Introduction
Inflammatory bowel disease (IBD) is a chronic
disease that consists of ulcerative colitis (UC) and Crohn's
disease (1–4). The clinical course of IBD varies
markedly, from frequent relapses, to chronic active disease, to
years of complete remission (5).
At present, the etiology of IBD is not completely understood
(6–8). There are at least five distinct types
of enteroendocrine cell in the large intestine, which are arranged
between the epithelial cells lining the intestinal lumen (9,10).
These cells regulate intestinal motility, secretion and absorption,
as well as visceral sensitivity, local immune defense, cell
proliferation and appetite (9,11–26).
The enteroendocrine cells in the large intestine are abnormal in
patients with IBD and in animal models of IBD (24,27–42).
Interactions between the hormones secreted by the large intestine
enteroendocrine cells and the immune system have previously been
debated, and it has been speculated that these interactions serve a
critical role in the pathophysiology of IBD (43–45).
The cause of abnormalities in the large intestine
enteroendocrine cells in IBD is not currently known. Abnormal
intestinal enteroendocrine cells have been reported in congenital
malabsorptive diarrhea alongside mutated transcription factor
Neurogenin 3 (Neurog3), and in mutant mice with ablation of Neurog3
(46). The present study aimed to
investigate whether the abnormalities observed in intestinal
enteroendocrine cells in dextran sulfate sodium (DSS)-induced
colitis are associated with abnormalities in the clonogenic and/or
proliferative activities of stem cells (47). Furthermore, it was investigated
whether the alterations in enteroendocrine cells and stem cells may
be restored by treatment with two anti-inflammatory agents:
3-[(dodecylthiocarbonyl)-methyl]-glutarimide (DTCM-G) and
dehydroxymethylepoxyquinomicin (DHMEQ). These agents have been
demonstrated to exert potent anti-inflammatory activity in animal
models (48,49).
Materials and methods
Rats
A total of 48 male Wistar rats (6 weeks old;
Hannover GALAS; Taconic Europe A/S, Lille Skensved, Denmark) with a
mean body weight of 290 g (range, 238–385 g) were housed in
Macrolon III cages with ad libitum access to food and water. The
rats were fed a standard diet (B&K Universal Limited, Hull,
UK), and were maintained under the following conditions:
Temperature between 20 and 22°C, relative humidity between 50 and
60%, and 12/12-h light/dark cycle.
The animals were allowed to acclimate in the animal
house for ≥1 week prior to experimentation, and were then divided
into 4 groups, each containing 12 rats. Rats in the control group
were provided with normal drinking water for 7 days, whereas
colitis was induced in the other three groups using DSS, as
previously described (50,51). Briefly, the rats were provided with
distilled drinking water containing 5% DSS (40 kD; TDB Consultancy
AB, Uppsala, Sweden) for 7 days. The rats with DSS-induced colitis
were randomized into the following three groups: i) DSS group,
which received 0.5 ml 0.5% carboxymethyl cellulose (CMC; vehicle);
ii) DSS-G group, which was treated with DTCM-G at 20 mg/kg body
weight in 0.5% CMC; and iii) DSS-Q group, which was treated with
DHMEQ at 15 mg/kg body weight in 0.5% CMC. Treatments were
administered intraperitoneally twice daily for 5 days in all
groups. DTCM-G and DHMEQ were synthesized as described previously
(52–55). The rats were monitored frequently,
and those that showed any signs of pain were injected
subcutaneously with 1 ml Temgesic solution (containing 0.3 g/ml
Temgesic; Merck & Co., Inc., Kenilworth, NJ, USA) as an
analgesic.
At the end of the 5-day treatment period, rats were
sacrificed by CO2 inhalation, the colon was collected,
and tissue samples were obtained from the lower part of the colon
for subsequent examinations. The present study was approved by the
local ethical committee at the University of Bergen for the
Protection of Vertebrate Animals used for Experimental and Other
Scientific Purposes (Bergen, Norway; project no. 20124629).
Histopathology and
immunohistochemistry
The tissue samples were fixed in 4% buffered
paraformaldehyde, embedded in paraffin, and cut into 5 mm sections.
