Introduction
The causes of spinal cord injury, in response to
hypoxic-ischemic insults, are multifactorial, and affect the
central nervous system (CNS) (1,2). In
the developing CNS, lack of oxygen results in an initial depletion
of high-energy phosphates, in particular adenosine triphosphate and
phosphocreatine (3,4). These levels transiently return to
baseline, followed by a second, more prolonged depletion of
cellular energy reserves accompanied by the progression of spinal
cord and brain injury (2).
Previous studies have revealed that mitochondria determine cell
fate in neuronal cells (4–6). They may induce cell death via the
release of pro-apoptotic proteins, which occurs following
mitochondrial permeabilization (MP) (7). MP may occur through selective opening
of the outer mitochondrial membrane, mitochondrial outer membrane
permeabilization (MOMP), or through opening of the mitochondrial
permeability transition pore, which permeabilizes the outer and
inner mitochondrial membranes (7,8).
MOMP appears to predominantly induce apoptosis, whereas
mitochondrial permeability transition pore opening results in
mitochondrial swelling and may lead to necrotic cell death
(7,8).
Protein folding in the endoplasmic reticulum (ER) is
impaired under various physical and pathological conditions, termed
endoplasmic reticulum stress (ERS) (9). ERS, induced by the activation of the
unfolded protein response (UPR), is characterized by the
upregulation of molecular chaperone glucose-related protein 78
(GRP78) and the activation of apoptosis (9,10).
GRP78 associates with various critical transmembrane ER signaling
proteins (9,11). The UPR induces signals via three
distinct stress sensors located at the ER membrane: Protein kinase
RNA-like ER kinase (PERK), inositol-requiring protein-1 (IRE-1) and
activating transcription factor-6 (ATF-6) (11). Of these, PERK, whose intrinsic
kinase activity is induced by oligomerization, regulates the
phosphorylation of the eukaryotic translation initiation factor 2α,
which induces the suppression of global mRNA translation to protect
cells against ERS (12). Various
ERS-associated signaling pathways have been proposed to be involved
with this programmed cell death, including the activation of
CCAAT/enhancer-binding protein homologous protein (CHOP) and
caspase-12 (10,13). These studies suggested that the
induction of ERS was closely associated with apoptosis. However,
whether ERS is involved in hypoxia-induced apoptosis in rat dorsal
root ganglion (DRG) neurons remains to be elucidated.
Recent studies have focused on the activity of
non-nutritional dietary compounds that have protective or disease
preventive properties (14–16).
Myristicin (Myr; 1-allyl-5-methoxy-3,4-methylenedioxybenzene) is an
active aromatic compound present in nutmeg (the seed of
Myristica fragrans), carrot, basil, cinnamon and parsley
(15). Myr has been revealed to
have antibacterial (16),
hepatoprotective (17),
anti-inflammatory (18) and
anticancer (15) effects.
Additionally, Myr has exhibited significant effects on the CNS. Myr
may induce neurotoxicity in SK-N-SH human neuroblastoma cells
(19). However, whether Myr
induces this effect against hypoxia-induced apoptosis in rat DRG
neurons remains to be elucidated.
The present study observed that Myr enhanced cell
viability significantly in hypoxia-induced rat DRG neurons in a
dose-dependent manner. In addition, Myr reduced the terminal
deoxynucleotidyl transferase-mediated dUTP nick end-labeling
(TUNEL)-positive DRG neurons and influenced the expression of
apoptotic genes in the hypoxia-induced group. Furthermore, Myr
exhibited a protective effect against hypoxic injury in DRG
neurons, the underlying mechanism of which may be associated with
the inhibition of the ERS pathway.
Materials and methods
Cell culture
Myr (CAS no. 607 91 0) was obtained from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). The cell
culture materials and reagents were obtained from Invitrogen;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). DRG neuron
culture was based on previous studies, including our own (20–22).
Sprague-Dawley (SD) rats (n=24; weight, 25–30 g; male) were
purchased from the Animal Laboratory of Wannan Medical College
(Wuhu, China). Rats were kept on a 12 h/12 h light-dark cycle and
freely given food and water in a pathogen-free area. All procedures
that involved animals were approved by the Institutional Animal
Care and Use Committee of Yijishan Hospital (Wuhu, China). DRG
neurons were harvested from rats at day 15. The DRG neurons were
digested with 0.25% trypsin in Hank's Balanced Salt solution
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 20 min,
and added to 10% fetal bovine serum to prevent digestion. Cells
were passed through a 74-µm filter and centrifuged in 1,500 × g at
4°C for 5 min. The pellet was resuspended in a neurobasal medium of
2% B-27 supplement, 10 ng/ml nerve growth factor, 1 mmol/l
L-glutamine and 1000 U/ml penicillin/streptomycin/neomycin
solution. Dissociated DRG neurons were cultured in
poly-D-lysine-precoated 6-well culture clusters at 2×106
cells/well in 2 ml. DRG neurons were cultured in media at 37°C with
5% CO2 and maintained in media containing 20 µmol/l
floxuridine for another 24 h to inhibit the growth of non-neuronal
cells. The purity of neuronal cells was confirmed by fluorescent
labeling of neurofilament protein 200 (cat. no. orb18247; dilution,
1:600; Biorbyt Ltd., Cambridge, UK) and NeuN (cat no. 104225;
dilution, 1:500, Abcam, Cambridge, UK) (data not shown). All
experimental procedures were performed in accordance with the
National Institute of Health Guidelines for the Care and Use of
Laboratory Animals and were approved by the Animal Experimentation
Committee of Wannan Medical College (Wuhu, China).
