Introduction
As a neurotropic substance, ethanol can damage nerve
cells through increasing the production of free radicals,
interference of neurotrophic factor signaling pathways, activation
of endogenous apoptotic signals and other molecular mechanisms
(1,2). Alcohol abuse has become a public
health issue (3). In 2014, a World
Health Organization survey estimated that ~3,300,000 individuals in
2012 succumbed to alcohol consumption-associated mortality, of
which ~132,000 were directly associated with neuropsychiatric
disorders (4). Chronic alcoholism
is a common worldwide disease and risks to human health are
worsening. Increasing evidence indicates that alcoholism can reduce
cell numbers in several neuronal populations within the developing
brain, including the hippocampus, cerebellum and cortex (5–7).
There are several drugs, which are currently used
for the clinical treatment of nerve damage caused by alcohol,
including naloxone for acute alcoholism, meclofenoxate as a central
nervous system stimulant, D-Ala2-met-enkephalinamide and
ondansetron, which can prevent alcoholism by reducing ethanol
intake (8). However, these drugs
are not adequate for the treatment of chronic nerve damage caused
by alcoholism, and 90% of alcoholic patients may have used aversive
drugs during a certain period of the treatment (9). As a result, it is important to
identify and develop novel drugs, which promote neuronal cell
regeneration or inhibit apoptosis for the treatment of
alcohol-induced nerve injury.
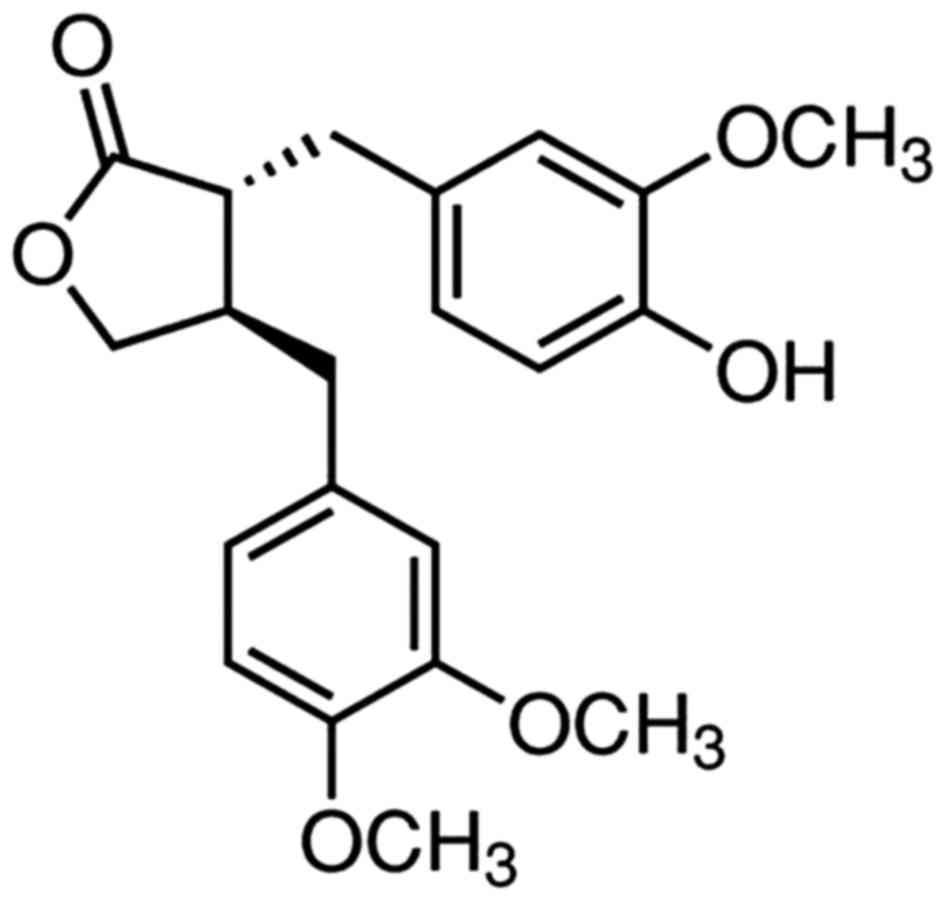
Arctigenin (ATG) is a type of lignin with low
toxicity and few side effects, the chemical structure of which is
shown in Fig. 1. It is the primary
active constituent isolated from the fruit of Arctium lappa
L. ATG has been reported to possess several bioactivities and a
number of important pharmacological properties, including
antioxidant, antitumor, anti-inflammatory and immunomodulatory
effects (10–14). Previous studies have found that ATG
provides a neuroprotective effect on cultured cortical neurons from
glutamate-induced neurodegeneration and acts on scopolamine-induced
memory deficit in mice through mechanisms, which remain to be fully
elucidated (15,16). The present study aimed to
investigate whether ATG promotes cell proliferation and inhibits
apoptosis in ethanol-damaged PC12 cells, to determine whether ATG
may be available for the clinical treatment of ethanol-induced
neurotoxicity.
