Introduction
Allergic rhinitis (AR) is an immunoglobulin (Ig)
E-mediated, non-infectious inflammation of the nasal mucosa,
characterized by paroxysmal sneezing, rhinorrhea, nasal itching and
nasal obstruction when the susceptible individuals are exposed to
allergens (1). The incidence of AR
has risen significantly and, in 2010, the disease affected an
estimated 10–20% of the global population (2). AR directly exacerbates other
inflammatory airway diseases, including asthma, which threatens the
lives of patients (3). The most
effective drugs for the treatment of AR are antihistamines and
topical glucocorticoids (1), but
while these drugs temporarily alleviate AR symptoms they cannot
cure AR altogether. Thus, it is important to further understand the
mechanisms underlying AR development, as this will assist the
exploration of novel AR therapies.
Mast cells are directly and pathologically involved
in AR (4). While AR pathology is
dominated by Th2 cells, it remains dependent on the ability of
antigen-specific IgE to bind to FcεRI, which is expressed on mast
cells. Cross-linkage of FcεRI results in the activation of the mast
cell and initiation of a signal transduction cascade, leading to
the release of tumor necrosis factor-α, interleukin (IL)-4,
histamine, heparin, serotonin, kinins and proteases, which in turn
lead to inflammatory cell activation and recruitment, and allergic
disease-associated smooth muscle contraction (5). Furthermore, mast cells respond to
multiple inflammatory factors, including IgG, cytokines,
chemokines, adenosine and sphingosine-1-1phosphate (6–10).
The direct relationship between the activation of mast cells and AR
pathological responses is well documented, but it is necessary to
further elucidate the process of mast cell activation.
The matrix metalloproteinase (MMP) family consists
of zinc-dependent endopeptidases (11). MMPs are primarily involved in the
cleavage of the extracellular matrix (ECM), but are also involved
in a range of biological and pathological processes, including
fibrosis, inflammation and wound healing (12). MMP9, a member of the MMP family,
has been categorized as a pro-inflammatory factor. Mast cells
synthesize ECM components and their adhesive interactions with
fibroblasts result in MMP9 release. MMP9 release has been
demonstrated to further increase in the presence of IgE (13). In turn, MMP9 induces the release of
cytokines and chemokines from EMC, facilitating the infiltration of
immune cells into the inflammation site (14). MMP9 is also closely associated with
mast cells. Interactions between mast cells and fibroblast induce
MMP9 release from fibroblasts (15) and myocardial mast cells are
involved in the regulation of MMP9 activity (16). Mast cell chymase is involved in the
activation of pro-MMP9 and MMP2 (17), and tryptase-producing mast cells
may be associated with MMP2 and MMP9 expression (18). Notably, human mast cells produce
MMP9 themselves (19). However, to
the best of our knowledge, the regulation of MMP9 production in
mast cells and its effects on mast cell activation remains
unknown.
In the present study, MMP9 expression was
demonstrated to increase in activated mast cells in an AKT and ERK
signaling pathway-dependent manner, and increased MMP9 levels were
implicated in the activation of mast cells. Furthermore, the
increased expression of MMP9 in activated mast cells was inhibited
by IL-4.
Materials and methods
Reagents
Phorbol ester (PMA) and ionomycin (ION) were
purchased from Sigma-Aldrich, Merck Millipore (Darmstadt, Germany).
Murine IL-4 and IL-6 ELISA kits were purchased from eBioscience,
Inc. (San Diego, CA, USA). TRIzol reagent was purchased from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Antibodies against ERK2/1 (MK1; cat. no. sc-135900; 1:400), AKT
(C-20; cat. no. sc-1618; 1:200), phosphorylated (p)-ERK (E-4) (cat.
no. sc-7383; 1:400), p-AKT-Thr308 (cat. no. sc-16646; 1:200) and
MMP9 (cat. no. sc-6841; 1:400), the ERK/MAPK inhibitor U0126, the
AKT inhibitor MK2206 and the MMP9 inhibitor CTK8G1150, and MMP9
small interfering RNA (siRNA) (cat. no. sc-29401; 1:400) were all
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
For transient silencing of MMP9, 21-nt sequences of siRNA duplexes
were synthesized (GenePharma, Shanghai, China): sense
5′-CAUUCAGGGAGACGCCCAUUUTT-3′ and antisense
5′-AAAUGGGCGUCUCCCUGAAUGTT-3′; scramble control siRNA sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. A total of 40 nM siRNA duplexes was
transfected reagent on a 24-well plate. The efficiency of MMP9
transient silencing was confirmed by western blotting. Recombinant
murine IL-4 (cat. no. 404-ML; 1:200) and murine IL-4 antibodies
(cat. no. MAB404; 1:200); were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA).
