Introduction
Osteosarcoma is the most common primary tumor of
bone tissue, is more common in young individuals and exhibits a
high degree of malignancy (1,2). The
treatment of osteosarcoma comprises neoadjuvant chemotherapy and
surgery. The five-year survival rate remains at a level of 60–70%,
and has not increased significantly (3). The primary reason for this is that
the mechanism underlying its pathogenesis remains to be fully
elucidated. The formation and development of the tumor is
multifactorial, multistage and a gradual evolution process. Early
diagnosis and timely intervention is essential for improving the
prognosis. Investigation of the association between cell
proliferation and cell signaling has become an area of increased
interest (4,5).
As the axis of several types of signal channels,
mitogen-activated protein kinase (MAPK) cascade activation is a key
member in the receipt of signals, which are transferred and carried
by membrane receptors into the nucleus (6,7),
which is key to numerous signaling channels associated with cell
proliferation.
The overexpression of cyclin D1, which is referred
to as one of the regulatory factors in the cell cycle, is a
characteristic of several types of human primary tumor, and is of
vital significance for the diagnoses and prognoses of tumors
(8,9).
In order to elucidate molecular abnormalities of the
signaling cascade in the growth inhibition and growth of
osteosarcoma, cell proliferation and malignant
transformation-associated mechanisms, the present study detected
MAPK, phosphorylated (p)-MAPK and cyclin D1 using
immunohistochemical staining in tissues of osteosarcoma and benign
bone tumors, and in normal bone tissues as a control. The
association between p-MAPK and expression of cyclin D1, and the
mechanism underlying the formation of osteosarcoma were examined to
identify novel techniques for its early diagnosis.
Materials and methods
Patients and controls
In the last three years, samples of human
osteosarcoma and benign bone tumor tissues were collected from 60
patients (30 patients with human osteosarcoma and 30 patients with
benign bone tumor), who received surgical resection at The First
People's Hospital of Yancheng City (Yancheng, China), and had been
diagnosed via pathological confirmation. Detailed clinical and
pathological data were collected from each patient, and none of the
patients had received preoperative chemotherapy or radiotherapy.
The patients with osteosarcoma included 20 men and 10 women, aged
between 25 and 73 years (mean 40.9±11.6 years). The benign bone
tumor patient group included 19 men and 11 women, aged between 40
and 73 years (mean 54.8±12.2 years). Normal bone tissue specimens
were collected by surgical resection from 10 individuals to serve
as a control group. These included five men and five women, aged
between 36 and 68 years (mean 49.4±10.3 years). No statistically
significant differences were detected in age or gender among the
three groups. All specimens were obtained following the provision
of informed patient consent and were approved by the Ethics
Committee of The First People's Hospital of Yancheng City [ID no.
HMU (Ethics) 20121103].
Immunohistochemical staining
techniques
The EnVision immunohistochemical staining method was
used to detect the distributions of p-MAPK and cyclin D1. The
immunohistochemical procedures were performed in strict accordance
with the manufacturer's protocols. The EnVision and DAB chromogenic
reagent kits (Antibody Diagnostic, Inc., New York, NY, USA) were
used for immunohistochemical staining. All staining was performed
under the same conditions, in which the tissue specimen was sliced
into 4 µm sections, dehydrated and dewaxed, and antigen retrieval
was performed using 0.01 mol/l citric acid (pH 6.0). Normal goat
serum (Toyobo Co., Ltd., Osaka, Japan) was added to the tissue
sections and incubated for 10 min at room temperature, following
which the corresponding specific antibody (cat. no. 7074; Cell
Signaling Technology, Inc., Danvers, MA, USA; dilution, 1:1,000)
were added to the tissue section and incubated for 1.5 h at room
temperature. The sections were washed three times with PBS for 3
min. The secondary antibody (cat. no. 4370; Cell Signaling
Technology, Inc.; dilution, 1:1,000) was added and incubated for 30
min at room temperature. Staining was performed using DAB and
nuclei were stained using hematoxylin. The tissue sections were
then dehydrated in gradient ethanol, cleared using xylene and
sealed using natural gum. Each group stained had a positive
control, with the known positive section reagent provided by the
reagent company (p-MAPK; cat. no. 9216; Cell Signaling Technology,
Inc.; dilution, 1:1,000) (10),
and a negative control, in which the corresponding specific
antibody was replaced with PBS.
