Introduction
Endometriosis, which is defined by the presence of
endometrial tissue outside of the uterus, occurs in ~10% women, and
is associated with persistent pelvic pain and infertility. Despite
extensive research, the etiology of endometriosis remains elusive.
Inflammation of the surrounding pelvic microenvironment is thought
to have an important role in the initiation and progression of
endometriosis, in addition to ovarian steroid hormones (1). The ectopic growth of ‘lesions’,
consisting of endometrial cells outside the uterine cavity,
stimulate an inflammatory response, which initiates the activation
of macrophages and leads to increased concentrations of cytokines
and growth factors in the peritoneal fluid (2,3).
Indoleamine 2,3-dioxygenase (IDO1) is an
intracellular heme enzyme that catalyzes the initial and
rate-limiting step in the metabolism of the essential amino acid
tryptophan in the kynurenine pathway. In the last decade, numerous
studies have demonstrated that IDO1 produces a marked tolerance
effect in fetal rejection, organ transplantation, autoimmune
disorders and cancer (4–6). We previously demonstrated that
elevated IDO1 expression in eutopic and ectopic endometrial stromal
cells (ESCs) promoted the expression of cyclooxygenase-2 (COX-2)
and matrix metalloproteinase-9 (MMP-9), which further induced
abnormal ESC growth, and initiated the invasion and implantation of
the shed endometrium to the peritoneum (7,8).
Macrophages have a key role in regulating and
executing the immune response under various conditions. They are
considered to be involved in highly complex immunological
gynecological processes, including endometriosis, preeclampsia and
miscarriage (9,10). The activation and differentiation
of macrophages is altered in these types of immune reactions.
Higher levels of IDO1 in ectopic ESCs modulate adjacent macrophages
through soluble factors, including interleukin (IL)-33, to generate
a supportive microenvironment in endometriosis. Dysfunctional
macrophages display reduced expression of Human Leukocyte
Antigen-antigen D Related (HLA-DR) and CD11c, and increased
secretion of modified cytokine IL-10 and transforming growth
factor-β1 (TGF-β1) (11). Thus,
the cross-talk between ESCs and macrophages within the peritoneal
cavity remains unclear. The present study aimed to investigate the
effect of IDO1-induced tolerant macrophages on the survival of ESCs
involved in the pathogenesis of endometriosis.
Materials and methods
Sample collection and cell
culture
Patients (age, 23–40 years) that underwent
laparoscopy and additional curettage for treatment of endometriosis
(n=16) or ovary dermoid cyst (n=14) were originally enrolled in
this study. Patients that were later considered to be negative for
endometriosis (n=4) or ovary dermoid cyst (n=2) following
laparoscopy and histological diagnosis were excluded from the
study. In total, 12 endometrial and 12 endometriotic samples were
obtained from patients who underwent laparoscopy for treatment of
endometriosis or ovary dermoid cyst. Inclusion criteria were as
follows: Reproductive age (23–40 years old); in the secretory phase
of the menstrual cycle; absence of systemic pathologies; and no
drug therapy in the past 6 months. Diagnosis was confirmed visually
by laparoscopy and histological analysis. All of the women with
endometriosis were classified as stage III/IV, according to the
revised America Fertility Society classification of endometriosis
(12). Peripheral blood samples
(15 ml) were collected sterilely in women with dermoid cyst (n=12;
mean ± SD: 33.6±6.2 years old) as controls prior to the
administration of general anesthesia with tracheal intubation in
heparinized Hank's buffer solution (Gibco; Thermo Fisher
Scientific, Inc.; Waltham, MA, USA). Endometriotic cyst wall tissue
(ectopic endometrium) was obtained from ovarian endometriosis
patients (n=12; mean ± SD: 30.2±8.1 years old) during surgery, and
normal endometrial samples were collected from control groups. The
protocol was approved by the Research Ethics Committee of Nanjing
Drum Tower Hospital (Nanjing, China) and informed written consent
was obtained from all participants. All tissue samples, which were
≥200 mg, were collected under sterile conditions and transported to
the laboratory on ice in Dulbecco's modified Eagle's medium
(DMEM)/F-12 (Gibco; Thermo Fisher Scientific, Inc.). ESCs were
purified as described previously (13). Immunocytochemistry identified
>95% vimentin-positive and cytokeratin-negative ESCs (13).
IDO1 overexpression or short hairpin
RNA (shRNA) plasmid transfection
Normal ESCs were cultured in DMEM/F-12 with 10%
fetal bovine serum in a 6-well plate (FBS; Gibco; Thermo Fisher
Scientific, Inc.). When cells had reached confluency, 10 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), 2 ml OPTI-MEM™ (Gibco, Thermo Fisher Scientific,
Inc.) and 4 µg plasmid pEGFP-N1-IDO1 (Gene Chem Co., Ltd.,
Shanghai, China) or 4 µg SD11-IDO1 shRNA (Gene Chem Co., Ltd.) were
mixed and incubated at room temperature for 20 min, and then added
to the cells at room temperature according to manufacturer's
protocol. The vector-only plasmid pEGFP-N1 and SD11 (Gene Chem Co.,
Ltd.) were used as negative controls, respectively. After 6 h
incubation, cells were cultured in DMEM/F-12 containing 10% FBS in
5% CO2 at 37°C.
