Introduction
A total of 375,000 hip and knee endoprostheses
implants are performed in Germany each year (1). In 1.5% of cases, implant-associated
infections (IAI) occur following total joint arthroplasty (2). Despite the relatively low prevalence
of IAI, it may have severe consequences for patients, physicians
and the health care system (3). In
the majority of cases, infections are initiated by
Staphylococcus aureus, a biofilm-forming coagulase-negative
Staphylococcus epidermidis (S. epidermidis), or
gram-negative species (4). Early
infections occur within weeks following surgery and are usually
acquired intraoperatively. In addition, IAI may manifest as a late
infection, occurring between 3 and 24 months following surgery,
resulting from hematogenous spreading of the bacteria from other
foci (5,6). IAI requires local and systemic
antimicrobial therapy, which necessitates the removal of the
contaminated endoprosthetic implant and insertion of a temporary
cement implant (spacer) loaded with antibiotics (7–9).
There is currently a requirement for novel
endoprosthetic materials with optimal surface and mechanical
characteristics that prevent bacterial biofilm formation avoid
bacterial resistance mechanisms and mediate biocompatibility and
osseointegration (10). In recent
years, magnesium has emerged as a promising implant material
solution. Magnesium has various favourable properties, as it is a
freely accessible metal and an essential cation serves an important
role in the metabolism of human cells (11,12).
The biocompatibility of magnesium as a coating is influenced by the
corrosion rate, corrosion products and the varying pH-value of the
solution (13). A previous in
vitro study revealed that rapidly corroding magnesium in a thin
layer on titanium (Ti6Al4V) was a suitable candidate for implant
coatings with antimicrobial and biocompatible properties (14). Monocultures of S.
epidermidis and human osteoblasts (hOB) revealed that magnesium
coatings may prevent initial bacterial adhesion while remaining
biocompatible according to the cytotoxicity test standards of the
International Organisation for Standardisation (15). In contrast to monocultures,
co-cultures provide a more comprehensive model of the development
of the infection, as demonstrated in a previous study (16).
The aim of the present study was to investigate the
antibacterial potential and cytotoxic effects of a thin, rapidly
corroding magnesium coating on titanium samples in a co-culture
model of S. epidermidis and hOBs.
Materials and methods
Titanium samples coated with pure
magnesium
The present study used Ti6Al4V discs (DOT GmbH,
Rostock, Germany) coated with pure magnesium for the experiments.
Uncoated Ti6Al4V discs (a common implant material) served as a
control. The discs were 11 mm in diameter and 2.5 mm in thickness.
Magnesium-sputtering targets (diameter, 20 cm; 3.5 mm thickness;
purity, 3N5) were produced by Fhr Anlagenbau GmbH
(Ottendorf-Okrilla, Germany). The magnetron sputtering process was
conducted with a Von Ardenne CS730 cluster machine (Von Ardenne
GmbH, Dresden, Germany) with a 200 W sputtering power and 2.3×103
mbar pressure. The samples were coated with layers of magnesium
(5-µm thick) by breaking the vacuum and turning the samples. The
temperature of the substrate holder did not surpass 76°C, and all
process parameters were performed in accordance with those
described by Schlüter et al (17). The roughness (Rz) of the
surfaces was measured via 3D laser scanning microscopy (VK-X260,
Keyence Corporation, Osaka, Japan). The Ti6Al4V disc roughness was
between 8 and 12.7 µm for the magnesium-coated discs as determined
in our previous study (14). The
samples were Υ-sterilized at 25 kGy following the sputtering
process.