Cells and cell culture
The murine mast cell P815 cell line and human mast
cell HMC-1 cell line were obtained from the American Type Culture
Collection (Manassas, VA, USA). RPMI-1640 supplemented with
L-glutamine, sodium pyruvate, non-essential amino acids, a 2-fold
vitamin solution, and penicillin-streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.). Fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) or horse serum
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to the
media. All cultures were performed at 37°C in a 5% CO2
atmosphere.
Cytokine assay and β-hexosaminidase
release
A total of 2×105 P815 or HMC-1 cells were
stimulated with 50 nM PMA and 500 nM ION for 24 h, and were
subsequently centrifuged at 400 × g for 5 min at 25°C. Cell
supernatant was collected and IL-4 and IL-6 levels were measured by
ELISA, according to the manufacturer's protocol. To detect
degranulation, 50 µl supernatant was removed for β-hexosaminidase
measurement, and deionized water was added to the remaining cell
pellets. The samples were frozen, thawed, and a second 50 µl was
removed to determine the total β-hexosaminidase content.
β-hexosaminidase samples (50 µl) were incubated in 0.04 M citric
acid with 0.02 M Na2HPO4 containing 10 mM
p-nitrophenyl N-acetyl-α-D-glucosaminide for 90 min at 37°C. The
reaction was developed using 0.4 M glycine and the absorbance was
determined at 405 nm. The release percentage was calculated as
follows: [β-hexosaminidase in supernatant/(β-hexosaminidase in
supernatant + total β-hexosaminidase in pellet)] ×100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted using TRIzol reagent.
and cDNA was synthesized using a PrimeScript™ RT reagent kit
(Takara Bio, Inc., Otsu, Japan), according to the manufacturer's
protocol. The following primers were used: β-actin, forward:
5′-CGTTGACATCCGTAAAGACC-3′ and reverse: 5′-AACAGTCCGCCTAGAAGCAC-3′;
MMP9, forward: 5′-CGGCACGCCTTGGTGTAGCA-3′ and reverse:
5′-GGCGCACCAGCGGTAACCAT-3′. The following PCR conditions were used:
1 cycle of 95°C for 30 sec followed by 40 cycles of 95°C for 5 sec
and 60°C for 34 sec. RT-qPCR and the 2−∆∆Cq method was
performed on an Applied Biosystems 7500 real time PCR system
(Thermo Fisher Scientific, Inc.) with version 2.0.6 software
(20).
Inhibition of ERK, AKT and MMP9
activity
To inhibit the ERK and AKT signaling pathways, the
2×105 P815 cells were pre-treated for 2 h with either
100 nM U0126 or 10 nM MK2206, or both, and were then activated
using 50 nM PMA and 500 nM ION for 24 h. To inhibit MMP9, the
2×105 P815 cells were pre-treated with 10 nM CTK8G1150
for 2 h and subsequently activated by 50 nM PMA and 500 nM ION for
24 h. The control group was treated with DMSO.
Western blot analysis
The total proteins were extracted from cells (HMC-1
and P815) using protein extracting buffer [20 mmol/l Tris-Cl
buffer, pH 7.5, containing 1 mmol/l ethylenediamine tetraacetic
acid, a protease inhibitor cocktail (complete, Mini,
ethylenediamine tetraacetic acid-free, 1 tablet of 10-ml buffer;
Sigma-Aldrich, Merck Millipore), 1% sodium dodecyl sulfate, 10%
Triton X-100, and 2 mol/l dithiothreitol]. After 30 min on ice, the
samples were centrifuged at 17,600 × g for 10 min at 4°C). A total
of 20 µg crude proteins extracted from cell lysates from HMC-1 and
P815 cells were separated on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were
subsequently transferred onto polyvinylidene difluoride membranes
(Merck Millipore). The membranes were blocked with 5% FBS in
Tris-buffered saline, pH 8.0, plus 0.05% Tween-20, for 1 h at room
temperature, and were then incubated with the corresponding primary
antibodies at 4°C overnight. Following washing with Tris-buffered
saline plus 0.05% Tween-20, the membranes were incubated with
secondary antibody conjugated to horseradish peroxidase (cat. nos.
P-0217, P-0161, P-0160; 1:1,000; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) was incubated for 1 h at room temperature.
Proteins were visualized using SuperSignal West Femto Maximum
Sensitivity Chemiluminescence substrate (Thermo Fisher Scientific,
Inc.).
