Introduction
Embryonic implantation is a complex reproductive
process involving reciprocal interactions between the blastocyst
and uterus. Successful embryonic implantation is dependent on
implantation of normal embryos, synchronous development of
endometrial receptivity, and molecular communication between the
embryo and the mother (1,2). The cellular dialogue precisely
regulates endometrial decidualization, as well as the proliferation
and differentiation of trophoblast cells. During development, the
endometrial stromal cells are stimulated by inducing factors, and
undergo proliferation and differentiation; a process referred to as
decidualization. This process serves a crucial role in the
establishment and maintenance of pregnancy (3–5).
Recently, microRNA (miRNA/miR) regulation of endometrial gene
expression during early pregnancy has received a lot of attention.
Chakrabarty et al (6)
demonstrated that mRNA expression of cyclooxygenase-2, which is
critical for embryonic implantation, was post-transcriptionally
regulated by mmu-miR-101a and mmu-miR-199a. Furthermore, Hu et
al (7) demonstrated
differential expression of miRNAs in the mouse uterus between
implantation sites (IS) and inter-implantation sites (IIS) by miRNA
microarray. Shen et al (8)
reported that mmu-miR-200a has an important role in embryonic
implantation and that phosphatase and tensin homolog is the target
gene of mmu-miR-200a. However, in recent years, few studies have
investigated the involvement of miRNAs in the regulation of
decidualization.
miRNAs are a class of non-coding small RNA
molecules, 18–24 nucleotides in length, which cause either the
degradation of target mRNA, or translational inhibition through
target mRNA-specific base pairing (9). They affect various biological
processes, including development, cell growth, differentiation,
apoptosis and maintenance of tissue identity (10). A previous study investigated the
dynamic alterations in miRNA and mRNA levels during the
pre-receptivity, receptivity and implantation phases, which reflect
the mechanism by which miRNAs regulate their target mRNAs.
Mmu-miR-96 and mmu-miR-200 were revealed to act as hub miRNAs, and
were verified to share the target gene B-cell lymphoma 2 (Bcl2),
and to exert synergistic regulation (11).
Previous in house miRNA microarray results
demonstrated that mouse uterine expression of mmu-miR-96 was
upregulated at IS on day five of pregnancy compared with at IIS,
indicating that it may participate in the regulation of
implantation (11). Mmu-miR-96,
which maps to chromosomal region 17, is one of the members of the
miR-183 family, other members of which include microRNA-183 and
microRNA-182, and has recently been found to have a role in cancer.
Its expression is upregulated in various tumors, including bladder
cancer (12,13), lung cancer (14), breast cancer (15), endometrioid carcinomas (16) and liver cancer (17). A previous study identified that
miR-96 promoted suppression of forkhead box protein O1 (FOXO1) and
may serve a key role in transitional cell carcinoma tumorigenesis
by avoiding cell apoptosis (18).
Furthermore, the upregulation of miR-96 has been reported to induce
downregulation of the transcription factors FOXO3a and FOXO1, and
thus promote cell proliferation in human breast cancer (15,19).
It has also been revealed that in non-small cell lung
cancer-derived cell lines, downregulation of miR-96 inhibits
proliferative and invasive capacities, and promotes apoptosis
(20). Therefore, the present
study hypothesized that mmu-miR-96 may serve a role in
decidualization by regulating the proliferation and apoptosis of
endometrial stromal cells. This study aimed to investigate the
target gene of mmu-miR-96. The results revealed the expression
pattern and function of mmu-miR-96 in the endometrium during early
pregnancy in mice. In addition, the anti-apoptotic protein Bcl2 was
identified as the target gene of mmu-miR-96 in the endometrium.
Materials and methods
Animals and tissue preparation
A total of 52 female and 13 male Kunming mice (age,
6–8 weeks; weight, 25–30 g) were provided by the Laboratory Animal
Center of Chongqing Medical University, [Chongqing, China;
Certificate No.: SCXK (YU) 20070001]. The mice were housed in the
Chongqing Medical University Animal Care Facility with ad
libitum access to food and water under a 14 h light/10 h dark
cycle, at constant temperature (22±2°C) and humidity (50%). Ethical
approval for this study was provided by the Ethics Committee of
Chongqing Medical University [Certification No: SCXK (YU)
20110016]. Female mice were mated with fertile or vasectomized
males of the same strain to induce pregnancy or pseudopregnancy,
respectively. The presence of a vaginal plug was regarded as the
first day of pregnancy (D1). The mice were sacrificed by cervical
dislocation and the tissue samples of mice were obtained on
pregnancy day 1, 4, 5, 6 and 7. IS and IIS tissues were collected
according to a previous study (21). The artificially induced
decidualization mouse model was generated according to standard
criteria. Briefly, on day 4 of pseudopregnancy, 25 µl corn oil was
infused into one uterine horn to induce artificial decidualization
and another horn without any infusion served as the control. On day
8 of pseudopregnancy the mice were sacrificed by cervical
dislocation.
