Introduction
Nasopharyngeal carcinoma (NPC) is a common head and
neck malignancy in southern China, with >20,404 associated cases
of mortality reported in 2012 (1–3). NPC
is highly metastatic, as evidenced by the observation that 30–60%
of patients diagnosed with local-advanced disease will develop
distant metastases (4–6). Despite the majority of patients with
metastatic NPC typically experiencing a good initial response to
chemotherapy, frequent recurrences can occur due to the development
of multidrug resistance (MDR) against chemotherapeutic agents
(7). As a result, the prognosis
for patients with metastatic NPC remains poor, with a 3-year
overall survival rate of 30–40% (6,8),
which emphasizes the requirement for novel therapies that target
drug-resistant NPC.
MicroRNAs (miRNA) are small non-coding nucleotides
that regulate the expression of target genes at a
post-transcriptional level. miRNAs reportedly affect tumorigenesis
(9) and NPC prognosis by
functioning as oncogenes (10,11)
or tumor suppressor genes (12,13).
Indeed, the role of miRNAs in MDR has recently received
considerable attention, as previous reports have suggested that
miRNAs may regulate tumor cell responses to chemotherapy, and the
manipulation of miRNAs may modulate MDR (14–16).
There is accumulating interest in understanding the
role of miR-125b in cancer. miR-125b was one of the first miRNAs
identified, and studies have demonstrated that miR-125b serves a
functional role in MDR, tumorigenesis and in the prognosis of
different cancer types (17–19).
Consistent with these reports, the upregulation of miR-125b in
Taxol-resistant cells, inhibited Taxol-induced cytotoxicity and
apoptosis, and promoted a subsequent increase in Taxol resistance
in breast cancer cells (20).
However, the effects of miR-125b in the development of MDR in NPC
remain elusive.
The aims of the present study were to characterize
miR-125b expression levels in the cisplatin (DDP)-resistant
CNE2/DDP human NPC cell line compared with the drug-sensitive
parental CNE2 cell line, and to investigate the role of miR-125b in
the development of MDR in NPC.
Materials and methods
Cell lines and culture conditions
The human NPC cell line CNE2 was provided by
Professor MuSheng Zeng (Sun Yat-sen University Cancer Center
(Guangzhou, China). CNE2 cells were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and maintained in
a humidified incubator at 37°C and 5% CO2.
The DDP-resistant cell line CNE2/DDP was generated
in our laboratory (Guangzhou, China) by supplementing culture
medium with increasing concentrations of 0.5–10.0 µg/ml DDP (Mayne
Pharma, Salisbury South, SA, Australia) over the course of 12
months until a significant increase in DDP-resistance was achieved
compared with the CNE2 parental cell line. CNE2/DDP cells were
cultured in the presence of DDP (0.5 µg/ml) to maintain the
DDP-resistant phenotype, however, cells were cultured in drug-free
medium for a minimum of 2 weeks prior to downstream
experiments.
miRNA transfection
CNE2 and CNE2/DDP cells were seeded in 6-well plates
at a density of 5×105 cells/plate 1 day prior to transfection. CNE2
cells were transfected with 100 nM miR-125b inhibitors or control
miRNA inhibitors, and CNE2/DDP cells were transfected with 10 nM
miR-125b mimics or 10 nM control miRNA mimics using HiPerfect
Transfection Reagent (Qiagen, Inc., Valencia, CA, USA). Cells were
harvested for downstream, analysis 48 h following transfection.
miR-125b mimics, inhibitors and controls were purchased from
Shanghai GenePharma Co., Ltd., (Shanghai, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for analysis of miRNA
expression levels
Total RNA was isolated from cells using the TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA,
USA) according to the manufacturer's instructions. miRNA was
purified using a mirVana™ miRNA Isolation kit (Ambion; Thermo
Fisher Scientific, Inc., Foster City, CA, USA). RNA sample
concentrations and purities were determined spectroscopically.
RT-qPCR was performed using the GoTaq® qPCR Master Mix
(Promega Corporation, Madison, WI, USA) and the MiniOpticon™
Real-Time PCR detection instrument (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) in addition to the SYBR-Green detection protocol
(Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's instructions. Sequence-specific primers for miR-125b
and hsa-U6 small nuclear RNA gene transcripts are listed in
Table I. All PCR reactions were
run in triplicate, and non-template controls were included.
miRNA-125b expression levels in CNE2/DDP cells were calculated
relative to control CNE2 cells using the 2-ΔΔCq method
where ΔCq = CqmiRNA-125b - CqU6, and ΔΔCq =
ΔCqCNE2/DDP - ΔCqCNE2.
 | Table I.Details of primer sequences used to
detect miR-125b expression levels by RT-qPCR. |
Table I.
