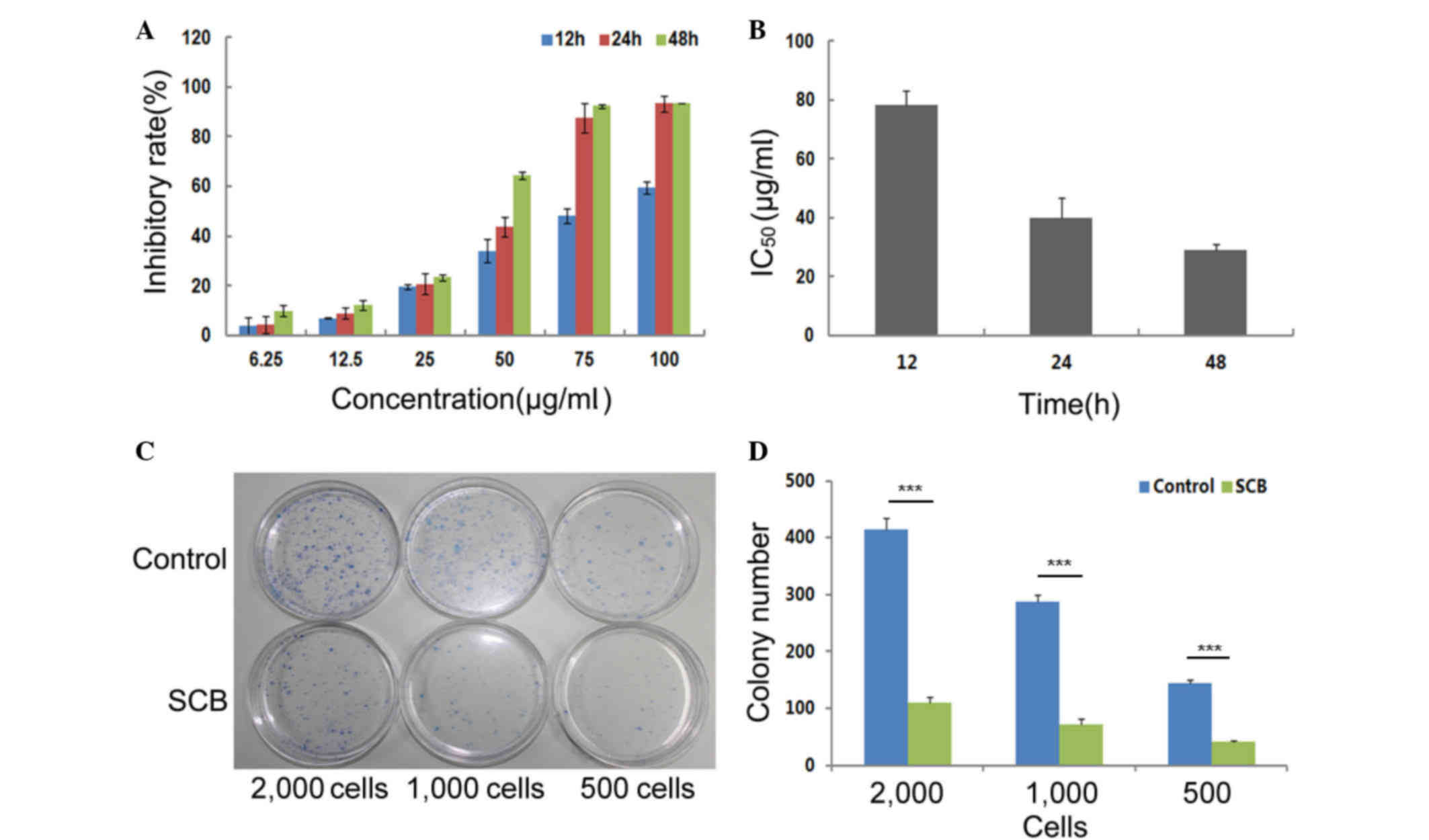
NCI-H1299 cells (Fig. 1A). Based
on the cell inhibitory rates, it was determined that SCB led to
complete and prolonged inhibition of NCI-H1299 cells growth up to
48 h, with a decreasing IC50 from 78.25 to 29.02 µg/ml
(Fig. 1B).
The effect of SCB on the colony-forming ability of
NCI-H1299 cells was also investigated. NCI-H1299 cells are normal
cancer cells, which are capable of adherence. An individual
adherent cell may grow and develop into a single colony on the
plastic surface of tissue culture dish. In the present assay,
different quantities of cells (500, 1,000 and 2,000) were seeded on
10 cm dishes and divided into two groups. The experimental group
was treated with 40 µg/ml SCB for 2 weeks. The control group was
treated with normal medium. Based on the colony numbers that were
counted, the colony-forming efficiency of the experimental group
was significantly reduced compared with the control group
(P<0.01; Fig. 1C and D).