In situ hybridization
The mmu-miR-96-specific probe and the negative
control (scramble) were purchased from Exiqon A/S (Vedbaek,
Denmark). Probe sequence: AAG CAA AAA TGT GCT AGT GCC AAA. An in
situ hybridization kit (Dingguo Biotechnology Co. Ltd.,
Beijing, China) was used for hybridization according to the
manufacturer's protocol. Briefly, frozen endometrial tissues were
sectioned (10 µm) and fixed with 4% paraformaldehyde at room
temperature for 10 min. The uterine sections were treated with
protease K at 37°C for 5 min, and then incubated with
prehybridization solution for 4 h at 50°C, followed by incubation
with hybridization solution including probes (40 mmol/l) overnight
at 50°C. After washing with standard saline citrate, sections were
incubated with bovine serum albumin (1:100 dilution) at 37°C for 1
h, and then with alkaline phosphatase-labeled goat anti-rabbit
immunoglobulin G (1:100 dilution) for 1 h. Nitro-blue tetrazolium
chloride/5-bromo-4-chloro-3′-indolylphosphate was employed to
indicate a positive signal and the nuclei were stained with Nuclear
Fast Red. All slides were viewed directly under a microscope (BX43;
Olympus Corporation, Tokyo, Japan).
Cell culture and treatment
Primary cells were isolated from the uteri of mice
as previously described (22,23).
The mice were sacrificed on day 4 (stromal cells) or day 8
(decidual cells) of pregnancy by cervical dislocation, and the
uteri were split longitudinally. After washing with D-Hank's
Balanced Salt Solution (HBSS) (Boster Systems, Inc., Pleasanton,
CA, USA), uterine tissues were cut into small pieces and treated
with 7.5 ml HBSS containing 1% (w/v) trypsin (Boster Systems, Inc.)
and 6 mg/ml dispase (Roche Diagnostics, Indianapolis, IN, USA). The
tissues were rinsed 3 times with HBSS and incubated in 2 ml HBSS
containing 0.5 mg/ml collagenase I (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 30 min. The suspension was purified
through a 70-µm nylon filter and centrifuged at 1,500 × g for 5
min. The cells were seeded in 50 ml culture flasks and cultured in
phenol red-free culture medium (DMEM/Ham's F-12; 1:1;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) containing 10%
charcoal-stripped fetal bovine serum (FBS) (Sigma-Aldrich; Merck
Millipore). The cells were transfected with miR-96 mimic
(5′-UUUGGCACUAGCACAUUUUUGCU-3′ 5′AGCAAAAAUGUGCUAGUGCCAAA-3′) or a
negative control (5′UUUGUACUACACAAAAAGUACUG3′
5′CAGUACUUUUGUGUAGUACAAA3′) (Guangzhou RiboBio Co. Ltd., Guangzhou,
China) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The medium was replaced with fresh DMEM/F12
medium (supplemented with 10% FBS) after transfection for 4 to 6 h,
and then collected 48 h later.
In vitro decidualization
Decidualization was performed as described
previously (24,25), with modification. Briefly,
endometrial stromal cells isolated from the uteri of mice on day 4
of pregnancy were treated with 10 nmol/l estradiol-17β
(Sigma-Aldrich; Merck Millipore) and 1 µmol/l progesterone
(Sigma-Aldrich; Merck Millipore) for 96 h, whereas endometrial
stromal cells not treated with E2P4 served as the control group.
Culture medium was changed each day.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mouse endometrial
tissues or cultured cells using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. RT of cDNA was conducted using the
miScript II Reverse Transcription kit with miScript HiSpec Buffer
(Qiagen, Inc., Valencia, CA, USA,). Briefly, RNA was mixed with
miScript HiSpec Buffer, RNAse-free water and miscript Reverse
Transcriptase Mix, and was incubated at 37°C for 60 min and 95°C
for 5 min. qPCR was conducted with the miScript SYBR-Green Real
Time PCR kit (Qiagen, Inc.) for miRNA detection and SYBR Premix Ex
Taq. The specific primers for mmu-miR-96, U6, Bcl2,
decidual/trophoblast prolactin-related protein (dtPRP) and β-actin
were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China)
(Table I). The qPCR master mix (15
µl) contained 7.5 µl SYBR Premix Ex Taq, 0.6 µl primers, 1.2 µl
cDNA and 5.1 µl diethylpyrocarbonate-treated H2O. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 30 sec; 40 cycles at 95°C for 5 sec (denaturation) and
60°C for 30 sec, followed by 72°C for 5 sec. Experiments were
performed in triplicate. Data obtained from qPCR were analyzed
using the 2-ΔΔCq method (26).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer | Sequence
(5′-3′) |
|---|
| Mmu-miR-96 | RT primer |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGCAAA |
|
| Forward |
TTTGGCACTAGCACATTTTTG |
|
| Reverse |
GTGCAGGGTCCGAGGT |
| U6 | RT primer |
CGCTTCACGAATTTGCGTGTCAT |
|
| Forward |
GCTTCGGCAGCACATATACTAAA AT |
|
| Reverse |
CGCTTCACGAATTTGCGTGTCAT |
| Bcl2 | Forward |
CGATTGTGGCAGTCCCTTA |
|
| Reverse |
CAGGATGAAGTGCTCAGGTG |
| dtPRP | Forward |
AGCCAGAAATCACTGCCACT |
|
| Reverse |
TGATCCATGCACCCATAAAA |
| β-actin | Forward |
TCGTGCGTGACATCAAAGAC |
|
| Reverse |
CAAGAAGGAAGGCTGGAAAA |
Flow cytometric analysis
The stromal cells transfected with mmu-miR-96 mimics
and negative controls were collected. At 48 h post-transfection,
the cells were harvested and washed three times with PBS. Flow
cytometry was used to analyze cell cycle progression and apoptosis
according to a previous report (27). The experiment was repeated three
times.
