Introduction
Spinal cord injury (SCI) is characterized by
sensorimotor deficits below the injury and suffers from a lack of
effective treatment. Previous studies have aimed to evaluate the
severity and the potential for recovery in patients with traumatic
SCI; however, reliable methods for this and prediction of the
outcome following traumatic SCI, particularly in the acute stages
remain to be elucidated (1,2). The
current evaluation methods of injury severity of traumatic SCI is
predominantly limited to neurological evaluation and imaging
studies, which is often imprecise due to the unstable condition of
patients, with occurrences such as spinal shock and the artifacts
of metal implants following spinal operations (3–6).
This contributes to the difficulty of developing novel treatments
for patients with SCI.
Recently, increasing experimental evidence has
demonstrated that SCI should be considered a neurodegenerative
disease (7,8). A previous study determined that there
was degenerative evidence for structural changes in the brain
during the early stage of SCI using high-field structural magnetic
resonance imaging (MRI) (9).
Previous studies revealed that neuron degeneration may be part of
SCI pathology. In degenerative diseases, such as multiple sclerosis
(MS), amyotrophic lateral sclerosis (ALS), and Alzheimer's disease,
amyloid-β (Aβ) deposition occurs as a marker for neuron
degeneration (10–12). Changes in Aβ concentration in serum
and cerebrospinal fluid (CSF) were considered to be an indication
of the severity of injury to the blood-brain barrier and neuron
degeneration in the central nervous system (CNS) (13,14).
A recent study reported an increase in Aβ protein levels in a
lesion site of spinal cord in SCI model of rats (15). However, to the best to our
knowledge the present study is the first to determine whether Aβ
protein levels in serum and CSF are associated with injury severity
in SCI. The present study quantified Aβ protein levels in serum and
CSF in rats with traumatic SCI. The primary aims were to determine
whether Aβ protein was detectable in serum and CSF samples of
traumatic SCI and whether Aβ protein level reflected the severity
of the injury.
Materials and methods
Experimental animals and grouping
All experiments were performed in accordance with
the guidelines established by the Animal Ethics Committee of
Chinese PLA Beijing Army General Hospital (Beijing, China). A total
of 140 adult female Sprague-Dawley rats (age, 8–9 months; weight,
220–260 g) were purchased from Beijing Haidian Thriving
Experimental Animal Center (Beijing, China). All rats were housed
in a climate-controlled closed facility with 12 h light/dark cycles
at 24±2°C and free access to food and water for 1 week prior to the
experimental procedures. All rats were randomly and equally divided
into four groups (n=35 per group).
SCI model induction
Rats were anesthetized via intraperitoneal injection
of 10% chloral hydrate (3.0 mg/kg body weight; Shanghai Golden
Harvest Biotechnology Co., Ltd., Shanghai, China). The graded
contusion model of SCI was performed using the New York
University-Multicenter Animal Spinal Cord Injury Study (NYU-MASCIS)
impactor as previously described (16). Briefly, a laminectomy was performed
at T8 level to expose the spinal cord, and a contusion lesion was
produced with the NYU-MASCIS impactor by dropping a 10 g rod onto
the exposed dorsal surface of the spinal cord from the following
specific heights: i) Mild group (SCI-M), 12.5 mm; ii) moderate
group (SCI-Mo), 25 mm; and iii) severe group (SCI-S), 50 mm. Rats
in the sham group received laminectomy only. Following the
operation, rats were placed back to their cages with heating pads
and closely observed until they woke up. As prophylactic for
infections, 200,000 U/animal/day penicillin was administered
subcutaneously for 3 consecutive days following the operation.
Saline was injected subcutaneously immediately following
development of the lesion and then daily for 7 days. Food and water
were provided ad libitum. Postoperative care included manual
expression of bladders twice a day until the rats were sacrificed
or bladder function recovered.