Gelatin zymography
A gelatin zymography assay was performed to
investigate the secretion of active MMP9. A total of
4×104 of HMC-1 and P815 cells were grown to 70%
confluence, washed twice with PBS and incubated in serum-free
medium. Conditioned medium was collected 24 h following this and
was concentrated with a centrifugal filter (Merck Millipore) at
6,000 × g for 15 min at 4°C Concentrated samples were prepared in
non-reducing sample buffer (Invitrogen; Thermo Fisher Scientific,
Inc.). Proteins (20 µl/lane) were separated by SDS-PAGE on gels
containing 1 mg/ml gelatin (Novex 10% gelatin gel; Invitrogen;
Thermo Fisher Scientific, Inc.). The gels were renatured for 1 h at
room temperature in 1X renaturing buffer (Invitrogen; Thermo Fisher
Scientific, Inc.). Following this, gels were incubated overnight at
37°C in 1X developing buffer (Invitrogen; Thermo Fisher Scientific;
Inc.). Gels were stained with Coomassie blue for 2 h at room
temperature. The brightness of the clear bands, where MMP9 was
located and the gelatin was degraded, was analyzed according the
optical density using Bio-Rad Image Lab version 4.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
RNA interference assay
MMP9 and negative control siRNA duplexes (40 nM)
were transfected into cells of HMC-1 and P815
(5×105/well) using 3 µl INTERFER in siRNA transfection
reagent (Santa Cruz Biotechnology, Inc.) in a 24-well plate. The
efficiency of MMP9 silencing was confirmed by western blot
analysis.
Statistical analysis
Data are presented as the mean ± standard error. The
results were compared using one-way analysis of variance in SPSS
version 16 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
MMP9 expression is upregulated in
activated mast cells
Following stimulation with 50 nM PMA and 500 nM ION,
P815 cells released significantly increased levels of IL-4, IL-6
and β-hexosaminidase compared with control cells or DMSO-treated
cells (P=0.0018, P=0.0014 and P=0.0002, respectively; Fig. 1A). HMC-1 cells also had higher
levels of IL-4, IL-6 and β-hexosaminidase (data not shown).
Following activation, increased mRNA (Fig. 1B) and protein (Fig. 1C) expression levels of MMP9 were