SCB caused cell cycle arrest and
induced apoptotic cell death in NCI-H1299 cells
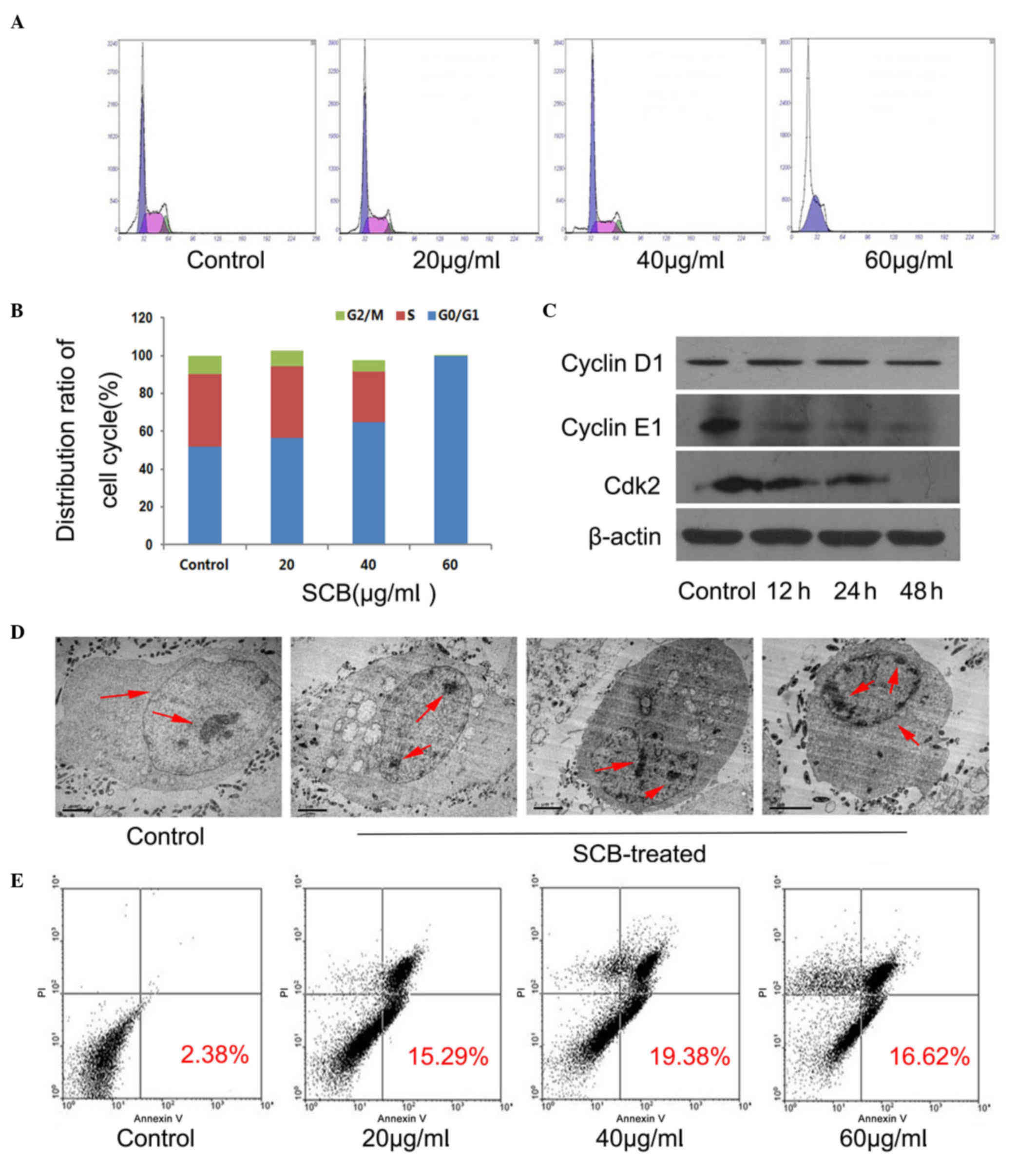
In order to determine whether the inhibition of
proliferation by SCB in NCI-H1299 cells was associated with cell
cycle arrest, cells were treated with different concentrations of
SCB (20, 40, 60 µg/ml) for 48 h. DNA content was detected by PI and
cell cycle distribution was analyzed by flow cytometry. The flow
cytometry results revealed that SCB may regulate the G1 phase and
arrest cell cycle at the G0/G1 phase in NCI-H1299 cells (Fig. 2A). Additionally, with increasing
concentrations of SCB, the cell population increased at G0/G1 phase
(almost 99% for 60 µg/ml SCB) and decreased at S and G2/M phase
accordingly (Fig. 2B). In order to
identify the underlying mechanism of SCB regulation of cell cycle
progression in NCI-H1299 cells, the protein expression levels of
three cell cycle-associated proteins that have been previously
identified as rate limiting for G0/G1 to S phase transition were
investigated (12). Western
blotting revealed that the expression levels of cyclin E1 and Cdk2
were reduced in NCI-H1299 cells after treatment (60 µg/ml for 48
h), whereas the expression of cyclin D1 remained unchanged
(Fig. 2C). Therefore, these
findings confirmed that SCB may arrest the cell cycle at G0/G1
phase and suppress cellular proliferation.
Apoptosis is the process of programmed cell death
that may occur in multicellular organisms (13); cells undergo apoptosis, the
morphology changes. Two different samples were prepared (control
and 40 µg/ml SCB treated for 48 h) and the morphological
characteristics of the cells in the samples were examined by TEM
and the ultrastructure of NCI-H1299 cells was clearly observed.
Normal cell morphology was evident in the control sample, with an
integrated cell nucleus and nuclear envelope. The nucleus was
hypertrophied and chromatin was diffuse. However, in the
SCB-treated sample, the typical apoptotic morphology was observed,
with cell body and nucleus shrinkage, condensed chromatin that was
separated and moved to the inside edge of nuclear envelope;
however, the nuclear membrane, plasma membrane and organelles were
intact (Fig. 2D, red arrows).