Western blotting
Cells were harvested for protein extraction using
cell lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China). The protein concentration was determined using the
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. Total
proteins (50 µg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% nonfat milk in PBS with
0.1% Tween-20 (PBST) for 1 h at room temperature. Immunoblotting
was performed by incubating the membranes in 5% milk-PBST overnight
at 4°C with rabbit monoclonal anti-Bcl2 (1:600; cat. no. sc-509;
Santa Cruz, Biotechnology, Inc., Dallas, TX, USA). After washing
three times in PBST, the membranes were incubated for 1 h with goat
anti-rabbit immunoglobulin G antibody (1:1,000; cat. no. BA1055;
Wuhan Boster Biological Technology Co., Ltd., Wuhan, China). Bcl2
expression was normalized to β-actin (1:1,000; cat. no. A5441;
Sigma-Aldrich; Merck Millipore). The immunoreactive bands were
visualized using ChemiDoc™ XRS+ (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and chemiluminescent reagents (cat. no.
WBKLS0500; EMD Millipore). The image collection and densitometric
analysis was performed using Quantity One analysis software,
version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein expression levels were normalized against β-actin.
Fluorescence reporter vector
analysis
Fluorescence reporter vector analysis, including
generation of the mutated sequence of Bcl2, was conducted with
assistance from Tianjin Saierbio Technology Incorporation (Tianjin,
China). Enhanced green fluorescent protein (EGFP) cDNA was removed
from the pEGFP-N2 vector (Clontech Laboratories Inc., Mountainview,
CA, USA) using restriction enzymes and subcloned into the pcDNA3.1
vector (Invitrogen; Thermo Fisher Scientific, Co. Ltd.). The
3′-untranslated region fragments from Bcl2 containing the predicted
mmu-miR-96 binding site were cloned into pcDNA3.1/EGFP constructs
at the BamHI and EcoRI sites. Four types of vector
were constructed in the present study: i) pcDNA3/EGFP/Bcl2; ii)
pcDNA3.1(+), iii) pcDNA3.1/pri-miR-96 and iv) mutant vector
Mu-pcDNA3/EGFP/Bcl2. Six groups were subsequently established: i)
pcDNA3/EGFP/Bcl2; ii) pcDNA3/EGFP/Bcl2 + pcDNA3.1 (+); iii)
pcDNA3/EGFP/Bcl2 + pri-96; iv) Mu-pcDNA3/EGFP/Bcl2; v)
Mu-pcDNA3/EGFP/Bcl2 + pcDNA3.1 (+); and vi) Mu-pcDNA3/EGFP/Bcl2 +
pri-96. The Mu-pcDNA3/EGFP/Bcl2 plasmid included a mutated sequence
of Bcl2 that was predicted to interact with mmu-miR-96. The mouse
uterine stromal cells were co-transfected in 24-well plates with
0.5 mg EGFP reporter vector and 0.1 mg control vector containing
pDsRed-C1 (Invitrogen; Thermo Fisher Scientific, Inc.). EGFP and
red fluorescent protein activities were measured using a
fluorospectrometer. Plasmids containing the EGFP sequence coded
green fluorescence. Measuring the fluorescence intensity can be
used to measure the activity of EGFP.
Digital gene expression (DGE) library
preparation and sequencing
Total RNA isolated from each sample was used for DGE
library preparation. The DGE libraries were established as
described previously (28).
Briefly, mRNA was reverse transcribed to cDNA, ligated to
sequencing adapters, and the products were purified and enriched
with PCR to create the final cDNA libraries. Subsequently, the
libraries were sequenced using the Illumina GAII system (Illumina,
San Diego, CA, USA). Statistical analyses were performed, and the
significantly differentially expressed genes were determined at a
threshold false discovery rate (FDR) and absolute value of the log2
ratio. DGE data analysis was conducted by BGI Tech Solutions Co.,
Ltd. (Shenzhen, China).
Statistical analysis
The data were analyzed using SPSS13.0 (SPSS Inc.,
Chicago, IL, USA). Student's t-test was used to analyze
differences between two groups, whereas the differences between
numerous groups were detected using one-way analysis of variance
followed by Student-Newman-Keuls test. P<0.05 was considered to
indicate a statistically significant difference. Data were
expressed as the mean ± standard error of the mean of experiments
repeated at least three times.
Results
Differential expression of mmu-miR-96
in mouse uterus during early pregnancy
The present study detected the temporal and spatial
distribution of uterine mmu-miR-96 during early pregnancy. The
results of the qPCR analysis were consistent with the miRNA array
(data not shown). On day 5 of pregnancy, the expression levels of
mmu-miR-96 were much higher at the IS compared with the IIS
(Fig. 1A). In situ
hybridization demonstrated that mmu-miR-96 was mainly located in
the luminal epithelia on day 1 (Fig.