Magnesium ion release and pH of the
supernatants
The magnesium-coated Ti6Al4V samples were
pre-incubated for 24 h in Modified Eagle's osteogenic cell culture
medium (MEM; EMD Millipore, Billerica, MA, USA) without calcium or
antibiotics, containing 10% foetal calf serum (EMD Millipore), 1%
HEPES buffer (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and the following osteogenic additives: 100 nM dexamethasone,
50 µg/ml L-ascorbic acid and 10 mM β-glycerophosphate, which were
all obtained from Sigma-Aldrich; Merck Millipore (Darmstadt,
Germany). Following 24 h pre-incubation and at 2, 4 and 7 days
following infection, supernatants were transferred to 1.5-ml
Eppendorf reaction tubes (Eppendorf, Hamburg, Germany). Digestion
was achieved using 65% concentrated nitric acid (1.0% v/v;
Sigma-Aldrich, Merck Millipore). The concentration of magnesium
ions released from magnesium coatings was quantified using atomic
absorption spectrometry ZEEnit 650P in combination with a graphite
tube atomizer (all from Analytik Jena AG, Jena, Germany). The
analysis process started with evaporation steps at 80, 90 and
110°C. This was followed by a pyrolysis phase at 900°C in the
platform tube, heated to 2,000°C to evaporate and transform compact
fragments into atoms. This step involved elemental analysis using a
resonating cathode lamp and a magnesium cathode at a wavelength of
202.6 nm. Following 0, 2 and 7 days, the pH value of the
supernatants was determined using an InoLab® pH 720 pH
meter (WTW GmbH, Weilheim, Germany).
Isolation and cultivation of hOBs
The isolation of hOBs was conducted according to the
protocol previously described by Jonitz et al (18). The osteoblasts were obtained from
the cancellous femoral head of 6 patients that underwent total hip
replacement surgery (3 male donors, mean age, 72±8.6 years; 3
female donors, mean age, 78±3.8 years) at the Department of
Orthopaedics University Medicine Rostock (Rostock, Germany). The
present study was approved by The Local Ethics Committee of Rostock
(Rostock, Germany; registration number, A2010-10) and written
informed consent was obtained from each patient.
Co-culture of hOBs and S.
epidermidis
The procedures used to co-culture hOBs and S.
epidermidis were the same as those described by Zaatreh et
al (16). hOBs (25,000
cells/ml) at passage three were transferred to a 24-well-plate
(Sarstedt, Nümbrecht, Germany) and cultured on the Ti6Al4V sample
discs in MEM with adjunctions for 24 h. Similarly, hOBs and S.
epidermidis were cultured on Ti6Al4V sample discs coated with
magnesium for 24 h. A prepared overnight culture of S.
epidermidis (RP62A; ref. no. 35984; American Type Culture
Collection, Manassas, VA, USA) was subsequently used for
mono-species infections with a multiplicity of infection of 0.04
(25,000 osteoblasts/ml, 1,000 colony forming units/ml). Co-cultures
were incubated for a period of 7 days under standard aerobic
conditions (37°C and 5% CO2). At 2 and 4 days following
infection the medium was replenished.
Determination of hOB viability
hOBs that adhered to the discs were washed with 1X
phosphate-buffered saline (PBS; EMD Millipore), followed by the
addition of 200 µl trypsin/EDTA (1X; Gibco; Thermo Fisher
Scientific, Inc.) for 3 min under aerobic conditions (37°C and 5%
CO2). The hOBs were then mechanically relocated from the
discs with a pipette tip (Eppendorf). The solution with the
osteoblasts was transferred to a 1.5 ml Eppendorf reaction tube
(Eppendorf) centrifuged at 169 × g for 4 min at 4°C, and washed
with 1X PBS. Quantification of the number of viable primary
osteoblasts on the test samples was determined by trypan blue
staining (Sigma-Aldrich; Merck Millipore). Trypan blue enters the
damaged membranes of dead cells, leaving viable cells unstained.
The viable cells were counted using a Thoma cell counting chamber,
obtained from Paul Marienfeld GmbH & Co. KG (Lauda-Königshofen,
Germany) according to manufacturer's protocol (19) and using an Olympus CKX41SF optical
light microscope, (Olympus Soft Imaging Solutions GmbH, Hamburg,
Germany). Measurements were performed at 2 and 7 days of
co-culture.