detected in both P815 and HMC-1 cells. To confirm an increase of
MMP9 release, MMP9 activity was detected by gelatin zymography.
Activated P815 and HMC-1 cells degraded visibly more gelatin
compared with the control or DMSO-treated cells (Fig. 1D). These results indicated that
MMP9 expression is upregulated in activated P815 and HMC-1 mast
cells.
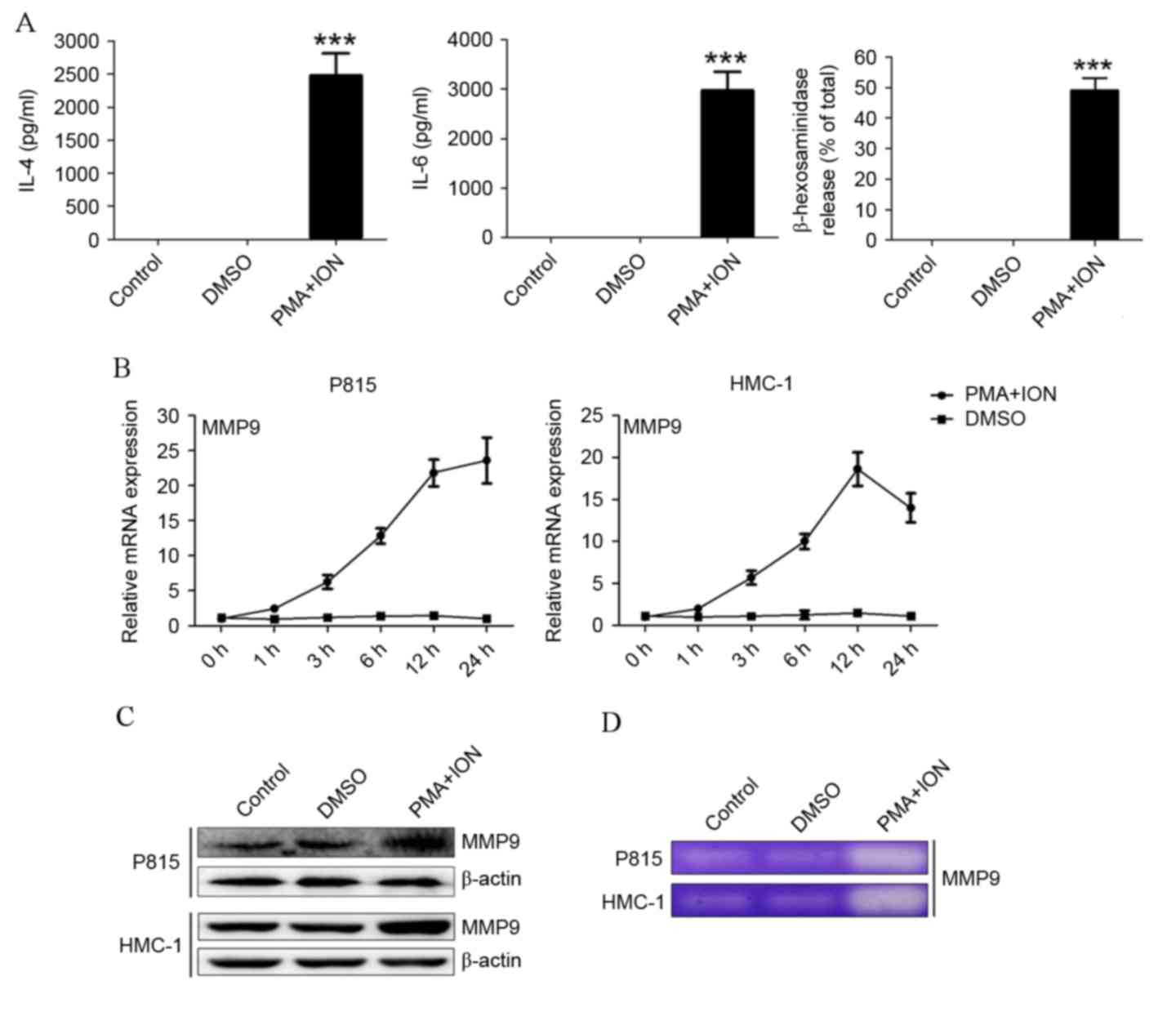 | Figure 1.MMP9 expression is upregulated in
activated mast cells. (A) IL-4 and IL-6 levels, as determined by
ELISA, and degranulation, as determined by β-hexosaminidase
release, in PMA+ION-treated activated P815 cells. (B) The mRNA
expression levels of MMP9 in P815 and HMC-1 cells, as detected by
reverse transcription-quantitative polymerase chain reaction. (C)
The protein expression levels of MMP9 in P815 and HMC-1 cells, as
detected by western blotting. (D) The secretion level of MMP9 in
activated P815 and HMC-1 cells, as detected by gelatin zymography.
The data are represented as the results of three independent
experiments ***P<0.001 vs. DMSO. MMP9, matrix metalloproteinase
9; IL, interleukin; PMA, phorbol ester; ION, ionomycin; DMSO,
dimethyl sulfoxide. |
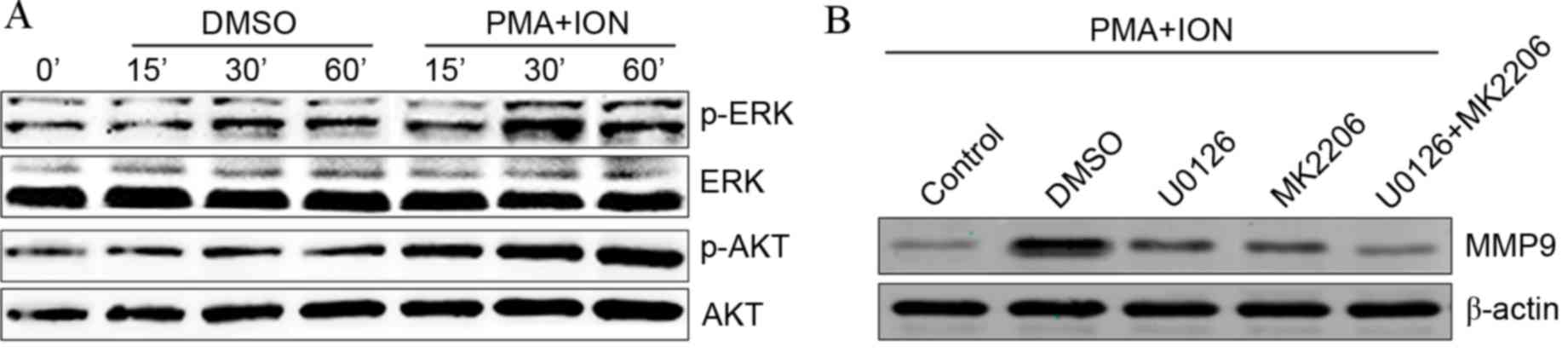
Upregulation of MMP9 in activated mast
cells is dependent on the ERK and AKT signaling pathways
The ERK and AKT signaling pathways are involved in
the activation of mast cells (21)
and have also been reported to induce the expression of MMP9
(22). The present study aimed to
establish whether the ERK and AKT signaling pathways are involved
in MMP9 upregulation in activated mast cells. Phosphorylated ERK
and AKT expression levels were visibly increased in activated P815
cells (Fig. 2A). Following
treatment with the ERK-specific inhibitor U0126 or the AKT-specific
inhibitor MK2206, PMA+ION treatment-induced increases in MMP9
protein levels were partially inhibited in activated P815 cells
compared with DMSO-treated activated P815 cells (Fig. 2B). Combined treatment with both
U0126 and MK2206 appeared to completely abolish increased MMP9
protein levels in activated P815 cells compared with DMSO-treated
activated P815 cells (Fig. 2B).