Staining of the nucleus in yellow or tan reactant
particles indicated positivity. The staining was examined in four
independent experiments for random detection using an Olympus
optical microscope (BH-2; Olympus Corporation, Tokyo, Japan) at
high magnification (magnification, ×200). According to the degree
of positive staining and the percentage of tumor cells, the
criteria for assessment were as follows: Negative expression (−),
marginal cell shading, expression of <5%; low expression (+),
pale yellow or positive staining of 5–29%; moderate expression
(2+), yellow or positive staining of 30–59%; high expression (3+),
tan colored or positive staining of >60%.
Statistical analysis
SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. The χ2 test
was used to compare the distribution of p-MAPK and cyclin D1 among
osteosarcoma tissues, benign bone tumor tissues and normal bone
tissues, and Spearman's correlation was used to analyze the
association between the distribution of p-MAPK and cyclin D1.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Distribution of nuclear staining of
p-MAPK in human osteosarcoma, benign bone tumor and normal bone
tissues
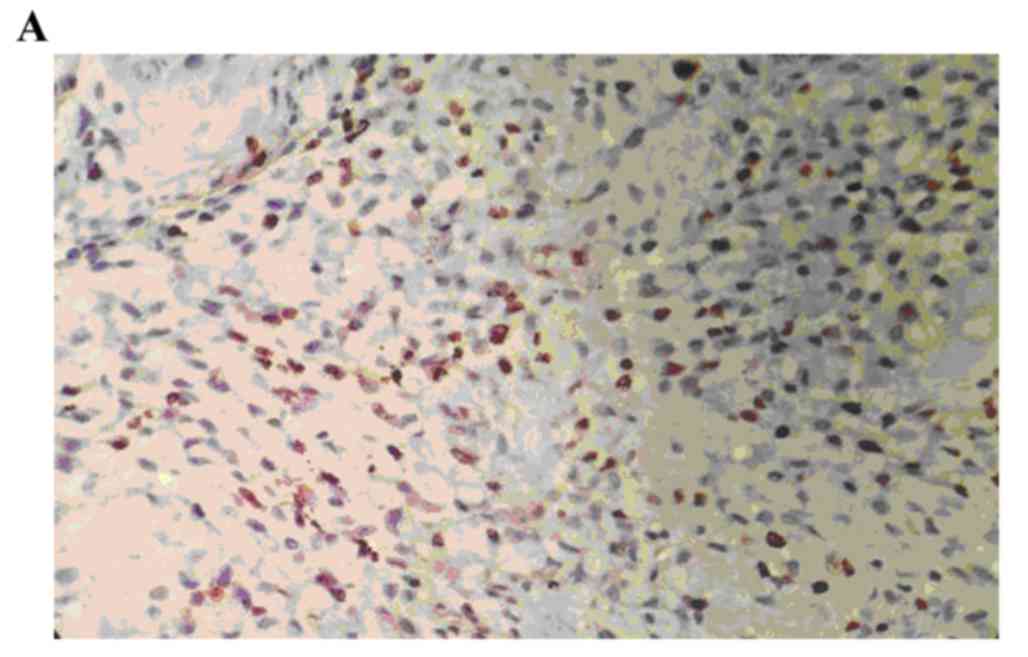
The positive rate of p-MAPK staining in the human
osteosarcoma tissues was 86.67% (26/30). The positive rate of
p-MAPK in the benign bone tumor tissues was 10.00% (3/30), and,
staining was significantly higher, compared with that in the normal
bone tissue (0; P<0.05). The staining intensity of p-MAPK in the
human osteosarcoma tissues was significantly higher, compared with
the staining intensity of p-MAPK in the benign bone tumor tissues
(P<0.05; Fig. 1A-C; Table I).
 | Table I.EnVision immunohistochemical staining
for p-MAPK in human osteosarcoma, benign bone tumor and normal bone
tissues. |
Table I.