Quantification of biofilm-bound S.
epidermidis
The test samples were transferred to glass test
tubes (Greiner Bio-One International GmbH, Kremsmünster, Austria)
containing 1 ml PBS (1X). S. epidermidis were removed by
ultrasonic treatment with device settings at 100% for 4 min
(BANDELIN BactoSonic, GmbH & Co. KG, Berlin, Germany) at days 2
and 7 of co-culture. The solution in the glass test tube was
diluted in 1X PBS and plated onto tryptic soy broth (TSB)-agar
plates (Thermo Fisher Scientific, Inc.). Following 24 h incubation
at 37°C and 5% CO2, the number of colony forming units
were quantified.
Quantification of planktonic S.
epidermidis
The co-culture supernatants containing planktonic
S. epidermidis were collected in 15 ml centrifuge tubes
(Greiner Bio-One International AG, Kremsmünster, Austria) with 1 ml
PBS (1X), and centrifuged at 3,345 × g for 10 min at 4°C on day 2
and 7 of co-culture. The number of planktonic bacteria were
quantified by serial dilution in 1X PBS and then counting the
number of colony forming units on TSB-agar plates (Thermo Fisher
Scientific, Inc.) following 24 h incubation at 37°C and 5%
CO2.
Scanning electron microscopy
(SEM)
Test samples were fixed in a 2.5% glutaraldehyde
solution at 4°C for 24 h. Specimens were subsequently washed with
0.1 M sodium acetate buffer, and dried using a graded ethanol
series (5 min in 30% ethanol, 5 min in 50% ethanol, 10 min in 70%
ethanol, 15 min in 90% ethanol and twice for 10 min in 100%
ethanol). The samples were then dried by critical point drying with
CO2 (Critical Point Dryer, Emitech Ltd., Ashford, UK),
sputter-coated with gold, and examined with a scanning electron
microscope (Zeiss DSM 960A; Zeiss GmbH, Jena, Germany).
Statistical analysis
Data are expressed as the mean ± standard error for
three independent experiments. Statistical significance was
evaluated with an unpaired two-tailed t-test using SPSS software
(version, 20.0; IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Magnesium ion release of the test
samples and pH-values of the media
Ion release data were examined in order to
characterize the strength of the corrosion process as it progresses
over the 7 days. Fig. 1 shows the
release of magnesium ions in the medium following one day of
pre-incubation and at 2, 4 and 7 days following infection of the
co-culture and the test sample alone. The magnesium-coated titanium
alloy released 24 mM/day magnesium ions during pre-incubation and
11 mM/day over the first 2 days, decreasing to 3 mM/day during days
4–7. The magnesium-coated surface in the co-culture with
osteoblasts and bacteria released 24 mM/day during pre-incubation
and 8 mM/day over the first 2 days, which decreased to 2 mM/day
during days 4 to 7. Media were replenished following 1 day of
pre-incubation and after 2 and 4 days of co-culture. Magnesium ion
release levels in the magnesium-coated samples maintained in medium
without bacteria and cells were not significantly greater when
compared with the hOB and S. epidermis co-culture samples.
This indicates that hOB adhesion and S. epidermidis
infection did not significantly alter the release of magnesium.
Bacterial growth is influenced by pH, therefore the
pH-values of the media were monitored. Table I indicates the pH-values in the
co-culture at 0, 2, 4 and 7 days. The magnesium-coated Ti6Al4V
altered the pH-value to an alkaline environment whereas the
uncoated Ti6Al4V control demonstrated a slightly acidic pH
value.
 | Table I.pH-values for Ti6Al4V and Mg-coated
Ti6A14V test samples in co-culture with hOB and S.
epidermidis for 0, 2, 4 and 7 days. |
Table I.
pH-values for Ti6Al4V and Mg-coated
Ti6A14V test samples in co-culture with hOB and S.
epidermidis for 0, 2, 4 and 7 days.