Materials and methods
Cell culture
The PC12 cells purchased from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China) were grown in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
heat-inactivated FBS, at 37°C in a 5% CO2 humidified
incubator. In all experiments, the cells were seeded at densities
of 8,000 cells/well (96-well plate), 1×105 cells/well
(24-well plate) or 2.5×105 cells/well (6-well plate).
ATG was purchased from Sigma-Aldrich (St. Lous, MO, USA), dissolved
in dimethyl sulfoxide (DMSO) and stored frozen until use. The
treatment procedures were performed when the cells had adhered to
the wall.
Measurement of cell viability
The protective effect of ATG on the ethanol-induced
reduction of PC12 cell viability was detected using a conventional
MTT reduction assay. The cells were seeded onto 96-well plates at
the density of 8,000 cells/well. On adhering to the wall, the cells
were incubated with various concentrations of ATG (0, 1, 5, 10, 30,
50 and 100 µM) and 500 mM of ethanol for 24, 48 and 72 h at 37°C,
respectively. In order to maintain a constant ethanol
concentration, a methodology to deliver ethanol to the cultured
cells was developed according to a method described by Adickes
et al (17). Subsequently
10 µl of the MTT solution (5 mg/ml) was added to each well and
incubated at 37°C for 4 h. Finally, 150 µl DMSO was added to each
well and shaken for 10 min prior to removal of the medium. The
absorbance was measured at 490 nm using an enzyme-linked
immunosorbent assay. Cell viability was calculated by cell
proliferation index as a percentage of the value against the
control group. An untreated PC12 cell group was included and
detected as a positive control.
Cell cycle analysis
PC12 cells cultured with 500 mM ethanol, 10 µM ATG,
and 500 mM ethanol combined with 10 µM ATG were separately
harvested by trypsinization and washed twice with cold PBS prior to
fixation in 70% ethanol overnight at 4°C. The cells were then
incubated with RNase A (100 µg/ml; Fermentas; Thermo Fisher
Scientific, Inc.) in PBS at 37°C for 30 min. Propidium iodide (PI;
25 µg/ml; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was
added at 4°C for 30 min in the dark. The cells were then detected
using flow cytometry (FCM) with a BD FACSCalibur system (BD
Biosciences, San Jose, CA, USA) and were analyzed at 488 nm
excitation using ModFit software version 4.1 (Verity Software
House, Inc., Topsham, ME, USA). At least 20,000 cells were analyzed
in the cell sorting. The untreated PC12 cells were also analyzed as
a positive control.
Hoechst33342/PI fluorescence
staining
Following the treatments described above, the cells
plated in 24-well plates were stained with 2 mg/ml Hoechst for 5
min, followed by the addition of PI at a final concentration of 5
mg/ml for 10 min. The stained nuclei were observed using a
fluorescence photomicroscope (Olympus, Tokyo, Japan). To evaluate
the percentage of positive cells, four non-overlapping images were
captured and at least 600 cells were calculated in each well.
FCM for analysis of apoptosis
The apoptotic rates were quantified using an Annexin
V-PI apoptotic assay kit. Following the incubation with ATG and
ethanol, the PC12 cells were harvested, washed twice with cold PBS,
suspended in binding buffer, and incubated with PI and Annexin
V-fluorescein isothiocyanate (FITC) for 30 min at room temperature.
The samples were examined using FCM (BD FACSCalibur; BD
Biosciences) and analyzed using the Cell Quest version 5.1 (BD
Biosciences). At least 10,000 cells were collected in cell
sorting.
Statistical analysis
Data obtained from the experiments are presented as
the mean ± standard error of the mean from at least three
independent experiments. Statistical analyses were performed with
SPSS version 10.0 (SPSS, Inc., Chicago, IL, USA) using one-way
analysis of variance followed by Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
ATG protects PC12 cells from
ethanol-induced reduction of cell viability
Nerve cell regeneration is limited, and exposure to
ethanol increases apoptosis and necrosis. As a result, it is
essential to improve cell viability in the presence of ethanol
in vitro. Therefore, the present study examined the
protective effect of ATG on the ethanol-induced reduction of PC12
cell viability. Following treatment for 24, 48 and 72 h with
ethanol (500 mM) and ATG (1, 5 and 10 µM), cell proliferation rates
were investigated using an MTT assay. As shown in Fig. 2A, the viability of the
ethanol-treated cells increased significantly when treated with
different concentration of ATG for 24, 48 and 72 h, respectively
(P<0.05). Treatment with ATG at a concentration of 10 µM
effectively promoted the viability of the ethanol-damaged
cells.