Generation of human macrophages
Peripheral blood mononuclear cells (PBMC) were
isolated from blood samples by Ficoll-Hypaque density gradient
centrifugation (11). CD14+ cells
were obtained through positive selection by CD14+ micromagnetic
beads according to the manufacturer's instructions (Miltenyi Biotec
GmbH, Bergisch Gladbach, Germany). Cell purity was identified as
>95% by flow cytometry identification using a fluorescein
isothiocyanate (FITC)-anti CD14 monoclonal antibody (catalogue no.
555397; BD Biosciences, Franklin Lakes, NJ, USA). CD14+ cells were
harvested at a final concentration of 2×107 cells/ml and washed in
cold PBS. Then, 5 µl CD14 monoclonal antibody was added into each
100 µl of cell suspension for 15 min in the dark at room
temperature. Following staining, cells were washed twice with cold
PBS and then analyzed by Facs Calibur BD flow cytometry (BD
Biosciences). Data were acquired in the list mode, and the relative
proportions of cells within different areas of the fluorescence
profile were quantified using the FlowJo 7.6 software program
(FlowJo, LLC., Ashland, OR, USA). Monocytes were subsequently
cultured with granulocyte macrophage colony-stimulating factor
(GM-CSF; 5 ng/ml; catalog no. 300-03-100, PeproTech, Rocky Hill,
NJ, USA) and macrophage colony-stimulating factor (M-CSF; 20 ng/ml;
R&D Systems, Inc., Minneapolis, MN, USA) in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS and 2 mM
L-glutamine for up to 6 days. The medium containing GM-CSF and
M-CSF was changed every 3 days.
Cell co-culture unit
Normal, ectopic and transfected normal ESCs (ESCs
transfected with pEGFP-N1-IDO1 plasmid or SD11-IDO1 shRNA) were
cultured in 24-well plates (Corning Incorporated, Corning, NY, USA)
at a density of 2×105 cells/well. After ESCs had reached
confluency, the monocyte-generated macrophages were subsequently
added to the wells directly at the same density as ESCs. After 48
h, following gentle scattering, the macrophages were collected.
Some were immediately analyzed by flow cytometry for the
phagocytosis assay; others were further co-cultured directly with
normal ESCs for another 36 h, and the normal ESCs were subsequently
analyzed in cell viability, cell proliferation and Annexin
V/propidium iodide (PI) apoptosis assays.
Phagocytosis assay
A total of 2×106 ESC-pretreated macrophages were
mixed with fluoresbrite carboxy NYO-labeled beads in a ratio of
10:1 (1 µm-diameter microspheres; Polysciences Inc., Warrington,
PA, USA) for 30 min with shaking at 37°C. The unbound beads were
washed away by cold PBS (Corning Incorporation) twice and cells
were then resuspended in 2% bovine serum albumin (Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany). Then, the cell suspension was
added to the upper layer of Ficoll-hypaque solution (GE Healthcare,
Sunnyvale, CA, USA) at a ratio of 1:1, and centrifuged at 300 × g
at room temperature for 10 min. Using density-gradient
centrifugation, the macrophages were separated in the
Ficoll-hypaque solution layer. Following the resuspension of
macrophages in PBS, the ratio of macrophages that ingested
fluorescent beads was directly determined using Facs Calibur BD
flow cytometry (BD Biosciences) and analyzed using the FlowJo 7.6
software program (FlowJo, LLC., Ashland, OR, USA).
Cell viability assay
To detect cell viability, an MTT (Sigma-Aldrich,
Merck KGaA) assay was used. Normal ESCs (2×104 cell/per well in
96-well plate) were co-cultured with ESC-pretreated macrophages for
36 h. Normal ESCs were subsequently incubated with 2.5 mg/ml MTT
for 4 h, and 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck, KGaA)
was added. Absorbance (450 nm) was determined using the DigiScan
Microplate Reader (ASYS Hitech GmbH, Eugendorf, Austria). These
values were normalized to the measurement in normal ESCs
co-cultured with macrophages that had not been previously cultures
with ESCs, in which the absorbance was set at 1.
Cell proliferation assay
Normal ESCs co-cultured with pretreated-macrophages
were separated and detection by BrdU (5-bromo-2-deoxyuridine) Cell
Proliferation Kit (Merck Millipore, Billerica, MA, USA) was
performed for cell proliferation according to the manufacturer's
instructions. The absorbance values (at 450 nm) were detected by
the DigiScan Microplate Reader (ASYS Hitech GmbH) and represent the
rate of DNA synthesis, which corresponds to the number of
proliferating cells. The values were normalized to the absorbance
of normal ESCs co-cultured with untreated macrophages, which was
set at 1.
Measurement of apoptosis
The rate of apoptosis of co-cultured normal ESCs was
analyzed by flow cytometry with Cell Apoptosis kit with Annexin
V-FITC and propidium iodide for flow cytometry according to the
manufacturer's protocol (Invitrogen, Thermo Fisher Scientific,
Inc.). The relative proportions of cells within different areas of
the fluorescence profile were quantified using the FlowJo 7.6
software program (FlowJo, LLC.).