EnVision immunohistochemical staining
for p-MAPK in human osteosarcoma, benign bone tumor and normal bone
tissues.
|
|
| p-MAPK positive
|
|
|
|
|---|
| Group | n | + | 2+ | 3+ | p-MAPK negative | Positive rate
(%) | Strong positive rate
(%) |
|---|
| Normal bone
tissue | 10 | 0 | 0 | 0 | 10 | 0 | 0 |
| Benign bone
tumor | 30 | 1 | 1 | 1 | 27 | 10.00 | 3.33 |
| Human
osteosarcoma | 30 | 4 | 10 | 12 | 4 | 86.67 | 40.00 |
Cell nuclear staining distribution of
cyclin D1 in human osteosarcoma, benign bone tumor and normal bone
tissues
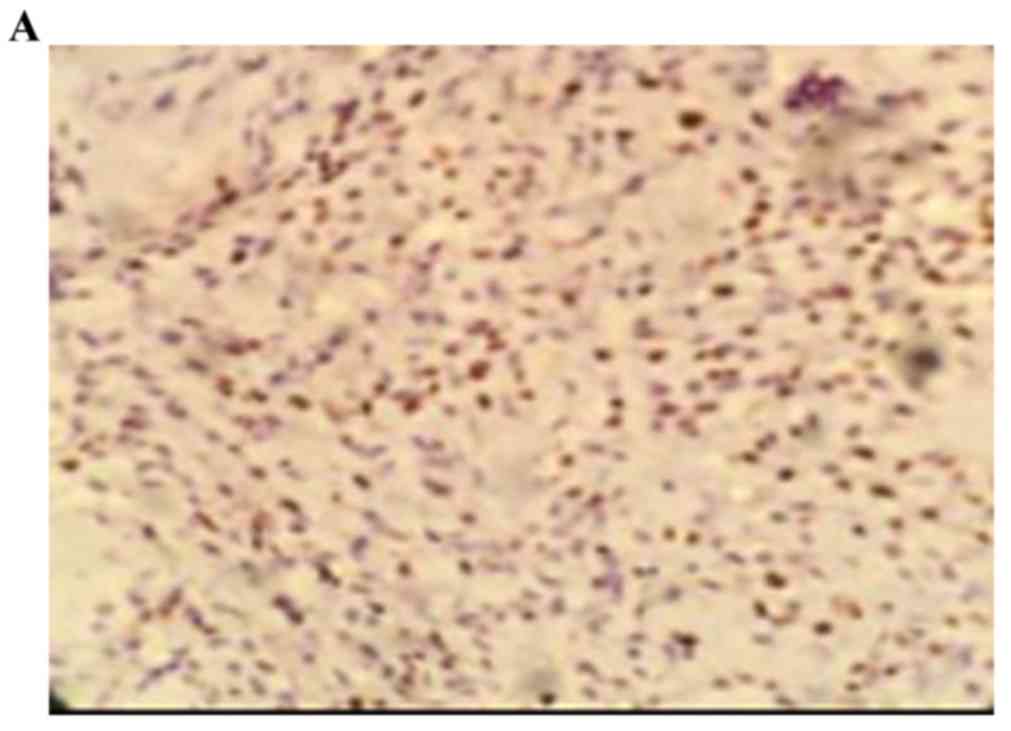
The positive rate of cyclin D1 staining in the human
osteosarcoma tissues was 73.00% (22/30). The positive rate of
cyclin D1 in the benign bone tumor was 3.30% (1/30), and staining
was negative in normal bone tissues. The staining intensity of
cyclin D1 in the benign bone tumor was significantly higher,
compared with that in normal bone tissue (0; P<0.05). The
staining intensity of cyclin D1 in the human osteosarcoma tissues
was significantly higher, compared with the staining intensity of
cyclin D1 in the benign bone tumor tissues (P<0.05; Fig. 2A-C; Table II).
 | Table II.EnVision immunohistochemical staining
for cyclin D1 in human osteosarcoma, benign bone tumor and normal
bone tissues. |
Table II.