| Co-culture of hOB
and S. epidermidis | Day 0 | Day 2 | Day 4 | Day 7 |
|---|
| MEM+10% FCS | 7.94±0.07 | 8.03±0.15 | 8.01±0.07 | 8.05±0.03 |
| Ti6Al4V | 7.88±0.06 | 7.83±0.08 | 7.81±0.03 | 7.64±0.04 |
| Mg-coating | 8.82±0.08 | 8.56±0.08 | 8.19±0.01 | 8.12±0.04 |
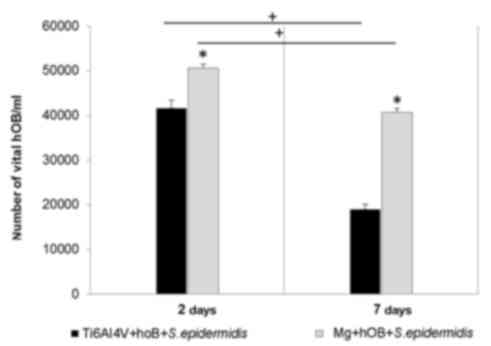
Determination of hOB viability in
co-culture with S. epidermidis
The number of viable hOBs was examined by trypan
blue staining (Fig. 2). A total of
25,000 cells were seeded on the two samples. The number of
osteoblasts on the magnesium-coated sample was 50,000 following 2
days (P=0.01) and 40,000 at 7 days (P=0.01). For the control, a
value of 40,000 cells at day 2 and 20,000 at day 7 were observed.
The number of viable osteoblasts was greater on the
magnesium-coated sample when compared with the control by ~10,000
at 2 days and ~20,000 at 7 days.
Evaluation of the viability of S.
epidermidis in co-culture with hOBs
Measurements of bacterial colonization in the test
samples revealed that a greater number of S. epidermidis
colonies survived on the control TiAI6V4 surface when compared with
the magnesium-coated Ti6Al4V discs (Fig. 3). The magnesium-coated sample
demonstrated a significant antibacterial effect, as indicated by
the significant decrease in biofilm population by three orders of
magnitude following 2 days (P=0.02), and 4 orders of magnitude
following 7 days (P=0.03; Fig. 3).
The effect was approximately 1 order of magnitude lower for the
planktonic population. Complete results of the measurements for
S. epidermidis are presented in Fig. 3.
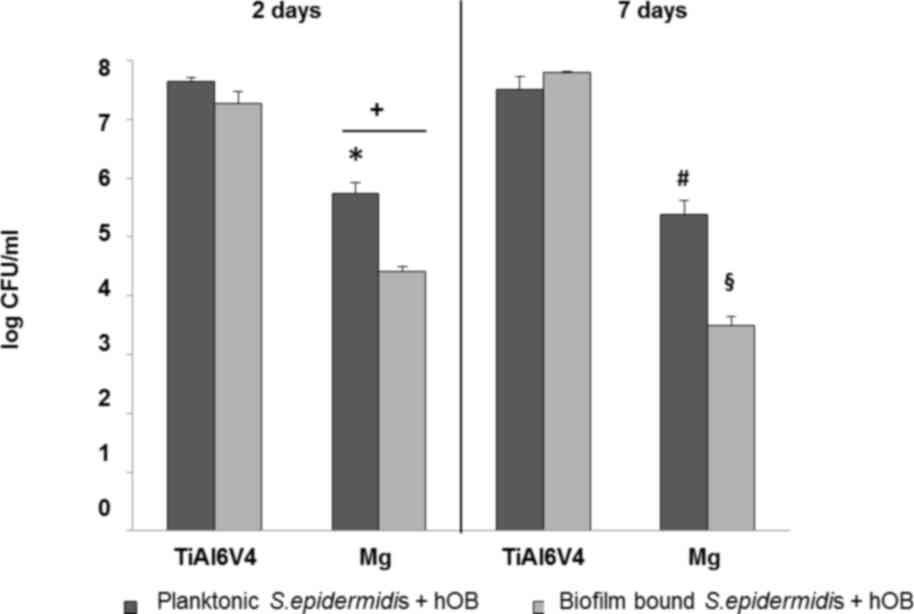 | Figure 3.Quantification of viable biofilm-bound