Subsequently, FITC-conjugated Annexin V and PI were used to
distinguish apoptotic and necrotic cells using flow cytometry. With
increased duration of SCB treatment, the cell population of early
and late apoptotic cells increased compared with the control
treatment (19.38% for 40 µg/ml 24 h); however, the necrotic cells
also increased at higher concentrations of SCB (Fig. 2E). Therefore, it was demonstrated
that SCB may induce apoptotic cell death in NCI-H1299 cells.
SCB caused mitochondrial dysfunction
in NCI-H1299 cells
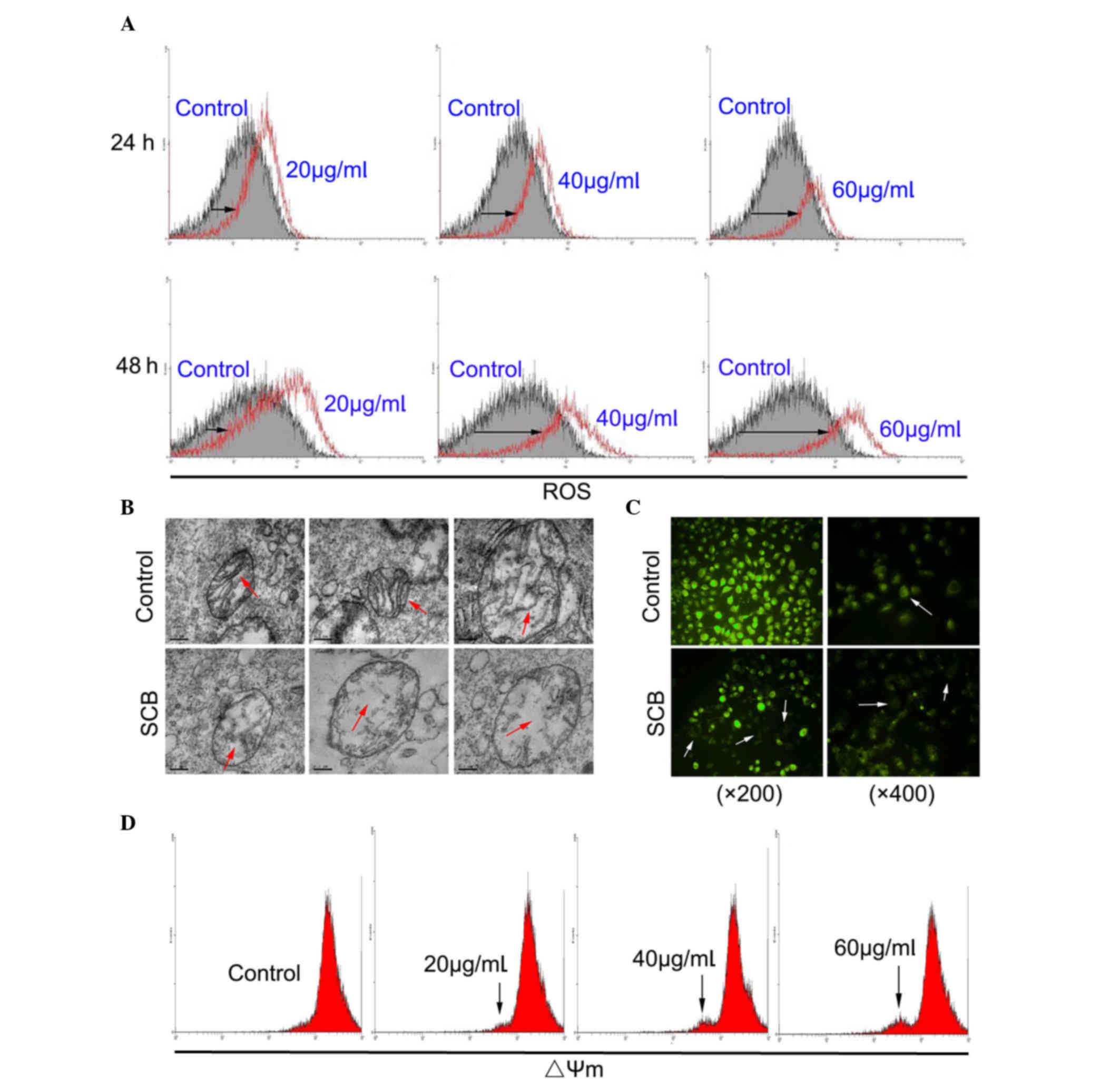
ROS are implicated in the mediation of apoptotic
cell death (14). In order to
investigate whether SCB-induced apoptosis of NCI-H1299 cells may be
associated with ROS generation, the intracellular ROS level was
examined by flow cytometry. ROS level of SCB-treated cells was
increased compared with the control in a time- and dose-dependent
manner (Fig. 3A; black arrows).
These findings demonstrated that SCB enhanced the intracellular ROS
level in NCI-H1299 cells. Mitochondria are the primary site of ROS
production; therefore, they are uniquely vulnerable to oxidative
damage (15). Oxidative damage
stimulates and leads to mitochondrial dysfunction (16). The ultrastructure of mitochondria
in NCI-H1299 cells was observed by TEM. Without SCB treatment, the
morphology of the mitochondria was normal, with a double membrane
and distinct cristae structure, which expands the inner
mitochondrial membrane. Following SCB treatment, the mitochondria
were damaged, with the organelle swollen and the cristae partially
fractured (Fig. 3B; red
arrows).