Statistical analysis
One-way analysis of variance with the S-N-K method
post hoc test was used for multiple comparisons, using SPSS
software version 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
IDO1 in ectopic ESCs modulates
macrophage phagocytosis
Our previous study demonstrated that IDO1 expression
was higher in ectopic endometrial tissues compared with normal ones
(7). To investigate the effect of
IDO1 in ESCs on PBMC-derived macrophages, macrophages were
pretreated with normal ESCs, ectopic ESCs, normal ESCs transfected
with plasmid pEGFP-N1-IDO1, SD11-IDO1 shRNA, or vector-only
plasmids. The aim of the current study was to determine whether
IDO1 expression in ESCs is responsible for the phagocytic ability
of macrophages. Compared with normal ESCs that had not been
transfected (blank controls), pEGFP-N1 and SD11 vector-transfected
ESCs (negative controls) had the same effect on macrophage
phagocytosis (P>0.05; data not shown). IDO1 overexpression in
normal ESCs significantly reduced the phagocytosis of co-cultured
macrophages, compared with normal ESCs (P<0.05; group c vs.
group b; Fig. 1), whereas IDO1
interference demonstrated the opposite effects (P<0.01; group d
vs. group b; Fig. 1). Ectopic ESCs
significantly inhibited the phagocytic capacity of co-cultured
macrophages, compared with normal ESCs (P<0.01; group e vs.
group b; Fig. 1).
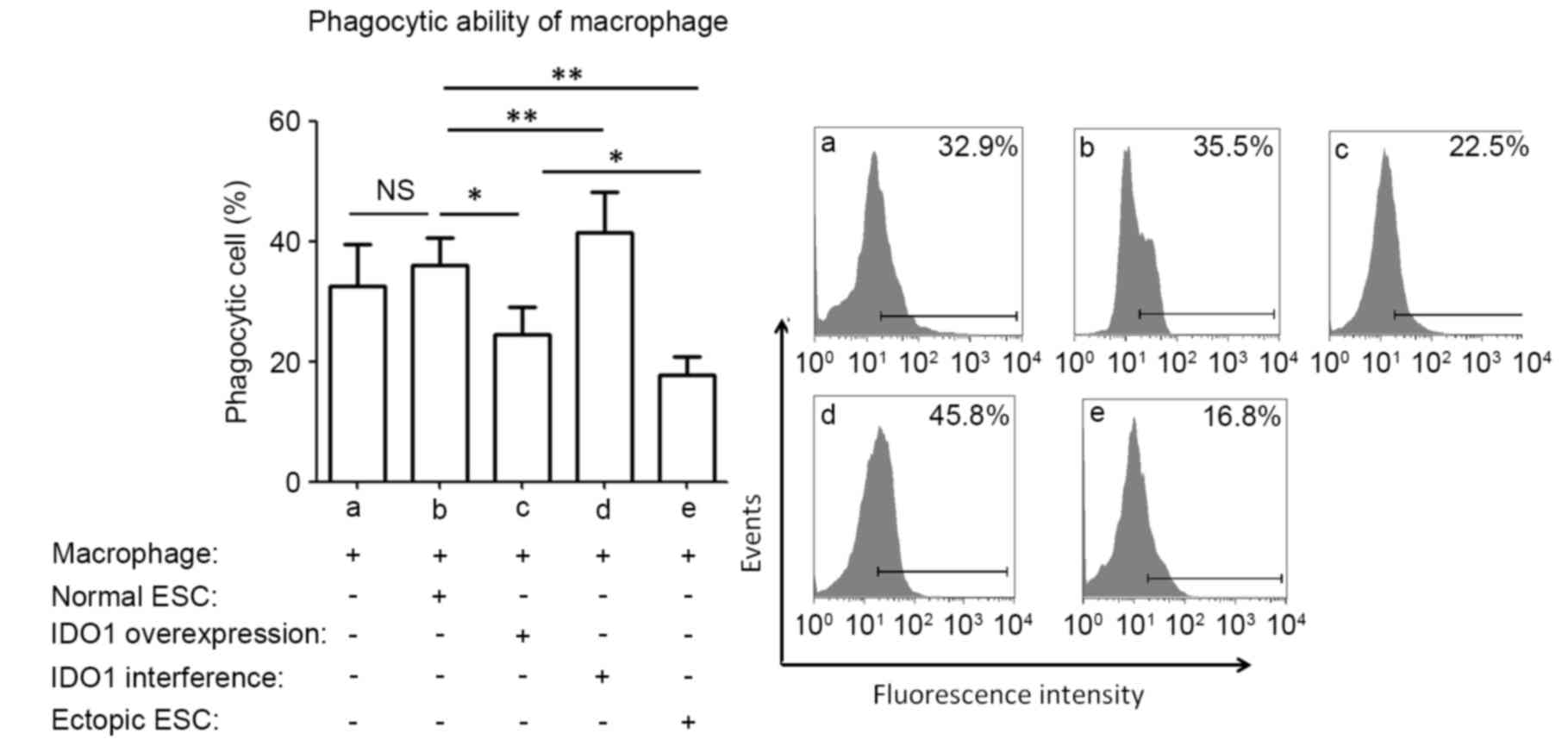 | Figure 1.Phagocytic ability of macrophages in
various ESC-macrophage co-culture groups. Normal ESCs, ectopic ESCs
and normal ESCs treated with pEGFP-N1-IDO1 or SD11-IDO1 short
hairpin RNA were co-cultured with human macrophages directly. The
phagocytosis of macrophages was detected by flow cytometry.