and planktonic S. epidermidis in co-culture with hOB cells
on the test samples following 2 and 7 days. The number of viable
biofilm-bound S. epidermidis on the Mg-coated specimen was
significantly decreased when compared with the titanium control
over a period of 7 days. Data are expressed as the mean ± standard
error of the mean (n=3). *P<0.05 Mg vs. titanium alloy control,
day 2, planktonic. +P<0.05 biofilm vs. planktonic,
day 2, on Mg. #P<0.05 Mg vs. titanium alloy control,
day 7, planktonic. §P<0.05 Mg vs. titanium alloy
control, day 7, biofilm. S. epidermidis, Staphylococcus
epidermidis; hOBs, human osteoblasts; Mg, magnesium; CFU,
colony forming units. |
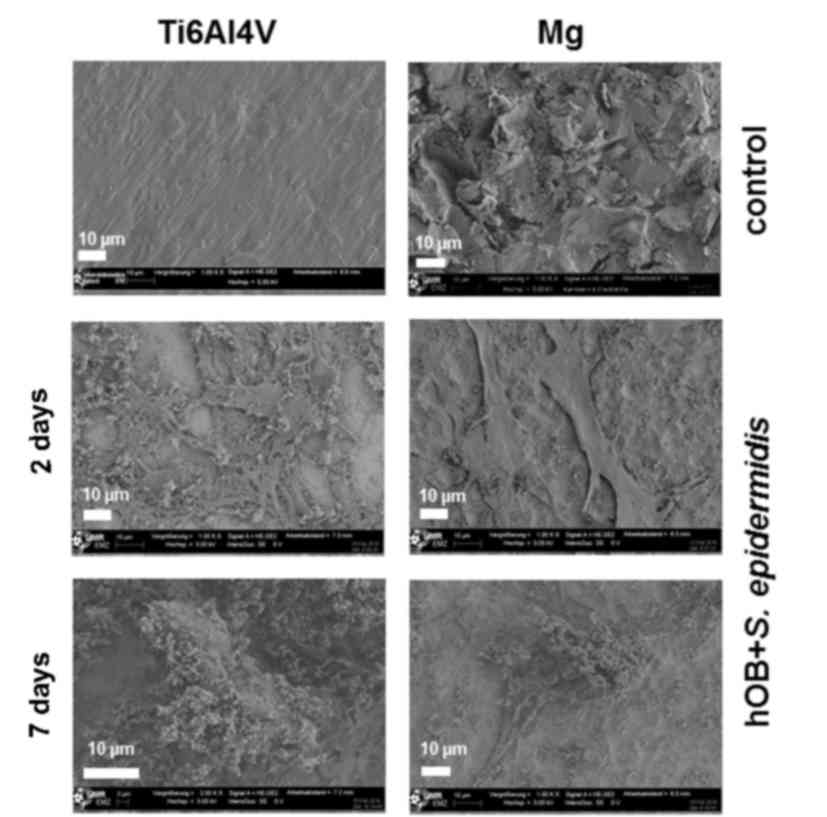
SEM imaging
SEM was performed to qualitatively assess the layer
structure and morphology of osteoblasts, as well as the growth
patterns and distribution of bacteria on the test specimen. The SEM
images shown in Fig. 4 confirm the
results of the quantitative measurements of the biofilm-bound
bacteria. S. epidermidis colonization was observed to be
lower on the magnesium-coated surface when compared with the
control on both days 2 and 7 (Fig.
4). The bacteria form biofilm-like structures on the human
primary osteoblasts on the two surfaces.
Discussion
The aim of the present study was to investigate the
antimicrobial properties of a rapidly corroding magnesium coating
on titanium against S. epidermidis, as well as its effects
on hOBs, in a co-culture model. Measurements of hOB and S.
epidermidis populations revealed that hOB growth was improved
and S. epidermidis growth was inhibited; these alterations
differed by several orders of magnitude when compared with the
uncoated titanium control.