Mitochondrial dysfunction is frequently accompanied
by alteration of ΔΨm. Therefore, a reporter dye for mitochondria
was used to detect the ΔΨm in NCI-H1299 cells. Rh123 is a reporter
dye for mitochondria of living cells. The yellow-green fluorescence
intensity under the microscope reflected the ΔΨm level of
mitochondria (white arrows). As presented in Fig. 3C, SCB led to a marked reduction in
fluorescence intensity in NCI-H1299 cells, indicating a reduction
of highly energized mitochondria. The effect of SCB on the ΔΨm in
NCI-H1299 cells was also examined by flow cytometry. The result
revealed that with the increased dose of SCB (48 h) there was a
fluorescence peak (black arrows), which may indicate a collapse of
ΔΨm in NCI-H1299 cells in a dose-dependent manner (Fig. 3D).
SCB induces apoptosis in NCI-H1299
cells via an intrinsic pathway
The present study aimed to assess whether
SCB-induced apoptotic cell death occurred due to an intrinsic
pathway; therefore, the effects of SCB on intrinsic
pathway-associated factors were examined. The expression of Bax and
Bcl-2 following SCB treatment (40 µg/ml) was determined at
different time points. Western blotting revealed that SCB treatment
increased the ratio of Bax/Bcl-2 at the protein expression level
(Fig. 4A). The release of
cytochrome c may induce the activation of caspases and lead to
apoptotic cell death (17).
Therefore, cytochrome c expression level in NCI-H1299 cells was
examined by western blotting. Following SCB treatment, cytochrome c
expression level increased in the cytosol and decreased in
mitochondria in a time-dependent manner (Fig. 4B).
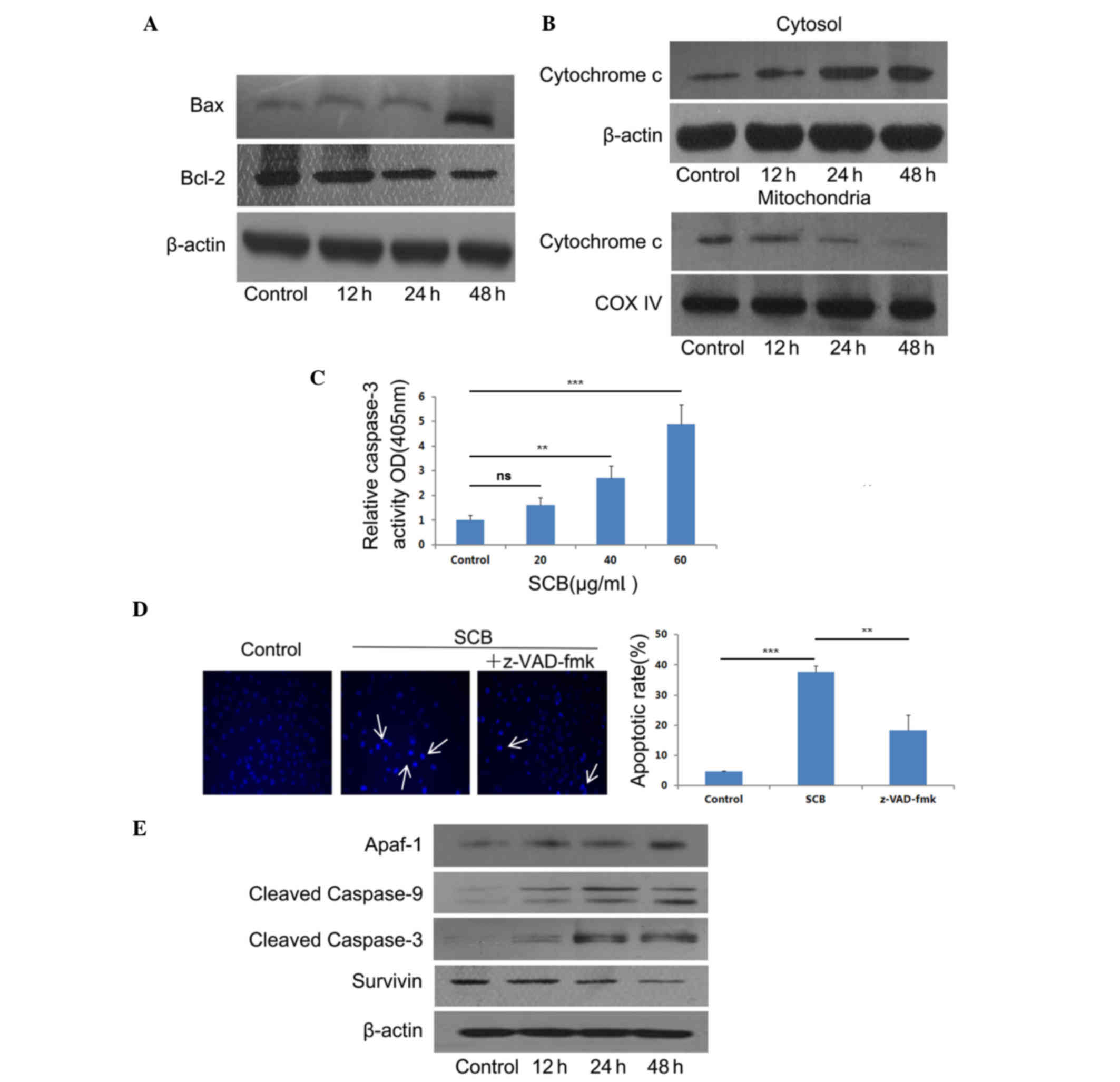 | Figure 4.SCB induces apoptotic cell death in
NCI-H1299 cells through the intrinsic pathway. (A) Expression of
Bax and Bcl-2 following SCB treatment was detected by western
blotting. β-actin was used as an internal control. (B) Cytosolic
and mitochondrial fractions were prepared and the level of
cytochrome c in cytosol and mitochondria were detected by western
blotting. β-actin and COX IV were used as internal controls. (C)
Effect of SCB on the activity of pro/cleaved caspase-3.