1B). Weak positive signals were observed in the stromal cells
on day 4, whereas its expression increased in stromal cells and in
the embryo on day 5 of pregnancy. Mmu-miR-96 was widely expressed
in the second decidual zone (SDZ) on day 6, which was further
expanded on day 7 (Fig. 1B). To
explore the expression of mmu-miR-96 during decidualization, whilst
avoiding influence from the blastocyst, artificial in vivo
and in vitro models of decidualization using stromal cells
were employed. Compared with the control horn, the oil-infused horn
presented a robust deciduoma (Fig.
1C), and the weight of the deciduoma was greater than that of
the control horn (Fig. 1D).
Furthermore, expression of the decidualization marker dtPRP was
increased in the infused horn (Fig.
1E), which confirmed the model was successfully established.
Finally, mmu-miR-96 expression was much higher in the deciduoma, as
compared with the control horn (Fig.
1F). These data indicated that mmu-miR-96 may participate in
the transformation of stromal cells into decidual cells, or in the
regulation of decidual cells to support the subsequent development
of the fetus.
 | Figure 1.Expression of mmu-miR-96 in mouse
endometrium. (A) mRNA expression levels of mmu-miR-96, as detected
by RT-qPCR (**P<0.01, ***P<0.001, vs. D1;
#P<0.05, vs. D5IS). (B) Location of mmu-miR-96
detected by in situ hybridization. Blue represents positive
signals. Scrambled represents a negative control. Scale bar, 100
µm. (C) Typical uterine appearance of artificial decidualization.
(D) Statistical analysis of uterine horn weight (***P<0.001).
(E) Expression of dtPRP detected by RT-qPCR (***P<0.001). (F)
Expression of mmu-miR-96 between the infused (oil) and non-infused
(control) uterine horns. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; miR-96,
microRNA-96; IS, implantation site; IIS, inter-implantation site;
dtPRP, decidual/trophoblast prolactin-related protein; le, luminal
epithelium; ge, gland epithelium; s, stromal; em, embryo; sdz,
secondary decidual zone. |
Effects of mmu-miR-96 on endometrial
decidualization
To identify the role of mmu-miR-96 in the
endometrium during early pregnancy, further functional experiments
were conducted in primary endometrial stromal cells. Compared with
the control group, the mRNA expression levels of dtPRP in the
hormone treatment group (E2P4) increased significantly, whereas
expression of dtPRP decreased following transfection with the
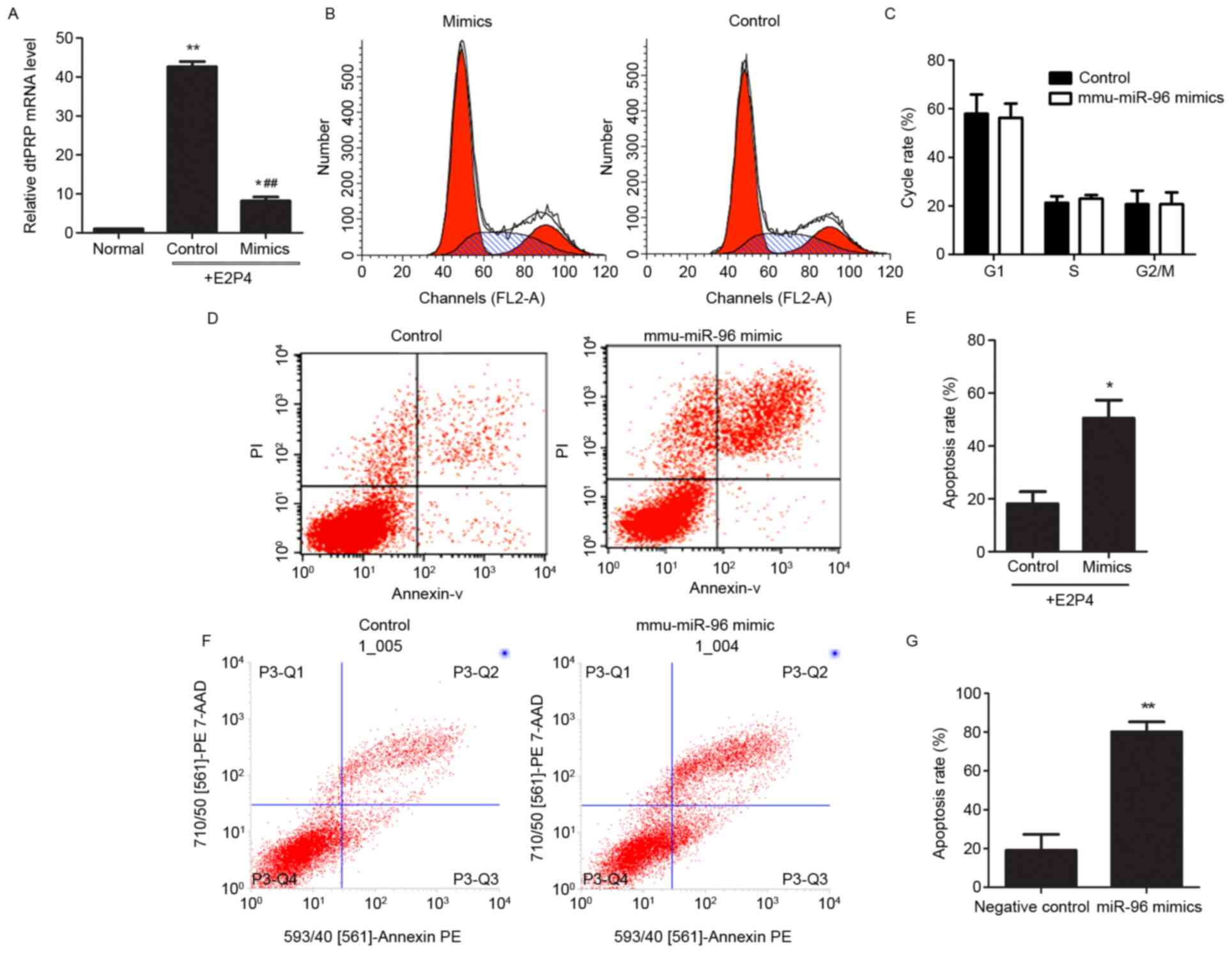
mmu-miR-96 mimic (Fig. 2A).