Phagocytic ability was significantly lower when macrophages were
cultured with ectopic ESCs compared with normal ESCs. IDO1
overexpression in normal ESCs significantly decreased the
phagocytosis of macrophages in the normal ESC-macrophage co-culture
group, while IDO1 interference significantly increased it, compared
with normal ESC-treated macrophages. Results are presented as the
ratio of macrophages that ingested fluorescent beads, as the mean +
standard deviation of 12 different experiments (*P<0.05 and
**P<0.01). ESC, endometrial stromal cells; IDO1, indoleamine
2,3-dioxygenase-1; NS, not significant; normal ESC, ESCs from
patients without endometriosis; IDO1 overexpression, normal ESCs
transfected with pEGFP-N1-IDO1; IDO1 interference, normal ESCs
transfected with SD11-IDO1 short hairpin RNA; ectopic ESC, ESC from
endometriosis-derived endometriotic tissue. |
Ectopic ESC-pretreated macrophages
increase the survival of ESCs via higher IDO1 expression in
ESCs
To determine the effect of ESC-treated macrophages
on viability and proliferation of ESCs, ESC-pretreated macrophages
were separated and co-cultured with normal ESCs. pEGFP-N1 and SD11
vector-transfected ESC-educated macrophage (negative controls) had
the same effect on ESC survival as normal ESCs (blank controls;
data not shown). The viability and proliferation index of ESCs
co-cultured with ectopic ESC-educated macrophages were 1.9- and
2.2-fold of the control, respectively (P<0.01; ectopic ESC vs.
normal ESC; Fig. 2). Significant
increases in the viability and proliferation index of ESCs were
observed when co-cultured with pEGFP-N1-IDO1-transfected
ESC-induced macrophages compared with normal ones (P<0.05; IDO1
overexpression vs. normal ESC; Fig.
2). However, the viability and proliferation index of ESCs
co-cultured with pEGFP-N1-IDO1-transfected ESC-induced macrophage
were significantly lower compared with those cultured with ectopic
ESC-induced macrophages (P=0.017, IDO1 overexpression vs. ectopic
ESC in Fig. 2A; P=0.021, IDO1
overexpression vs. ectopic ESC in Fig.
2B which indicated that ectopic ESC-educated macrophages may
promote the growth of ESCs through a mediator other than IDO1.
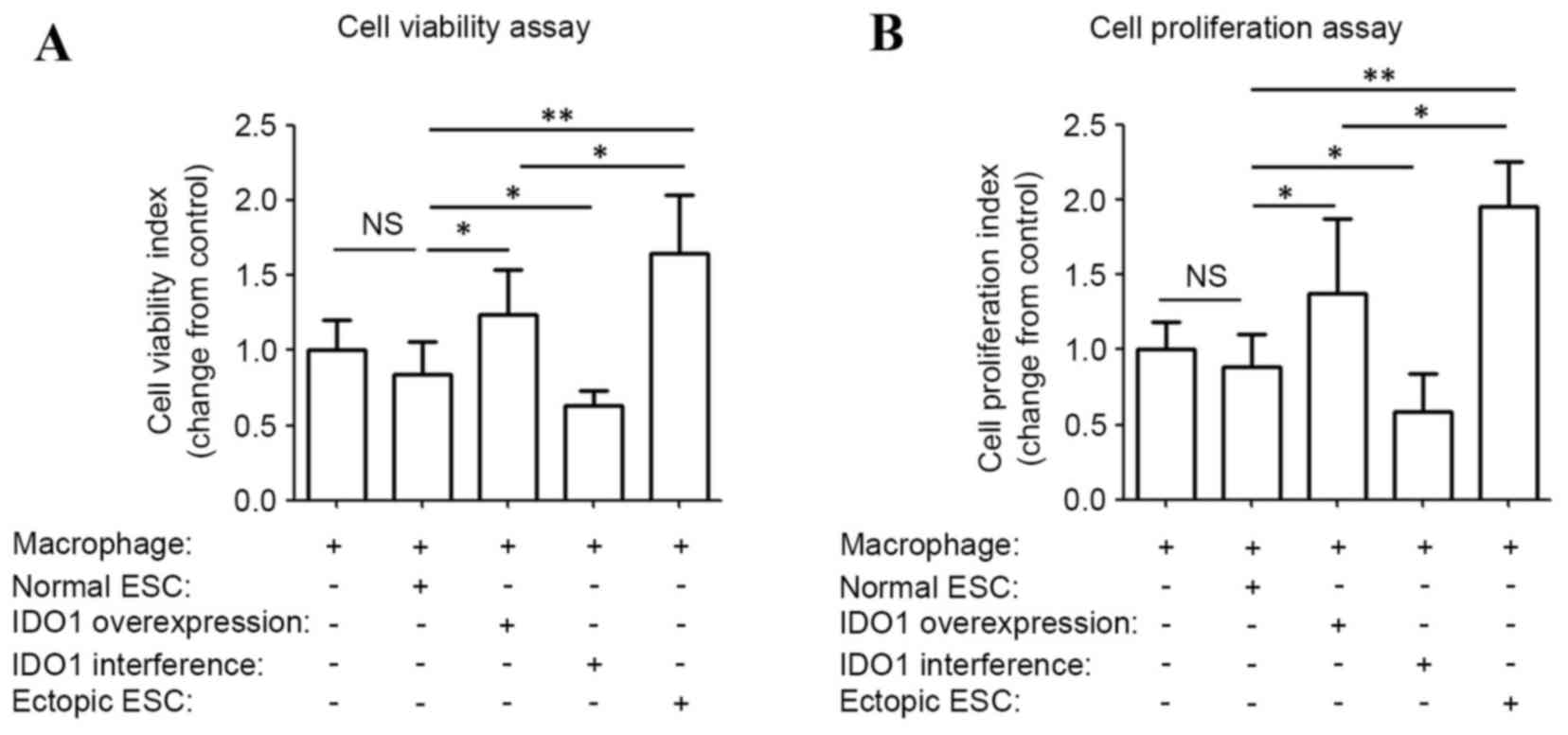 | Figure 2.ESC-pretreated macrophages increase
the survival of ESCs by IDO1. Peripheral blood mononuclear
cell-derived macrophages were pretreated with normal ESCs, ectopic
ESCs, IDO1-overexpressing and IDO1-deficient ESCs for 48 h, and
subsequently co-cultured with normal ESCs (2×104 cells/well in a
96-well plate) for 36 h. (A) MTT assay and (B)
5-bromo-2-deoxyuridine ELISA assay were subsequently performed to
analyze the viability and proliferation of normal ESCs,
respectively. Results are presented as the mean ± standard
deviation of 12 different experiments (*P<0.05 and **P<0.01).