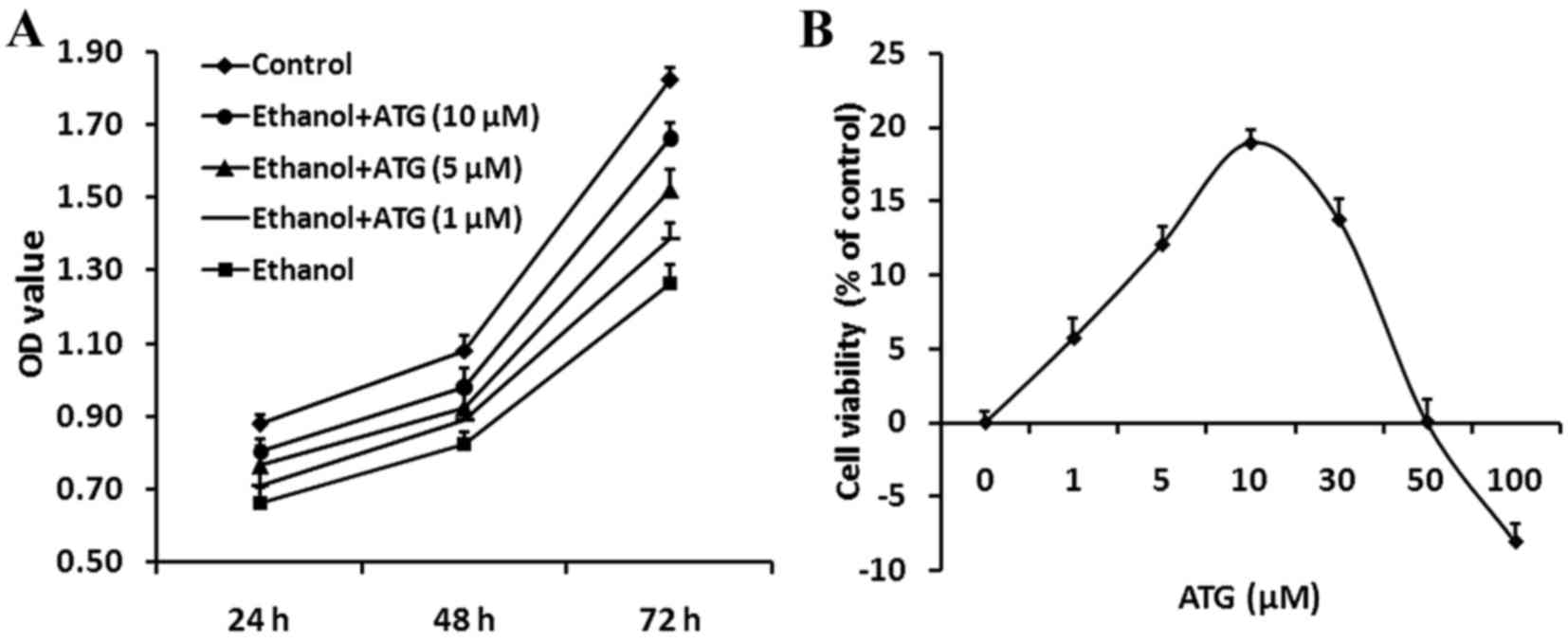 | Figure 2.MTT assay of PC12 cells. (A)
Proliferation of differently treated PC12 cells was assessed using
an MTT assay. The proliferation of the ethanol-treated cells
increased significantly when treated with different concentrations
of ATG, with a concentration of 10 µM effectively promoting the
proliferation of damaged cells. Data are presented as the mean ±
standard error of the mean from a minimum of three independent
experiments, *P<0.05, vs. control; #P<0.05, vs.
ethanol. (B) Dose-response investigation of ATG. PC12 cells were
incubated with ethanol (500 mM) and ATG (0, 1, 5, 10, 30, 50 and
100 µM) for 24 h. ATG (5–30 µM) markedly promoted the proliferation
of ethanol-treated cells (12–18%). The most marked increase in cell
viability was observed at an ATG concentration of 10 µM. Cell
viability was examined using an MTT assay, and was calculated by
cell proliferation index as a percentage of the value against the
control group. ATG, arctigenin; OD, optical density. |
Subsequently, the effect of ATG concentration on
ethanol-damaged PC12 cells was investigated further. The PC12 cells
were incubated with various concentrations of ATG (0, 1, 5, 10, 30,
50 and 100 µM) and 500 mM ethanol for 24 h, and cell viability was
assessed using an MTT assay. Cells incubated with ethanol alone
were used as a negative control. Incubation with ATG (5–30 µM)
promoted the viability of the ethanol-treated cells (12–18%) and
the ATG concentration of 10 µM led to the most marked increase in
cell viability. However, the concentration >50 mM had a
contrasting effect (Fig. 2B).