Furthermore, the effects of mmu-miR-96 on the proliferation and
apoptosis of stromal cells were investigated. Flow cytometric
analysis indicated that mmu-miR-96 expression in stromal cells did
not result in changes to the cell cycle (Fig. 2B and C), whereas the rate of
apoptosis was markedly increased following overexpression of
mmu-miR-96 (Fig. 2D and E). These
data indicated that upregulation of mmu-miR-96 in the deciduoma may
be involved in the regulation of decidual cells to maintain
pregnancy. To address this, primary decidual cells were transfected
with mmu-miR-96 mimics, and then the apoptotic rate was observed.
Consistently, excessive mmu-miR-96 induced apoptosis in decidual
cells (Fig. 2F and G). These data
indicated that overexpression of mmu-miR-96 may inhibit the
transformation of stromal cells into decidual cells by inducing
apoptosis, and that mmu-miR-96 may serve a role in the apoptosis of
decidual cells to support the subsequent development of the
fetus.
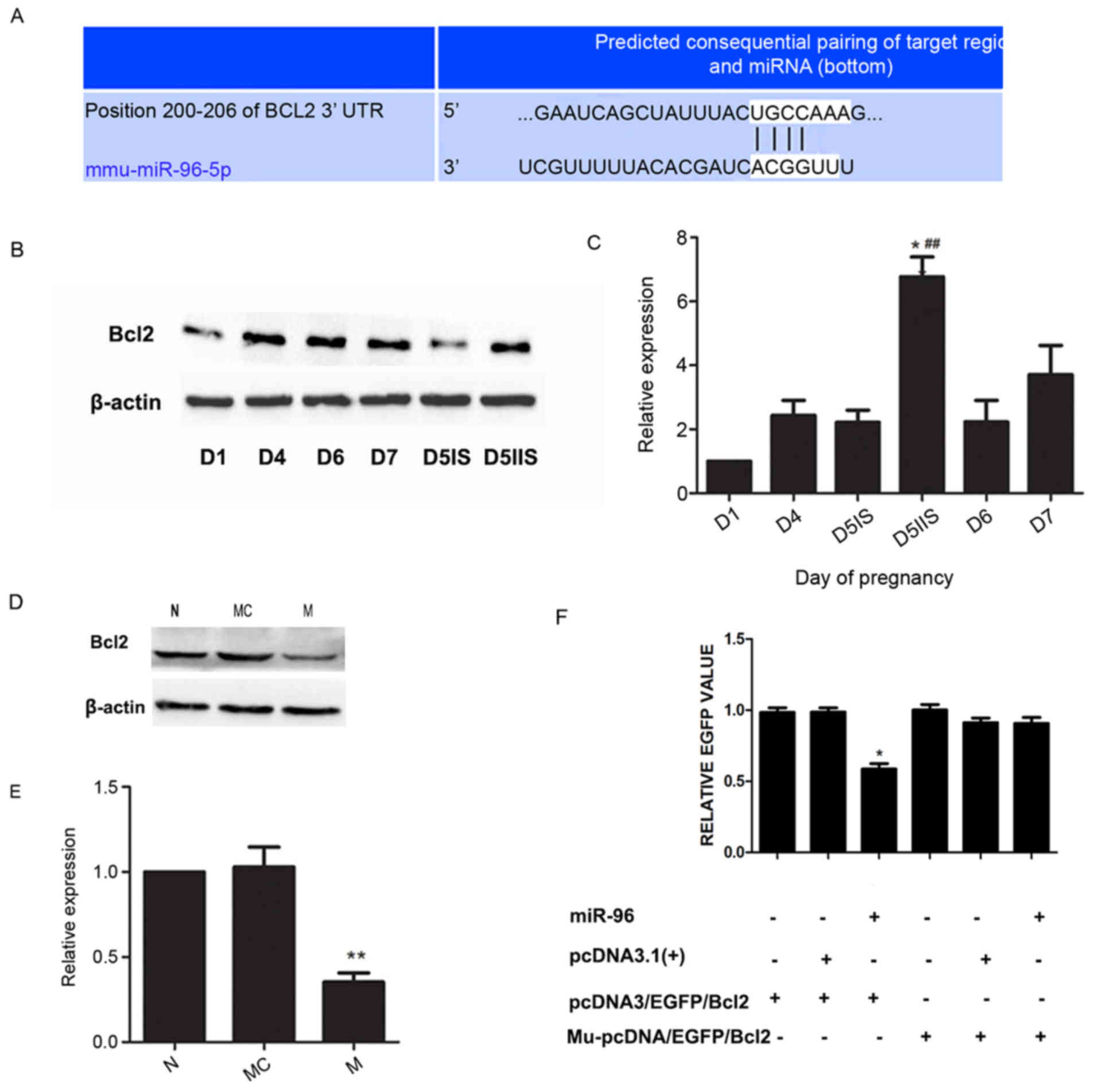
Identification of the target gene of
mmu-miR-96
To further determine the molecular mechanism by
which mmu-miR-96 induces apoptosis, the target genes of mmu-miR-96
were predicted using TargetScan (http://www.targetscan.org/vert_71/), miRGen
(http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=mirgenv3%2Findex)
and Pictar (www.pictar.org). Three predicted target
genes, namely Bcl2, Bcl212, and Bcl213, which are associated with
cell apoptosis, were of interest. From this information, combined
with our recent gene expression data (data not shown) on day 5
endometrium by DGE analysis, the present study focused on Bcl2. DGE
analysis demonstrated differential endometrial expression of Bcl2
between the IS and the IIS (Table
II and Fig. 3A), whereas there
was no significant difference in Bcl2112 and Bcl2113 expression
(data not shown). To verify expression of Bcl2, the levels of Bcl2
protein in the endometrium on days 1, 4, 5, 6 and 7 of pregnancy
were detected by western blotting. As shown in Fig. 3B, Bcl2 expression was lowest on day
1 and increased thereafter. On day 5, the levels of Bcl2 protein
were higher at IIS compared with at IS (Fig. 3B and C). This finding suggested
that the expression of mmu-miR-96 is inversely correlated with Bcl2
protein levels. Levels of Bcl2 expression were also assessed in the
mmu-miR-96 mimic-transfected endometrial stromal cells. The results
revealed that Bcl2 protein levels were reduced when mmu-miR-96 was
overexpressed in stromal cells (Fig.
3D and E). Finally, a fluorescent reporter gene assay was
performed (Fig. 3F). The