ESC, endometrial stromal cells; IDO1, indoleamine
2,3-dioxygenase-1; NS, not significant; normal ESC, ESCs from
patients without endometriosis; IDO1 overexpression, normal ESCs
transfected with pEGFP-N1-IDO1; IDO1 interference, normal ESCs
transfected with SD11-IDO1 short hairpin RNA; ectopic ESC: ESCs
from endometriosis-derived endometriotic tissue. |
Effect of IDO1-induced tolerant
macrophages on the apoptosis of ESCs
ESCs co-cultured with ectopic ESC or pEGFP-N1-IDO1
transfected ESC-pretreated macrophages exhibited a significantly
lower apoptosis rate compared with ESCs co-cultured with normal
ESC-pretreated macrophages (P<0.01; group e vs. group b and
group c vs. group b; Fig. 3), and
the apoptosis of ESCs increased when co-cultured with macrophages
that were pretreated with SD11-IDO1 shRNA-transfected ESCs
(P<0.01; group d vs. group b; Fig.
3).
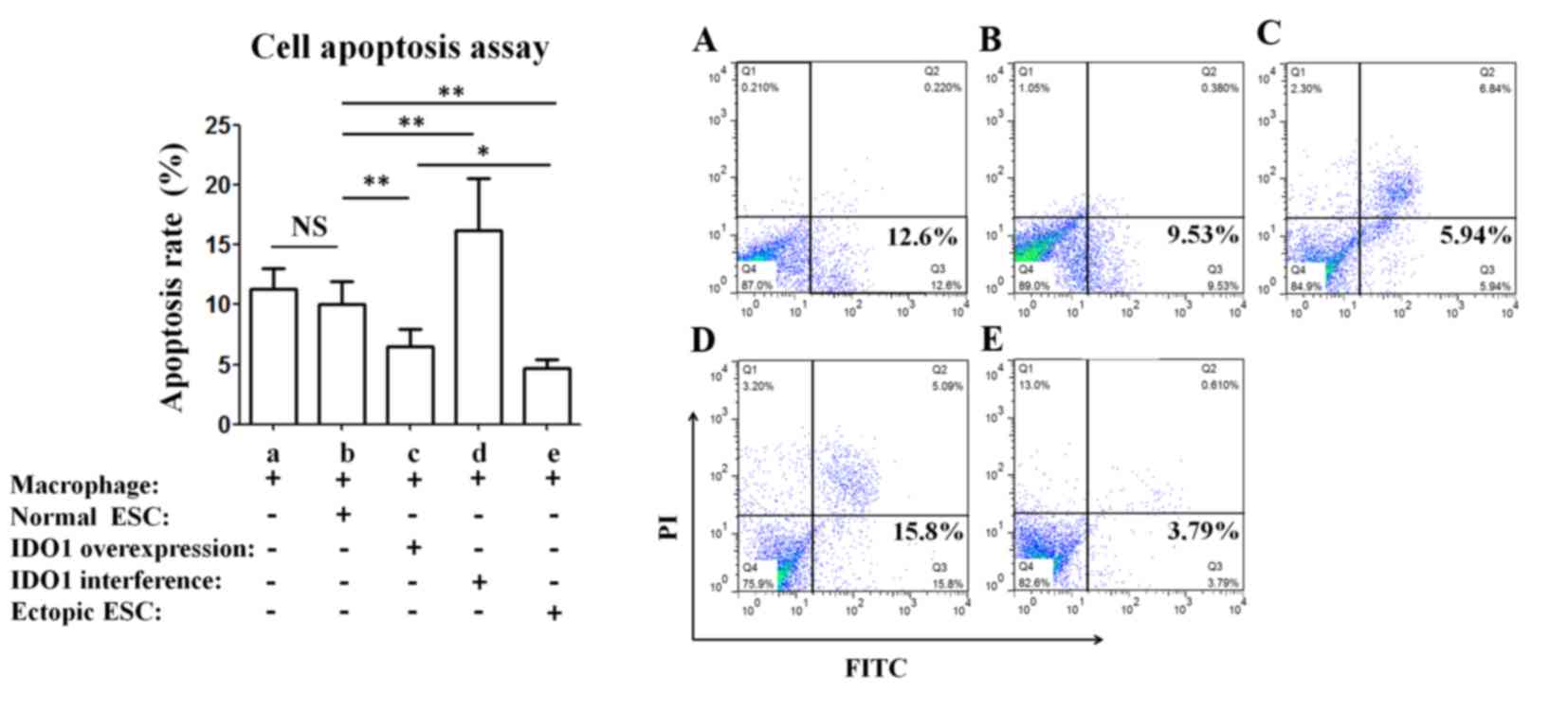 | Figure 3.IDO1-induced tolerant macrophages
inhibit the apoptosis of ESCs. Normal ESCs were co-cultured with
peripheral blood mononuclear cell-derived macrophages, which were
pretreated with normal ESCs, ectopic ESCs, normal ESCs transfected
with plasmid pEGFP-N1-IDO1 or SD11-IDO1 short hairpin RNA.
Subsequently, ESCs were collected and apoptosis was evaluated by
flow cytometry. Results are presented as the mean + standard
deviation of 12 different experiments (*P<0.05, **P<0.01).
ESC, endometrial stromal cells; IDO1, indoleamine
2,3-dioxygenase-1; NS, not significant; PI, propidium iodide;
normal ESC, ESCs from patients without endometriosis; IDO1
overexpression, normal ESCs transfected with pEGFP-N1-IDO1; IDO1
interference, normal ESCs transfected with SD11-IDO1 short hairpin
RNA; ectopic ESC, ESCs from endometriosis-derived endometriotic
tissue. |
Discussion
Endometriosis-associated inflammation is chronic and
long lasting (14). An increasing
number of studies have focused on the importance of immunological
imbalances in women with endometriosis. It has been confirmed that,
a permissive peritoneal environment may be associated with the
initiation and development of endometriosis (1–3,11).
Rather than effectively removing the retrograde endometrial
fragments in the pelvic cavity, the tolerant environment
facilitates the implantation, neo-angiogenesis and proliferation of
ectopic endometrial tissue (15,16).
Conditions in the tolerant environment may include elevated levels
of activated peritoneal macrophages, reduced natural killer cell
activity, an abnormal T lymphocyte response and an increased number
of regulatory T cells in endometriotic tissue and peritoneal fluid
(17–20).
Cells of the monocyte-macrophage lineage are
characterized by diversity and plasticity, which respond to
environmental stimuli by acquiring diverse phenotypes. In response
to external cues, M1 macrophage activation occurs with microbicidal