Behavioral testing
Locomotor functions of all animals were assessed
using the Basso, Beattie and Bresnahan (BBB) locomotor rating scale
(17). BBB is a 21-point scale
used to assess and analyze the hindlimb movements of rats in
open-field, at 12 h and 1, 3, 7, 14, 21 and 28 days after the
operation. Two investigators, who were blinded to experimental
design, assessed the motor function in all the animals.
ELISA and western blotting
Rats were sacrificed by intraperitoneal injection of
lethal dose of 10% chloral hydrate (30 mg/kg body weight), at 12 h,
1, 3, 7, 14, 21 and 28 days post injury (n=4) after surgery, for
the sham group, laminectomy only. Blood samples (5 ml) were taken
from the tail vein of the injured rats at 12 h, 1, 3, 7, 14, 21 and
28 days. CSF samples (100 µl) were collected by lumbar puncture at
the same time point. The blood samples were anticoagulated using
ethylenediamine tetraacetic acid and centrifuged at 1,368 × g for
10 min at room temperature to obtain plasma. Plasma and CSF samples
were stored at −80°C prior to analysis of the protein levels of Aβ
by ELISA. Proteins for ELISA and western blotting were sequentially
extracted as previously described (18), from a total 5 mm spinal cord tissue
(2.5 mm rostral and 2.5 mm caudal to the injury site) using
diethylamine (DEA) and radioimmunoprecipitation assay buffer. A
commercially available kit (rat Aβ1–42 ELISA kit) from
Xinfan Biotechnology Corporation (Shanghai, China) was used to
detect endogenous soluble rat Aβ1–42, as per
manufacturer's protocol from the DEA extraction.
Anti-Aβ1–42 antibody (cat. no. ab10148) for western
blotting was purchased from Abcam (Cambridge, UK). Western blot
analysis was performed as previously described (19). Total protein (20 µg for each) was
separated on 10% sodium dodecyl sulfate polyacrylamide gels and
transferred onto nitrocellulose membranes. The membranes were first
blocked with 5% skimmed milk and then incubated with rabbit anti-Aβ
(ab10148; 1:500) or anti-β-actin (ab129348; 1:1,000; Abcam)
overnight at 4°C. After 5 washes in 1X PBST, membranes were
incubated with appropriate horseradish peroxidase conjugated
affinity purified secondary antibody (AP187P; 1:1,000; EMD
Millipore, Billerica, MA, USA) for 1 h at 37°C, developed using an
enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for detection of proteins. Pixel intensity for
each sample and its corresponding β-actin was determined on three
different blots using ImageJ software (imagej.nih.gov/ij/). To quantify the results, samples
were normalized to β-actin and a bar chart was produced using
GraphPad Prism version 5 (GraphPad Software, Inc., San Diego, CA,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using SPSS version 19.0 (IBM SPSS, Armonk, NY,
USA). One-way analysis of variance, followed by the
Student-Newman-Keuls test were used to determine the differences
between groups. The association between variables was determined by
the correlation coefficient method according to the Spearman or
Pearson methods. P<0.05 was considered to indicate a
statistically significant difference.
Results
Behavioral scoring
Significant differences in the behavioral score
using the BBB locomotor rating scale between the SCI groups and the
sham group were identified (P<0.05). Higher SCI injury groups
had significantly lower behavioral scores (P<0.05; Fig. 1). Over the period of four weeks,
gradual recovery was observed in all the SCI groups. Significant
motor functional improvement was detected in the mild SCI group
when compared with moderate and severe SCI group 12 at 7, 10, 14
and 28 days after SCI (P<0.05).
Aβ protein levels in serum and CSF of
SCI rats
Serum and CSF Aβ protein levels observed were
similar (Figs. 2 and 3). In the sham group, the serum and CSF
Aβ levels were low for all time points. The Aβ levels in SCI-S
group at 12 h after injury were significantly increased compared
with the sham group (P<0.05; Fig.