expression levels of EGFP were lowest in pcDNA3/EGFP/Bcl2 and
mmu-miR-96 transfected cells, indicating that mmu-miR-96
significantly reduced EGFP expression compared with the control,
whereas the EGFP expression remained unaffected in the
Mu-pcDNA3/EGFP/Bcl2 transfected cells. This result confirmed that
mmu-miR-96 targets the 3′-UTR of Bcl2 mRNA in stromal cells. These
results suggested that mmu-miR-96 regulates expression of Bcl2 in
stromal cells and Bcl2 is a target gene of mmu-miR-96. The data
indicated that mmu-miR-96 may participate in decidualization by
regulating endogenous Bcl2.
 | Table II.Levels of mmu-miR-96 and Bcl2 mRNA in
endometrium on day 5 detected by digital gene expression tag
profiling. |
Table II.
Levels of mmu-miR-96 and Bcl2 mRNA in
endometrium on day 5 detected by digital gene expression tag
profiling.
|
| Relative expression
on day 5 |
|---|
|
|
|
|---|
| Gene | IS | IIS | log2 Ratio
(D5IS/D5IIS) | P-value |
|---|
| Mmu-miR-96 | 21.6031 | 3.0448 | 2.82681898 | 2.15E-37 |
| Bcl2 | 30.05 | 89.4 |
−1.57290984047213 | 7.39E-33 |
Discussion
Following implantation of the blastocyst, the
endometrium thickens and the stromal cells differentiate; their
morphological transformation from fibroblast cells to larger,
multinuclear cells is associated with increasing proliferation and
degradation, and this series of changes in the endometrium is
called the decidual reaction (29,30).
A previous study provided the miRNA signature of human endometrial
stromal cells (hESC) during the decidualization process in
vitro and analyzed the role of the enzyme Dicer during this
process (31). Research also
illustrated that miR-181a serves an important role in the
decidualization of human endometrial stromal cells by inhibiting
kruppel-like factor 12 (KLF12) (32). These studies suggested that miRNAs
participate in the regulation of decidualization. The present study
demonstrated that mmu-miR-96 is also involved in decidualization by
regulating cell apoptosis, and potentially affecting embryo
implantation.
miR-96 is generally considered to be an oncogene due
to its ability to promote cell proliferation and inhibit cell
apoptosis. The present study revealed that mmu-miR-96 expression at
IS was higher than at IIS, and was mainly located in stromal cells.
Mmu-miR-96 was widely expressed in the SDZ on day 6, which was
further expanded on day 7. The rate of apoptosis was markedly
increased following overexpression of mmu-miR-96; excessive
mmu-miR-96 was able to induce apoptosis of decidual cells. These
results suggested that it may be involved in the decidualization of
stromal cells or in the maintenance of decidual cells.