Therefore, an ATG concentration of 10 µM effectively increased the
viability of the ethanol-damaged cells and was used in the
subsequent experiments.
ATG improves the proliferation index
of ethanol-damaged cells
The proliferation status of cells is often evaluated
using a proliferation index, which is calculated using the
following formula: (S+G2/M)/(G0/G1+S+G2/M) (18). To investigate the possible
mechanism underlying the promoting effect of ATG on proliferation,
FCM was used to detect the cell cycle. Following treatment for 24,
48 and 72 h with 10 µM ATG, 500 mM ethanol, and 10 µM ATG combined
with 500 mM ethanol, respectively, the cells were collected and
incubated with PI. The FCM results showed that, compared with the
ethanol-damaged group of cells, ATG significantly increased the
number of cells in the G2/M phase (Fig. 3A; P<0.05), effectively promoted
the proliferation of damaged cells, and significantly increased the
distribution ratio of the cells at the G2/M and S phases (Fig. 3B).
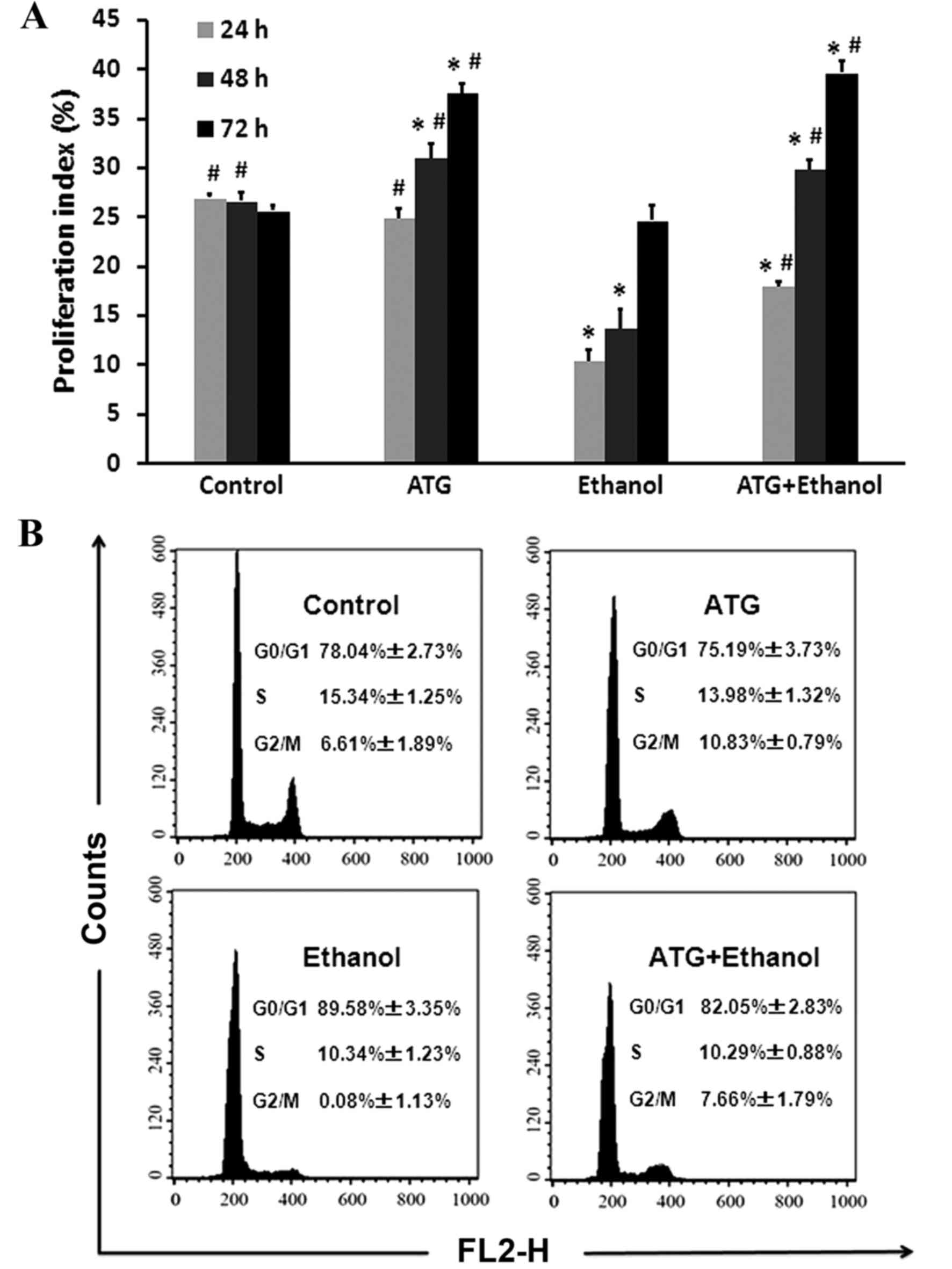 | Figure 3.Cell cycle analysis. (A) Cell cycle
analysis of different treatment groups at 24, 48 and 72 h. Data are
presented as the mean ± standard error of the mean from a minimum