2). At 1 and 3 days after injury, the serum Aβ protein levels
were increased significantly in all SCI groups (P<0.05; Fig. 2). It is of note that the serum Aβ
levels in the SCI-M group were significantly reduced compared with
the SCI-Mo and SCI-S groups at 3 days (P<0.05; Fig. 2). Subsequently, the serum Aβ levels
were gradually reduced in all SCI groups; however, Aβ expression
level was significantly higher in the SCI-S group compared with the
SCI-M group at 28 days after injury (P<0.05; Fig. 2). The CSF Aβ level in the SCI-S
group was significantly higher compared with SCI-M group
(P<0.01) and SCI-Mo group (P<0.05) at 1 and 3 days after
injury (Fig. 3). At 28 days after
injury, Aβ levels in the SCI-S group were significantly higher
compared with the SCI-M and SCI-Mo groups at 28 days after injury
(P<0.05; Fig. 3).
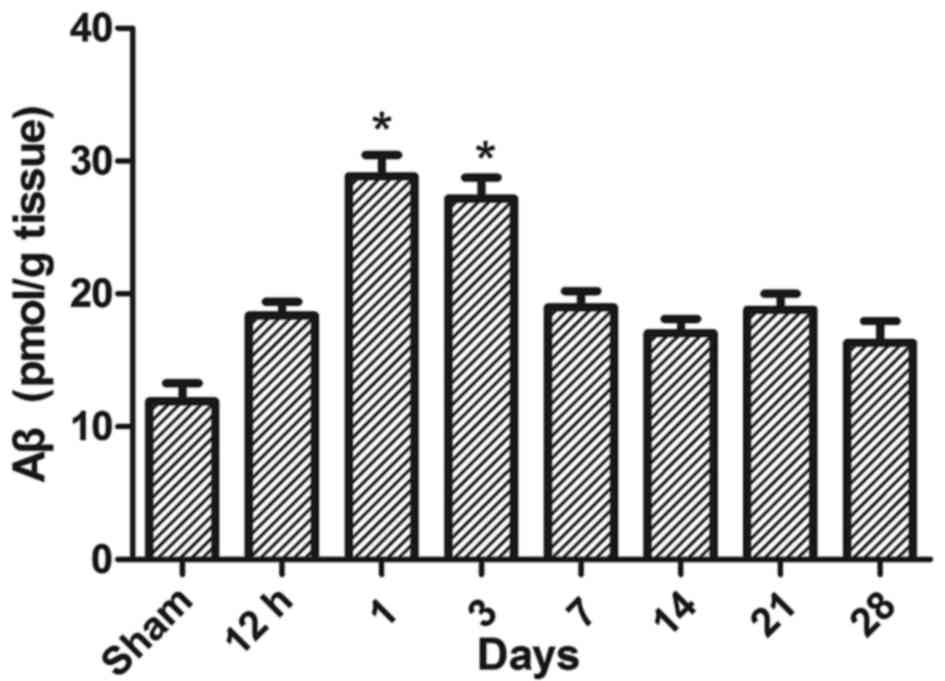
Aβ protein levels in injured spinal
cord tissue
To determine whether the increase of Aβ in injured
spinal cord tissue may be associated with the protein expression
levels observed in serum and CSF, rats in moderate group (n=4) were
sacrificed at 12 h, 1, 3, 7, 14, 21 and 28 days and an ELISA was
performed to detect the Aβ changes in tissues following injury.
Over the course of the experiment, changes of Aβ protein levels in
injured spinal cord tissue were similar to those occurred in CSF.
The Aβ protein levels in spinal cord tissues were significantly
higher in the SCI-M group at 1 and 3 days after injury compared
with the sham group (P<0.05; Fig.