The sections were stained with hematoxylin and eosin, or
immunostained using the ultraView Universal DAB Detection kit
(version 1.02.0018; Ventana Medical Systems, Inc., Tucson, AZ, USA)
and the BenchMark Ultra IHC/ISH staining module (Ventana Medical
Systems, Inc.). The sections were incubated with the following
primary antibodies for 32 min at 37°C: Monoclonal mouse
anti-N-terminal of purified chromogranin A (CgA; 1:1,500; cat. no.
M869; Dako Denmark A/S, Glostrup, Denmark); polyclonal rabbit
anti-residues 5–21 [APQPGLASPDSPHDPCK] of the human, mouse and rat
Musashi 1 (Msi1) protein (1:100; cat. no. NB100-1759; R&D
Systems Europe, Abingdon, UK); polyclonal rabbit anti-synthetic
peptide surrounding amino acid 190 of human Math-1 (1:50; code no.
3658-100; BioVision, Inc., Milpitas, CA, USA); polyclonal rabbit
anti-KLH-conjugated synthetic peptide between 40–69 amino acids
from the N-terminal region of human Neurog3 (1:50; cat. no.
PA5-11893, Thermo Fisher, Oslo, Norway); and polyclonal rabbit
anti-recombinant full-length human neurogenic differentiation D1
(NeuroD1; 1:50; cat. no. PA5-47381; Thermo Fisher). All of these
antibodies detect antigens in humans and rats.
Quantification
The number of CgA-, Msi1-, Math-1-, Neurog3- and
NeuroD1-immunoreactive cells, the number of crypts, and the area
containing epithelial cells were counted in ten randomly selected
microscopic fields using a light microscope (BX 43). Measurements
were performed using cellSens imaging software (version 1.7;
Olympus Corporation, Tokyo, Japan). This morphometric method has
previously been validated (56).
The number of immunoreactive cells and crypts in each field were
counted manually by pointing and clicking the computer mouse,
whereas the area of epithelial cells was determined by manual
drawing using the computer mouse. A ×40 objective was used, for
which each frame (field) on the monitor represented a tissue area
of 0.035 mm2. The density of CgA was expressed as the
number of immunoreactive endocrine cells per square millimeter of
epithelium, the density of Msi1 was expressed as the number of
immunoreactive cells per crypt, and the densities of Math-1,
Neurog3 and NeuroD1 were expressed as the number of immunoreactive
cells per field. Immunostained sections were coded, and
measurements were performed by the same individual (M.E-S.), who
was blinded to the identity of the sections.
Statistical analysis
The Kruskal-Wallis nonparametric test and Dunn's
post hoc test were used to compare between the control, DSS, DSS-G
and DSS-Q groups. Correlations between abnormalities/alterations in
the densities of CgA-, Neurog3-, and NeuroD1-immunoreactive cells
were determined using the nonparametric Spearman correlation test.
Data are presented as the mean ± standard error of the mean.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The colon samples collected from rats in the
control, DSS-G and DSS-Q groups appeared histopathologically
normal; however, in the DSS group, disturbed mucosal architecture,
crypt abscesses, edema, bleeding and immune cell infiltration were
observed (Fig. 1).
CgA immunostaining
CgA-immunoreactive cells were detected in crypts and
alongside the gland of Lieberkühn. The cell densities in the
control, DSS, DSS-G and DSS-Q groups were 113.0±20.4, 319.1±32.0,
123.9±22.6 and 141.3±14.3 cells/mm2 epithelium,
respectively (Kruskal-Wallis test, P<0.0001; Figs. 2 and 3). Dunn's test indicated that the density
of CgA-immunoreactive cells was significantly higher in the DSS
group compared with in the control group (P<0.0001). The
densities of CgA in DSS-G and DSS-Q did not differ from that of
controls (P=0.9, and 0.1, respectively). The CgA-immunoreactive
cell density was correlated with the densities of Neurog3- and
NeuroD1-immunoreactive cells (r=0.8; P=0.006 for both).
Msi1 immunostaining
Msi1-immunoreactive cells were observed exclusively
in the crypts of the gland of Lieberkühn. The cell densities in the
control, DSS, DSS-G and DSS-Q groups were 4.9±0.5, 4.8±0.5, 5.1±0.6
and 5.0±0.6 cells/crypt, respectively (Kruskal-Wallis test, P=0.98;
Fig. 4).