of three independent experiments, *P<0.05, vs. control;
#P<0.05, vs. ethanol. (B) Cell cycle distribution was
determined using FCM. Effects of ATG on ethanol-damaged PC12 cell
G1 phase arrest. Following 24, 48 and 72 h treatment with 10 µM
ATG, 500 mM ethanol, and 10 µM ATG combined with 500 mM ethanol,
respectively, the PC12 cells were stained with propidium iodide and
analyzed for DNA content using FCM. ATG, arctigenin; FCM, flow
cytometry. |
ATG efficiently reduces the apoptosis
and necrosis caused by ethanol
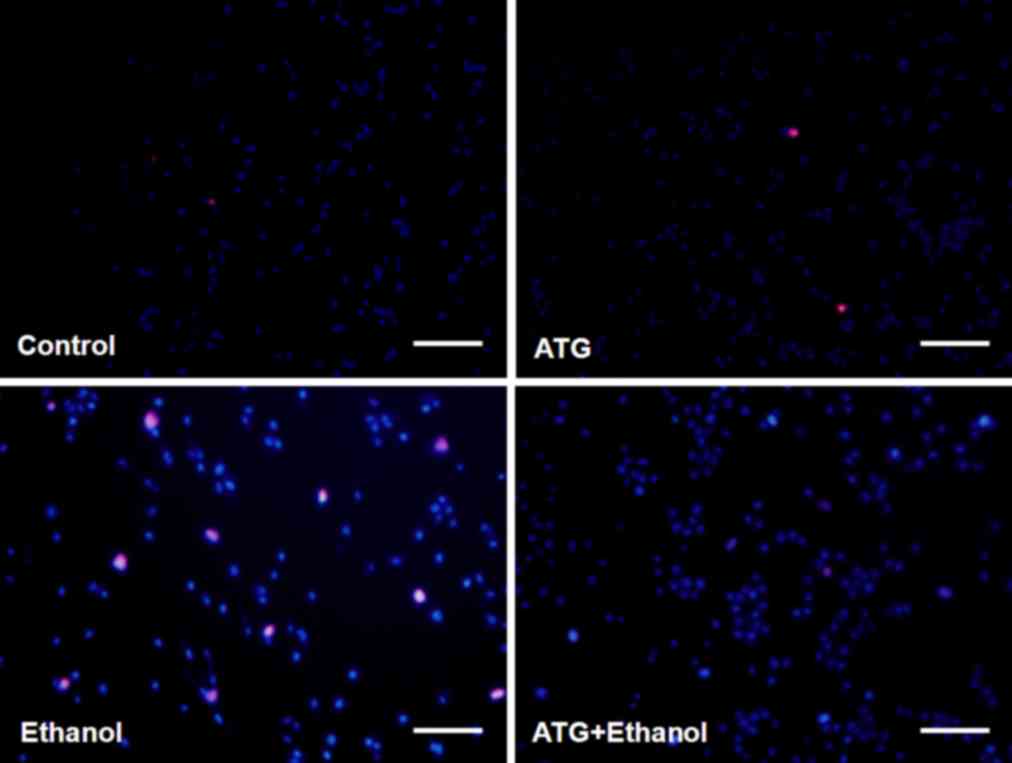
Following treatment of the cells with ethanol, the
cell membrane was found to shrink and several apoptotic bodies were
produced in the PC12 cells. To detect the apoptosis and necrosis of
PC12 cells, the cells were stained with Hoechst33342 and PI
following 48 h treatment with 10 µM ATG, 500 mM ethanol, and 500 mM
ethanol combined with 10 µM ATG, respectively. Following staining,
apoptotic cells were bright blue in color, whereas necrotic cells
were red and normal cells were dark blue. The results showed that
the apoptosis and necrosis of PC12 cells were significantly
decreased following treatment with ATG (Fig. 4).