Analysis of cell viability
Cell viability was measured via a quantitative
colorimetric assay with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich; Merck Millipore). Briefly, DRG neurons were seeded
into 96-well plates at 5×104 cells per well. A portion
of cells were treated with 1% O2 (hypoxia) for 24 h, and
incubated with 5 mg/ml MTT solution for a further 4 h. A total of
100 µl dimethyl sulfoxide was added to each well and the absorbance
was measured at a wavelength of 540 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Cell viability was
expressed as the ratio of clustered DRG neurons to control group
The control group were cultured in neurobasal medium with 2% B-27
supplement, 10 ng/ml nerve growth factor, 1 mmol/l L-glutamine and
1,000 U/ml penicillin/streptomycin/neomycin solution, which were
all purchased from were purchased from Sigma Aldrich; Merck
Millipore.
TUNEL assay
Dulbecco's Modified Eagle's medium/F12, FBS and
neurobasal cell medium were purchased from Gibco; Thermo Fisher
Scientific, Inc. The Apoptosis Detection System kit (Roche
Diagnostics GmbH, Mannheim, Germany) was used for the TUNEL assay,
according to the manufacturer's protocol. DRG neurons were seeded
into 96-well plates at a density of 1×105 cells/well.
After fixing with 4% paraformaldehyde for 1 h, DRG neurons in each
groups were washed with PBS and treated with 1% Triton X-100 in PBS
for 30 min on ice. And then incubated for 60 min at 37°C with 50 µl
of TUNEL reaction mixture. Cells were subsequently incubated with
DAPI (dilution, 1:800; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) for 2 h at room temperature. After washing with PBS, the cells
were analyzed by confocal microscopy (magnification, ×40; Zeiss
510; Zeiss AG, Oberkochen, Germany). DRG neurons untreated with
myristicin and/or hypoxia were used as a control group. To avoid
counting the same cell in more than one region, the authors counted
every fifth section (50 µm apart). For each plate, six fields of
view were examined.
Western blotting
DRG neurons were washed twice with cold PBS and
lysed in radioimmunoprecipitation assay buffer consisting of 50 mM
TRIS, 150 mM NaCl, 2% sodium dodecyl sulfate (SDS) and a protease
inhibitor mixture (Roche Diagnostics GmbH). Equal amounts of
protein (2.0 mg/ml, 10 µl in each lane) were separated by 10%
SDS-PAGE and electrophoretically transferred to polyvinylidene
difluoride membranes. Membranes were blocked with 5% nonfat milk
and incubated with primary antibodies at 4°C overnight. The
following primary antibodies were used: B cell lymphoma 2 (cat. no.
SAB4300339; dilution, 1:500; anti-rabbit; Sigma-Aldrich; Merck
Millipore), Bcl-2 associated X protein (cat. no. SAB4502549;
dilution, 1:800; anti-rabbit; Sigma-Aldrich; Merck Millipore),
cleaved caspase-3 (cat. no. sc-22171; dilution, 1:500; anti-rabbit;
Santa Cruz Biotechnology, Inc.), CHOP (cat. no. 2895P; dilution,
1:500; anti-rabbit; Cell Signaling Technology, Inc.), GRP78 (cat.
no. G9043; dilution, 1:800; anti-rabbit; Sigma-Aldrich; Merck
Millipore), cleaved caspase-12 (cat. no. sc-70227; dilution, 1:500;
anti-rabbit; Santa Cruz Biotechnology, Inc.) and β-actin (cat. no.
sc-47778; dilution, 1:1,000; anti-rabbit; Santa Cruz Biotechnology,
Inc.). Membranes were subsequently incubated with a horseradish
peroxidase-conjugated mouse anti-rabbit secondary antibody (cat.
no. sc-2357; dilution, 1:5,000, Santa Cruz Biotechnology, Inc.) for
2 h at room temperature. Following each incubation, membranes were
extensively washed in TBS containing Tween-20 and the
immunoreactive bands were detected using an enhanced
chemiluminescence kit (cat. no. orb90503; Biorbyt, Ltd.). The
quantification of Western blotting was conducted using a
computerized image-analysis system (Image Pro Plus; version, 6.0;
Media Cybernetics, Inc., Rockville, MD, USA) in duplicate.