**P<0.01, ***P<0.001 vs. control group. (D) Morphology
changes occurred in NCI-H1299 cells following SCB treatment. To
inhibit apoptosis, 20 µM z-VAD-fmk (a pan-capase inhibitor) was
used during SCB treatment. Hoechst 33258 was used to assess cell
apoptosis (white arrows). **P<0.01, ***P<0.001 vs. SCB group.
(E) Expression of Apaf-1, cleaved caspase-9, cleaved caspase-3 and
survivin following SCB treatment were detected by western blotting.
β-actin was used as internal control. Bcl-2, B cell
leukemia/lymphoma 2; Bax, Bcl-2 associated X; COX IV, cytochrome
c oxidase subunit 4; OD, optical density; SCB, Siamese
crocodile bile; Apaf-1, apoptotic peptidase activating factor
1. |
In order to determine whether caspase-3 was
activated during SCB treatment, the caspase-3 activity was examined
using a Caspase-3 Activity Assay kit. As presented in Fig. 4C, caspase-3 activity was increased
in a dose-dependent manner. There was a significant increase in
caspase-3 activity in the groups treated with 40 and 60 µg/ml SCB
when compared with the control (P<0.01 and P<0.001,
respectively). Additionally, the cells were treated with PBS only
(control), SCB (40 µg/ml) only, and SCB (40 µg/ml) added
pan-caspase inhibitor z-VAD-fmk (20 µM) together for 24 h.
Additionally, the pan-caspase inhibitor z-VAD-fmk (20 µM)
significantly reduced SCB-induced cell apoptosis compared with the
SCB only treatment (P<0.01; Fig.
4D). Therefore, the present study aimed to determine the
association of SCB-induced caspase-3 activation with the increased
Bax/Bcl-2 ratio in intrinsic pathway. The effect of SCB on the
expression levels of Apaf-1, cleaved caspase-9 and −3, and survivin
were examined (Fig. 4E). Western
blotting results revealed that the expression of Apaf-1 was
increased in NCI-H1299 cells treated with SCB. The procaspase-9 and
procaspase-3 were cleaved, the expression of cleaved caspase-9 and
cleaved caspase-3 also increased with SCB treatment. The expression
level of survivin was decreased by SCB treatment in NCI-H1299 cells
compared with the control treatment. Therefore, these findings
revealed that SCB may induce apoptosis in NCI-H1299 cells through
the intrinsic pathway with increased Bax/Bcl-2 ratio and release of
cytochrome c.
SCB inhibits the growth of NSCLC
xenograft tumors in athymic nude mice without observable
toxicity
To verify the in vitro findings, in
vivo experiments were performed. The efficacy of SCB against
NSCLC xenograft tumors in nude mice was investigated. The present
study used SCB powder mixed with normal saline at a dose of SCB 100
mg/kg in 100 µl/mouse and was administered via intraperitoneal
injection (control with normal saline only). After 4 weeks, the
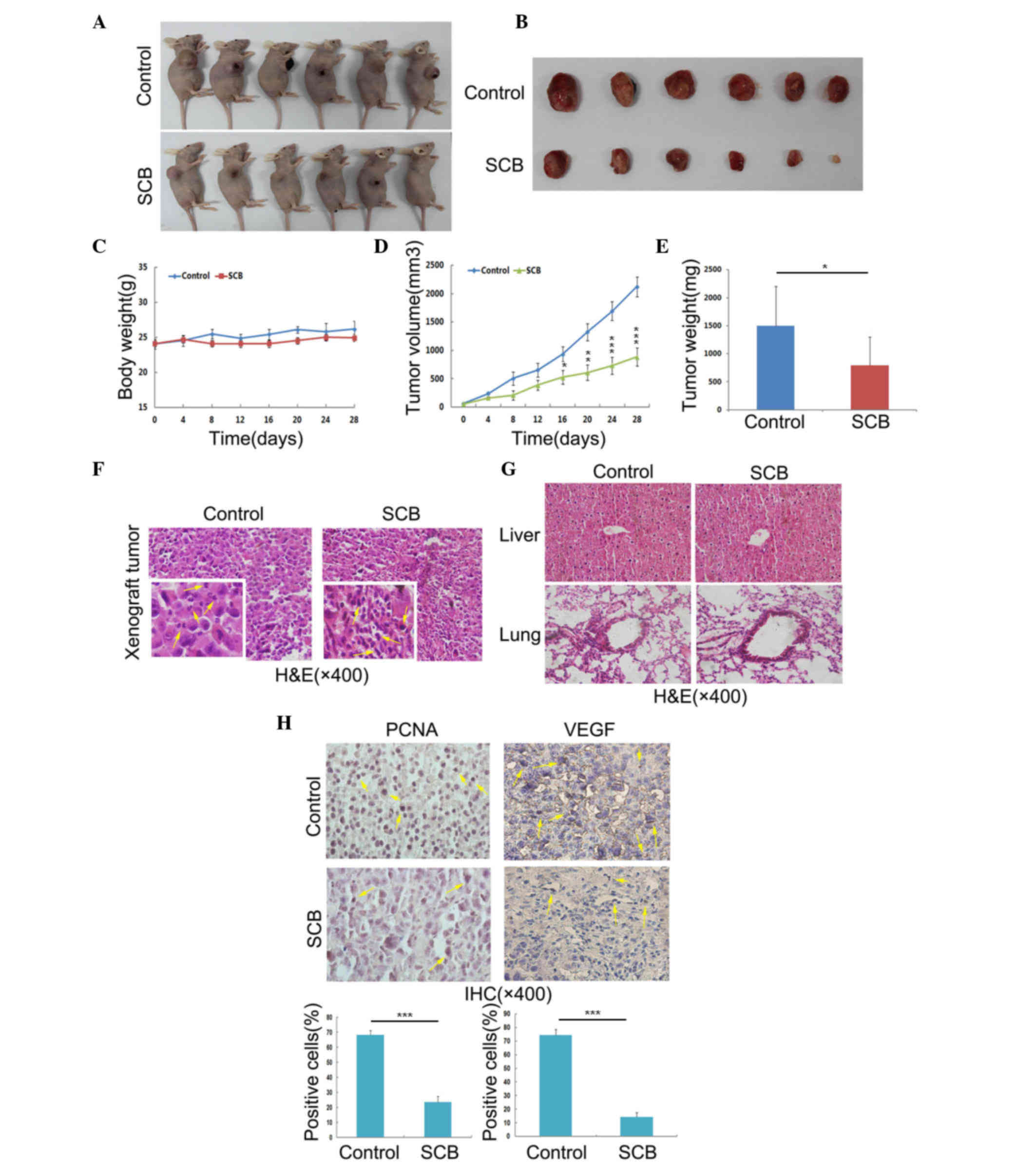
nude mice were sacrificed and the tumors were collected (Fig. 5A and B). During the experiment mice
were observed for general signs of toxicity, such as body weight
profile, and the tumor volume was also measured every 4 days
(Fig. 5). These findings indicated
that SCB administration at the aforementioned dose did not lead to
any body weight loss, which indicated that SCB was well-tolerated
by mice at this dose (Fig. 5C).
SCB treatment led to a significant reduction in NSCLC xenograft
tumor volume with time (Fig. 5D).