and tumoricidal features. Alternatively, the M2 pathway
predominantly participates in parasite containment, tissue
remodeling and immunomodulation (21). The activated peritoneal macrophages
have an important role in the onset and development of
endometriosis (22). Impaired
macrophages cannot effectively clear the ectopic endometrial cells
in endometriosis patients (23),
and inversely secrete various inflammatory mediators that may
contribute to the progression of endometriosis. However, the
understanding of the contribution of macrophages to the
endometriotic environment remains inadequate.
As it was previously demonstrated that the phenotype
of PBMCs did not differ in women with and without endometriosis
(24), PBMCs were obtained from
control women in the present study. The current study co-cultured
ESCs with macrophages to determine if IDO1 in ESCs had any effect
on the immune function of macrophages. Following incubation with
IDO1-overexpressing ESCs, macrophages exhibited a decreased
phagocytic ability. Wu et al (25) and Chuang et al (26) demonstrated that the master
regulator of the peritoneal microenvironment, prostaglandin E2
(PGE2), suppressed at least two aspects of the scavenger function
of macrophages, including decreased secretion and activation of
MMP-9, and reduced class B scavenger receptor (CD36) expression in
peritoneal macrophages. Furthermore, PGE2 also inhibited annexin A2
expression, which led to a reduced immunological response by
peritoneal macrophages (23). The
present study subsequently investigated how the tolerant
macrophages affected the growth of ESCs. The results demonstrated
that the viability and proliferation of ESCs were significantly
increased, and apoptosis index decreased, when co-cultured with
macrophages pretreated with IDO1-overexpressing ESCs compared with
macrophages treated with normal ESCs. The results indicate that
high levels of IDO1 expressed in the ectopic environment may induce
the formation of tolerant macrophages, which in turn may promote
ectopic ESC growth in the progression of endometriosis. There are
various solute cytokines in the peritoneal microenvironment that
are secreted by macrophages and may affect the survival of
endometrial tissue and the development of endometriosis. Shi et
al (27,28) demonstrated that estradiol and
2,3,7,8-tetrachlorodibenzo-p-dioxin coordinated to the excessive
growth of endometriotic cells in vitro by stimulating the
secretion of pro-inflammatory cytokines, IL-8 and chemokine (C-C
motif) receptor 8 (CCR8), by macrophages, which led to persistent
and severe inflammation. Furthermore, increased RANTES in eutopic
and ectopic ESCs recruited more macrophages into the environment,
and also induced tolerance in macrophages, which inhibited the
apoptosis and promoted the proliferation of ESCs in the
endometriotic environment (3).