ATG significantly inhibits early
apoptosis of ethanol-damaged cells
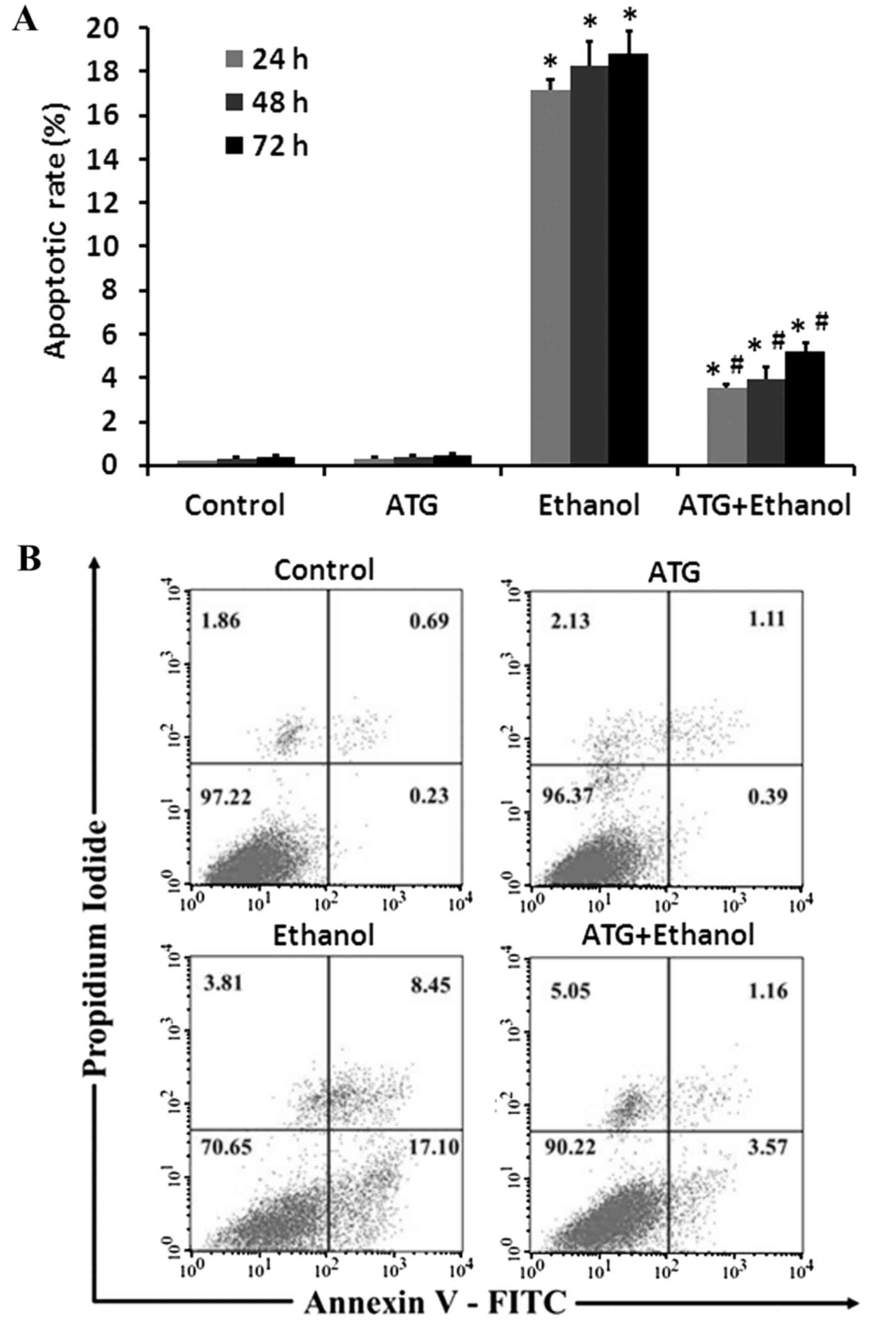
In order to detect the effects of ATG on apoptosis
and necrosis in ethanol-damaged PC12 cells, the apoptotic rates of
the PC12 cells and the protective effects of ATG were detected
using Annexin V/PI staining and FCM, respectively. Using Annexin
V/PI double staining, it was possible to separate normal cells
(Annexin V−/PI−), early apoptotic cells
(Annexin V+/PI−), late apoptotic and necrotic
cell (Annexin V+/PI+). Following treatment
with 24, 48 and 72 h with 10 µM ATG, 500 mM ethanol, and 10 µM ATG
combined with 500 mM ethanol, respectively, the cells were
collected and incubated with Annexin V-FITC and PI. The FCM results
indicated that, compared with the control group, ethanol treatment
significantly increased PC12 cell apoptosis, whereas apoptosis was
significantly inhibited by the addition of ATG (Fig. 5A; P<0.05), revealing a
protective effect of ATG on ethanol-induced injured PC12 cells. The
early apoptotic rate of the control cells was ~0.23%. Following
incubation with ethanol fror 48 h, the early apoptotic rate
increased to 17.10%. However, when treated with the ethanol and ATG
together, the early apoptotic rate of the PC12 cells reduced to
3.57% (Fig. 5B).
Discussion
As a lipid-soluble and water-soluble substance,
ethanol is rapidly absorbed in the body, and spreads to various
organs and body fluids. Its metabolites and free radicals can cause
a number of disorders and cytotoxic responses of the body's
metabolism (1,2). However, the mechanism underlying
chronic ethanol-induced nervous system damage remains to be fully
elucidated. It is generally considered to act in different ways,
including the activation of GABA receptors, inhibition of NMDA
glutamate receptors (19,20), interference of
Na+/K+, ATP enzymes and Ca2+
channels (21), and increasing the
generation of reactive oxygen species causing apoptosis (22).