Endometrial stromal cells undergo extensive
proliferation prior to implantation, followed by differentiation
and transformation into decidual cells following implantation. It
has previously been reported that miR-96 regulates FOXO1 expression
in decidualized hESCs (31). To
further investigate whether mmu-miR-96 is associated with
endometrial decidualization, artificial decidualization was
performed in vivo and in vitro, and the results
indicated that mmu-miR-96 expression was increased in decidual
cells compared with untreated groups. It was therefore hypothesized
that mmu-miR-96 may affect the process of decidualization through a
specific mechanism. In order to investigate this hypothesis, the
biological functions of mmu-miR-96 were examined at the cellular
level. Upregulation of mmu-miR-96 promoted stromal and decidual
cell apoptosis, which is a vital molecular event for stromal cell
decidualization, and which is a key factor for successful
implantation. Boeddeker and Hess (33) indicated that apoptosis served a
major role in the female reproductive tract with regards to
different requirements throughout the menstrual cycle, including
decidualization and implantation, or menstrual shedding of the
non-pregnant endometrium. Therefore, increased apoptosis of stromal
cells may have an effect on decidualization and implantation.
mmu-miR-96 may function by reverse regulating its
target genes. Chen et al (11) revealed a regulatory relationship
between miRNAs and mRNAs, and discovered that the miRNAs mmu-miR-96
and mmu-miR-200b target the anti-apoptotic protein Bcl2, as well as
Kruppel-like factor 13. Correia-da-Silva et al (34) investigated the spatial and temporal
pattern of expression of the Bcl2 family members in uterine tissues
at the IS, from the post-implantation period to parturition, and
demonstrated that the apoptotic mitochondrion-dependent pathway is
involved in decidual regression during pregnancy progression.
Joswig et al (35)
confirmed the presence of Bcl2-associated X protein and Bcl2 in
mouse decidua, and indicated that both were absent in the uterine
epithelium. These findings suggested that Bcl2 has an important
role in this process. The present study used computational analysis
to predict potential mmu-miR-96 target sequences and found three
potential anti-apoptosis-related gene targets: Bcl2, Bcl2112 and
Bcl2113. The results demonstrated that Bcl2 expression was higher
at IIS compared with at IS on day 5 of pregnancy through DGE
analysis, and this result is inverse to the expression pattern of
mmu-miR-96 on the same day of pregnancy. In addition, Bcl2 protein
expression was inhibited following the upregulation of mmu-miR-96.
Finally, a Bcl2-fluorescent reporter assay was used to further
confirm that Bcl2 is indeed a target gene of mmu-miR-96.
In conclusion, the present study indicated that
mmu-miR-96 affects the apoptosis of stromal cells and decidual
cells via regulation of Bcl2, a process that is important in the
establishment and maintenance of pregnancy. The mechanism of
mmu-miR-96 participation in embryonic implantation should be
investigated in greater detail in future studies.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 31501207) and by the
Science and Technology Commission of Chongqing, Chongqing, China
[grant no. SCXK (YU) 2012–0001].
References
|
1
|
Cha J, Sun X and Dey SK: Mechanisms of
implantation: Strategies for successful pregnancy. Nat Med.
18:1754–1767. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H and Dey SK: Roadmap to embryo
implantation: Clues from mouse models. Nat Rev Genet. 7:185–199.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkes AS: The Functions of the Corpus
Luteum. II.-The Experimental Production of Placentomata in the
Mouse. Proceedings of the Royal Society of London Series B
Containing Papers of a Biological Character. 104:183–188. 1929.
View Article : Google Scholar
|
|
4
|
Shimizu A, Maruyama T, Tamaki K, Uchida H,
Asada H and Yoshimura Y: Impairment of decidualization in
SRC-deficient mice. Biol Reprod. 73:1219–1227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eyal O, Jomain JB, Kessler C, Goffin V and
Handwerger S: Autocrine prolactin inhibits human uterine
decidualization: A novel role for prolactin. Biol Reprod.
76:777–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chakrabarty A, Tranguch S, Daikoku T,
Jensen K, Furneaux H and Dey SK: MicroRNA regulation of
cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci
USA. 104:15144–15149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu SJ, Ren G, Liu JL, Zhao ZA, Yu YS, Su
RW, Ma XH, Ni H, Lei W and Yang ZM: MicroRNA expression and
regulation in mouse uterus during embryo implantation. J Biol Chem.
283:23473–23484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen LJ, He JL, Yang DH, Ding YB, Chen XM,
Geng YQ, Liu SJ, Liu XQ and Wang YX: Mmu-microRNA-200a
overexpression leads to implantation defect by targeting
phosphatase and tensin homolog in mouse uterus. Reprod Sci.
20:1518–1528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh SK, Pal Bhadra M, Girschick HJ and
Bhadra U: MicroRNAs-micro in size but macro in function. FEBS J.
275:4929–4944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y and Srivastava D: A developmental
view of microRNA function. Trends Biochem Sci. 32:189–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen K, Chen X, He J, Ding Y, Geng Y, Liu
S, Liu X and Wang Y: Mouse endometrium temporal and spatial
expression mRNA and microRNA associated with embryo implantation.
Reprod Sci. 22:1399–1408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han Y, Chen J, Zhao X, Liang C, Wang Y,
Sun L, Jiang Z, Zhang Z, Yang R, Chen J, et al: MicroRNA expression
signatures of bladder cancer revealed by deep sequencing. PLoS One.
6:e182862011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamada Y, Enokida H, Kojima S, Kawakami K,
Chiyomaru T, Tatarano S, Yoshino H, Kawahara K, Nishiyama K, Seki N
and Nakagawa M: MiR-96 and miR-183 detection in urine serve as
potential tumor markers of urothelial carcinoma: Correlation with
stage and grade, and comparison with urinary cytology. Cancer Sci.