These results suggested that the increased MMP9 levels observed in
activated mast cells are dependent on the ERK and AKT signaling
pathways.
MMP9 promotes the activation of mast
cells
To establish the effect of MMP9 on mast cell
activation, the MMP9 inhibitor CTK8G1150 was used. PMA and ION
treatment-induced IL-4, IL-6 and β-hexosaminidase release
significantly decreased in CTK8G1150-treated P815 cells compared
with the untreated P815 cells (P=0.0495, P=0.0128, P=0.0067,
respectively; Fig. 3A).
Furthermore, similar results were demonstrated in activated P815
cells transfected with siRNA, with IL-4, IL-6 and β-hexosaminidase
release significantly decreased in MMP9 siRNA-transfected P815
cells compared with control siRNA-transfected P815 cells (P=0.0490,
P=0.0099 and P=0.0073, respectively; Fig. 3B). These results suggested that
MMP9 is involved in the activation of mast cells.
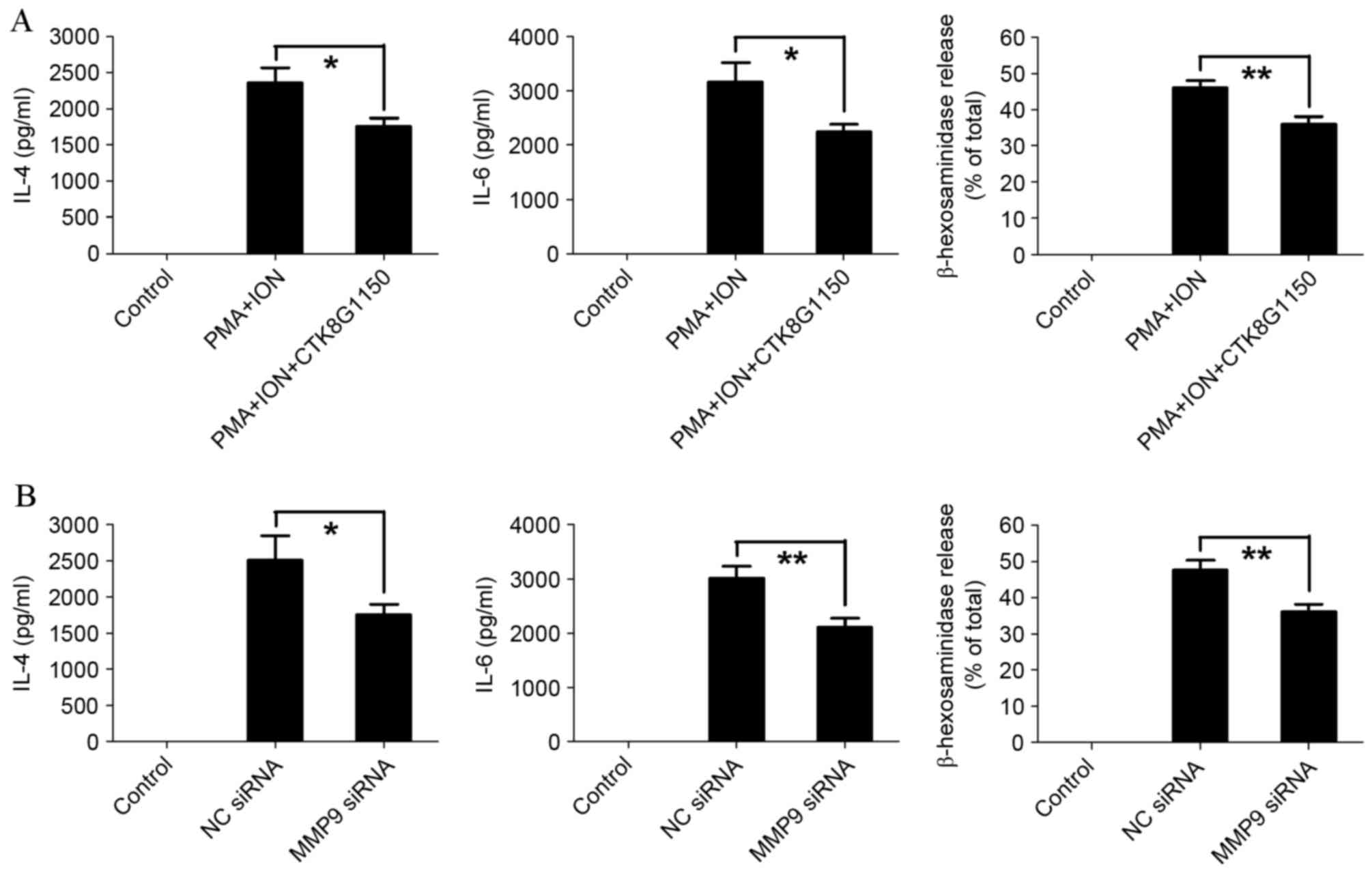 | Figure 3.MMP9 promotes the activation of mast
cells. (A) The levels of IL-4 and IL-6 were determined by ELISA,
and degranulation was determined by β-hexosaminidase release in
activated P815 cells following treatment with the MMP9 specific
inhibitor, CTK8G1150 (10 nM). (B) The levels of IL-4 and IL-6 were
determined by ELISA, and degranulation as determined by
β-hexosaminidase release in activated P815 cells following
transfection with MMP9 specific siRNA. The data are representative
of three independent experiments, and comparisons are indicated by
lines. *P<0.05, **P<0.01. MMP9, matrix metalloproteinase 9;
IL, interleukin; siRNA, small interfering RNA; PMA, phorbol ester;
ION, ionomycin; NC, negative control. |
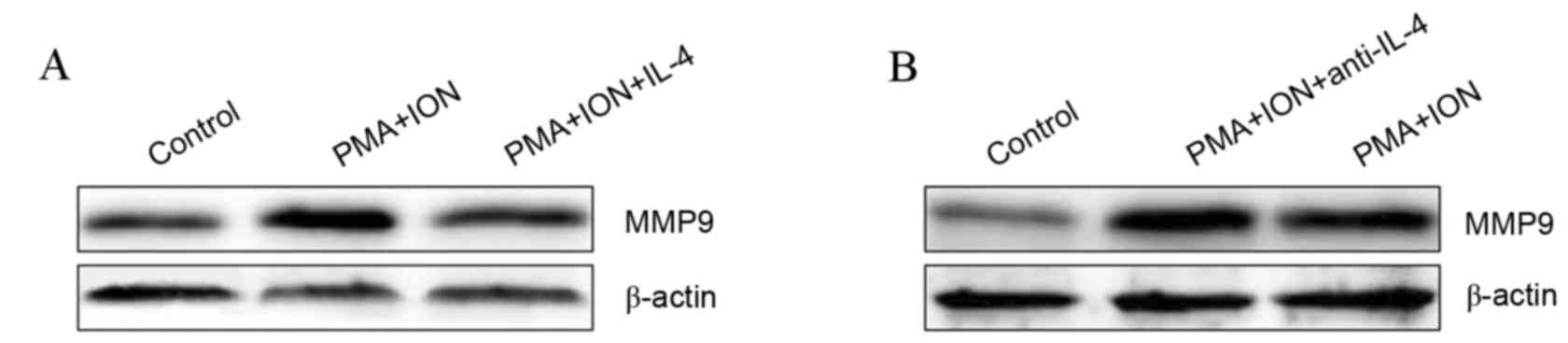
IL-4 inhibits MMP9 upregulation in
activated mast cells