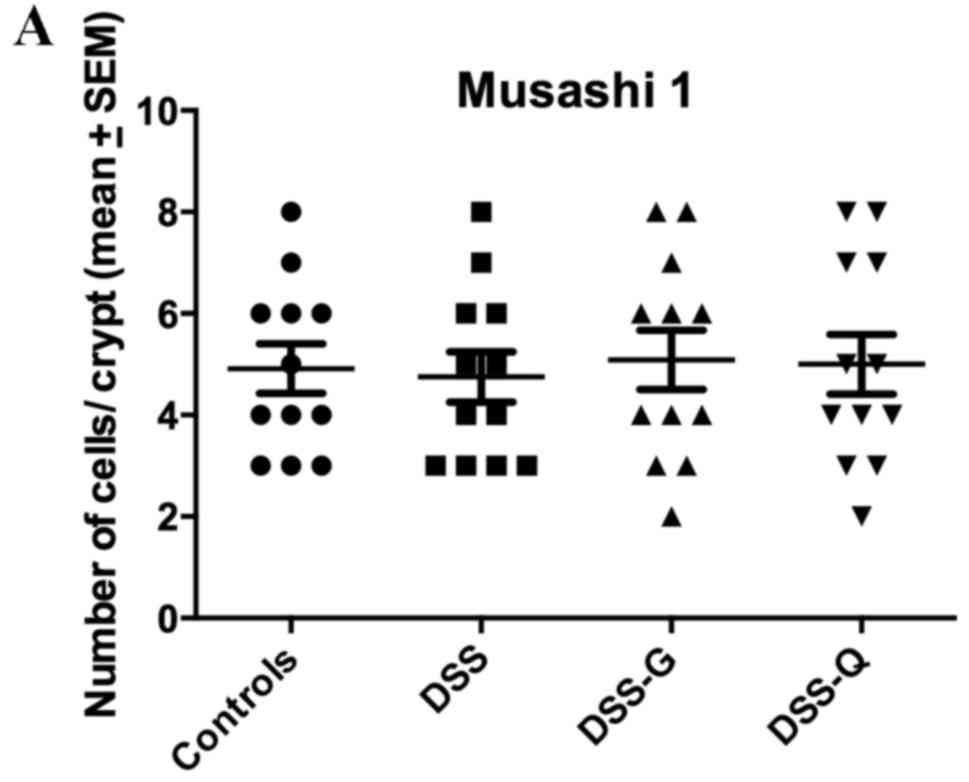 | Figure 4.Densities of (A) Msi1-, (B) Math-1,
(C) Neurog3- (D) and NeuroD1-immunoreactive cells in the control,
DSS, DSS-G and DSS-Q groups. *P<0.001. DSS, dextran sulfate
sodium-induced colitis vehicle-treated group; DSS-G,
3-[(dodecylthiocarbonyl)-methyl]-glutarimide-treated DSS group;
DSS-Q, dehydroxymethylepoxyquinomicin-treated DSS group; Msi1,
Musashi 1; Neurog3, neurogenin 3; NeuroD1, neurogenic
differentiation D1. |
Math-1 immunostaining
Math-1-immunoreactive cells were observed in the
crypts and alongside the gland of Lieberkühn. The cell densities in
the control, DSS, DSS-G and DSS-Q groups were 80.2±10.4,
101.6±10.7, 99.1±8.3 and 100.1±11.3 cells/field, respectively
(Kruskal-Wallis test, P=0.41; Fig.
4).
Neurog3 immunostaining
Neurog3-immunoreactive cells were detected in the
crypts and alongside the gland of Lieberkühn (Figs. 4 and 5). The cell densities were 79.1±11.1,
223.1±36.0, 103.8±12.4 and 77.3±10.9 cells/field in the control,
DSS, DSS-G and DSS-Q groups, respectively (Kruskal-Wallis test,
P=0.002). The Neurog3-immunoreactive cell density was significantly
higher in the DSS group compared with in the control group (Dunn's
test: P=0.0002; Fig. 4C). There
was no statistically significant difference between controls and
DSS-G and DSS-Q regarding Neurog3 cell density (P=0.1, and 0.7,
respectively).
NeuroD1 immunostaining
Similar to Neurog3, NeuroD1-immunoreactive cells
were observed in the crypts and alongside the gland of Lieberkühn.
The cell densities were 73.3±10.7, 217.3±24.4, 105.8±11.8 and
79.1±10.7 cells/field in the control, DSS, DSS-G and DSS-Q groups,
respectively (Kruskal-Wallis test, P=0.0001). The density of
NeuroD1-immunoreactive cells was significantly higher in the DSS
group compared with in the control group (Dunn's test, P=0.0002;
Fig. 4). The densities of NeuroD1
in DSS-G, and DSS-Q did not differ from that of controls (P=0.07,
and 0.9, respectively).