Details of primer sequences used to
detect miR-125b expression levels by RT-qPCR.
| Primers | Sequence (5′-3′) |
|---|
|
Has-miR-125b-Reverse |
GTCGTATCCAGTGCAGGGTC |
|
|
CGAGGTATTCGCACTGGATA |
|
| CGACTCACAA |
|
Has-miR-125b-Forward |
GCTCCCTGAGACCCTAAC |
| hsa-U6-Forward |
CTCGCTTCGGCAGCACA |
| hsa-U6-Reverse |
AACGCTTCACGAATTTGCGT |
Cell proliferation assay
At 48 h following transfection, cells were seeded
onto 96-well plates (2×103 cells/well) and allowed to adhere to the
culture vessel surface overnight. The following day, newly prepared
DDP (Mayne Pharma), 5-fluorouracil (5-FU) (Shanghai Xudong Haipu
Pharmaceutical Co., Ltd., Shanghai, China), vincristine (VCR)
(Zhejiang Hisun Chemical Co., Ltd., Zhejiang, China) and etoposide
(VP-16) (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) were
added at final concentrations of 0.001-, 0.01-, 0.1-, 1-, 10- and
100-fold higher than the human peak plasma concentrations for each
drug. Following 70 h, cell viability was assessed using a
3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxy-phenyl)-2-4-sulfophenyl)-2H-tetrazolium
assay (Promega Corporation) according to the manufacturer's
instructions. For each well, the absorbance was read at 490 nm
using a spectrophotometer. The concentration at which each drug
produced a 50% inhibition of cell growth (IC50) was
estimated by constructing a relative survival curve. Experiments
were performed in triplicate.
Apoptosis assay
Cells were seeded on 6-well plates (5×105/well),
transfected as described previously and exposed to DDP (0.8 µg/ml)
at 48 h following transfection. Apoptosis was then detected using a
flow cytometry-directed annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) assay (KayGen Biotechnology, Co.,
Ltd., Nanjing, China) according to the manufacturer's instructions.
Apoptotic cells were considered to be positive if they were annexin