The tumor weight was significantly reduced the SCB-treated group
(797.2±500.54 mg) was compared with the control group (1498±506
mg), with inhibition of 46.8% (P<0.05; Fig. 5E). The tumor cell morphology was
also observed under a microscope by H&E staining. The tumor
section from control group revealed that the nuclei were split and
the cells were undergoing mitosis. By contrast, the enhanced
basophilic staining of chromatin in the tumor sections from
SCB-treated group indicated that the cells were undergoing
apoptosis and proliferation was inhibited (Fig. 5F, yellow arrows in left lower
corners). Additionally, the H&E staining of the liver and lung
sections revealed no adverse effects of SCB on these organs
(Fig. 5G). In order to confirm the
cell proliferation and metastasis in the xenograft tumor from
control and SCB-treated groups, IHC of PCNA and VEGF was performed.
The number of cells positively-stained for PCNA and VEGF was
significantly reduced in the SCB-treated mice compared with the
control group (P<0.001; Fig.
5H). Together, these findings suggested SCB treatment has in
vivo against NSCLC xenograft growth, without any evident side
effects.
Discussion
NSCLC is any type of epithelial lung cancer other
than SCLC. The majority of patients with NSCLC are relatively
insensitive to chemotherapy, compared to patients with SCLC
(18). Patients with NSCLC are
primarily treated by surgical resection, and subsequently
chemotherapeutic drugs with curative intent are required. Our
previous study determined that SCB has an anti-cancerous effect on
several forms of human cancer cell lines by inducing apoptosis
(9,10), including human NSCLC. The present
study aimed to investigate the mechanism of SCB-induced apoptosis
on NCI-H1299 human NSCLC cells in vitro and its anti-tumor
efficacy in vivo.
The present study demonstrated that SCB treatment
significantly inhibited the proliferation of NCI-H1299 cells in a
time- and dose-dependent manner and their colony-forming ability.
Control of cell cycle progression in cancer cells is considered to
be an effective method to inhibit tumor growth (19). The findings of the present study
revealed that SCB treatment arrested the cell cycle at G0/G1 phase
and blocked entry into S phase in NCI-H1299 cells by reduction of
the expression of cyclin E1 and Cdk2, which are required for the
G1/S transition (20). These
findings suggested that cell cycle regulation contributed to the
anti-proliferative effect of SCB treatment in NCI-H1299 cells.
Apoptosis in response to chemotherapeutic drugs is
one of the common mechanisms in cancer cell death (21). The present study investigated
whether the inducing apoptotic cell death following SCB treatment
was responsible for inhibition of the growth of NCI-H1299 cells.
Changes in cell morphology are primary indicators of apoptosis
(22,23), involving cell shrinkage, nuclei
chromatin condensation and the formation of apoptotic bodies
(23), which were observed in
SCB-treated NCI-H1299 cells under TEM. Additionally, the proportion
of apoptotic NCI-H1299 cells increased with prolonged SCB
treatment. The findings of the present study demonstrated that SCB
treatment inhibited the growth of NCI-H1299 cells by inducing
apoptotic cell death.