PC12 cells are from Rattus norvegicus adrenal
tumors and are an ideal model for examining in vitro
neurochemistry, neurobiology and nervous system diseases. Previous
studies have demonstrated that chronic ethanol exposure can inhibit
the proliferation of cultured PC12 cells (23) and promote the apoptosis of PC12
cells by affecting the protein expression of B-cell lymphoma 2 and
Caspase-3 (24). Therefore,
promoting nerve cell proliferation or inhibiting apoptosis may be
an effective treatment for ethanol-induced neurotoxicity.
The use of a single natural active ingredient of a
traditional Chinese medicine to promote proliferation and inhibit
apoptosis has increased in popularity. As a natural medicine, ATG
exerts several bioactivities and a number of important
pharmacological properties, including antioxidant, antitumor,
anti-inflammatory and immunomodulatory effects (10–14).
Studies have found that ATG has a neuroprotective effect on
cultured cortical neurons from glutamate-induced neurodegeneration
and acts on mice with scopolamine-induced memory deficit through
mechanisms, which remain to be fully elucidated (15,16).
ATG has been found to have protective effects on a selective
pharmacological protein kinase A inhibitor in H89-induced neuronal
injury in rats (15). In addition,
a previous study provided evidence that ATG can inhibit the
synthesis of β-amyloid and accelerate its degradation, which was
found to improve memory impairment in mice with Alzheimer's disease
(16). However, the protective
effect of ATG on ethanol-induced neurotoxicity remains to be fully
elucidated.
In the present study, in order to investigate the
protective effect of ATG on alcohol-induced neurotoxicity and
apoptosis in rat pheochromocytoma PC12 cells, a cell model of
ethanol damage was first established using ethanol (500 mM) in a
series of doses in preliminary experiments, which induced
significant apoptosis of the PC12 cells. Subsequently, a variety of
detection methods were used to investigate whether ATG had a
protective effect on ethanol-induced neurotoxicity in PC12 cells.
The results showed that ATG at a concentration of 10 µM
significantly promoted the proliferation of damaged cells, reduced
the proportion of cells in the G0/G1 phase and increased the cell
proliferation index. Following exposure of the cells to 500 mM
ethanol, the number of cells was reduced and a substantial number
of apoptotic bodies were formed. Data from the FCM analysis
indicated that 10 µM ATG significantly reduced apoptosis and
necrosis in the ethanol-treated cells. This experiment demonstrated
that ATG reduced the ability of a high concentration of ethanol to
inhibit well-differentiated PC12 cells, the specific mechanism of
which requires further investigation (25,26).
In conclusion, the data obtained in the present
study indicated that ATG at a concentration of 10 µM increased the
distribution ratio of the damaged cells at the G2/M and S phases,
and significantly reduced apoptosis and necrosis in ethanol-treated
cells. Therefore, it was concluded that ATG had a protective effect
on the ethanol-damaged PC12 cells. The results of the present study
provide a novel theoretical basis and therapeutic strategy for ATG
in the clinical treatment of ethanol-induced neurotoxicity and
damage.
Acknowledgements
This study was supported by the Innovative Research
Team Development Program in University of Chongqing (grant no.
CXTDX201601031), the Top Innovative Talents Training Fund for
College Students from Chongqing University of Technology (grant
nos. BC201305 and BC201409) and the Undergraduate Training Programs
for Innovation and Entrepreneurship of Chongqing University of
Technology (grant no. 2014CX03).
References
|
1
|
Baker RC and Kramer RE: Cytotoxicity of
short-chain ethanols. Annu Rev Pharmacol Toxicol. 39:127–150. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith AM, Zeve DR, Dohrman DP and Chen WJ:
The interactive effect of alcohol and nicotine on NGF-treated
pheochromocytoma cells. Alcohol. 39:65–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grant BF, Dawson DA, Stinson FS, Chou SP,
Dufour MC and Pickering RP: The 12-month prevalence and trends in
DSM-IV alcohol abuse and dependence: United States, 1991–1992 and
2001-2002. Drug Alcohol Depend. 74:223–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
World Health Organization, . Global status
report on alcohol and health. Geneva, Switzerland: World Health
Organization; 2014
|
|
5
|
Heaton MB, Paiva M, Madorsky I and Shaw G:
Ethanol effects on neonatal rat cortex: Comparative analyses of
neurotrophic factors, apoptosis-related proteins, and oxidative
processes during vulnerable and resistant periods. Brain Res Dev
Brain Res. 145:249–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dikranian K, Qin YQ, Labruyere J, Nemmers
B and Olney JW: Ethanol-induced neuroapoptosis in the developing
rodent cerebellum and related brain stem structures. Brain Res Dev
Brain Res. 155:1–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green CR, Kobus SM, Ji Y, Bennett BM,
Reynolds JN and Brien JF: Chronic prenatal ethanol exposure
increases apoptosis in the hippocampus of the term fetal guinea
pig. Neurotoxicol Teratol. 27:871–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jonas DE, Amick HR, Feltner C, Bobashev G,
Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ and Garbutt
JC: Pharmacotherapy for adults with alcohol use disorders in
outpatient settings: A systematic review and meta-analysis. JAMA.