EnVision immunohistochemical staining
for cyclin D1 in human osteosarcoma, benign bone tumor and normal
bone tissues.
|
|
| Cyclin D1 positive
|
|
|
|
|---|
| Group | n | + | 2+ | 3+ | Cyclin D1
negative | Positive rate
(%) | Strong positive rate
(%) |
|---|
| Normal bone
tissues | 10 | 0 | 0 | 0 | 10 | 0 | 0 |
| Benign bone
tumor | 30 | 0 | 1 | 0 | 29 | 3.30 | 0.00 |
| Human
osteosarcoma | 30 | 7 | 5 | 10 | 8 | 73.00 | 33.33 |
Correlation between p-MAPK and cyclin
D1 in human osteosarcoma
To analyze the mutual associations among the
proteins according to the expression of each antigen in human
osteosarcoma, Spearman's correlation coefficient analysis was used.
p-MAPK and cyclin D1 were positively associated with the intensity
of positive staining (r=0.714; P<0.05; Table III).
 | Table III.Staining of p-MAPK and cyclin D1 in
human osteosarcoma cases. |
Table III.
Staining of p-MAPK and cyclin D1 in
human osteosarcoma cases.
|
| Positive staining
rate (%)
|
|---|
| n | p-MAPK | Cyclin D1 |
|---|
| 1 | 5 | 5 |
| 2 | 8 | 5 |
| 3 | 10 | 2 |
| 4 | 30 | 35 |
| 5 | 12 | 20 |
| 6 | 10 | 6 |
| 7 | 15 | 8 |
| 8 | 65 | 60 |
| 9 | 4 | 3 |
| 10 | 8 | 57 |
| 11 | 7 | 5 |
| 12 | 4 | 3 |
| 13 | 5 | 3 |
| 14 | 65 | 60 |
| 15 | 8 | 30 |
| 16 | 45 | 60 |
| 17 | 35 | 30 |
| 18 | 8 | 3 |
| 19 | 3 | 4 |
| 20 | 5 | 5 |
| 21 | 75 | 15 |
| 22 | 2 | 4 |
| 23 | 90 | 50 |
| 24 | 30 | 15 |
| 25 | 10 | 10 |
| 26 | 10 | 8 |
| 27 | 70 | 55 |
| 28 | 5 | 1 |
| 29 | 12 | 30 |
| 30 | 28 | 6 |
Discussion
The occurrence and development of cancer is closely
associated with abnormalities in the transfer and regulation of
cellular signaling (11,12). Several signaling channels of
carcinogenic tyrosine kinase have a convergence point with MAPK.
MAPK remains static in unstimulated cells, however, it is activated
following the receipt of activation signals from MAPK kinase (MKK)
and MKK kinase (13), and is
phosphorylated in a stepwise manner in cells stimulated by growth
factors (14). When it is
activated, MAPK transfers into the nucleus and activates certain
oncogenes to stimulate the proliferation of cells and inhibit
apoptosis.
The results of the present study showed that the
positive rates of MAPK staining in osteosarcoma and benign bone
tumors were higher, compared with that in normal bone tissues, and
the expression of p-MAPK was significantly higher, compared with
that in normal bone tissues. These results suggested that
activation of the MAPK cascade is important in the occurrence of
osteosarcoma. In addition, the degree of malignant bone tumor
tissue p-MAPK staining intensity was significantly higher, compared
with that of benign tumor tissue. Therefore, the results suggested
that the overactivation of MAPK may be closely associated with the
invasive growth potential of osteosarcoma.