Detection of lactate dehydrogenase
(LDH), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX)
and malondialdehyde (MDA)
The DRG neurons were randomly divided into four
groups: Control group, control plus Myr group, hypoxia group, and
hypoxia plus Myr group. DRG neurons were seeded at 5×104
per well in 6-well plates, cells were harvested and washed twice
with cold PBS, before being lysed in radioimmunoprecipitation assay
buffer consisting of 50 mM TRIS, 150 mM NaCl, 2% SDS and a protease
inhibitor mixture (Roche Diagnostics GmbH). DRG neurons were
quantified using the bicinchoninic acid kit for protein
determination (cat. no. BCA1-1KT; Sigma-Aldrich; Merck Millipore).
SOD (cat. no. 20080829), GSH-PX (cat. no. 20080801) and MDA (cat.
no. 20080801) levels in the supernatant were measured using
commercially available kits purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). All assays were
conducted according to the manufacturer's protocol.
LDH release assay
LDH activity was evaluated using a colorimetric LDH
assay. DRG neurons were seeded onto a 96-well plate under a 1%
O2 environment for 24 h. Briefly, 100 µl supernatant was
transferred from each well to a new 96-well plate and 100 µl fresh
reaction mixture was added to each well. After 30 min of incubation
at room temperature in the dark, the optical density values were
detected at a wavelength of 490 nm using a microplate reader
(Thermo Labsystems, Santa Rosa, CA, USA). The quantity of LDH was
calculated as a percentage compared with the total amount of LDH
present in cells treated with 1% Triton-X 100 (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). LDH levels
were measured using a commercially available kit (cat. no.
20071129) purchased from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol®
solution (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The quantity and quality of isolated
RNA were evaluated using absorbance at wavelengths of 260 and 280
nm. Following this, RT was performed in a 20 µl reaction mixture
using the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). Primer sequences were as follows: Forward,
5′-GGACCTGACCTGCCGTCTAG-3′ and reverse, 5′-GTAGCCCAGGATGCCCTTGA-3′
for β-actin; forward, 5′-GGUAUGAGGACCUGCAAGA-3′ and reverse,
5′-CACCAAGCAUGAACAAUUG-3′ for CHOP; forward
5′-CUACCCAAACAUCGGGAAA-3′ and reverse, 5′-CUCCAGAGAUGCUGAGCGA-3′
for GRP78. qPCR with SYBR® Green I (Shanghai Hi-Tech
Enterprise Bio Co., Shanghai, China) (http://china.53trade.com/web0/disp_company_info.asp?userid=166543)
was performed using a 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with thermocycling
conditions as follows: An initial denaturation step at 95°C for 5
min, followed by 40 cycles of denaturation at 95°C for 15 sec,
annealing at 60°C for 60 sec and extension at 72°C for 30 sec. All
samples were processed in triplicate. The mRNA expression levels of
each gene were calculated using the relative quantitative method