102:522–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nymark P, Guled M, Borze I, Faisal A,
Lahti L, Salmenkivi K, Kettunen E, Anttila S and Knuutila S:
Integrative analysis of microRNA, mRNA and aCGH data reveals
asbestos- and histology-related changes in lung cancer. Genes
Chromosomes Cancer. 50:585–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin H, Dai T, Xiong H, Zhao X, Chen X, Yu
C, Li J, Wang X and Song L: Unregulated miR-96 induces cell
proliferation in human breast cancer by downregulating
transcriptional factor FOXO3a. PLoS One. 5:e157972010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Myatt SS, Wang J, Monteiro LJ, Christian
M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S and Lam EW:
Definition of microRNAs that repress expression of the tumor
suppressor gene FOXO1 in endometrial cancer. Cancer Res.
70:367–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pineau P, Volinia S, McJunkin K, Marchio
A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM and
Dejean A: miR-221 overexpression contributes to liver
tumorigenesis. Proc Natl Acad Sci USA. 107:264–269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo Y, Liu H, Zhang H, Shang C and Song Y:
miR-96 regulates FOXO1-mediated cell apoptosis in bladder cancer.
Oncol Lett. 4:561–565. 2012.PubMed/NCBI
|
|
19
|
Guttilla IK and White BA: Coordinate
regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast
cancer cells. J Biol Chem. 284:23204–23216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Li P, Chen T, Gao G, Chen X, Du Y,
Zhang R, Yang R, Zhao W, Dun S, et al: Expression of microRNA-96
and its potential functions by targeting FOXO3 in non-small cell
lung cancer. Tumour Biol. 36:685–692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma XH, Hu SJ, Ni H, Zhao YC, Tian Z, Liu
JL, Ren G, Liang XH, Yu H, Wan P and Yang ZM: Serial analysis of
gene expression in mouse uterus at the implantation site. J Biol
Chem. 281:9351–9360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan Y, Li M, Cox S, Davis MK, Tawfik O,
Paria BC and Das SK: HB-EGF directs stromal cell polyploidy and
decidualization via cyclin D3 during implantation. Dev Biol.
265:181–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao XG, Li YL, Gao RF, Geng YQ, Chen XM,
Liu XQ, Ding YB, Mu XY, Wang YX and He JL: Folate deficiency
decreases apoptosis of endometrium decidual cells in pregnant mice
via the mitochondrial pathway. Nutrients. 7:1916–1932. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Q, Kannan A, Wang W, Demayo FJ, Taylor
RN, Bagchi MK and Bagchi IC: Bone morphogenetic protein 2 functions
via a conserved signaling pathway involving Wnt4 to regulate
uterine decidualization in the mouse and the human. J Biol Chem.
282:31725–31732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang XH, Zhao ZA, Deng WB, Tian Z, Lei W,
Xu X, Zhang XH, Su RW and Yang ZM: Estrogen regulates
amiloride-binding protein 1 through CCAAT/enhancer-binding
protein-beta in mouse uterus during embryo implantation and
decidualization. Endocrinology. 151:5007–5016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu R, Tang S, Wang M, Xu X, Yao C and Wang
S: MicroRNA-497 induces apoptosis and suppresses proliferation via
the Bcl-2/Bax-Caspase9-caspase3 pathway and cyclin D2 protein in
HUVECs. PLoS One. 11:e01670522016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Z, Jiang R, Yuan S, Wang N, Feng Y, Hu
G, Zhu X, Huang K, Ma J, Xu G, et al: Integrated analysis of DNA
methylation and RNA transcriptome during in vitro differentiation
of human pluripotent stem cells into retinal pigment epithelial
cells. PLoS One. 9:e914162014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nashta AA Fouladi, Andreu CV, Nijjar N,
Heath JK and Kimber SJ: Role of leukemia inhibitor factor (LIF) in
decidualisation of murine uterine stromal cells in vitro. J
Endocrinol. 181:477–492. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Evans MJ and Kaufman MH: Establishment in
culture of pluripotential cells from mouse embryos. Nature.
292:154–156. 1981. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Estella C, Herrer I, Moreno-Moya JM,
Quiñonero A, Martínez S, Pellicer A and Simón C: miRNA signature
and Dicer requirement during human endometrial stromal
decidualization in vitro. PLoS One. 7:e410802012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Zhang H, Jiang Y, Xue B, Diao Z,
Ding L, Zhen X, Sun H, Yan G and Hu Y: MicroRNA-181a is involved in
the regulation of human endometrial stromal cell decidualization by
inhibiting Kruppel-like factor 12. Reprod Biol Endocrinol.
13:232015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boeddeker SJ and Hess AP: The role of
apoptosis in human embryo implantation. J Reprod Immunol.
108:114–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Correia-da-Silva G, Bell SC, Pringle JH
and Teixeira NA: Patterns of expression of Bax, Bcl-2 and Bcl-x(L)
in the implantation site in rat during pregnancy. Placenta.
26:796–806. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Joswig A, Gabriel HD, Kibschull M and
Winterhager E: Apoptosis in uterine epithelium and decidua in
response to implantation: Evidence for two different pathways.
Reprod Biol Endocrinol. 1:442003. View Article : Google Scholar : PubMed/NCBI
|