Studies have indicated that the overexpression of
cyclin D1 may result in a reduction of the G1 phase of the cell
cycle (15–17), leading to progression into the S
phase and completing the duplication of DNA. The increase in the
protein expression of cyclin D1 is observed in certain primary
malignant tumors, including parathyroid adenoma, neck squamous-cell
carcinoma, breast cancer, esophageal cancer, and hepatocellular
carcinoma (18–21). As MAPK is expressed as an upstream
gene of cyclin D1, the increase in the level of p-MAPK in
osteosarcoma was directly proportional to the intensity of cyclin
D1-positive staining.
The results of the present study also demonstrated
that the positive rate of cyclin D1 staining was significantly
higher in the osteosarcoma tissues, compared with rates in normal
bone tissues and benign bone tumors. In addition, there was
positive correlation between the two genes in osteosarcoma,
determined by cyclin D1 and p-MAPK positive intensity. MAPK is
expressed upstream of cyclin D1, and overexpression of the cyclin
D1 may be induced by the p-MAPK signaling pathway in osteosarcoma,
which can lead to increased proliferation of the tumor cells.
In conclusion, the present study demonstrated that
the expression of p-MAPK and cyclin D1 were suitable for use as
markers for osteosarcoma to assist in early diagnosis and
prognosis. This was achieved by examining the expression levels of
p-MAPK and cyclin D1 in osteosarcoma, benign bone tumor tissues and
normal bone tissues, and examining the association between the two.
By interfering with the p-MAPK signal transduction pathway in human
osteosarcoma, it is possible to prevent the overexpression of
cyclin D1 in the tissue, which may assist in preventing the
occurrence of osteosarcoma. This provides a novel technique and
offers potential for clinical use in the treatment of
osteosarcoma.
References
|
1
|
Huang J, Liu K, Song D, Ding M, Wang J,
Jin Q and Ni J: Krüppel-like factor 4 promotes high-mobility group
box 1-induced chemotherapy resistance in osteosarcoma cells. Cancer
Sci. 107:242–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li YS, Deng ZH, Zeng C and Lei GH: JNK
pathway in osteosarcoma: Pathogenesis and therapeutics. J Recept
Signal Transduct Res. 36:465–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chakravarthi PS, Kattimani VS, Prasad LK
and Satish PR: Juxtacortical osteosarcoma of the mandible:
Challenges in diagnosis and management. Natl J Maxillofac Surg.
6:127–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou W, Zhu Y, Chen S, Xu R and Wang K:
Fibroblast growth factor receptor 1 promotes MG63 cell
proliferation and is associated with increased expression of
cyclin-dependent kinase 1 in osteosarcoma. Mol Med Rep. 13:713–719.
2016.PubMed/NCBI
|
|
5
|
Yang J, Cheng D, Zhou S, Zhu B, Hu T and
Yang Q: Overexpression of X-Box Binding Protein 1 (XBP1) Correlates
to Poor Prognosis and Up-Regulation of PI3K/mTOR in Human
Osteosarcoma. Int J Mol Sci. 16:28635–28646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shao Y, Wang C, Hong Z and Chen Y:
Inhibition of p38 mitogen-activated protein kinase signaling
reduces multidrug transporter activity and anti-epileptic drug
resistance in refractory epileptic rats. J Neurochem.
136:1096–1105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Liu Q, Fang Z, Hu X, Huang F,
Tang L and Zhou S: Hypoxia induces the proliferation of endothelial
progenitor cells via upregulation of Apelin/APLNR/MAPK signaling.