(23).
Immunofluorescence
DRG neurons were fixed with 4% PFA for 1 h, washed
with PBS containing 0.1% Triton X-100 (PBST), and blocked for 30
min in PBST supplemented with 10% FBS. DRG neurons were
subsequently incubated with cleaved antibodies against caspase-12
(cat. no. sc-70227; dilution, 1:800; anti-rabbit; Santa Cruz
Biotechnology, Inc.) in the same solution overnight at 4°C, washed
and incubated with a mouse anti-rabbit IgG-HRP conjugated secondary
antibody (cat. no. sc-2357; dilution, 1:5,000, Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature. Nuclei were
stained with DAPI (1:800; Santa Cruz Biotechnology, Inc.). The
cells were examined under a Leica fluorescence microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent experiments. All
statistical analyses were performed using SPSS software version
11.0 (SPSS, Inc., Chicago, IL, USA). Statistical comparisons
between the different treatments were performed using one-way
analysis of variance with Tukey's multiple comparison post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Myr enhances the cell viability of
hypoxia-induced DRG neurons
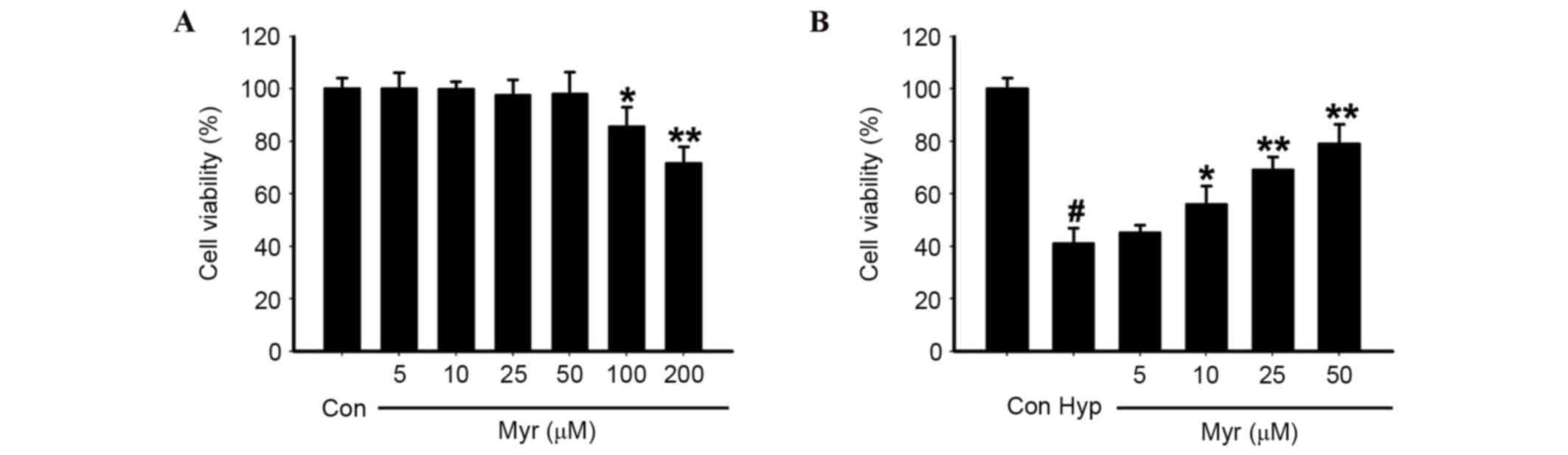
DRG neurons were exposed to 10–200 µM Myr, the
chemical structure of which is presented in Fig. 1, for 24 h. Concentrations of 10–50
µM did not affect cell viability; however, viability was reduced
with concentrations >50 µM (Fig.
2A). Therefore, 10–50 µM Myr was selected to treat the 1%
O2 (hypoxia)-exposed DRG neurons for 24 h. Myr inhibited
the hypoxia-induced decreased cell viability of DRG neurons in a
dose-dependent manner (Fig. 2B).
The results confirmed that the optimal concentration of Myr was 50
µM.
Myr reverses the apoptosis of
hypoxia-induced DRG neurons
TUNEL staining was used to assess apoptotic cells.
As presented in Fig. 3A, increased
TUNEL staining was observed in the hypoxia-induced DRG neurons
compared with the normal group. The number of TUNEL-positive cells
was reduced in DRG neurons following Myr treatment; however, Myr
treatment had no effect on the normal group. Quantification of
TUNEL staining revealed that Myr significantly reduced the
percentage of TUNEL-positive DRG neurons following hypoxia
(Fig. 3B).
To determine the possible involvement of the
mitochondrial pathway of apoptosis in hypoxia-induced DRG neurons,
protein expression levels of the pro-apoptotic Bax, the apoptosis
protease cleaved caspase-3 and the anti-apoptotic Bcl-2 were
detected by western blot analysis (Fig
4A). Increased protein expression levels of Bax and cleaved
caspase-3, and reduced protein expression levels of Bcl-2, were
detected in the hypoxia-induced group compared with the normal
group; these effects were significantly abrogated by Myr treatment
(Fig. 4B-D). Therefore, Myr may
reverse hypoxia-induced apoptosis in DRG neurons by affecting the
protein expression levels of apoptosis-associated molecules.