ROS form as a byproduct of oxygen metabolism
(24). Excess ROS induces
oxidative stress, which functions as a trigger for signaling
molecules to initiate downstream events in apoptosis (14). Following exposure of SCB, the
intracellular ROS level increased in NCI-H1299 cells. Mitochondria
serve a crucial role in the apoptotic process by integrating
numerous apoptotic signals from the intracellular space (25). Therefore, the present study focused
on the mitochondria in NCI-H1299 cells. The images of mitochondria
obtained from TEM revealed that the cristae of the mitochondria
were gradually degraded. The regular function of the cristae is to
expand the surface area of the inner mitochondrial membrane,
enhancing and thus increase the production of ATP via the electron
transport chain (25). The
yellow-green fluorescent intensity observed under the inverted
fluorescence microscope in the present study indicated that the
electron transport chain was interrupted and the ATP level was
reduced. Therefore, it was concluded that mitochondrial dysfunction
occurred following SCB treatment of NCI-H1299 cells. The
mitochondria are essential to multicellular life. Once they suffer
damage, apoptosis-associated proteins target the mitochondria and
increase the permeability of the mitochondrial membrane which
causes apoptotic effectors to leak out (26). Therefore, the ΔΨm was further
investigated and the release of cytochrome c following SCB
treatment was quantified. The findings revealed that the ΔΨm was
collapsed and cytochrome c was released into the cytoplasm
from the mitochondria of NCI-H1299 cells. These events are very
closely associated to intrinsic pathway, which arises more
frequently in tumors (27). The
release of cytochrome c and the activation of caspases are
usually under the control of the Bcl-2 family proteins through the
intrinsic pathway (28). The Bcl-2
family are involved in the formation of pores on the mitochondrial
membrane (29,30). The family consists of the
pore-stabilizing protein Bax, as well as the anti-apoptotic
pore-destabilizing protein, Bcl-2 (31). The present study determined that
the SCB treatment increased the ratio of Bax/Bcl-2 and caspase-3
activity in NCI-H1299 cells. Therefore, it is possible that SCB
induced apoptosis in NCI-H1299 cells through this intrinsic
pathway. In the intrinsic pathway, once cytochrome c is
released it binds with Apaf-1 to produce a protein complex termed
the apoptosome (32). The
apoptosome possesses a caspase recruitment domain, which allows it
to bind and process the crucial initiator, caspase-9 (33). Activated caspase-9 then cleaves and
activates the downstream effector, caspase-3, finally initiating
specific caspase cascades to induce apoptosis (34). The western blotting findings of the
present study indicated that SCB treatment promoted the expression
of Apaf-1 and then activated caspase-9 and −3. Conversely, SCB
treatment reduced the expression of survivin. Survivin is a member
of the inhibitor of apoptosis family, which functions to inhibit
caspase activation, thereby negatively regulating apoptosis
(35). Therefore, it is possible
that SCB treatment induces apoptosis in NCI-H1299 cells apoptotic
via a mitochondria-mediated intrinsic pathway.
Finally, the in vivo animal experimental
findings suggested that SCB may inhibit tumor growth in a xenograft
model. The tumors in nude mice that received SCB treatment alone
were ~46.8% smaller than the control group. Changes in body weight
change were monitored and any changes in liver and lung tissues
were assessed using H&E staining. No toxicity was observed in
the nude mice following SCB treatment. IHC analysis revealed that
the number of PCNA and VEGF-positive cells were markedly reduced in
the xenograft tumors from SCB-treated group compared with the
control group. These in vivo findings confirmed the previous
results in cell culture.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time, that the inhibitory
mechanism of SCB on NCI-H1299 human NSCLC cells in vitro and
therapeutic efficacy against xenograft tumors in vivo. These
findings support the use of SCB in future clinical studies of human
NSCLC.
Acknowledgements
The present study was supported by the Science and
Technology Foundation of the City of Xiamen (grant no.
3502Z20133009), the Natural Science Foundation of China (grant no.
81101502) and the National Science Foundation for Fostering Talents
in Basic Research of the National Natural Science Foundation of
China (grant no. J1310027).
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
SCB
|
siamese crocodile bile
|
|
TEM
|
transmission electron microscopy
|
|
ROS
|
reactive oxygen species
|
|
IC50
|
half maximal inhibitory
concentration
|
References
|
1
|
National Lung Screening Trial Research
Team, ; Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan
F, Fagerstrom RM, Gareen IF, Gierada DS, et al: Results of initial
low-dose computed tomographic screening for lung cancer. N Engl J
Med. 368:1980–1991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type:
Male:female differences diminishing and adenocarcinoma rates
rising. Int J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morgensztern D, Ng SH, Gao F and Govindan
R: Trends in stage distribution for patients with non-small cell
lung cancer: A National Cancer Database survey. J Thorac Oncol.
5:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tint GS, Dayal B, Batta AK, Shefer S,
Joanen T, McNease L and Salenet G: Biliary bile acids, bile
alcohols, and sterols of Alligator mississippiensis. J Lipid Res.
21:110–117. 1980.PubMed/NCBI
|
|
5
|
Hofmann AF and Roda A: Physicochemical
properties of bile acids and their relationship to biological
properties: An overview of the problem. J Lipid Res. 25:1477–1489.