V-FITC-positive-PI-negative. Cells stained with annexin V-FITC or
PI alone were used as controls.
Western blot analysis
Transfected cells were harvested and homogenized
using Cell Lysis Buffer (Abcam, Cambridge, MA, USA). Then, 50 µg
total protein was separated using 10% SDS-PAGE followed by transfer
to nitrocellulose membranes. Membranes were blocked using 5%
non-fat dry milk in Tris-buffered saline (TBS) for 1 h at 37°C and
then incubated overnight at 4°C with B-cell CLL/lymphoma 2 (Bcl-2;
1:500; cat. no. 2872), ATP binding cassette subfamily B member 1
(MDR1; 1:200; cat. no. 13978) and α-tubulin (1:1,000; cat. no.
2144) primary antibodies purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). The following day, membranes were washed
three times with 1X TBS-Tween 20 (TBST) and probed with horseradish
peroxidase-conjugated goat anti-rabbit IgG antibodies (cat. no.
sc-2301; 1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 1 h at room temperature. Membranes were then washed with 1X
TBST, and protein bands were visualized using an enhanced
chemiluminescence assay according to the manufacturer's
instructions (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
Statistical analysis
All experiments were conducted in triplicate, and
the data are presented as the mean ± standard deviation.
Differences between means were analyzed using the Student's t-test.
All statistical analyses were performed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). All statistical tests were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-125b is differentially expressed
in MDR NPC cell lines
In order to investigate whether miR-125b is involved
in the MDR of NPC cells, miR-125b expression levels in the MDR NPC
cell line (CNE2/DDP) and the parental CNE2 cell line were
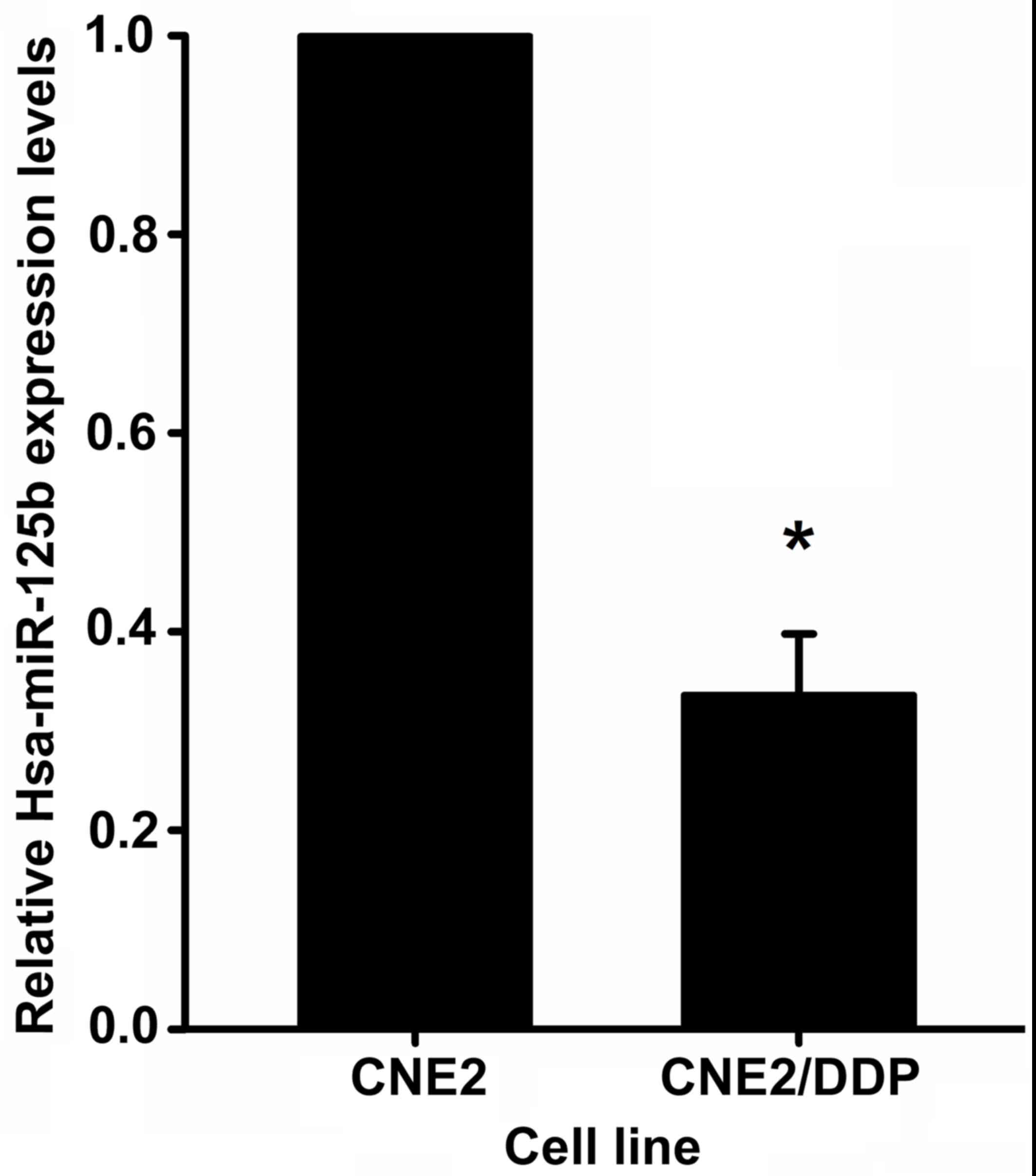
determined using RT-qPCR analysis. As demonstrated in Fig. 1, miR-125b expression was observed