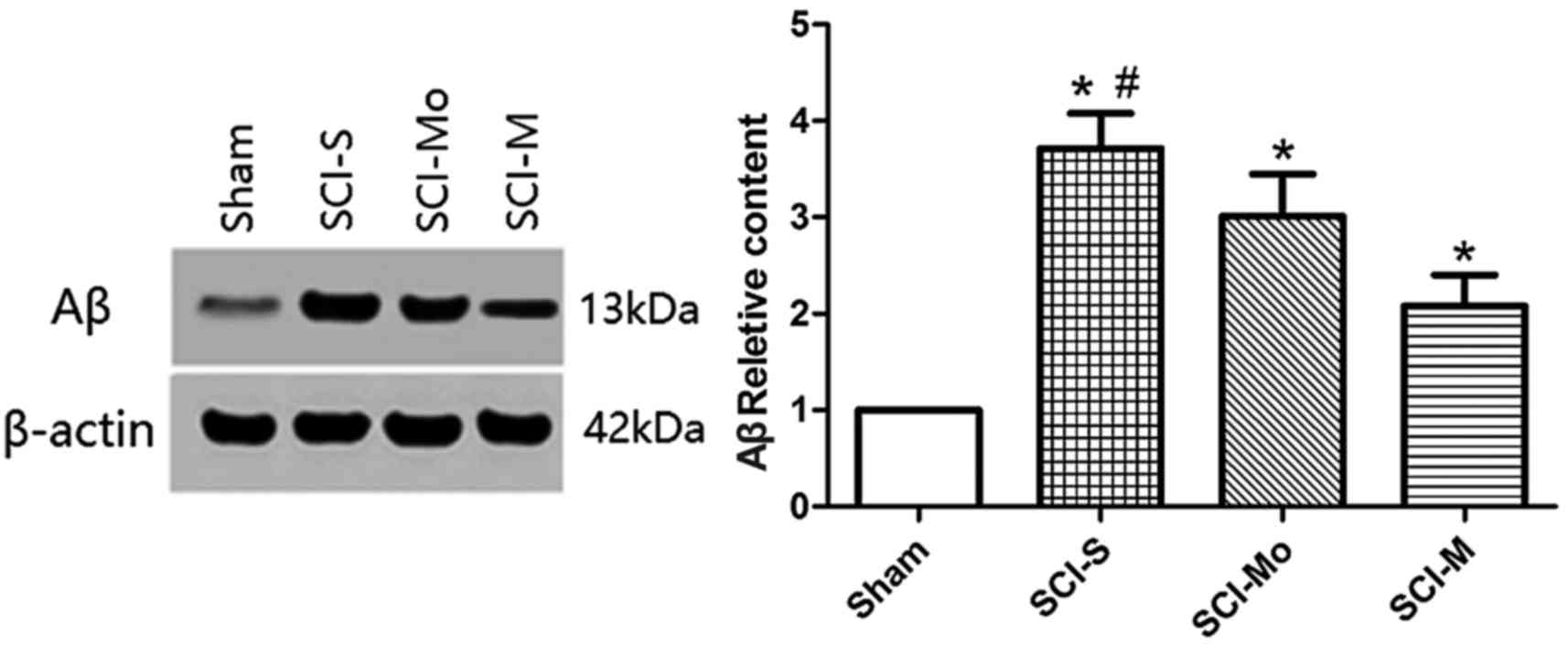
4). Rats in all the SCI groups and the sham group (n=4) were
then sacrificed 3 days after injury and western blots were
performed to detect changes in injured spinal cord tissue. The Aβ
protein level in the SCI-S group was significantly higher compared
with the SCI-M group at 3 days after injury (P<0.05; Fig. 5).