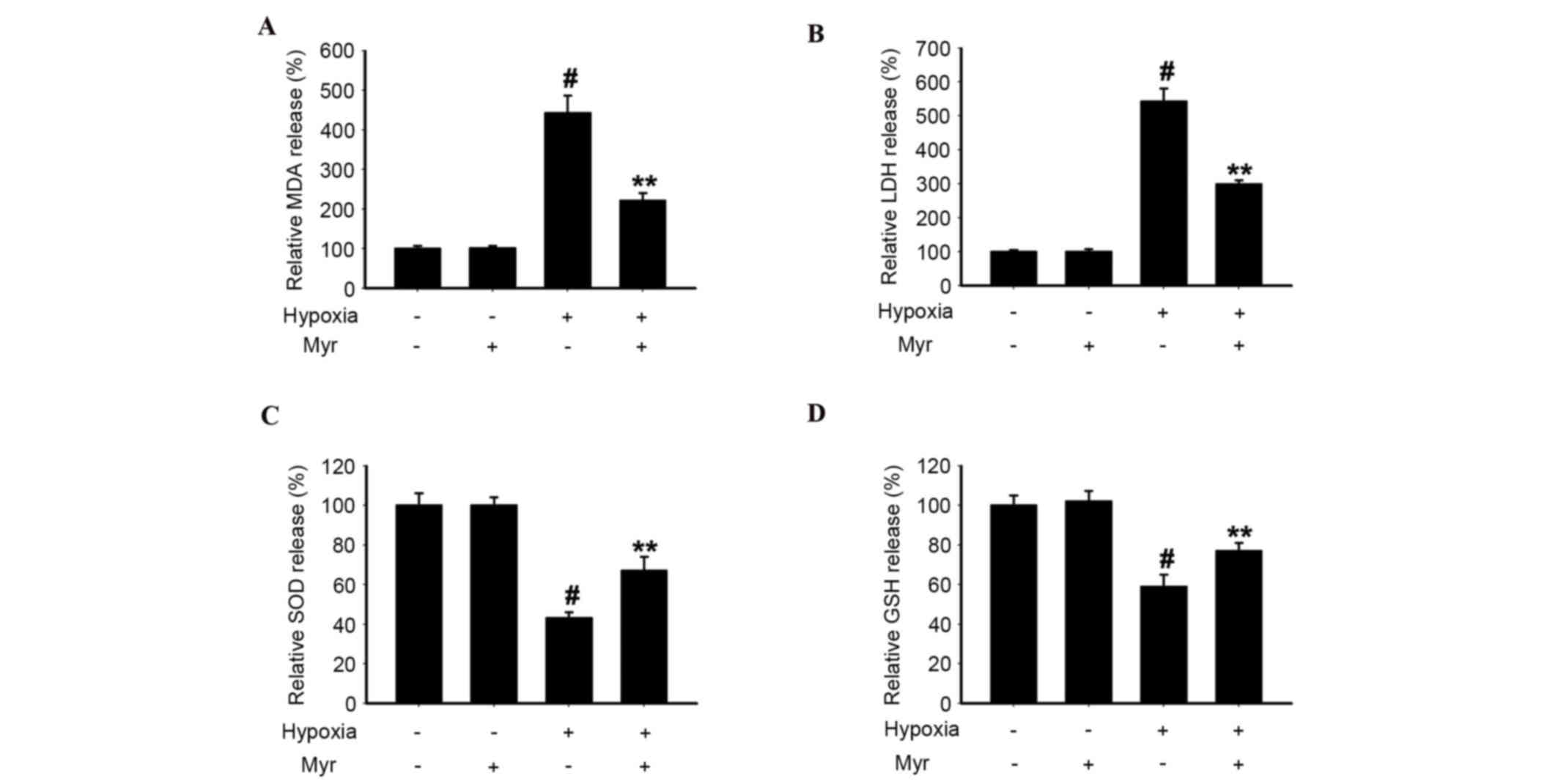
Myr decreases MDA content and LDH
release, and upregulates SOD and GSH-PX activities, in
hypoxia-induced DRG neurons
To investigate the effect of hypoxic injury on DRG
neurons, the present study analyzed MDA content, LDH release and
SOD and GSH-PX activities in the four groups. The MDA content
(Fig. 5A) and LDH release
(Fig. 5B) in hypoxia-induced DRG
neurons was greater compared with the normal group, whereas SOD
(Fig. 5C) and GSH-PX (Fig. 5D) activities were reduced.
Following treatment with Myr, these hypoxia-induced effects were
significantly attenuated. Therefore, Myr exhibited a protective
effect against hypoxic injury in DRG neurons.
Myr reduces the expression levels of
CHOP, GRP78 and cleaved caspase-12 in hypoxia-induced DRG
neurons
The levels of CHOP and GRP78 were analyzed by
western blot analysis (Fig. 6A) to
determine whether ERS was involved in apoptosis of hypoxia-induced
DRG neurons. CHOP (Fig. 6B) and
GRP78 (Fig. 6C) protein expression
levels were significantly increased in DRG neurons treated with
hypoxia, and this increase was significantly inhibited by Myr.
Similarly, CHOP (Fig. 6D) and
GRP78 (Fig. 6E) mRNA expression
levels in the hypoxia group were significantly increased compared
with the normal group, as assessed by RT-qPCR; however, this was
significantly downregulated by Myr. The protein expression levels
of cleaved caspase-12 were analyzed as it is a specific marker of
ERS-induced apoptosis (Fig. 7A).