Discussion
CgA is a general marker for enteroendocrine cells
(57). In the present study, the
density of CgA-immunoreactive cells in the large intestine was
significantly elevated in rats with DSS-induced colitis, which is
in agreement with previously reported observations (47). DSS-induced colitis is an animal
model that is very similar, but not identical, to human UC
(58). The density of
CgA-immunoreactive cells in the large intestine has also been
reported to be higher in patients with UC compared with in healthy
subjects (27).
The intestine contains between 4 and 6 stem cells
per crypt, and these cells exhibit two types of activity: i)
Dividing into new stem cells (self-renewal, clonogeny) and ii)
differentiating into all types of epithelial cell (differentiation)
(59–71). The differentiating stem cell
progeny includes two lineages: Secretory and absorptive. The
secretory lineage gives rise to goblet, endocrine and Paneth cells,
whereas the absorptive lineage gives rise to absorptive enterocytes
(59–71). Msi1 is a transcription factor
expressed by intestinal stem cells and their early progeny
(71–74). In the present study, the density of
Msi1-immunoreactive cells did not differ between rats in the DSS
group and those in the control group, thus indicating that the
clonogenic activity of the stem cells was not affected by
inflammation.
Math-1 is expressed by an early progenitor in the
secretory lineage, and Math−/− mice lack secretory cells
(75). The present study indicated
that the density of Math-1-immunoreactive cells did not
significantly differ between rats in the DSS group and those in the
control group. These findings suggested that inflammation does not
interfere with early secretory lineage differentiation.
Neurog3 is expressed in endocrine progenitor cells,
which direct the differentiation of secretory progenitors into
endocrine cells (46).
Neurog3−/− mice possess normal densities of goblet and
Paneth cells; however, they possess no pancreatic endocrine or
enteroendocrine cells (46,76,77).
NeuroD1 is a transcription factor that is expressed by cells
derived from Neurog3 progenitors (78,79).
Mice deficient in NeuroD1 do not possess a subgroup of
enteroendocrine cells (46,80).
In the present study, the densities of Neurog3- and
NeuroD1-immunoreactive cells were higher in DSS-induced rats
compared with in control rats. Furthermore, this elevation was
strongly correlated with the increased CgA-immunoreactive cell
density. This finding provided evidence to suggest that the
increased density of enteroendocrine cells observed following
DSS-induced colitis may be caused by an increase in the
differentiation of early enteroendocrine progenitors during the
secretory lineage. Intestinal stem cell proliferation is regulated
by numerous signaling pathways (71). It is probable that the DSS-induced
inflammatory processes trigger certain signaling pathways, which
control the differentiation of the stem-cell secretory lineage into
mature enteroendocrine cells.
The present study confirmed the findings of previous
studies, that DTCM-G and DHME exhibit potent anti-inflammatory
activity in animal models of UC (48,49).
Stem cells differentiate rapidly into mature intestinal cells; this
process typically takes 2–3 days (72). This may explain why, in the present
study, treating rats with DSS-induced colitis with the
anti-inflammatory agents DTCM-G and DHME for only 5 days restored
the densities of CgA, Neurog3- and NeuroD1-immunoreactive cells to
those of the control group. The rapid proliferation and
differentiation of epithelial cells are disturbed by inflammation,
which causes impairment in epithelial barrier function (81–84).
Polyphenols, which is quite different from DTCM-G and DHME, exert a
protective effect on epithelial cells and consequently suppress the
inflammatory response (81–83).
In conclusion, the present study demonstrated that
the elevated densities of enteroendocrine cells detected in
DSS-induced colitis are probably due to increased differentiation
of early enteroendocrine progenitors during the secretory lineage.
It is likely that inflammatory processes trigger certain signaling
pathways that control differentiation of the stem-cell secretory
lineage into mature enteroendocrine cells. In addition, this
process appears to be responsive to short-term anti-inflammatory
treatment. It is probable that stem cell transplantation may be an
effective treatment for patients with IBD, that have not responded
to current available treatment.
Acknowledgements
The present study was supported by grants from
Helse-Fonna (grant no. 40415) and Helse-Vest (grant no. 911978),
Norway.