to be significantly downregulated (3.04±0.59-fold, P=0.017) in
CNE2/DDP cells compared with CNE2 cells.
miR-125b modulates MDR in NPC
cells
To investigate an association between miR-125b and
MDR in NPC cells, the expression levels of miR-125b in CNE2 and
CNE2/DDP cells, which express relatively high and low levels of
miR-125b respectively, were altered, and the effects of specific
anticancer agents on the MDR phenotype were determined. As shown in
Fig. 2A, transfection of CNE2/DDP
cells with miR-125b mimics significantly increased the
chemosensitivity of cells to DDP (IC50: 12.16±1.23 vs.
1.62±0.85 µg/ml, P=0.002), 5-FU (IC50: 85.00±8.67 vs.
44.40±6.40 µg/ml, P=0.025), VCR (IC50: 15.84±2.56 vs.
2.88±1.15 µg/ml, P=0.001) and VP-16 (IC50: 53.49±7.43
vs. 19.87±4.67 µg/ml, P=0.017) compared with cells transfected with
miRNA mimic controls. By contrast, suppression of miR-125b
expression in CNE2 cells by transfecting cells with miR-125b
inhibitors, significantly decreased the sensitivity of cells to DDP
(P=0.011), 5-FU (P=0.001), VCR (P=0.034) and VP-16 (P=0.010)
treatment (Fig. 2B). Together,
these results suggest that modulation of miR-125b expression
affects MDR in NPC cells.
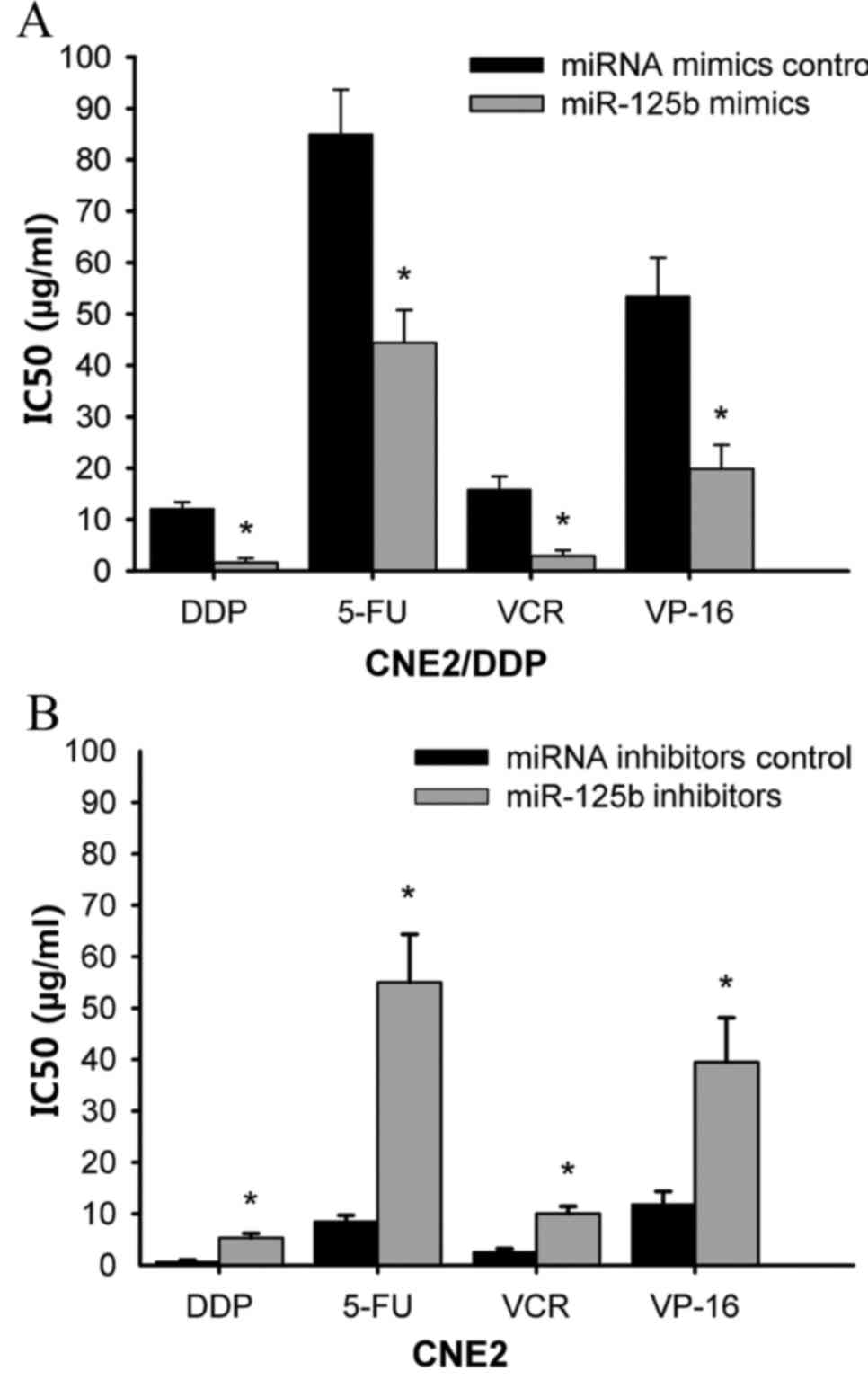 | Figure 2.Modulation of miR-125b expression
alters the sensitivity of nasopharyngeal carcinoma cells to
anticancer drugs. (A) CNE2/DDP and (B) CNE2 cells were transfected
with miR-125b mimics and miR-125b inhibitors respectively.
Following incubation with DDP, 5-FU, VCR and VP-16 anticancer
agents for 70 h, cell viability was assessed using an
3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxy-phenyl)-2-4-sulfophenyl)-2H-tetrazolium
assay, and the IC50 was calculated. The data represent
the mean ± standard deviation from three independent experiments.
*P<0.05 vs. CNE2/DDP and CNE2 cells transfected with control