1984.PubMed/NCBI
|
|
6
|
Yeh YH, Wang DY, Liau MY, Wu ML, Deng JF,
Noguchi T and Hwang DF: Bile acid composition in snake bile juice
and toxicity of snake bile acids to rats. Comp Biochem Physiol C
Toxicol Pharmacol. 136:277–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng Y, Siu K, Wang N, Ng KM, Tsao SW,
Nagamatsu T and Tong Y: Bear bile: Dilemma of traditional medicinal
use and animal protection. J Ethnobiol Ethnomed. 5:22009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baillie J and Groombridge B: IUCN red list
of threatened animals. The IUCN Species Survival Commission, Gland,
Switzerland. 1996.
|
|
9
|
Kang JH, Zhang WQ, Song W, Shen DY, Li SS,
Tian L, Shi Y, Liang G, Xiong YX and Chen QX: Apoptosis mechanism
of human cholangiocarcinoma cells induced by bile extract from
crocodile. Appl Biochem Biotechnol. 166:942–951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song W, Li SS, Qiu PP, Shen DY, Tian L,
Zhang QY, Liao LX and Chen QX: Apoptosis induced by aqueous
extracts of crocodile bile in human heptacarcinoma SMMC-7721. Appl
Biochem Biotechnol. 170:15–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu ZH, Lu MK, Hu LY and Li X: Praziquantel
synergistically enhances paclitaxel efficacy to inhibit cancer cell
growth. Plos One. 7:e517212012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Resnitzky D and Reed SI: Different roles
for cyclins D1 and E in regulation of the G1-to-S transition. Mol
Cell Biol. 15:3463–3469. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lo AC, Woo TT, Wong RL and Wong D:
Apoptosis and other cell death mechanisms after retinal detachment:
Implications for photoreceptor rescue. Ophthalmologica. 226 Suppl
1:S10–S17. 2011. View Article : Google Scholar
|
|
14
|
Ozben T: Oxidative stress and apoptosis:
Impact on cancer therapy. J Pharm Sci. 96:2181–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waldbaum S and Patel M: Mitochondrial
dysfunction and oxidative stress: A contributing link to acquired
epilepsy? J Bioenerg Biomembr. 42:449–455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blattner JR, He L and Lemasters JJ:
Screening assays for the mitochondrial permeability transition
using a fluorescence multiwell plate reader. Anal Biochem.
295:220–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kroemer G, Dallaporta B and Resche-Rigon
M: The mitochondrial death/life regulator in apoptosis and
necrosis. Annu Rev Physiol. 60:619–642. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Graham MV, Purdy JA, Emami B, Harms W,
Bosch W, Lockett MA and Perez CA: Clinical dose-volume histogram
analysis for pneumonitis after 3D treatment for non-small cell lung
cancer (NSCLC). Int J Radiat Oncol Biol Phys. 45:323–329. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mork CN, Faller DV and Spanjaard RA: A
mechanistic approach to anticancer therapy: Targeting the cell
cycle with histone deacetylase inhibitors. Curr Pharm Des.
11:1091–1104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohtsubo M, Theodoras AM, Schumacher J,
Roberts JM and Pagano M: Human cyclinE, a nuclear protein essential
for the G1-to-S phase transition. Mol Cell Biol. 15:2612–2624.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chow KU, Nowak D, Boehrer S, Ruthardt M,
Knau A, Hoelzer D, Mitrou PS and Weidmann E: Synergistic effects of
chemotherapeutic drugs in lymphoma cells are associated with
down-regulation of inhibitor of apoptosis proteins (IAPs),
prostate-apoptosis-response-gene 4 (Par-4), death-associated
protein (Daxx) and with enforced caspase activation. Biochem
Pharmacol. 66:711–724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hunot S and Flavell RA: Apoptosis. Death
of a monopoly? Science. 292:865–866. 2001.PubMed/NCBI
|
|
23
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Devasagayam TP, Tilak JC, Boloor KK, Sane
KS, Ghaskadbi SS and Lele RD: Free radicals and antioxidants in
human health: Current status and future prospects. J Assoc
Physicians India. 52:794–804. 2004.PubMed/NCBI
|
|
25
|
Mannella CA: Structure and dynamics of the
mitochondrial inner membrane cristae. Biochim Biophys Acta.
1763:542–548. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weinberg F and Chandel NS: Mitochondrial
metabolism and cancer. Ann N Y Acad Sci. 1177:66–73. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mohan S, Abdul AB, Abdelwahab SI,
Al-Zubairi AS, Sukari MA, Abdullah R, Taha MM Elhassan, Ibrahim MY
and Syam S: Typhonium flagelliforme induces apoptosis in CEMss
cells via activation of caspase-9, PARP cleavage and cytochrome c
release: Its activation coupled with G0/G1 phase cell cycle arrest.
J Ethnopharmacol. 131:592–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brenner C and Kroemer G: Apoptosis.
Mitochondria-the death signal integrators. Science. 289:1150–1151.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dejean LM, Martinez-Caballero S and
Kinnally KW: Is MAC the knife that cuts cytochrome c from
mitochondria during apoptosis? Cell Death Differ. 13:1387–1395.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martindale JL and Holbrook NJ: Cellular
response to oxidative stress: Signaling for suicide and survival. J
Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cecconi F, Alvarez-Bolado G, Meyer BI,
Roth KA and Gruss P: Apaf1 (CED-4 homolog) regulates programmed
cell death in mammalian development. Cell. 94:727–737. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan G, O'Rourke K and Dixit VM: Caspase-9,
Bcl-XL, and Apaf-1 form a ternary complex. J Biol Chem.
273:5841–5845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pop C, Timmer J, Sperandio S and Salvesen
GS: The apoptosome activates caspase-9 by dimerization. Mol Cell.
22:269–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sah NK, Khan Z, Khan GJ and Bisen PS:
Structural, functional and therapeutic biology of survivin. Cancer
lett. 244:164–171. 2006. View Article : Google Scholar : PubMed/NCBI
|