IL-4 has been previously demonstrated to inhibit
MMP9 expression in rat synovial membranes incubated in vitro
(23) and activated mast cells
produce high levels of IL-4 (24).
Therefore, it was hypothesized that IL-4 was involved in the
regulation of MMP9 expression in activated mast cells. The protein
expression of MMP9 was visibly inhibited in 10 ng/ml IL-4-treated
activated P815 cells compared with untreated activated P815 cells
(Fig. 4A). Furthermore, inhibiting
IL-4 signaling using 10 µg/ml IL-4 neutralizing antibody resulted
in visibly increased MMP9 protein levels in activated P815 cells
compared with untreated activated P815 cells (Fig. 4B). These results indicated that
IL-4 negatively regulates MMP9 expression in activated mast
cells.
Discussion
AR is an important health problem due to its
prevalence and its impact on the social life, school performance
and work productivity of patients (25). Mast cells are the most important
effector cells in AR (26). In
general, mast cells are activated through cross-linkage of FcεRI by
antigen-specific IgE. They are key effectors in IgE-dependent
hypersensitivity reactions (5).
Ligation of FcεRI, constitutively expressed on mast cells, promotes
cell activation and immediate release and production of
pro-inflammatory mediators, characterized by sneezing, itching,
rhinorrhea and nasal obstruction, and AR may negatively impact AR
patient's quality of life (27,28).
HMC-1 (27) and P815 (28) cell lines were often used to explore
the mechanism of AR development, whereas there is no recognition
about their involvement in mastocytoma. The present study,
determined that MMP9 was implicated in PMA and ION-induced
activation of mast cells. This finding provided a novel insight
into the mechanisms underlying mast cell activation.