In previously conducted monoculture experiments, the
number of hOBs that colonized the magnesium-coated surface
increased to ~80% when compared with the uncoated titanium control,
due to the slightly less favourable growth conditions on the
corroding surface (14). However,
in the present co-culture experiments, hOB growth on the
magnesium-coated samples increased to ~125% following 2 days and
200% following 7 days. The antibacterial surface significantly
reduced the negative influence of S. epidermidis on the hOB.
This result was achieved despite the presence of the magnesium
coating, which demonstrates a negative effect on the growth
potential of the hOBs. The negative influence of S.
epidermidis on hOB may be due to competition for nutrients and
pathogenicity (20). The
uninhibited negative effects was observed on the control sample,
where hOB numbers were markedly reduced following 7 days when
compared with 2 days (a decrease of 20,000 from 40,000).
The increased growth of the hOBs on magnesium-coated
samples, and the observed antibacterial effect, may be explained by
the following factors: Prevention of bacterial adherence due to the
corrosive dissolution process, osmotic stress in the strongest
phase of initial corrosion, the microstructure of the surface, an
unfavourable increase in pH-value for the bacteria or the
inhibitory effects of magnesium ions on the bacteria (21–28).
These effects are most prominent in the local environment close to
the surface. The effects of dissolution and osmotic pressure
decline over 2 days, however the pH shift may also have a negative
effect on S. epidermidis growth over the 7-day period. The
impact on the planktonic population may be less significant due to
reduced exposure to these factors (13,29).
The antibacterial effects observed in the
monoculture experiments were reproduced in the co-culture setup,
however, they were observed to be one order of magnitude less
prominent (14). These
observations are in agreement with a prior comparison of a
monoculture to co-culture experiment conducted by Zaatreh et
al (16). Putative reasons for
the diminished effect may include the favourable environment that
the hOBs present for S. epidermidis and the protection that
they may provide from antibacterial effects of the surface. The
planktonic population of S. epidermidis depends on the
biofilm population, as this is where most of the bacterial
reproduction occurs (30).
In previous studies, the authors demonstrated that
monocultures may capture only a limited number of interactions
among human cells, bacteria and the tested surface (14,16).
Unpredicted behaviour may arise from the complex interaction among
human cells, bacteria and implant surfaces. Therefore, the results
of co-culture experiments are regarded as more robust (31,32).
In the present study, the co-culture model captured the apparent
protective effects of the hOB cells, whereby this layer of cells
protects bacteria from the adverse effects of the surface. The
antibacterial effects were diminished in the co-culture system,
however a significant antibacterial effect persisted (three orders
of magnitude following 7 days).
The investigation of different experimental
parameters must be limited. Future work will aim to test different
growth media, a greater number of bacterial strains and species, as
well as different osteoblast cell lines. In addition, the length of
time for infection to occur, and the ratio of initial hOB and
bacterial numbers will be explored. The results of the present
study provide significant observations with promising implications
for implant material research.
In conclusion, novel implant materials are in high
demand due to the severity of IAI, which may be life-threatening
for patients with lengthy and demanding treatment procedures and
may result in a marked decrease in quality of life. It has been
previously demonstrated that titanium coated with a thin, rapidly
corroding layer of magnesium may be a suitable candidate material,
based on monoculture studies involving primary hOBs and S.
epidermidis. The present study applied a more rigorous test
system with hOBs and S. epidermidis in co-culture on the
magnesium-coated titanium surface. The favourable qualities of this
surface as an antibacterial and biocompatible material were
verified. These results provide evidence for progression towards
future animal model testing.
Acknowledgements
The authors would like to thank Professor Andreas
Podbielski (Institute of Medical Microbiology, Virology and
Hygiene, University Medicine Rostock) for his help in performing
the tests. In addition, the authors would like to thank Dr Marcus
Frank, Mr. Wolfgang Labs, Mr. Gerhard Fulda (Electron Microscopy
Centre, University Medicine Rostock) Mr. Philipp Pisowocki
(Department of Orthopaedics, University Medicine Rostock) and Mrs.