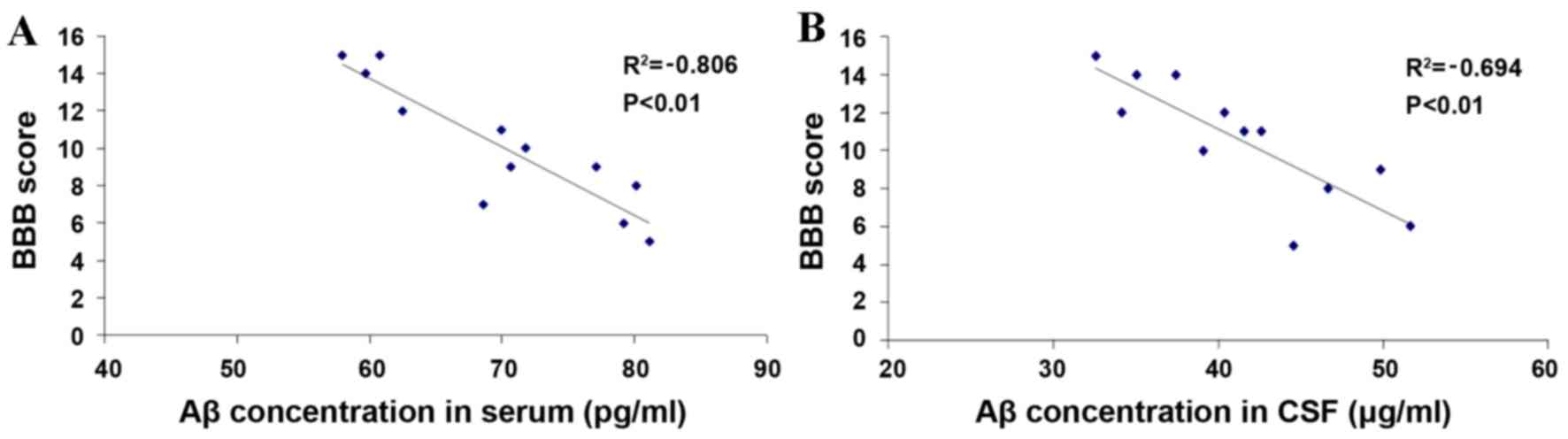
Correlation analysis between the serum
and CSF Aβ levels and BBB score
The serum and CSF Aβ levels peaked at 3 days after
injury and a significant difference between the SCI-S group and the
SCI-M group was identified (P<0.01; Figs. 2 and 3). The BBB score improved gradually with
time and reached the peak at 28 days after injury with a
significant difference between the SCI-S group and SCI-M group
(P<0.01; Fig. 1). Therefore, a
correlation analysis was performed between Aβ levels in serum or
CSF and recovery hind limb function (BBB score). The serum and CSF
Aβ levels at 3 days after injury were plotted against the BBB score
at 28 days after injury for each sample respectively (for the
SCI-S, SCI-Mo and SCI-M groups). A significant negative correlation
was identified between the two parameters in the Aβ levels in serum
(P<0.01; Fig. 6A) and CSF
(P<0.01; Fig. 6B).
Discussion
The present study determined that there was a
significant negative correlation between Aβ protein levels in the
CSF and serum and the BBB score of rats with traumatic spinal cord
injury.
Spinal cord injury results in mortality and
morbidity and currently there are a limited number of effective
treatments. The association between inflammation and secondary
damage led to the use of a high dose of methylprednisolone therapy
within 8 h of injury, and the common surgical procedures made by
physicians are stabilization and decompression of spinal cord
(2). Successful management of SCI
requires an appropriate diagnostic standard following injury,
particularly in the first 3 days, which is the acute phase.
Neurological examinations provide a general indication of spinal
cord neurological function; however, they may be unreliable,
particularly during the acute phase following SCI due to spinal
shock in patients (2).
Neurophysiological examinations, such as determination of
somatosensory evoked potentials and motor evoked potentials, are
rarely performed in the acute phase due to their low sensitivity at
this time (1). MRI has been
established as the gold standard for anatomical SCI diagnosis;
however, it may not always be able to distinguish neuronal tissue
necrosis from apoptosis and edema (5,6).
Serum biomarker examination may provide a
quantitative approach for the assessment of real-time tissue
injuries and may be useful for making clinical diagnoses. CSF is in
direct contact with the CNS and the spinal cord, which may reflect
the biochemical state of the central and periphery nervous system
under different physiological and pathological conditions (4). Therefore, CSF may be regarded as a
potential source for identifying biomarkers for neurological
diseases and trauma. A previous study demonstrated that quantifying
the blood or CSF level of neuron- or astroglia-specific proteins
may be a more sensitive way to assess the severity of traumatic
brain injury (TBI) (7). It has
been proposed that CSF, which surrounds and protects the brain and
spinal cord from traumatic injury, may be a potential target for
the diagnosis of a variety of conditions, including TBI and SCI, in
addition to Alzheimer's disease, amyotrophic lateral sclerosis
(ALS) and multiple sclerosis (MS) (10–12).