References
|
1
|
Prantera C and Marconi S:
Glucocorticosteroids in the treatment of inflammatory bowel disease
and approaches to minimizing systemic activity. Therap Adv
Gastroenterol. 6:137–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cosnes J, Gower-Rousseau C, Seksik P and
Cortot A: Epidemiology and natural history of inflammatory bowel
diseases. Gastroenterology. 140:1785–1794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Podolsky DK: The current future
understanding of inflammatory bowel disease. Best Pract Res Clin
Gastroenterol. 16:933–943. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carter MJ, Lobo AJ and Travis SP: IBD
Section, British Society of Gastroenterology: Guidelines for the
management of inflammatory bowel disease in adults. Gut 53 Suppl.
5:V1–V16. 2004. View Article : Google Scholar
|
|
6
|
Danese S and Fiocchi C: Etiopathogenesis
of inflammatory bowel diseases. World J Gastroenterol.
12:4807–4812. 2006.PubMed/NCBI
|
|
7
|
Nunes T, Fiorino G, Danese S and Sans M:
Familial aggregation in inflammatory bowel disease: Is it genes or
environment? World J Gastroenterol. 17:2715–2722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Clinical presentation, diagnosis, pathogenesis and
treatment options for lymphocytic colitis (Review). Int J Mol Med.
32:263–270. 2013.PubMed/NCBI
|
|
9
|
El-Salhy M, Seim I, Chopin L, Gundersen D,
Hatlebakk JG and Hausken T: Irritable bowel syndrome: The role of
gut neuroendocrine peptides. Front Biosci (Elite Ed). 4:2783–2800.
2012.PubMed/NCBI
|
|
10
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable bowel syndrome: Diagnosis, pathogenesis, and
treatment options. Nova Science Publishers, Inc., New York;
2012
|
|
11
|
El-Salhy M: Irritable bowel syndrome:
Diagnosis and pathogenesis. World J Gastroenterol. 18:5151–5163.
2012.PubMed/NCBI
|
|
12
|
El-Salhy M, Ostgaard H, Gundersen D,
Hatlebakk JG and Hausken T: The role of diet in the pathogenesis
and management of irritable bowel syndrome (Review). Int J Mol Med.
29:723–731. 2012.PubMed/NCBI
|
|
13
|
Mawe GM, Coates MD and Moses PL: Review
article: Intestinal serotonin signalling in irritable bowel
syndrome. Aliment Pharmacol Ther. 23:1067–1076. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wade PR, Chen J, Jaffe B, Kassem IS,
Blakely RD and Gershon MD: Localization and function of a 5-HT
transporter in crypt epithelia of the gastrointestinal tract. J
Neurosci. 16:2352–2364. 1996.PubMed/NCBI
|
|
15
|
Gershon MD and Tack J: The serotonin
signaling system: From basic understanding to drug development for
functional GI disorders. Gastroenterology. 132:397–414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gershon MD: 5-Hydroxytryptamine
(serotonin) in the gastrointestinal tract. Curr Opin Endocrinol
Diabetes Obes. 20:14–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gershon MD: Serotonin is a sword and a
shield of the bowel: Serotonin plays offense and defense. Trans Am
Clin Climatol Assoc. 123:268–280. 2012.PubMed/NCBI
|
|
18
|
El-Salhy M, Mazzawi T, Gundersen D,
Hatlebakk JG and Hausken T: The role of peptide YY in
gastrointestinal diseases and disorders (review). Int J Mol Med.
31:275–282. 2013.PubMed/NCBI
|
|
19
|
Dubrasquet M, Bataille D and Gespach C:
Oxyntomodulin (glucagon-37 or bioactive enteroglucagon): A potent
inhibitor of pentagastrin-stimulated acid secretion in rats. Biosci
Rep. 2:391–395. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schjoldager B, Mortensen PE, Myhre J,
Christiansen J and Holst JJ: Oxyntomodulin from distal gut. Role in
regulation of gastric and pancreatic functions. Dig Dis Sci.
34:1411–1419. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schjoldager BT, Baldissera FG, Mortensen
PE, Holst JJ and Christiansen J: Oxyntomodulin: A potential hormone
from the distal gut. Pharmacokinetics and effects on gastric acid
and insulin secretion in man. Eur J Clin Invest. 18:499–503. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dakin CL, Small CJ, Batterham RL, Neary
NM, Cohen MA, Patterson M, Ghatei MA and Bloom SR: Peripheral
oxyntomodulin reduces food intake and body weight gain in rats.