Regina Lange (Institute for Electronic Appliances and Circuits,
University Medicine Rostock) for their help with SEM. Furthermore,
a special thanks to Professor Regine Willumeit-Römer (Institute of
Materials Research, Division of Metallic Biomaterials,
Helmholtz-Zentrum Geesthacht) for her support and expertise in the
field of magnesium as a biomaterial. This study was financially
supported by the Helmholtz Virtual Institute MetBioMat (grant no.
VH-VI-523) titled ‘In vivo studies of biodegradable
magnesium-based implant materials’.
References
|
1
|
Fallpauschalenbezogene
Krankenhausstatistik (DRG-Statistik): Operationen und Prozeduren
der vollstationären Patientinnen und Patienten der Krankenhäuser
(4-Steller). Statistisches Bundesamt (Destatis), Wiesbaden.
2015.
|
|
2
|
Darouiche RO: Treatment of infections
associated with surgical implants. N Engl J Med. 350:1422–1429.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walter RP and Blake SM: Re: Two-stage
exchange for infected resurfacing arthroplasty use of a novel
cement spacer technique. J Arthroplasty. 26:9792011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harris LG and Richards RG: Staphylococci
and implant surfaces: A review. Injury. 37 Suppl 2:S3–S14. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Casey AL, Lambert PA and Elliott TSJ:
Staphylococci. Int J Antimicrob Agents. 29 Suppl 3:S23–S32. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arciola CR, Campoccia D, Speziale P,
Montanaro L and Costerton JW: Biofilm formation in Staphylococcus
implant infections. A review of molecular mechanisms and
implications for biofilm-resistant materials. Biomaterials.
33:5967–5982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilde AH and Ruth JT: Two-stage
reimplantation in infected total knee arthroplasty. Clin Orthop.
23–35. 1988.PubMed/NCBI
|
|
8
|
Vorndran E, Spohn N, Nies B, Rössler S,
Storch S and Gbureck U: Mechanical properties and drug release
behavior of bioactivated PMMA cements. J Biomater Appl. 26:581–594.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uchiyama K, Takahira N, Fukushima K,
Moriya M, Yamamoto T, Minegishi Y, Sakai R, Itoman M and Takaso M:
Two-stage revision total hip arthroplasty for periprosthetic
infections using antibiotic-impregnated cement spacers of various
types and materials. ScientificWorldJournal. 2013:1472482013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Connaughton A, Childs A, Dylewski S and
Sabesan VJ: Biofilm disrupting technology for orthopedic implants:
What's on the Horizon? Front Med (Lausanne). 1:222014.PubMed/NCBI
|
|
11
|
Staiger MP, Pietak AM, Huadmai J and Dias
G: Magnesium and its alloys as orthopedic biomaterials: A review.
Biomaterials. 27:1728–1734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luthringer BJ, Feyerabend F and
Willumeit-Römer R: Magnesium-based implants: A mini-review. Magnes
Res. 27:142–154. 2014.PubMed/NCBI
|
|
13
|
Robinson DA, Griffith RW, Shechtman D,
Evans RB and Conzemius MG: In vitro antibacterial properties of
magnesium metal against Escherichia coliPseudomonas aeruginosa and
Staphylococcus aureus. Acta Biomater. 6:1869–1877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaatreh S, Haffner D, Pasold J,
Kreikemeyer B, Mittelmeier W, Podbielski A, Quandt E and Bader R:
Track A. Biomaterials and biocompatibility 1. Biomed Tech (Berl).