Additionally, Khan et al (29) demonstrated that an inflammatory
reaction in the intrauterine environment stimulated the expression
of the stress reaction marker, human heat shock protein 70 (HSP70),
which further stimulated the production of IL-6 and tumor necrosis
factor α by macrophages, and promoted the proliferation of ESCs.
However, these effects of HSP70 were more prominent in cells
derived from women with endometriosis compared with normal ones. It
may be inferred that macrophages from control women were less
responsive to the inflammatory surrounding. This may be due to
variations in cytokine secretion and the receptor-ligand binding
affinity in macrophages of control women. The evidence discussed
indicates that ESC-educated macrophages may regulate the growth of
ESCs by secreting soluble cytokines. Further studies investigating
the mechanism of the cross-talk between endometrial tissues and
macrophages in the pelvis of women with endometriosis are required
to strengthen the results of the present study.
In conclusion, the progression of endometriosis may
be recognized as the product of evolving cross-talk between ectopic
ESCs and macrophages within the peritoneal cavity. Increased IDO1
protein expression in eutopic and ectopic endometria of women with
endometriosis is of biological importance. It may directly promote
the proliferation and invasion of endometrial tissue by regulating
the expression of COX-2 and MMPs. Additionally, it may also
modulate adjacent macrophages to generate a supportive
microenvironment. Dysfunctional macrophages exhibit impaired
phagocytic abilities, which may lead to decreased clearance of
endometriotic tissue outside the uterus. Furthermore, the altered
polarization of macrophages may promote endometrial tissue growth
and suppress its apoptosis by releasing a modified profile of
cytokines, including IL-10 and TGF-β1. Due to the potential role of
IDO1 in endometriosis and its application in clinical trials of
cancer therapy (30), further
investigation is required to identify potential IDO1-based
therapies for endometriosis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81601354), the
National Science Foundation of Jiangsu Province, China (grant no.
BK20160128), and the Fundamental Research Funds for the Central
Universities (grant no. 021414380180) (all to J. M.).
References
|
1
|
Burney RO and Giudice LC: Pathogenesis and
pathophysiology of endometriosis. Fertil Steril. 98:511–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pirdel L and Pirdel M: Role of iron
overload-induced macrophage apoptosis in the pathogenesis of
peritoneal endometriosis. Reproduction. 147:R199–R207. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y,
Wang L and Li DJ: The high level of RANTES in the ectopic milieu
recruits macrophages and induces their tolerance in progression of
endometriosis. J Mol Endocrinol. 45:291–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ravishankar B, Liu H, Shinde R, Chaudhary
K, Xiao W, Bradley J, Koritzinsky M, Madaio MP and McGaha TL: The
amino acid sensor GCN2 inhibits inflammatory responses to apoptotic
cells promoting tolerance and suppressing systemic autoimmunity.
Proc Natl Acad Sci USA. 112:10774–10779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noh KT, Son KH, Jung ID, Kang TH, Choi CH
and Park YM: Glycogen synthase kinase-3β (GSK-3β) inhibition
enhances dendritic cell-based cancer vaccine potency via
suppression of interferon-γ-induced Indoleamine 2,3-dioxygenase
expression. J Biol Chem. 290:12394–12402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sucher R, Fischler K, Oberhuber R,
Kronberger I, Margreiter C, Ollinger R, Schneeberger S, Fuchs D,
Werner ER, Watschinger K, et al: IDO and regulatory T cell support
are critical for cytotoxic T lymphocyte-associated Ag-4 Ig-mediated
long-term solid organ allograft survival. J Immunol. 188:37–46.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mei J, Jin LP, Ding D, Li MQ, Li DJ and
Zhu XY: Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix
metalloproteinase-9 expression and decreases proliferation,
adhesion and invasion of endometrial stromal cells. Mol Hum Reprod.
18:467–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mei J, Li MQ, Ding D, Li DJ, Jin LP, Hu WG
and Zhu XY: Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival
and invasiveness of endometrial stromal cells via the activation of
JNK signaling pathway. Int J Clin Exp Pathol. 6:431–444.
2013.PubMed/NCBI
|
|
9
|
Tang MX, Hu XH, Liu ZZ, Kwak-Kim J and
Liao AH: What are the roles of macrophages and monocytes in human
pregnancy? J Reprod Immunol. 112:73–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee CL, Guo Y, So KH, Vijayan M, Guo Y,
Wong VH, Yao Y, Lee KF, Chiu PC and Yeung WS: Soluble human
leukocyte antigen G5 polarizes differentiation of macrophages
toward a decidual macrophage-like phenotype. Hum Reprod.