miRNA mimics and control miRNA inhibitors respectively. miR,
microRNA; DDP, cisplatin; 5-FU, 5-fluorouracil; VCR, vincristine;
VP-16, etoposide; IC50, the concentration at which each
drug produced a 50% inhibition of cell growth. |
miR-125b sensitizes CNE2/DDP cells to
DDP-induced apoptosis
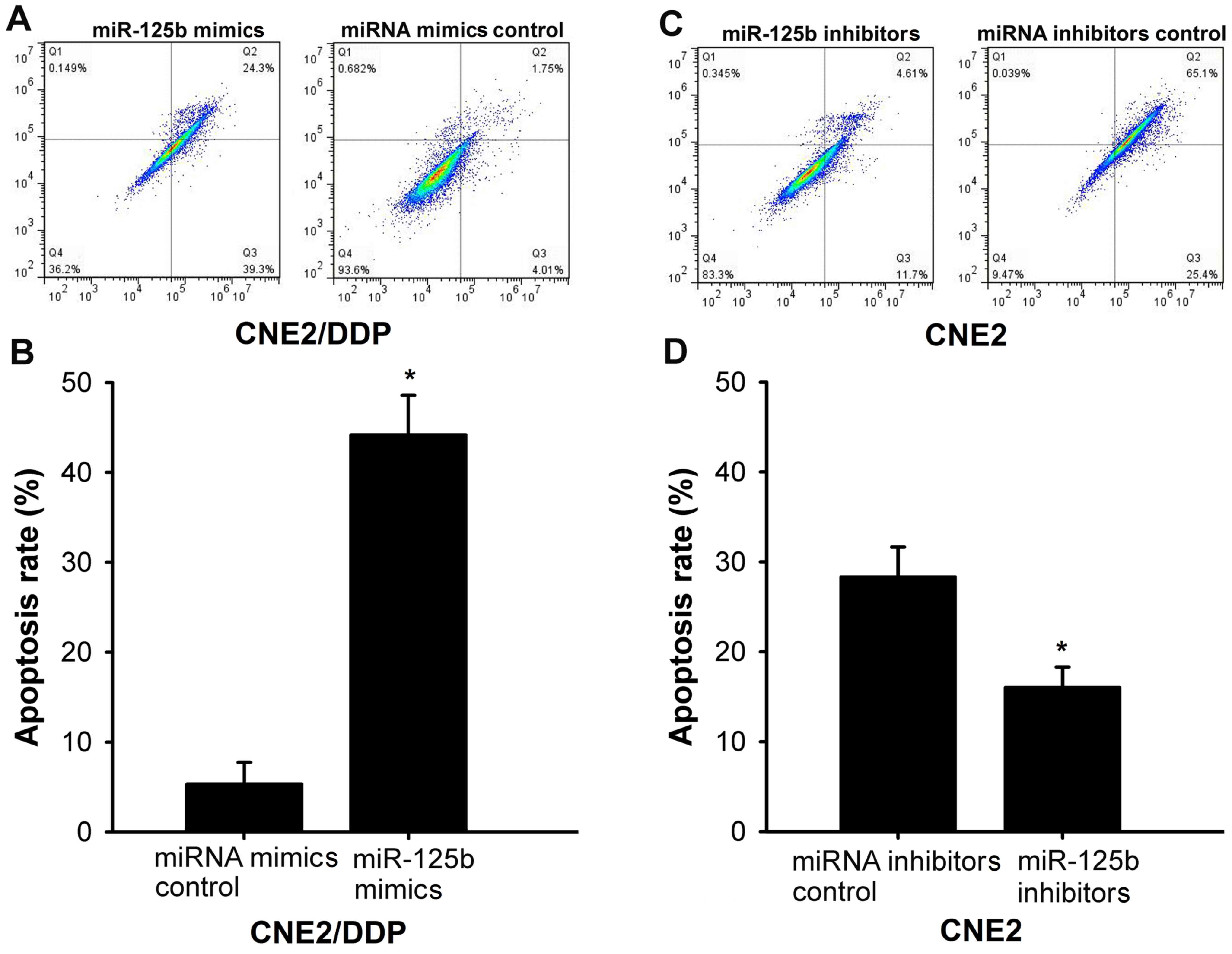
To investigate the possibility that miR-125b
upregulation increases DDP-induced apoptosis, CNE2/DDP cells were
transfected with either miR-125b mimics or miRNA mimic controls and
treated with 0.8 µg/ml DDP for 24 h. Transfection of CNE2/DDP cells
with miR-125b mimics significantly increased the percentage of
apoptotic cells, as determined using annexin-V staining, compared
with cells transfected with miRNA mimic controls (P<0.001;
Fig. 3A and B). Additionally, by
transfecting CNE2 cells with an miR-125b inhibitor, repression of
endogenous miR-125b significantly inhibited DDP-induced apoptosis
compared with cells transfected with control miRNA inhibitors
(P=0.006; Fig. 3C and D).
Together, these results demonstrate that miR-125b serves an
important role in modulating DDP resistance in NPC cells.
miR-125b regulates MDR by targeting
Bcl-2 in NPC
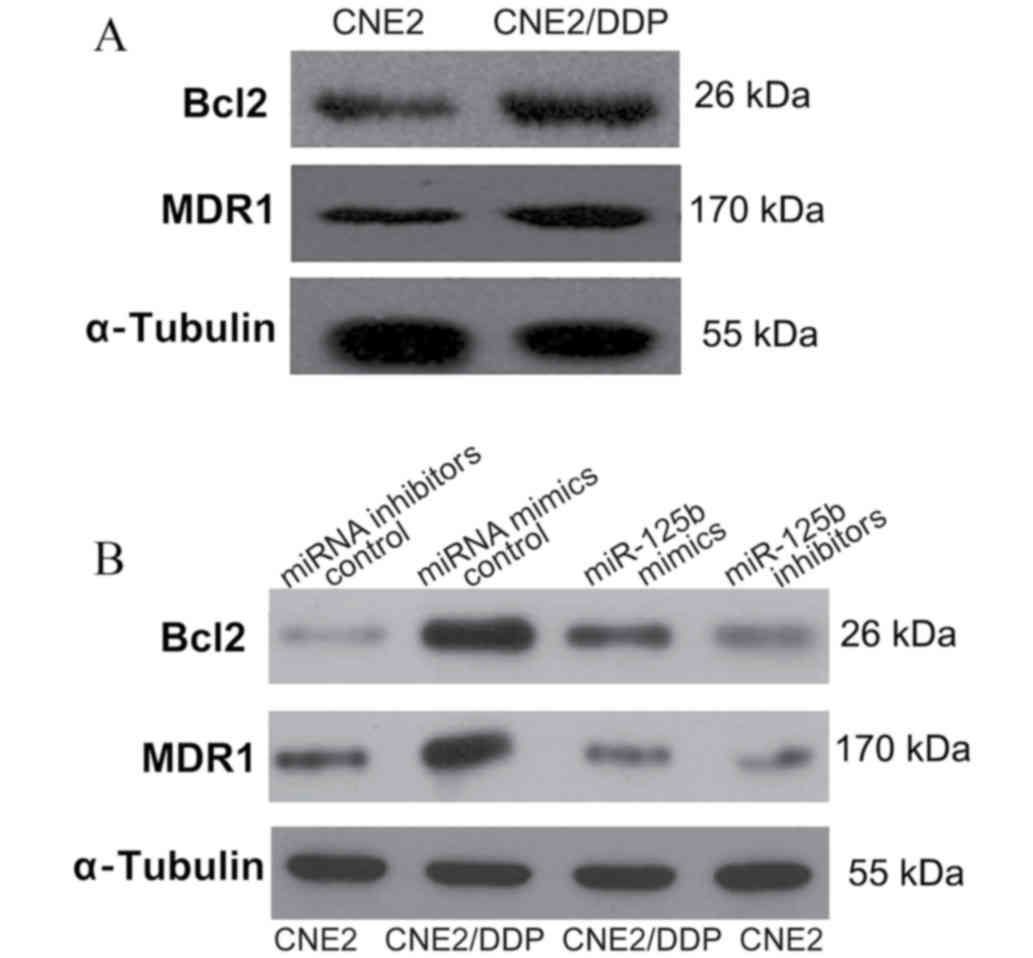
A previous study demonstrated an association between
MDR and the upregulation of antiapoptotic proteins, such as Bcl-2
(21). Taking the role of Bcl-2 in
apoptosis and chemoresistance into account, the function of
miR-125b in modulating MDR through regulating Bcl-2 expression was
investigated in the present study. Consistent with this hypothesis,
CNE2/DDP cells were found to display a marked increase in MDR1 and
Bcl-2 expression levels compared with CNE2 cells (Fig. 4A). Furthermore, CNE2/DDP cells
transfected with miR-125b mimics, notably inhibited Bcl-2 and MDR1
protein expression levels compared with cells transfected with
control miRNA mimics (Fig. 4B).