60 Suppl 1:S1–S30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cytotoxicity test according to ISO
10993-5. CYTOX. 1999.
|
|
16
|
Zaatreh S, Wegner K, Strauß M, Pasold J,
Mittelmeier W, Podbielski A, Kreikemeyer B and Bader R: Co-culture
of S. epidermidis and human osteoblasts on implant surfaces: An
advanced in vitro model for implant-associated infections. PloS
One. 11:e01515342016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schlüter K, Zamponi C, Piorra A and Quandt
E: Comparison of the corrosion behaviour of bulk and thin film
magnesium alloys. Corros Sci. 52:3973–3977. 2010. View Article : Google Scholar
|
|
18
|
Jonitz A, Lochner K, Lindner T, Hansmann
D, Marrot A and Bader R: Oxygen consumption, acidification and
migration capacity of human primary osteoblasts within a
three-dimensional tantalum scaffold. J Mater Sci Mater Med.
22:2089–2095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anleitungen-Marienfeld-Superior.
[Internet]. [cited 3 Nov 2016]. http://www.marienfeld-superior.com/index.php/anleitungen.html
|
|
20
|
Von Eiff C, Peters G and Heilmann C:
Pathogenesis of infections due to coagulasenegative staphylococci.
Lancet Infect Dis. 2:677–685. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang HX, Guan SK, Wang X, Ren CX and Wang
LG: In vitro degradation and mechanical integrity of Mg-Zn-Ca alloy
coated with Ca-deficient hydroxyapatite by the pulse
electrodeposition process. Acta Biomater. 6:1743–1748. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirkland NT, Lespagnol J, Birbilis N and
Staiger MP: A survey of bio-corrosion rates of magnesium alloys.
Corros Sci. 52:287–291. 2010. View Article : Google Scholar
|
|
23
|
Fischer J, Prosenc MH, Wolff M, Hort N,
Willumeit R and Feyerabend F: Interference of magnesium corrosion
with tetrazolium-based cytotoxicity assays. Acta Biomater.
6:1813–1823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fischer J, Pröfrock D, Hort N, Willumeit R
and Feyerabend F: Reprint of: Improved cytotoxicity testing of
magnesium materials. Mater Sci Eng B. 176:1773–1777. 2011.
View Article : Google Scholar
|
|
25
|
Schlüter K, Zamponi C, Hapke J, Hort N,
Kainer KU and Quandt E: Mechanical properties and corrosion
behaviour of freestanding, precipitate-free magnesium WE43 thin
films. Int J Mater Res. 104:286–292. 2013. View Article : Google Scholar
|
|
26
|
Schlüter K, Shi Z, Zamponi C, Cao F,
Quandt E and Atrens A: Corrosion performance and mechanical
properties of sputter-deposited MgY and MgGd alloys. Corros Sci.
78:43–54. 2014. View Article : Google Scholar
|
|
27
|
Sanchez AH Martinez, Luthringer BJC,
Feyerabend F and Willumeit R: Mg and Mg alloys: How comparable are
in vitro and in vivo corrosion rates? A review. Acta Biomater.
13:16–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Willumeit R, Feyerabend F and Huber N:
Magnesium degradation as determined by artificial neural networks.
Acta Biomater. 9:8722–8729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Charyeva O, Neilands J, Svensäter G and
Wennerberg A: Bacterial biofilm formation on resorbing magnesium
implants. Open J Med Microbiol. 5:1–11. 2015. View Article : Google Scholar
|
|
30
|
Gasol JM and Del Giorgio PA: Using flow
cytometry for counting natural planktonic bacteria and
understanding the structure of planktonic bacterial communities.
Sci Mar. 64:197–224. 2000. View Article : Google Scholar
|
|
31
|
Lee JH, Wang H, Kaplan JB and Lee WY:
Microfluidic approach to create three-dimensional tissue models for
biofilm-related infection of orthopaedic implants. Tissue Eng Part
C Methods. 17:39–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaji H, Camci-Unal G, Langer R and
Khademhosseini A: Engineering systems for the generation of
patterned co-cultures for controlling cell-cell interactions.
Biochim Biophys Acta. 1810:239–250. 2011. View Article : Google Scholar : PubMed/NCBI
|