As a member of the MMP family, the primary function
of MMP9 is to degrade the ECM, which facilitates the invasion and
metastasis of tumors (29). MMP9
has also been demonstrated to regulate the release of inflammatory
factors and cytokines. Binding of MMP9 to CD44 promotes the release
of activated transforming growth factor-β1 (30). MMP9 has also been previously
reported to increase vascular endothelial growth factor release and
promote angiogenesis (31). A
variety of inflammatory factors and cytokines activate mast cells
(32) and it is possible that MMP9
promotes PMA and ION-induced mast cell activation through the
regulation of the inflammatory factors and cytokines involved in
mast cell activation. However, further investigation is required to
elucidate the precise mechanism.
High IL-4 levels are produced by activated mast
cells. In the present study, it was demonstrated that IL-4
decreases MMP9 protein levels in activated mast cells, forming a
negative feedback loop. In this manner, mast cells are able to
limit self-activation. Inhibition of IL-4 expression has been
proposed as an effective strategy for the treatment of airway
inflammation (33). Therefore,
attempts to negatively regulate IL-4 in mast cells must take this
into consideration. Without further study, the comprehensive
outcome of IL-4 inhibition to control airway inflammation remains
difficult to determine.
In conclusion, MMP9 was upregulated during mast cell
activation, and this upregulation was dependent upon normal
function of the ERK and AKT signaling pathways. Increased MMP9
levels were demonstrated to further activate mast cells, whereas
IL-4 inhibited the increase of MMP9 in activated mast cells. These
findings revealed a novel mechanism underlying mast cell
activation, which enhances current understanding and may provide
novel targets for the treatment of AR.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant no. LY12H13003) and
the Medical Scientific Research Foundation of Zhejiang Province
(nos. 2010KYA104 and 2012KYA098).
References
|
1
|
Ridolo E, Montagni M, Melli V, Bonzano L,
Incorvaia C and Canonica GW: A role for the intranasal formulation
of azelastine hydrochloride/fluticasone propionate in the treatment
of allergic rhinitis. Ther Deliv. 6:653–659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brozek JL, Bousquet J, Baena-Cagnani CE,
Bonini S, Canonica GW, Casale TB, van Wijk RG, Ohta K, Zuberbier T,
Schünemann HJ, et al: Allergic Rhinitis and its Impact on Asthma
(ARIA) guidelines: 2010 Revision. J Allergy Clin Immunol.
126:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hadley JA, Derebery MJ and Marple BF:
Comorbidities and allergic rhinitis: Not just a runny nose. J Fam
Pract. 61(2 Suppl): S11–S15. 2012.PubMed/NCBI
|
|
4
|
Williams CM and Galli SJ: The diverse
potential effector and immunoregulatory roles of mast cells in
allergic disease. J Allergy Clin Immunol. 105:847–859. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robbie-Ryan M and Brown M: The role of
mast cells in allergy and autoimmunity. Curr Opin Immunol.
14:728–733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woolhiser MR, Okayama Y, Gilfillan AM and
Metcalfe DD: IgG-dependent activation of human mast cells following
up-regulation of FcgammaRI by IFN-gamma. Eur J Immunol.
31:3298–3307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang JX, Kaieda S, Ameri S, Fishgal N,
Dwyer D, Dellinger A, Kepley CL, Gurish MF and Nigrovic PA:
IL-33/ST2 axis promotes mast cell survival via BCLXL. Proc Natl
Acad Sci USA. 111:10281–10286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Juremalm M and Nilsson G: Chemokine
receptor expression by mast cells. Chem Immunol Allergy.
87:130–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okayama Y, Saito H and Ra C: Targeting
human mast cells expressing g-protein-coupled receptors in allergic
diseases. Allergol Int. 57:197–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oskeritzian CA, Price MM, Hait NC,
Kapitonov D, Falanga YT, Morales JK, Ryan JJ, Milstien S and
Spiegel S: Essential roles of sphingosine-1-phosphate receptor 2 in
human mast cell activation, anaphylaxis, and pulmonary edema. J Exp
Med. 207:465–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bratcher PE, Weathington NM, Nick HJ,
Jackson PL, Snelgrove RJ and Gaggar A: MMP-9 cleaves SP-D and
abrogates its innate immune functions in vitro. PLoS One.