Western blot analysis indicated that hypoxia significantly
increased its protein expression levels; however, this was
significantly inhibited by treatment with Myr (Fig. 7B). Immunofluorescence verified this
(Fig. 7C). These results suggested
that Myr may inhibit hypoxia-ERS-induced apoptosis in DRG
neurons.
Discussion
The biochemical cascade initiated by conditions of
hypoxia is complex. Depletion of cellular energy reserves is a
major problem (24) and the
mitochondrial response to hypoxia is critical in determining
neuronal cell fate (25). Severe
tissue oxygen depletion may result in total mitochondrial failure
and necrotic cell death, whereas less severe oxygen deprivation may
trigger activation of the apoptotic pathway (26). Kerr et al (27) first described apoptosis as a
programmed cell death defined by the nuclear and cell morphological
alterations due to activation of signaling cascades. The results of
the present study demonstrated the involvement of ERS in the
apoptosis of hypoxia-induced DRG neurons.
Activation of the UPR protects cells under ERS via
upregulation of GRP78 (28).
Physiological processes that demand a high rate of protein
synthesis and secretion must sustain activation of the adaptive
programs of the UPR without triggering cell death pathways
(29). However, prolonged
activation of the UPR by excessive ERS may convert its role to a
cytotoxic one, by activation of multiple apoptotic pathways in
mammalian cells (30). The
underlying mechanisms initiating apoptosis under conditions of
irreversible ER damage are now partially understood and may involve
a series of complementary pathways (29). The ERS transducer proteins ATF-6,
IRE-1α and PERK constitute the core stress regulators of the UPR,
and transduce signals from the ER to the cytoplasm and nucleus
following ERS (29). A previous
study reported that the induction of ERS was closely associated
with apoptosis (30). Chronic ER
stress leads to Bax-dependent apoptosis through the transcriptional
upregulation of Bcl-2 homology 3-only proteins, including
Bcl-2-interacting mediator of cell death (BIM) and p53 upregulated
modulator of apoptosis, which are upstream Bcl-2 family members
(31). The transcription of one of
the key UPR pro-apoptotic genes, CHOP, is positively controlled by
the PERK-ATF4 axis (31). CHOP
promotes the transcription of BIM and the downregulation of Bcl-2
expression, contributing to the induction of apoptosis (31). The present study indicated that
CHOP and GRP78 were expressed at high levels in hypoxia-induced DRG
neurons, which increased the protein expression levels of Bax and
decreased that of Bcl-2. Caspases are a superfamily of cysteine
aspartyl-specific proteases that regulate numerous aspects of cell
survival and death (32).
Caspase-3 is a validated marker in detecting early neuronal
apoptosis (32). Its activation is
mediated via extrinsic (death ligand) and intrinsic (mitochondrial)
pathways (11,32). The zymogen caspase-3 has almost no
activity until it is cleaved by an initiator caspase following
apoptotic signaling events (11).
The present study revealed that cleaved caspase-3 was expressed at
high levels in hypoxia-induced DRG neurons. Caspase-12, which is
localized on the ER membrane, has been demonstrated to be
specifically activated by ER stress (11,13).
In the current study, the level of caspase-12 was significantly
increased in hypoxia-induced DRG neurons, suggesting the
involvement of caspase-12 in ERS-triggered cell apoptosis.
LDH is an enzyme that is present in the cell
cytoplasm and is important in energy metabolism. An increase in LDH
activity suggests cellular damage (33). Enhanced lipid peroxidation is a
hallmark of free radical-induced tissue damage. The β-oxidation of
lipid peroxides in cells leads to double bond rearrangement of
unsaturated fatty acids of the membrane, which results in
destruction of the lipid membrane culminating in tissue damage
(33). This activity is enhanced
due to stress exerted by free radicals (34). MDA is involved in the
downregulation of lipid peroxidation. SOD reduces O2- to
H2O, thus scavenging noxious free radicals (35). GSH is an indispensable member of
the antioxidant free radical scavenger family, which converts
H2O2 to H2O (10). GSH shields cells and tissues
against free radical generation during stressful conditions arising
following the administration of acetic acid (10). GSH is important in electrophile
detoxification, transport of amino acids and synthesis of DNA
(10). The results of the present
study demonstrated that hypoxia treatment significantly increased
MDA content and the release of LDH, but downregulated SOD and
GSH-PX activities in DRG neurons.
A variety of drugs targeting cell death pathways
have been assessed in animal models of spinal cord injury. The
amplitude of neuroprotection observed in these studies has been
variable, and often the results are inconsistent between models and
research groups. However, various compounds, including
erythropoietin, N-acetyl-cysteine, caspase-2 inhibitors, p53
inhibitors, melatonin and c-Jun N-terminal kinase inhibitors, have
demonstrated promising neuroprotective properties. Myr, as the
primary active constituent of nutmeg, mace and parsley leaf oil,
has not been investigated in in vivo animal models of spinal
cord injury or DRG neuron cell models. In conclusion, the present
study, to the best of our knowledge, first demonstrated that Myr
may protect against hypoxia-induced apoptosis in rat DRG neurons
via inhibition of the ERS pathway. However, limitations of the
present study include the fact that it remains unknown whether Myr
is able to cross the blood-brain barrier. In addition, the safety
of Myr needs to be carefully tested in long-term follow-up studies.