Consistent with these findings, CNE2 cells transfected with
miR-125b inhibitors exhibited notably increased Bcl-2 expression
levels (Fig. 4B). Together, these
results demonstrate that miR-125b regulates NPC resistance to DDP,
in part, by targeting Bcl-2 protein expression levels.
Discussion
In the present study, the role of miR-125b in the
development of MDR in NPC cells was investigated. Using RT-qPCR
analysis, miR-125b was demonstrated to be significantly
downregulated in MDR CNE2/DDP cells compared with chemosensitive
CNE2 counterparts. Transfection of CNE2/DDP cells with miR-125b
mimics was also observed to increase apoptosis and the
chemosensitivity of cells, in part, through downregulating Bcl-2
and MDR1 protein expression levels. These results suggest that
miR-125b may represent a novel therapeutic target to resensitize
MDR NPCs to chemotherapy.
MDR is a major obstacle in the treatment of cancer
patients with chemotherapy and is often associated with an
increased efflux of drugs due to some proteins such as
P-glycoprotein (P-gp) and multidrug resistance-associated protein 1
(MRP1), which belong to the ATP-binding cassette transporters
(22). The involvement of P-gp and
MRP1 in MDR is due to their overexpression in resistant tumor
cells. In particular, P-gp functions as a ‘protein scavenger’,
which is able to ‘capture’ drugs and transport them out of cells
(23). The putative role of miRNAs
in human cancers has become a focus of current research, with
specific reports indicating an association between miRNAs and MDR
development (24). miR-125b is a
member of the miR-125b family, which is located on chromosomes
11q24.1 (miR-125b-1) and 21q21 (miR-125b-2). miR-125b upregulation
has been reported in certain cancer types including colorectal
(25), non-small-cell lung
(26) and head and neck cancers
(27). However, a tumor
suppressive role of miR-125b has also been reported in specific
tumor types that display reduced miR-125b expression levels
(28,29). Notably, previous studies have
observed altered (increased and decreased) miR-125b expression
levels in various drug-resistant cancer cells. Chen et al
(30) demonstrated that miR-125b
was upregulated in temozolomide-resistant glioblastoma stem cells,
and miR-125b inhibition resulted in a significant increase in
temozolomide-induced cytotoxicity, apoptosis and enhanced
temozolomide sensitivity. By contrast, doxorubicin-resistant MCF-7
(MCF-7/DR) cells reportedly exhibit significantly lower levels of
miR-125b expression compared with parental MCF-7 cells (31), which is consistent with the results
observed in the present study demonstrating that miR-125b was
significantly downregulated in CNE2/DDP cells compared with CNE2
cells.
Despite the reported association between miR-125b
and poor prognosis (23,24), few reports have investigated the
specific role of miR-125b in the regulation of drug resistance. For
example, miR-125b overexpression in breast cancer correlates with
an increase in tumor stem cell properties, such as chemoresistance,
whereas knockdown of miR-125b is associated with a decrease in
tumor stem cell-like populations (32). In OV2008 ovarian cancer cells,
miRNA-125b upregulation has been observed to suppress DDP-induced
cytotoxicity and apoptosis, and increase DDP resistance (33). In the present study, the role of
miR-125b in regulating the drug resistance of CNE2/DDP cells was
evaluated. miRNA-125b upregulation was observed to promote a
significant increase in cell cytotoxicity and apoptosis. This is
consistent with the findings presented by Xie et al
(31) demonstrating that
transfection of miR-125b significantly increases the cytotoxicity
of doxorubicin in MCF-7/DR cells. Collectively, these results
suggest that miR-125b serves a functional role in modulating
chemotherapy resistance in cancer cells.
The mitochondrial pathway of apoptosis reportedly
serves an important role in chemotherapy-induced apoptosis, and
Bcl-2 is recognized as a critical regulator of this pathway
(34). Results of the present
study demonstrated that transfection of CNE2/DDP cells with
miR-125b mimics increased DDP-induced apoptosis. To elucidate the
mechanisms by which miR-125b modulates MDR in NPC cells, the role
of miR-125b in regulating Bcl-2 protein expression levels was then
investigated. The results suggest that miR-125b modulates the
susceptibility of NPC cells to DDP-induced apoptosis by regulating
Bcl-2 and MDR1 expression directly, which provides an explanation
for the observed differences in the sensitivity of NPC cells to
VCR, 5-FU, VP-16 and DDP chemotherapeutic agents.