Mol Med Rep. 13:1801–1806. 2016.PubMed/NCBI
|
|
8
|
Xu W, Yang Z, Zhou SF and Lu N:
Posttranslational regulation of phosphatase and tensin homolog
(PTEN) and its functional impact on cancer behaviors. Drug Des
Devel Ther. 8:1745–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang N, Zhang C, Dill P, Panasyuk G, Pion
D, Koka V, Gallazzini M, Olson EN, Lam H, Henske EP, et al:
Regulation of YAP by mTOR and autophagy reveals a therapeutic
target of tuberous sclerosis complex. J Exp Med. 211:2249–2263.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Lu WY and Cui LL: Clinical
significance of STAT3 and MARK Phosphorylation, and the protein
expression of cyclin D1 in skin squamous cell carcinoma tissues.
Mol Med Rep. 12:8129–8134. 2015.PubMed/NCBI
|
|
11
|
Talbot JJ, Song X, Wang X, Rinschen MM,
Doerr N, LaRiviere WB, Schermer B, Pei YP, Torres VE and Weimbs T:
The cleaved cytoplasmic tail of polycystin-1 regulates
Src-dependent STAT3 activation. J Am Soc Nephrol. 25:1737–1748.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang YH, Li B, Shen L, Shen Y and Chen
XD: The role and clinical significance of YES-associated protein 1
in human osteosarcoma. Int J Immunopathol Pharmacol. 26:157–167.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng ZP, Yan Y, Xia J, Zhang S, Wang M,
Chen J and Xu Y: A phenylacetaldehyde-flavonoid adduct,
8-C-(E-phenylethenyl)- norartocarpetin, exhibits intrinsic
apoptosis andMAPK pathways-related anticancer potential on HepG2,
SMMC-7721 and QGY-7703. Food Chem. 197:1085–1092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cursons J, Angel CE, Hurley DG, Print CG,
Dunbar PR, Jacobs MD and Crampin EJ: Spatially transformed
fluorescence image data for ERK-MAPK and selected proteins within
human epidermis. Gigascience. 4:632015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beauvais S, Drevelle O, Lauzon MA, Daviau
A and Faucheux N: Modulation of MAPK signalling by immobilized
adhesive peptides: Effect on stem cell response to BMP-9-derived
peptides. Acta Biomater. 31:241–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dreyer JH, Hauck F, Barros MH and
Niedobitek G: pRb and CyclinD1 Complement p16 as
Immunohistochemical Surrogate Markers of HPV Infection in Head and
Neck Cancer. Appl Immunohistochem Mol Morphol. 2015.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Lv S, An J and Lu C: Pre-miR-149
rs71428439 polymorphism is associated with increased cancer risk
and AKT1/cyclinD1 signaling in hepatocellular carcinoma. Int J Clin
Exp Med. 8:13628–13633. 2015.PubMed/NCBI
|
|
18
|
Yang Y, Zhao LH, Huang B, Wang RY, Yuan
SX, Tao QF, Xu Y, Sun HY, Lin C and Zhou WP: Pioglitazone, a PPARγ
agonist, inhibits growth and invasion of human hepatocellular
carcinoma via blockade of the rage signaling. Mol Carcinog.
54:1584–1595. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi QQ, Zuo GW, Feng ZQ, Zhao LC, Luo L,
You ZM, Li DY, Xia J, Li J and Chen DL: Effect of trichostatin a on
anti HepG2 liver carcinoma cells: Inhibition of HDAC activity and
activation of Wnt/β-catenin signaling. Asian Pac J Cancer Prev.
15:7849–7855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Liu H, Wang X, Zeng Z, Xie LQ, Sun
ZG and Wei MX: Preventive effect of Actinidia valvata Dunn extract
on N-methyl-N'-nitro-N-nitrosoguanidine-induced gastrointestinal
cancer in rats. Asian Pac J Cancer Prev. 15:6363–6377. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gopalakrishnan N, Saravanakumar M,
Madankumar P, Thiyagu M and Devaraj H: Colocalization of β-catenin
with Notch intracellular domain in colon cancer: A possible role of
Notch1 signaling in activation of CyclinD1-mediated cell
proliferation. Mol Cell Biochem. 396:281–293. 2014. View Article : Google Scholar : PubMed/NCBI
|