Furthermore, the majority of drugs examined are non-specific and
have multiple effects in addition to anti-apoptotic/antinecrotic
effects. Further in vivo studies are required to test the
effects of Myr in animal models and in humans.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272048) and the
Natural Science Foundation of Anhui Province (grant no.
1308085MHl52).
References
|
1
|
Astorino TA, Harness ET and White AC:
Efficacy of acute intermittent hypoxia on physical function and
health status in humans with spinal cord injury: A brief review.
Neural Plast. 2015:4096252015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Navarrete-Opazo A, Vinit S, Dougherty BJ
and Mitchell GS: Daily acute intermittent hypoxia elicits
functional recovery of diaphragm and inspiratory intercostal muscle
activity after acute cervical spinal injury. Exp Neurol. 266:1–10.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kesherwani V, Atif F, Yousuf S and Agrawal
SK: Resveratrol protects spinal cord dorsal column from hypoxic
injury by activating Nrf-2. Neuroscience. 241:80–88. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poltl D, Schildknecht S, Karreman C and
Leist M: Uncoupling of ATP-depletion and cell death in human
dopaminergic neurons. Neurotoxicology. 33:769–779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xavier JM, Morgado AL, Sola S and
Rodrigues CM: Mitochondrial translocation of p53 modulates neuronal
fate by preventing differentiation-induced mitochondrial stress.
Antioxid Redox Signal. 21:1009–1024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown JE, Zeiger SL, Hettinger JC, Brooks
JD, Holt B, Morrow JD, Musiek ES, Milne G and McLaughlin B:
Essential role of the redox-sensitive kinase p66shc in determining
energetic and oxidative status and cell fate in neuronal
preconditioning. J Neurosci. 30:5242–5252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo C, Li Q, Gao Y, Shen X, Ma L, Wu Q,
Wang Z, Zhang M, Zhao Z, Chen X and Tao L: Poloxamer 188 attenuates
cerebral hypoxia/ischemia injury in parallel with preventing
mitochondrial membrane permeabilization and autophagic activation.
J Mol Neurosci. 56:988–998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y, Kazumura K, Maruyama W, Osawa T and
Naoi M: Rasagiline and selegiline suppress calcium efflux from
mitochondria by PK11195-induced opening of mitochondrial
permeability transition pore: A novel anti-apoptotic function for
neuroprotection. J Neural Transm (Vienna). 122:1399–1407. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu X, Zelmer A, Kapfhammer JP and
Wellmann S: Cold-inducible RBM3 inhibits PERK phosphorylation
through cooperation with NF90 to protect cells from endoplasmic
reticulum stress. FASEB J. 30:624–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goswami P, Gupta S, Biswas J, Sharma S and
Singh S: Endoplasmic reticulum stress instigates the rotenone
induced oxidative apoptotic neuronal death: A study in rat brain.
Mol Neurobiol. 53:5384–5400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012.PubMed/NCBI
|
|
12
|
Bernales S, Soto MM and McCullagh E:
Unfolded protein stress in the endoplasmic reticulum and
mitochondria: A role in neurodegeneration. Front Aging Neurosci.
4:52012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu B, Hu S, Liu L, Chen M, Wang L, Zeng X
and Zhu S: CART attenuates endoplasmic reticulum stress response
induced by cerebral ischemia and reperfusion through upregulating
BDNF synthesis and secretion. Biochem Biophys Res Commun.
436:655–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farzaei MH, Abbasabadi Z, Ardekani MR,
Rahimi R and Farzaei F: Parsley: A review of ethnopharmacology,
phytochemistry and biological activities. J Tradit Chin Med.
33:815–826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martins C, Doran C, Silva IC, Miranda C,
Rueff J and Rodrigues AS: Myristicin from nutmeg induces apoptosis
via the mitochondrial pathway and down regulates genes of the DNA
damage response pathways in human leukaemia K562 cells. Chem Biol
Interac. 218:1–9. 2014. View Article : Google Scholar
|
|
16
|
Jabrane A, Ben Jannet H, Mastouri M,
Mighri Z and Casanova J: Chemical composition and in vitro
evaluation of antioxidant and antibacterial activities of the root
oil of Ridolfia segetum (L.) Moris from Tunisia. Nat Prod Res.