Due to the complexity of biofluids; however, considerable
developments are required in the analytical techniques used in
order to achieve comprehensive coverage of the proteins present in
CSF samples. An ideal biomarker should be exclusively intracellular
and present in high concentrations.
Aβ has long been used as potentially useful
diagnostic biomarker for brain injury. The Aβ peptide is produced
by proteolytic cleavage of amyloid precursor protein (APP) by two
different enzymes, β- and γ-secretase (20,21).
Aβ is derived from the transmembrane APP by proteolytic processing
during normal cell metabolism and secreted into the CSF, so the CSF
Aβ can serve as the foundation for an Aβ biomarker. The 42-amino
acid form of Aβ (Aβ1–42) is of special interest, as it
may have the greatest propensity to deposit into insoluble plaques,
which is the pathological characteristic of Alzheimer's disease.
Additionally, it may also aggregate into oligomeric Aβ species,
reported to be particularly neurotoxic (22–24).
Increased level of APP, β- and γ-secretase, and Aβ have been
observed with the onset of numerous other neurological disorders,
including ALS, MS and microglia activation and associated
neuroinflammation (10,11,25,26).
When the axon is severely injured, APP and Aβ are released into the
extracellular space via the damaged membrane. Brody et al
(13) reported an increase of APP
and Aβ levels in the CSF and peripheral blood following TBI and the
increases were positively associated with the severity of injury;
however, they were negatively correlated with the functional
outcome (13). The purpose of the
current study was to determine whether Aβ protein expression level
may be used as a diagnostic marker for SCI in an animal model.
The rat model of SCI in the present study was
modified from the methods described by Wrathall et al
(27). The severity of SCI was
positively associated with the height from which the weight was
dropped. The current study did not identify any significant
differences between the Aβ protein levels in the sham group and the
SCI-M and SCI-Mo groups 12 h after injury. However, a significant
increase in serum and CSF Aβ protein levels was observed in the
SCI-S group at 12 h. Aβ protein levels peaked at 3 days after
injury in all SCI groups, therefore it may accurately reflect the
severity during the acute phase of SCI. A previous study suggested
that the accumulation of β-APP and increase of Aβ peptide in
injured spinal cord tissue were accompanied by axonal dysfunction
within 1 day of SCI (28), with a
peak at 3 days after injury (29),
which is similar to the findings of the present study demonstrated
by ELISA and western blotting. The serum and CSF Aβ levels changed
similarly with the Aβ level in injured spinal cord tissue, which
determined that the Aβ levels in biofluids may predict the
alterations that occur in injured tissue. High levels of Aβ in the
CSF may reflect the reduced clearance of APP and the increased
deposition of Aβ in injured spinal cord tissue. At 12 h after
injury, although Aβ protein level in the SCI-S group was higher
compared with in the sham group, the graded SCI groups did not
differ significantly until 1 day after injury. These results are
consistent with previous studies on SCI (15,28,29).
SCI-S group was significantly different when compared with the
SCI-M and SCI-Mo groups at 28 days after injury in the serum and
CSF, which may indicate a long-lasting axonal transport destruction
and deposition of Aβ protein as a result of cleavage of APP in the
lesion site. By contrast to Pajoohesh-Ganji et al (15) where the Aβ expression in injured
spinal cord tissue returned to baseline levels at 7 days after
injury, the present study revealed the long-lasting increase of Aβ
levels in serum and CSF in the SCI-S group may be due to severe
destruction of spinal cord and blood-brain barrier, whereas Aβ
levels in SCI-M and SCI-Mo groups were consistent with the findings
of Pajoohesh-Ganji et al (15).