Endocrinology. 145:2687–2695. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wynne K, Park AJ, Small CJ, Patterson M,
Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA and
Bloom SR: Subcutaneous oxyntomodulin reduces body weight in
overweight and obese subjects: A double-blind, randomized,
controlled trial. Diabetes. 54:2390–2395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Salhy M and Hausken T: The role of the
neuropeptide Y (NPY) family in the pathophysiology of inflammatory
bowel disease (IBD). Neuropeptides. 55:134–144. 2016. View Article : Google Scholar
|
|
25
|
Camilleri M: Peripheral mechanisms in
irritable bowel syndrome. N Engl J Med. 367:1626–1635. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jianu CS, Fossmark R, Syversen U, Hauso Ø
and Waldum HL: A meal test improves the specificity of chromogranin
A as a marker of neuroendocrine neoplasia. Tumour Biol. 31:373–380.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Salhy M, Danielsson A, Stenling R and
Grimelius L: Colonic endocrine cells in inflammatory bowel disease.
J Intern Med. 242:413–419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Chromogranin a cell density as a diagnostic marker for
lymphocytic colitis. Dig Dis Sci. 57:3154–3159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: High densities of serotonin and peptide YY cells in the
colon of patients with lymphocytic colitis. World J Gastroenterol.
18:6070–6075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Salhy M, Lomholt-Beck B and Gundersen
TD: High chromogranin A cell density in the colon of patients with
lymphocytic colitis. Mol Med Rep. 4:603–605. 2011.PubMed/NCBI
|
|
31
|
Moran GW, Pennock J and McLaughlin JT:
Enteroendocrine cells in terminal ileal Crohn's disease. J Crohns
Colitis. 6:871–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Besterman HS, Mallinson CN, Modigliani R,
Christofides ND, Pera A, Ponti V, Sarson DL and Bloom SR: Gut
hormones in inflammatory bowel disease. Scand J Gastroenterol.
18:845–852. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Salhy M, Suhr O and Danielsson A:
Peptide YY in gastrointestinal disorders. Peptides. 23:397–402.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tari A, Teshima H, Sumii K, Haruma K,
Ohgoshi H, Yoshihara M, Kajiyama G and Miyachi Y: Peptide YY
abnormalities in patients with ulcerative colitis. Jpn J Med.
27:49–55. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sciola V, Massironi S, Conte D, Caprioli
F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT and Piodi L:
Plasma chromogranin a in patients with inflammatory bowel disease.
Inflamm Bowel Dis. 15:867–871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bishop AE, Pietroletti R, Taat CW,
Brummelkamp WH and Polak JM: Increased populations of endocrine
cells in Crohn's ileitis. Virchows Arch A Pathol Anat Histopathol.
410:391–396. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manocha M and Khan WI: Serotonin and GI
disorders: An update on clinical and experimental studies. Clin
Transl Gastroenterol. 3:e132012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stoyanova II and Gulubova MV: Mast cells
and inflammatory mediators in chronic ulcerative colitis. Acta
Histochem. 104:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamamoto H, Morise K, Kusugami K, Furusawa
A, Konagaya T, Nishio Y, Kaneko H, Uchida K, Nagai H, Mitsuma T and
Nagura H: Abnormal neuropeptide concentration in rectal mucosa of
patients with inflammatory bowel disease. J Gastroenterol.
31:525–532. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Payer J, Huorka M, Duris I, Mikulecky M,
Kratochvílová H, Ondrejka P and Lukác L: Plasma somatostatin levels
in ulcerative colitis. Hepatogastroenterology. 41:552–553.
1994.PubMed/NCBI
|
|
41
|
Watanabe T, Kubota Y, Sawada T and Muto T:
Distribution and quantification of somatostatin in inflammatory
disease. Dis Colon Rectum. 35:488–494. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koch TR, Carney JA, Morris VA and Go VL:
Somatostatin in the idiopathic inflammatory bowel diseases. Dis
Colon Rectum. 31:198–203. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Khan WI and Ghia JE: Gut hormones:
Emerging role in immune activation and inflammation. Clin Exp
Immunol. 161:19–27. 2010.PubMed/NCBI
|
|
44
|
Margolis KG and Gershon MD: Neuropeptides
and inflammatory bowel disease. Curr Opin Gastroenterol.