24:491–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morita T, Jinno K, Kawagishi H, Arimoto Y,
Suganuma H, Inakuma T and Sugiyama K: Hepatoprotective effect of
myristicin from nutmeg (Myristica fragrans) on
lipopolysaccharide/d-galactosamine-induced liver injury. J Agric
Food Chem. 51:1560–1565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JY and Park W: Anti-inflammatory
effect of myristicin on RAW 264.7 macrophages stimulated with
polyinosinic-polycytidylic acid. Molecules. 16:7132–7142. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee BK, Kim JH, Jung JW, Choi JW, Han ES,
Lee SH, Ko KH and Ryu JH: Myristicin-induced neurotoxicity in human
neuroblastoma SK-N-SH cells. Toxicol Lett. 157:49–56. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen F, Wu R, Zhu Z, Yin W, Xiong M, Sun
J, Ni M, Cai G and Zhang X: Wogonin protects rat dorsal root
ganglion neurons against tunicamycin-induced ER stress through the
PERK-eIF2a-ATF4 signaling pathway. J Mol Neurosci. 55:995–1005.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Xiong J, Zhan Y, Liu W and Wang X:
Wogonin inhibits LPS-induced inflammatory responses in rat dorsal
root ganglion neurons via inhibiting TLR4-MyD88-TAK1-mediated NF-aB
and MAPK signaling pathway. Cell Mol Neurobiol. 35:523–531. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu S, Zhao X, Zhao Q, Zheng Q, Fang Z,
Yang X, Wang H, Liu P and Xu H: Wogonin prevents rat dorsal root
ganglion neurons death via inhibiting tunicamycin-induced ER stress
in vitro. Cell Mol Neurobiol. 35:389–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ponsuksili S, Jonas E, Murani E, Phatsara
C, Srikanchai T, Walz C, Schwerin M, Schellander K and Wimmers K:
Trait correlated expression combined with expression QTL analysis
reveals biological pathways and candidate genes affecting water
holding capacity of muscle. BMC Genomics. 9:3672008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nijboer CH, Bonestroo HJ, Zijlstra J,
Kavelaars A and Heijnen CJ: Mitochondrial JNK phosphorylation as a
novel therapeutic target to inhibit neuroinflammation and apoptosis
after neonatal ischemic brain damage. Neurobiol Dis. 54:432–444.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rau TF, Lu Q, Sharma S, Sun X, Leary G,
Beckman ML, Hou Y, Wainwright MS, Kavanaugh M, Poulsen DJ and Black
SM: Oxygen glucose deprivation in rat hippocampal slice cultures
results in alterations in carnitine homeostasis and mitochondrial
dysfunction. PLoS One. 7:e408812012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ranganathan AC, Ojha S, Kourtidis A,
Conklin DS and Aguirre-Ghiso JA: Dual function of pancreatic
endoplasmic reticulum kinase in tumor cell growth arrest and
survival. Cancer Res. 68:3260–3268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Glover-Cutter KM, Lin S and Blackwell TK:
Integration of the unfolded protein and oxidative stress responses
through SKN-1/Nrf. PLoS Genet. 9:e10037012013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mishra R and Karande AA: Endoplasmic
reticulum stress-mediated activation of p38 MAPK, Caspase-2 and
Caspase-8 leads to abrin-induced apoptosis. PLoS One. 9:e925862014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woehlbier U and Hetz C: Modulating stress
responses by the UPRosome: A matter of life and death. Trends
Biochem Sci. 36:329–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanbak G, Kartkaya K, Ozcelik E, Guvenal
AB, Kabay SC, Arslan G and Durmaz R: The neuroprotective effect of
acute moderate alcohol consumption on caspase-3 mediated
neuroapoptosis in traumatic brain injury: The role of lysosomal
cathepsin L and nitric oxide. Gene. 512:492–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Venkataranganna MV, Rafiq M, Gopumadhavan
S, Peer G, Babu UV and Mitra SK: NCB-02 (standardized Curcumin
preparation) protects dinitrochlorobenzene- induced colitis through
down-regulation of NFkappa-B and iNOS. World J Gastroenterol.
13:1103–1107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kandhare AD, Raygude KS, Ghosh P, Ghule
AE, Gosavi TP, Badole SL and Bodhankar SL: Effect of hydroalcoholic
extract of Hibiscus rosa sinensis Linn. leaves in experimental
colitis in rats. Asian Pac J Trop Biomed. 2:337–344. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kruidenier L, Kuiper I, Van Duijn W,
Mieremet-Ooms MA, van Hogezand RA, Lamers CB and Verspaget HW:
Imbalanced secondary mucosal antioxidant response in inflammatory
bowel disease. J Pathol. 201:17–27. 2003. View Article : Google Scholar : PubMed/NCBI
|