In conclusion, the present study indicated that
serum and CSF Aβ protein level alterations are time-dependent. The
peak in the concentration of the biomarker may reflect the
mechanical disruption of the spinal cord and blood-brain barrier.
The concentrations of the biomarker were negatively correlated with
the BBB score. The current study suggested that Aβ protein may be
used as a specific biomarker for SCI diagnosis. This biomarker may
also be used for screening of degenerative changes following
SCI.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grants nos. 81301679 and
81671211).
References
|
1
|
Krishna V, Andrews H, Varma A, Mintzer J,
Kindy MS and Guest J: Spinal cord injury: How can we improve the
classification and quantification of its severity and prognosis? J
Neurotrauma. 31:215–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silva NA, Sousa N, Reis RL and Salgado AJ:
From basics to clinical: A comprehensive review on spinal cord
injury. Prog Neurobiol. 114:25–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pouw MH, Hosman AJ, van Middendorp JJ,
Verbeek MM, Vos PE and van de Meent H: Biomarkers in spinal cord
injury. Spinal Cord. 47:519–525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pouw MH, Kwon BK, Verbeek MM, Vos PE, van
Kampen A, Fisher CG, Street J, Paquette SJ, Dvorak MF, Boyd MC, et
al: Structural biomarkers in the cerebrospinal fluid within 24 h
after a traumatic spinal cord injury: A descriptive analysis of 16
subjects. Spinal Cord. 52:428–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheran S, Shanmuganathan K, Zhuo J, Mirvis
SE, Aarabi B, Alexander MT and Gullapalli RP: Correlation of MR
diffusion tensor imaging parameters with ASIA motor scores in
hemorrhagic and nonhemorrhagic acute spinal cord injury. J
Neurotrauma. 28:1881–1892. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokobori S, Zhang Z, Moghieb A, Mondello
S, Gajavelli S, Dietrich WD, Bramlett H, Hayes RL, Wang M, Wang KK,
Bullock MR, et al: Acute diagnostic biomarkers for spinal cord
injury: Review of the literature and preliminary research report.
World Neurosurg. 83:867–878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li K, Nicaise C, Sannie D, Hala TJ, Javed
E, Parker JL, Putatunda R, Regan KA, Suain V, Brion JP, et al:
Overexpression of the astrocyte glutamate transporter GLT1
exacerbates phrenic motor neuron degeneration, diaphragm compromise
and forelimb motor dysfunction following cervical contusion spinal
cord injury. J Neurosci. 34:7622–7638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L, Wang X, Bao J, Geng C, Xia Y and
Wang J: Direct comparison of cardiovascular magnetic resonance and
single-photon emission computed tomography for detection of
coronary artery disease: A meta-analysis. PLoS One. 9:e884022014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou JM, Yan RB, Xiang ZM, Zhang H, Liu J,
Wu YT, Zhao M, Pan QY, Song LH, Zhang W, et al: Brain sensorimotor
system atrophy during the early stage of spinal cord injury in
humans. Neuroscience. 266:208–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikonomovic MD, Uryu K, Abrahamson EE,
Ciallella JR, Trojanowski JQ, Lee VM, Clark RS, Marion DW,
Wisniewski SR and DeKosky ST: Alzheimer's pathology in human
temporal cortex surgically excised after severe brain injury. Exp
Neurol. 190:192–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bryson JB, Hobbs C, Parsons MJ, Bosch KD,
Pandraud A, Walsh FS, Doherty P and Greensmith L: Amyloid precursor
protein (APP) contributes to pathology in the SOD1 (G93A) mouse
model of amyotrophic lateral sclerosis. Hum Mol Genet.