25:503–511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bampton PA and Dinning PG: High resolution
colonic manometry-what have we learnt?-A review of the literature
2012. Curr Gastroenterol Rep. 15:3282013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang J, Cortina G, Wu SV, Tran R, Cho JH,
Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, et al: Mutant
neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med.
355:270–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
El-Salhy M and Umezawa K: Treatment with
novel AP-1 and NF-κB inhibitors restores the colonic endocrine
cells to normal levels in rats with DSS-induced colitis. Int J Mol
Med. 37:556–564. 2016.PubMed/NCBI
|
|
48
|
Funakoshi T, Yamashita K, Ichikawa N,
Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T,
Ichihara S, et al: A novel NF-kappaB inhibitor,
dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic
injury in mice. J Crohns Colitis. 6:215–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
El-Salhy M, Umezawa K, Gilja OH, Hatlebakk
JG, Gundersen D and Hausken T: Amelioration of severe TNBS induced
colitis by novel AP-1 and NF-κB inhibitors in rats. Scientific
World Journal. 2014:8138042014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Grimstad T, Bjørndal B, Cacabelos D,
Aasprong OG, Omdal R, Svardal A, Bohov P, Pamplona R, Portero-Otin
M, Berge RK and Hausken T: A salmon peptide diet alleviates
experimental colitis as compared with fish oil. J Nutr Sci.
2:e22013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Stucchi AF, Shofer S, Leeman S, Materne O,
Beer E, McClung J, Shebani K, Moore F, O'Brien M and Becker JM:
NK-1 antagonist reduces colonic inflammation and oxidative stress
in dextran sulfate-induced colitis in rats. Am J Physiol
Gastrointest Liver Physiol. 279:G1298–G1306. 2000.PubMed/NCBI
|
|
52
|
Ota E, Takeiri M, Tachibana M, Ishikawa Y,
Umezawa K and Nishiyama S: Synthesis and biological evaluation of
molecular probes based on the 9-methylstreptimidone derivative
DTCM-glutarimide. Bioorg Med Chem Lett. 22:164–167. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Takeiri M, Tachibana M, Kaneda A, Ito A,
Ishikawa Y, Nishiyama S, Goto R, Yamashita K, Shibasaki S, Hirokata
G, et al: Inhibition of macrophage activation and suppression of
graft rejection by DTCM-glutarimide, a novel piperidine derived
from the antibiotic 9-methylstreptimidone. Inflamm Res. 60:879–888.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ishikawa Y, Tachibana M, Matsui C, Obata
R, Umezawa K and Nishiyama S: Synthesis and biological evaluation
on novel analogs of 9-methylstreptimidone, an inhibitor of
NF-kappaB. Bioorg Med Chem Lett. 19:1726–1728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Umezawa N, Matsumoto N, Iwama S, Kato N
and Higuchi T: Facile synthesis of peptide-porphyrin conjugates:
Towards artificial catalase. Bioorg Med Chem. 18:6340–6350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
el-Salhy M, Sandstrom O, Näsström E,
Mustajbasic M and Zachrisson S: Application of computer image
analysis in endocrine cell quantification. Histochem J. 29:249–256.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Duodenal chromogranin a cell density as
a biomarker for the diagnosis of irritable bowel syndrome.
Gastroenterol Res Pract. 2014:4628562014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Elson CO, Sartor RB, Tennyson GS and
Riddell RH: Experimental models of inflammatory bowel disease.
Gastroenterology. 109:1344–1367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cardoso WV and Lü J: Regulation of early
lung morphogenesis: Questions, facts and controversies.
Development. 133:1611–1624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Darlington GJ: Molecular mechanisms of
liver development and differentiation. Curr Opin Cell Biol.