311:1889–1900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ojehagen A, Skjaerris A and Berglund M:
Long-term use of aversive drugs in outpatient alcoholism treatment.
Acta Psychiatr Scand. 84:185–190. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirano T, Gotoh M and Oka K: Natural
flavonoids and lignansare potent cytostatic agents against human
leukemic HL-60 cells. Life Sci. 55:1061–1069. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Umehara K, Nakamura M, Miyase T,
Kuroyanagi M and Ueno A: Studies on differentiation inducers VI.
Lignan derivatives from Arctium fructus. (2). Chem Pharm Bull
(Tokyo). 44:2300–2304. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho JY, Kim AR, Yoo ES, Baik KU and Park
MH: Immunomodulatory effect of arctigenin, a lignan compound, on
tumor necrosis factor and nitric oxide production, and lymphocyte
proliferation. J Pharm Pharmacol. 51:1267–1273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu JG, Wu JZ, Sun LN, Han T, Du J, Ye Q,
Zhang H and Zhang YG: Ameliorative effects of arctiin from Arctium
lappa on experimental glomerulonephritis in rats. Phytomedicine.
16:1033–1041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao F, Wang L and Liu K: In vitro
anti-inflammatory effects of arctigenin, alignan from Arctium lappa
L., through inhibition on iNOS pathway. J Ethnopharmacol.
122:457–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang N, Wen Q, Ren L, Liang W, Xia Y,
Zhang X, Zhao D, Sun D, Hu Y, Hao H, et al: Neuroprotective effect
of arctigenin via upregulation of P-CREB in mouse primary neurons
and human SH-SY5Y neuroblastoma cells. Int J Mol Sci.
14:18657–18669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Z, Yan J, Jiang W, Yao XG, Chen J,
Chen L, Li C, Hu L, Jiang H and Shen X: Arctigenin effectively
ameliorates memory impairment in Alzheimer's disease model mice
targeting both β-amyloid production and clearance. J Neurosci.
33:13138–13149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adickes ED, Mollner TJ and Lockwood SK:
Closed chamber system for delivery of ethanol to cell cultures.
Alcohol Alcohol. 23:377–381. 1988.PubMed/NCBI
|
|
18
|
Wang M, Shen J, Feng B, Gui L, Chen Q,
Zhang B, Tang J and Li X: Remote ischemic preconditioning promotes
early liver cell proliferation in a rat model of small-for-size
liver transplantation. J Surg Res. 179:e245–e253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moonat S, Starkman BG, Sakharkar A and
Pandey SC: Neuroscience of ethanolism: Molecular and cellular
mechanisms. Cell Mol Life Sci. 67:73–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramezani A, Goudarzi I, Lashkarboluki T,
Ghorbanian MT, Abrari K and Elahdadi Salmani M: Role of oxidative
stress in ethanol-induced neurotoxicity in the developing
cerebellum. Iran J Basic Med Sci. 15:965–974. 2012.PubMed/NCBI
|
|
21
|
Ramachandran V, Watts LT, Maffi SK, Chen
J, Schenker S and Henderson G: Ethanol-induced oxidative stress
precedes mitochondrially mediated apoptotic death of cultured fetal
cortical neurons. J Neurosci Res. 74:577–588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pettus BJ, Chalfant CE and Hannun YA:
Ceramide in apoptosis: An overview and current perspectives.
Biochim Biophys Acta. 1585:114–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pantazis NJ, Dohrman DP, Luo J, Goodlett
CR and West JR: Ethanol reduces the number of pheochromocytoma
(PC12) cells in culture. Alcohol. 9:171–180. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong F, Kim WH, Tian Z, Jaruga B, Ishac E,
Shen X and Gao B: Elevated interleukin-6 during ethanol consumption
acts as a potential endogenous protective cytokine against
ethanol-induced apoptosis in the liver: Involvement of induction of
Bcl-2 and Bcl-x(L) proteins. Oncogene. 21:32–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Messing RO, Henteleff M and Park JJ:
Ethanol enhances growth factor-induced neurite formation in PC12
cells. Brain Res. 565:301–311. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wooten MW and Ewald SJ: Ethanols synergize
with NGF to induce early differentiation of PC12 cells. Brain Res.
550:333–339. 1991. View Article : Google Scholar : PubMed/NCBI
|