21:3871–3882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uryu K, Laurer H, McIntosh T, Praticò D,
Martinez D, Leight S, Lee VM and Trojanowski JQ: Repetitive mild
brain trauma accelerates Abeta deposition, lipid peroxidation, and
cognitive impairment in a transgenic mouse model of Alzheimer
amyloidosis. J Neurosci. 22:446–454. 2002.PubMed/NCBI
|
|
13
|
Brody DL, Magnoni S, Schwetye KE, Spinner
ML, Esparza TJ, Stocchetti N, Zipfel GJ and Holtzman DM:
Amyloid-beta dynamics correlate with neurological status in the
injured human brain. Science. 321:1221–1224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mondello S, Buki A, Barzo P, Randall J,
Provuncher G, Hanlon D, Wilson D, Kobeissy F and Jeromin A: CSF and
plasma amyloid-β temporal profiles and relationships with
neurological status and mortality after severe traumatic brain
injury. Sci Rep. 4:64462014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pajoohesh-Ganji A, Burns MP, Pal-Ghosh S,
Tadvalkar G, Hokenbury NG, Stepp MA and Faden AI: Inhibition of
amyloid precursor protein secretases reduces recovery after spinal
cord injury. Brain Res. 1560:73–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agrawal G, Kerr C, Thakor NV and All AH:
Characterization of graded multicenter animal spinal cord injury
study contusion spinal cord injury using somatosensory-evoked
potentials. Spine (Phila Pa 1976). 35:1122–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loane DJ, Pocivavsek A, Moussa CE,
Thompson R, Matsuoka Y, Faden AI, Rebeck GW and Burns MP: Amyloid
precursor protein secretases as therapeutic targets for traumatic
brain injury. Nat Med. 15:377–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Byrnes KR, Stoica BA, Fricke S, Di
Giovanni S and Faden AI: Cell cycle activation contributes to
post-mitotic cell death and secondary damage after spinal cord
injury. Brain. 130:2977–2992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu X, Hicks CW, He W, Wong P, Macklin WB,
Trapp BD and Yan R: Bace1 modulates myelination in the central and
peripheral nervous system. Nat Neurosci. 9:1520–1525. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Selkoe DJ: American College of Physicians;
American Physiological Society: Alzheimer disease: Mechanistic
understanding predicts novel therapies. Ann Intern Med.
140:627–638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walsh DM, Klyubin I, Fadeeva JV, Cullen
WK, Anwyl R, Wolfe MS, Rowan MJ and Selkoe DJ: Naturally secreted
oligomers of amyloid beta protein potently inhibit hippocampal
long-term potentiation in vivo. Nature. 416:535–539. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lesné S, Koh MT, Kotilinek L, Kayed R,
Glabe CG, Yang A, Gallagher M and Ashe KH: A specific amyloid-beta
protein assembly in the brain impairs memory. Nature. 440:352–357.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shankar GM, Li S, Mehta TH, Garcia-Munoz
A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ and
Lemere CA: Amyloid-beta protein dimers isolated directly from
Alzheimer's brains impair synaptic plasticity and memory. Nat Med.
14:837–842. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuoka Y, Picciano M, Malester B,
LaFrancois J, Zehr C, Daeschner JM, Olschowka JA, Fonseca MI,
O'Banion MK, Tenner AJ, et al: Inflammatory responses to
amyloidosis in a transgenic mouse model of Alzheimer's disease. Am
J Pathol. 158:1345–1354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wyss-Coray T and Mucke L: Inflammation in
neurodegenerative disease-a double-edged sword. Neuron. 35:419–432.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wrathall JR, Pettegrew RK and Harvey F:
Spinal cord contusion in the rat: Production of graded,
reproducible, injury groups. Exp Neurol. 88:108–115. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kobayashi S, Sasaki T, Katayama T,
Hasegawa T, Nagano A and Sato K: Temporal-spatial expression of
presenilin 1 and the production of amyloid-beta after acute spinal
cord injury in adult rat. Neurochem Int. 56:387–393. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ward RE, Huang W, Kostusiak M, Pallier PN,
Michael-Titus AT and Priestley JV: A characterization of white
matter pathology following spinal cord compression injury in the
rat. Neuroscience. 260:227–239. 2014. View Article : Google Scholar : PubMed/NCBI
|