The primary focus of the present study was MDR,
which is a frequent occurrence in patients with cancer that are
treated with chemotherapy, and represents an obstacle to successful
treatment. Therefore, the targeting of MDR-promoting miRNAs, such
as miR-125b, may represent an improved treatment strategy for
patients with cancer compared with therapeutic agents that target a
single protein. However, it is important to note that the results
of the present study were limited to an in vitro setting,
which lacks important cues from the in vivo tumor
microenvironment and should not be considered as an accurate
representation of clinical tumors. Therefore, future studies that
aim to assess the functional role of miRNA-125b in NPC MDR should
be conducted using in vivo methods and in a clinical
setting.
In summary, the present study investigated the role
of miRNAs in the development of MDR in NPC cells. miR-125b was
observed to modulate the sensitivity of NPC cells to specific
anticancer agents by regulating Bcl-2 expression. Together, these
results provide a novel insight into the mechanisms of MDR in NPC,
and provide a rationale for the development of clinical strategies
to manipulate miRNA expression to reverse MDR in human cancer.
Acknowledgements
The present study was supported by grants from the
Health Department of Guangdong Province (grant no. B2013185) and
the special fund of Cancer Center of Guangzhou Medical University
(grant no. 2011-yz-09). Our laboratory is supported by FOCUSBIO
(Focusbio, Inc., Guangzhou, China).
References
|
1
|
Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou
ZX and Chen WQ: Nasopharyngeal carcinoma incidence and mortality in
China in 2010. Chin J Cancer. 33:381–387. 2014.PubMed/NCBI
|
|
2
|
Liu Q, Chen JO, Huang QH and Li YH: Trends
in the survival of patients with nasopharyngeal carcinoma between
1976 and 2005 in Sihui, China: A population-based study. Chin J
Cancer. 32:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
International Agency for Research on
Cancer, World Health Organization, . GLOBOCAN 2012: Estimated
cancer incidence, mortality and prevalence worldwide in 2012.
http://globocan.iarc.fr/Default.aspxJuly
1–2015.
|
|
4
|
Sun X, Su S, Chen C, Han F, Zhao C, Xiao
W, Deng X, Huang S, Lin C and Lu T: Long-term outcomes of
intensity-modulated radiotherapy for 868 patients with
nasopharyngeal carcinoma: An analysis of survival and treatment
toxicities. Radiother Oncol. 110:398–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao WW, Huang SM, Han F, Wu SX, Lu LX,
Lin CG, Deng XW, Lu TX, Cui NJ and Zhao C: Local control, survival,
and late toxicities of locally advanced nasopharyngeal carcinoma
treated by simultaneous modulated accelerated radiotherapy combined
with cisplatin concurrent chemotherapy: Long-term results of a
phase 2 study. Cancer. 117:1874–1883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang R, Cai XY, Yang ZH, Yan Y, Zou X,
Guo L, Sun R, Luo DH, Chen QY, Huang PY, et al: Elevated peripheral
blood lymphocyte-to- monocyte ratio predicts a favorable prognosis
in the patients with metastatic nasopharyngeal carcinoma. Chin J
Cancer. 34:237–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meijerman I, Beijnen JH and Schellens JH:
Combined action and regulation of phase II enzymes and multidrug
resistance proteins in multidrug resistance in cancer. Cancer Treat
Rev. 34:505–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li JX, Huang SM, Wen BX and Lu TX:
Prognostic factors on overall survival of newly diagnosed
metastatic nasopharyngeal carcinoma. Asian Pac J Cancer Prev.
15:3169–3173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu J, He ML, Wang L, Chen Y, Liu X, Dong
Q, Chen YC, Peng Y, Yao KT, Kung HF and Li XP: MiR-26a inhibits
cell growth and tumorigenesis of nasopharyngeal carcinoma through
repression of EZH2. Cancer Res. 71:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang LY, Ho-Fun Lee V, Wong AM, Kwong DL,
Zhu YH, Dong SS, Kong KL, Chen J, Tsao SW, Guan XY and Fu L:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of PTEN.