30:2263–2274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mei J, Xie XX, Li MQ, Wei CY, Jin LP, Li
DJ and Zhu XY: Indoleamine 2,3-dioxygenase-1 (IDO1) in human
endometrial stromal cells induces macrophage tolerance through
interleukin-33 in the progress of endometriosis. Int J Clin Exp
Pathol. 7:2743–2757. 2014.PubMed/NCBI
|
|
12
|
Revised American Fertility Society
classification of endometriosis: 1985. Fertil Steril. 43:351–352.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin
LP and Li DJ: CXCL8 enhances proliferation and growth and reduces
apoptosis in endometrial stromal cells in an autocrine manner via a
CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 27:2107–2116.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
González-Ramos R, Defrère S and Devoto L:
Nuclear factor-kappaB: A main regulator of inflammation and cell
survival in endometriosis pathophysiology. Fertil Steril.
98:520–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matarese G, De Placido G, Nikas Y and
Alviggi C: Pathogenesis of endometriosis: Natural immunity
dysfunction or auto-immune disease? Trends Mol Med. 9:223–228.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barrier BF: Immunology of endometriosis.
Clin Obstet Gynecol. 53:397–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Budiu RA, Diaconu I, Chrissluis R, Dricu
A, Edwards RP and Vlad AM: A conditional mouse model for human
MUC1-positive endometriosis shows the presence of anti-MUC1
antibodies and Foxp3+regulatory T cells. Dis Model Mech. 2:593–603.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berbic M, Hey-Cunningham AJ, Ng C,
Tokushige N, Ganewatta S, Markham R, Russell P and Fraser IS: The
role of Foxp3+ regulatory T-cells in endometriosis: A potential
controlling mechanism for a complex, chronic immunological
condition. Hum Reprod. 25:900–907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olkowska-Truchanowicz J, Bocian K, Maksym
RB, Białoszewska A, Włodarczyk D, Baranowski W, Ząbek J,
Korczak-Kowalska G and Malejczyk J: CD4+ CD25+ FOXP3+ regulatory T
cells in peripheral blood and peritoneal fluid of patients with
endometriosis. Hum Reprod. 28:119–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li MQ, Wang Y, Chang KK, Meng YH, Liu LB,
Mei J, Wang Y, Wang XQ, Jin LP and Li DJ: CD4+Foxp3+ regulatory T
cell differentiation mediated by endometrial stromal cell-derived
TECK promotes the growth and invasion of endometriotic lesion. Cell
Death Dis. 5:e14362014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khoufache K, Bazin S, Girard K,
Guillemette J, Roy MC, Verreault JP, Al-Abed Y, Foster W and Akoum
A: Macrophage migration inhibitory factor antagonist blocks the
development of endometriosis in vivo. PLoS One. 7:e372642012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu MH, Chuang PC, Lin YJ and Tsai SJ:
Suppression of annexin A2 by prostaglandin E2 impairs phagocytic
ability of peritoneal macrophages in women with endometriosis. Hum
Reprod. 28:1045–1053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamamoto Y, Maeda N, Izumiya C, Kusume T,
Oguri H, Kawashima M, Hayashi K, Nomura A, Yamashita C and Fukaya
T: Decreased human leukocyte antigen-DR expression in the lipid
raft by peritoneal macrophages from women with endometriosis.
Fertil Steril. 89:52–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu MH, Shoji Y, Wu MC, Chuang PC, Lin CC,
Huang MF and Tsai SJ: Suppression of matrix metalloproteinase-9 by
prostaglandin E(2) in peritoneal macrophage is associated with
severity of endometriosis. Am J Pathol. 167:1061–1069. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chuang PC, Lin YJ, Wu MH, Wing LY, Shoji Y
and Tsai SJ: Inhibition of CD36-dependent phagocytosis by
prostaglandin E2 contributes to the development of endometriosis.
Am J Pathol. 176:850–860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi YL, Luo XZ, Zhu XY, Hua KQ, Zhu Y and
Li DJ: Effects of combined 17beta-estradiol with TCDD on secretion
of chemokine IL-8 and expression of its receptor CXCR1 in
endometriotic focus associated cells in co-culture. Hum Reprod.
21:870–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi YL, Luo XZ, Zhu XY and Li DJ:
Combination of 17beta-estradiol with the environmental pollutant
TCDD is involved in pathogenesis of endometriosis via up-regulating
the chemokine I-309-CCR8. Fertil Steril. 88:317–325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan KN, Kitajima M, Inoue T, Tateishi S,
Fujishita A, Nakashima M and Masuzaki H: Additive effects of
inflammation and stress reaction on Toll-like receptor 4-mediated
growth of endometriotic stromal cells. Hum Reprod. 28:2794–2803.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soliman H, Mediavilla-Varela M and Antonia
S: Indoleamine 2,3-dioxygenase: Is it an immune suppressor? Cancer
J. 16:354–359. 2010. View Article : Google Scholar : PubMed/NCBI
|

