11:678–682. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fausto N, Campbell JS and Riehle KJ: Liver
regeneration. Hepatology. 43(2 Suppl 1): S45–S53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rawlins EL and Hogan BL: Ciliated
epithelial cell lifespan in the mouse trachea and lung. Am J
Physiol Lung Cell Mol Physiol. 295:L231–L234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zaret KS: Regulatory phases of early liver
development: Paradigms of organogenesis. Nat Rev Genet. 3:499–512.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
64
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ and Clevers H: Identification of stem cells in small
intestine and colon by marker gene Lgr5. Nature. 449:1003–1007.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Barker N, van de Wetering M and Clevers H:
The intestinal stem cell. Genes Dev. 22:1856–1864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cheng H and Leblond CP: Origin,
differentiation and renewal of the four main epithelial cell types
in the mouse small intestine. V. Unitarian theory of the origin of
the four epithelial cell types. Am J Anat. 141:537–561. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Le Douarin NM and Teillet MA: The
migration of neural crest cells to the wall of the digestive tract
in avian embryo. J Embryol Exp Morphol. 30:31–48. 1973.PubMed/NCBI
|
|
68
|
Rawdon BB and Andrew A: Origin and
differentiation of gut endocrine cells. Histol Histopathol.
8:567–580. 1993.PubMed/NCBI
|
|
69
|
Hoffman J, Kuhnert F, Davis CR and Kuo CJ:
Wnts as essential growth factors for the adult small intestine and
colon. Cell Cycle. 3:554–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Korinek V, Barker N, Moerer P, van
Donselaar E, Huls G, Peters PJ and Clevers H: Depletion of
epithelial stem-cell compartments in the small intestine of mice
lacking Tcf-4. Nat Genet. 19:379–383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Montgomery RK and Breault DT: Small
intestinal stem cell markers. J Anat. 213:52–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Potten CS, Booth C, Tudor GL, Booth D,
Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S and Okano H:
Identification of a putative intestinal stem cell and early lineage
marker; musashi-1. Differentiation. 71:28–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kayahara T, Sawada M, Takaishi S, Fukui H,
Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H and Chiba
T: Candidate markers for stem and early progenitor cells, Musashi-1
and Hes1, are expressed in crypt base columnar cells of mouse small
intestine. FEBS Lett. 535:131–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
He XC, Yin T, Grindley JC, Tian Q, Sato T,
Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et
al: PTEN-deficient intestinal stem cells initiate intestinal
polyposis. Nat Genet. 39:189–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang Q, Bermingham NA, Finegold MJ and
Zoghbi HY: Requirement of Math1 for secretory cell lineage
commitment in the mouse intestine. Science. 294:2155–2158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jenny M, Uhl C, Roche C, Duluc I,
Guillermin V, Guillemot F, Jensen J, Kedinger M and Gradwohl G:
Neurogenin3 is differentially required for endocrine cell fate
specification in the intestinal and gastric epithelium. EMBO J.
21:6338–6347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lee CS, Perreault N, Brestelli JE and
Kaestner KH: Neurogenin 3 is essential for the proper specification
of gastric enteroendocrine cells and the maintenance of gastric
epithelial cell identity. Genes Dev. 16:1488–1497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo
FJ, Leiter AB and Tsai MJ: Diabetes, defective pancreatic
morphogenesis and abnormal enteroendocrine differentiation in
BETA2/neuroD-deficient mice. Genes Dev. 11:2323–2334. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ahlgren U, Jonsson J and Edlund H: The
morphogenesis of the pancreatic mesenchyme is uncoupled from that
of the pancreatic epithelium in IPF1/PDX1-deficient mice.
Development. 122:1409–1416. 1996.PubMed/NCBI
|
|
80
|
Schonhoff SE, Giel-Moloney M and Leiter
AB: Minireview: Development and differentiation of gut endocrine
cells. Endocrinology. 145:2639–2644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang G, Bibi S, Du M, Suzuki T and Zhu MJ:
Regulation of the intestinal tight junction by natural polyphenols:
A mechanistic perspective. Crit Rev Food Sci Nutr. Mar
23–2016.(Epub ahead of print). View Article : Google Scholar
|
|
82
|
Yang G, Wang H, Kang Y and Zhu MJ: Grape
seed extract improves epithelial structure and suppresses
inflammation in ileum of IL-10-deficient mice. Food Funct.
5:2558–2563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang G, Xue Y, Zhang H, Du M and Zhu MJ:
Favourable effects of grape seed extract on intestinal epithelial
differentiation and barrier function in IL10-deficient mice. Br J
Nutr. 114:15–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yang GB and Lackner AA: Proximity between
5-HT secreting enteroendocrine cells and lymphocytes in the gut
mucosa of rhesus macaques (Macaca mulatta) is suggestive of a role
for enterochromaffin cell 5-HT in mucosal immunity. J Neuroimmunol.
146:46–49. 2004. View Article : Google Scholar : PubMed/NCBI
|