7:e418812012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vandenbroucke RE, Dejonckheere E and
Libert C: A therapeutic role for matrix metalloproteinase
inhibitors in lung diseases? Eur Respir J. 38:1200–1214. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gauchotte G, Marie B, Gallet P, Nguyen DT,
Grandhaye M, Jankowski R and Vignaud JM: Respiratory epithelial
adenomatoid hamartoma: A poorly recognized entity with mast cell
recruitment and frequently associated with nasal polyposis. Am J
Surg pathol. 37:1678–1685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Purwar R, Kraus M, Werfel T and Wittmann
M: Modulation of keratinocyte-derived MMP-9 by IL-13: A possible
role for the pathogenesis of epidermal inflammation. J Invest
Dermatol. 128:59–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abel M and Vliagoftis H: Mast
cell-fibroblast interactions induce matrix metalloproteinase-9
release from fibroblasts: Role for IgE-mediated mast cell
activation. J Immunol. 180:3543–3550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brower GL, Chancey AL, Thanigaraj S,
Matsubara BB and Janicki JS: Cause and effect relationship between
myocardial mast cell number and matrix metalloproteinase activity.
Am J Physiol Heart Circ Physiol. 283:H518–H525. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tchougounova E, Lundequist A, Fajardo I,
Winberg JO, Abrink M and Pejler G: A key role for mast cell chymase
in the activation of pro-matrix metalloprotease-9 and pro-matrix
metalloprotease-2. J Biol Chem. 280:9291–9296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abel M and Vliagoftis H: Mast
cell-fibroblast interactions induce matrix metalloproteinase-9
release from fibroblasts: Role for IgE-mediated mast cell
activation. J Immunol. 180:3543–3550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanbe N, Tanaka A, Kanbe M, Itakura A,
Kurosawa M and Matsuda H: Human mast cells produce matrix
metalloproteinase 9. Eur J Immunol. 29:2645–2649. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Silwal P, Shin K, Choi S, Kang SW, Park
JB, Lee HJ, Koo SJ, Chung KH, Namgung U, Lim K, et al: Adenine
suppresses IgE-mediated mast cell activation. Mol Immunol.
65:242–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dahiya S, Givvimani S, Bhatnagar S,
Qipshidze N, Tyagi SC and Kumar A: Osteopontin-stimulated
expression of matrix metalloproteinase-9 causes cardiomyopathy in
the mdx model of Duchenne muscular dystrophy. J Immunol.
187:2723–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hyc A, Osiecka-Iwan A, Niderla-Bielinska J
and Moskalewski S: Influence of LPS, TNF, TGF-ss1 and IL-4 on the
expression of MMPs, TIMPs, and selected cytokines in rat synovial
membranes incubated in vitro. Int J Mol Med. 27:127–137.
2011.PubMed/NCBI
|
|
24
|
McLeod JJ, Baker B and Ryan JJ: Mast cell
production and response to IL-4 and IL-13. Cytokine. 75:57–61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bousquet J, Khaltaev N, Cruz AA, Denburg
J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica
GW, van Weel C, et al: Allergic Rhinitis and its Impact on Asthma
(ARIA) 2008 update (in collaboration with the World Health
Organization, GA(2)LEN and AllerGen). Allergy. 63 Suppl 86:S86–S88.
2008. View Article : Google Scholar
|
|
26
|
Bernstein DI, Schwartz G and Bernstein JA:
Allergic Rhinitis: Mechanisms and Treatment. Immunol Allergy Clin
North Am. 36:261–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HY, Nam SY, Hwang SY, Kim HM and Jeong
HJ: Atractylone, an active constituent of KMP6, attenuates allergic
inflammation on allergic rhinitis in vitro and in vivo models. Mol
Immunol. 78:121–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin H, Zheng C, Li J, Yang C and Hu L:
Lentiviral shRNA against KCa3.1 inhibits allergic response in
allergic rhinitis and suppresses mast cell activity via PI3K/AKT
signaling pathway. Sci Rep. 5:131272015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang F, He W, Fanghui P, Wang L and Fan Q:
NF-kappaBP65 promotes invasion and metastasis of oesophageal
squamous cell cancer by regulating matrix metalloproteinase-9 and
epithelial-to-mesenchymal transition. Cell Biol Int. 37:780–788.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu Q and Stamenkovic I: Cell
surface-localized matrix metalloproteinase-9 proteolytically
activates TGF-beta and promotes tumor invasion and angiogenesis.
Genes Dev. 14:163–176. 2000.PubMed/NCBI
|
|
31
|
Gupta A, Zhou CQ and Chellaiah MA:
Osteopontin and MMP9: Associations with VEGF expression/secretion
and angiogenesis in PC3 prostate cancer cells. Cancers (Basel).
5:617–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu Y, Blokhuis BR, Garssen J and Redegeld
FA: Non-IgE mediated mast cell activation. Eur J Pharmacol.
778:33–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee CC, Huang HY and Chiang BL:
Lentiviral-mediated interleukin-4 and interleukin-13 RNA
interference decrease airway inflammation and hyperresponsiveness.
Hum Gene Ther. 22:577–586. 2011. View Article : Google Scholar : PubMed/NCBI
|