Carcinogenesis. 34:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo Z, Dai Y, Zhang L, Jiang C, Li Z, Yang
J, McCarthy JB, She X, Zhang W, Ma J, et al: miR-18a promotes
malignant progression by impairing microRNA biogenesis in
nasopharyngeal carcinoma. Carcinogenesis. 34:415–425. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Luo H, Liu X, Peng Y, Zhang B, Wang
L, Xu X, Peng X, Li G, Tian W, et al: miR-9 targets CXCR4 and
functions as a potential tumor suppressor in nasopharyngeal
carcinoma. Carcinogenesis. 35:554–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma L, Deng X, Wu M, Zhang G and Huang J:
Down-regulation of miRNA-204 by LMP-1 enhances CDC42 activity and
facilitates invasion of EBV-associated nasopharyngeal carcinoma
cells. FEBS Lett. 588:1562–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin W, Wang P, Wang X, Song W, Cui X, Yu H
and Zhu W: Identification of microRNAs and mRNAs associated with
multidrug resistance of human laryngeal cancer hep-2 cells. Braz J
Med Biol Res. 46:546–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong L, Yang Z, Ma J and Fan D: Function
of miRNA in controlling drug resistance of human cancers. Curr Drug
Targets. 14:1118–1127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai Y, Xie CH, Neis JP, Fan CY, Vural E
and Spring PM: MicroRNA expression profiles of head and neck
squamous cell carcinoma with docetaxel-induced multidrug
resistance. Head Neck. 33:786–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng SC, Liao CT, Peng CH, Cheng AJ, Chen
SJ, Huang CG, Hsieh WP and Yen TC: MicroRNAs MiR-218, MiR-125b and
Let-7g predict prognosis in patients with oral cavity squamous cell
carcinoma. PLoS One. 9:e1024032014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Z, Liu H, Desai S, Schmitt DC, Zhou M,
Khong HT, Klos KS, McClellan S, Fodstad O and Tan M: miR-125b
functions as a key mediator for snail-induced stem cell propagation
and chemoresistance. J Biol Chem. 288:4334–4345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feliciano A, Castellvi J, Artero-Castro A,
Leal JA, Romagosa C, Hernández-Losa J, Peg V, Fabra A, Vidal F,
Kondoh H, et al: miR-125b acts as a tumor suppressor in breast
tumorigenesis via its novel direct targets ENPEP CK2-α, CCNJ, and
MEGF9. PLoS One. 8:e762472013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi
Y, Xiong W, Li G, Lu J, Fodstad O, et al: MicroRNA-125b confers the
resistance of breast cancer cells to paclitaxel through suppression
of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J
Biol Chem. 285:21496–21507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reed JC: Regulation of apoptosis by bcl-2
family proteins and its role in cancer and chemoresistance. Curr
Opin Oncol. 7:541–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teodori E, Dei S, Martelli S, Scapecchi F
and Gualtieri F: The functions and structure of ABC transporters:
Implications for the design of new inhibitors of Pgp and MRP1 to
control multidrug resistance (MDR). Curr Drug Targets. 7:893–909.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levchenko A, Mehta BM, Niu X, Kang G,
Villafania L, Way D, Polycarpe D, Sadelain M and Larson SM:
Intercellular transfer of P-glycoprotein mediates acquired
multidrug resistance in tumor cells. Proc Natl Acad Sci USA.
102:1933–1938. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng T, Wang J, Chen X and Liu L: Role of
microRNA in anticancer drug resistance. Int J Cancer. 126:2–10.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishida N, Yokobori T, Mimori K, Sudo T,
Tanaka F, Shibata K, Ishii H, Doki Y, Kuwano H and Mori M: MicroRNA
miR-125b is a prognostic marker in human colorectal cancer. Int J
Oncol. 38:1437–1443. 2011.PubMed/NCBI
|
|
26
|
Yuxia M, Zhennan T and Wei Z: Circulating
miR-125b is a novel biomarker for screening non-small-cell lung
cancer and predicts poor prognosis. J Cancer Res Clin Oncol.
138:2045–2050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vriens MR, Weng J, Suh I, Huynh N,
Guerrero MA, Shen WT, Duh QY, Clark OH and Kebebew E: MicroRNA
expression profiling is a potential diagnostic tool for thyroid
cancer. Cancer. 118:3426–3432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferracin M, Bassi C, Pedriali M, Pagotto
S, D'Abundo L, Zagatti B, Corrà F, Musa G, Callegari E, Lupini L,
et al: miR-125b targets erythropoietin and its receptor and their
expression correlates with metastatic potential and ERBB2/HER2
expression. Mol Cancer. 12:1302013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakanishi H, Taccioli C, Palatini J,
Fernandez-Cymering C, Cui R, Kim T, Volinia S and Croce CM: Loss of
miR-125b-1 contributes to head and neck cancer development by
dysregulating TACSTD2 and MAPK pathway. Oncogene. 33:702–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Fu X, Wan Y, Wang Z, Jiang D and
Shi L: miR-125b inhibitor enhance the chemosensitivity of
glioblastoma stem cells to temozolomide by targeting Bak1. Tumor
Biol. 35:6293–6302. 2014. View Article : Google Scholar
|
|
31
|
Xie X, Hu Y, Xu L, Fu Y, Tu J, Zhao H,
Zhang S, Hong R and Gu X: The role of
miR-125b-mitochondria-caspase-3 pathway in doxorubicin resistance
and therapy in human breast cancer. Tumor Biol. 36:7185–7194. 2015.
View Article : Google Scholar
|
|
32
|
Wang HJ, Guo YQ, Tan G, Dong L, Cheng L,
Li KJ, Wang ZY and Luo HF: miR-125b regulates side population in
breast cancer and confers a chemoresistant phenotype. J Cell
Biochem. 114:2248–2257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kong F, Sun C, Wang Z, Han L, Weng D, Lu Y
and Chen G: miR-125b confers resistance of ovarian cancer cells to
cisplatin by targeting pro-apoptotic Bcl-2 antagonist killer 1. J
Huazhong Univ Sci Technolog Med Sci. 31:543–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|