Introduction
Retinoblastoma, a malignant intraocular tumor that
develops in the retina, is one of the most common ocular diseases
affecting children, particularly young children (<5 years of
age) (1). This tumor may be
monocular or binocular successively or simultaneously, and it can
cause serious damage to patient vision, even resulting in
blindness. Furthermore, if this cancer is not treated early it can
cause local or distant metastasis. This advanced form of
retinoblastoma can be life threatening. Currently, various
treatment strategies have been proposed, including surgery,
radiotherapy, chemotherapy or a combination of these treatments
(1). Chemotherapy is the major
conservative treatment; however, almost all types of chemotherapy
drugs are associated with severe systemic complications. Therefore,
identification of novel therapeutic medicines to supplement
chemotherapy in retinoblastoma is of urgently required. The ideal
drug should inhibit the proliferation of retinoblastoma cells and
also exert protective activity on neurons.
In recent years, accumulating evidence has suggested
that tetramethylpyrazine (TMP), an extract of the Chinese herbal
medicine Chuanxiong, administered in combination with other
treatments may significantly reduce the risk of multidrug
resistance during chemotherapy (2,3) and
inhibit the proliferation and metastasis of various types of cancer
cells, including ovarian carcinoma, hepatocellular carcinoma, and
lymphocytic leukemia cells (4–6).
Vascular endothelial growth factor, hypoxia inducible factor-1α,
the stromal cell-derived factor-1/C-X-C chemokine receptor type 4
(CXCR4) axis and P-glycoprotein may be involved in TMP-mediated
bioactivity (7–9). Currently, the function of TMP in
retinoblastoma remains unknown.
Additionally, Chuanxiong has been used for the
clinical treatment of neural diseases in Chinese medicine practices
for >2,000 years. Pharmacological studies have demonstrated that
TMP improves microcirculation through its anti-platelet aggregation
effects and arteriolar regulation. It also possesses anti-oxidant,
anti-free radical injury and calcium antagonist effects. Thus, TMP
has been used as a supplement to prevent and treat cerebral
ischemia and degenerative diseases of the central nervous system,
including Alzheimer's disease, Parkinson's disease and multiple
sclerosis. More importantly, it has been demonstrated to have only
mild side effects during clinical treatment (10–13).
Furthermore, our previous study demonstrated that
TMP protects primary rat brain neurocytes in vitro by
markedly reducing the intracellular calcium level and inhibiting
glutamate release via regulation of the expression of the chemokine
receptor, CXCR4. It was also demonstrated that the TMP-mediated
suppression of C6 glioma involves inhibition of CXCR4 expression
(14). CXCR4 is a
G-protein-coupled receptor with seven transmembrane-spanning
domains most widely expressed in various types of cancer cells. It
has been reported to mediate various processes that are essential
for cancer progression, including tumor cell proliferation,
metastasis, invasion and angiogenesis (15–17).
Notably, it was observed that TMP does not affect the cell cycle
when C6 glioma cells are at 50–80% confluency. However, it can
induce arrest in the S phase, significantly reducing the G1 and G2
populations of C6 glioma cells compared with controls, when cells
are at 100% confluency (18).
Therefore, TMP may have a dual role in the
inhibition of retinoblastoma growth and the protection of
neurocytes. The present study was undertaken to examine whether TMP
suppresses retinoblastoma cell growth by regulating CXCR4
expression and to determine whether its effect is associated with
cell density.
Materials and methods
Patients
Retinoblastoma tissue was obtained from patients
presenting at the Department of Pathology, Sun Yat-sen University
(Guangzhou, China). The details and clinical demographics of
patients are listed in Table I.
This study was approved by the ethics committee of Sun Yat-sen
University.
 | Table I.Clinical demographics of 12
retinoblastoma patients. |
Table I.
Clinical demographics of 12
retinoblastoma patients.
| Patient number | Age at diagnosis
(months) | Sex (M/F) | UL/BL |
|---|
| 1 | 48 | F | UL |
| 2 | 24 | F | UL |
| 3 | 12 | F | UL |
| 4 | 36 | F | UL |
| 5 | 48 | M | UL |
| 6 | 25 | M | BL |
| 7 | 20 | M | UL |
| 8 | 28 | M | UL |
| 9 | 11 | F | UL |
| 10 | 36 | M | UL |
| 11 | 14 | F | UL |
| 12 | 26 | M | BL |
Reagents
The WERI-Rb1 human retinoblastoma cell line and HeLa
cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). Sprague Dawley (SD) rats were obtained from
the animal center of Zhongshan Ophthalmic Center, Sun Yat-sen
University. TMP, AMD3100, DMSO and propidium iodide (PI) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Rabbit anti-CXCR4 and GAPDH, mouse anti-microtubule associated
protein-2 (MAP-2) primary polyclonal antibodies were purchased from
Abcam (Cambridge, UK; cat. no. ab2047), ProteinTech Group, Inc.
(Chicago, IL, USA; cat. no. 10494-1-AP), and Boster Biological
Technology, Ltd. (Wuhan, China; cat. no. BM1243), respectively.
Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG, Alexa
Fluor 555 anti-rabbit IgG and Alexa Fluor 488 anti-mouse IgG were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA;
cat. nos. 7074, 4413 and 4408, respectively). TRIzol Reagent and
all of the cell culture media/reagents and salt solutions were
obtained from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). A SYBR PrimeScript™ RT-PCR kit was purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). Amersham ECL reagents for
western blotting were obtained from GE Healthcare Bio-Sciences
(Pittsburgh, PA, USA).
Cell culture
WERI-Rb1 and HeLa cells were cultured in RPMI-1640
medium and Dulbecco's modified Eagle's medium (DMEM), respectively,
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 mg/ml streptomycin in a humidified atmosphere of 5% carbon
dioxide at 37°C. For primary retinal neurocyte culture, the
protocol was as follows: Briefly, P1-day-old SD rats (n=9) were
sacrificed by an intraperitoneal injection of Nembutal (60 mg/kg;
Sigma-Aldrich). The retinas that were separated from the enucleated
eyeballs were incubated for 20 min at 37°C in a solution containing
0.125% trypsin, to dissociate cells. To yield a suspension of
single cells, the tissue was then triturated sequentially through a
narrow-bore Pasteur pipette in a solution of DMEM supplemented with
10% fetal bovine serum. Cells were plated at a density of
~1×106 cells/ml on a culture plate pre-coated with 0.01%
poly-L-lysine and cultured in DMEM supplemented with 10% fetal
bovine serum, in a humidified atmosphere of 5% carbon dioxide at
37°C for 2 h. After 12 h, the cells were treated with 10 µM Ara-C
(Sigma-Aldrich) to suppress the growth of non-neurocytes. TMP was
dissolved in component solvent (DMSO: saline, 1:1) and AMD3100 was
dissolved in saline to the appropriate concentrations. The
component solvent and saline were applied as vehicle controls.
Cell viability assays using cell
counting Kit-8 (CCK-8)
Cell viability was measured using a CCK-8 (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). Exponentially
growing WERI-Rb1 cells (1×105 or 7.5×105
cells/ml) were seeded in a 96-well plate in 100 µl complete medium
for 4 h at 37°C prior to addition of 200 µM TMP, 10 µg/ml AMD3100
or a vehicle control. After 24 h of treatment, 10 µl CCK-8 reagent
was added to each well and incubated at 37°C for 1 h. Subsequently,
absorbance was measured at 450 nm using a fluorescence plate reader
(Power Wave XS; BioTek China, Beijing, China). Cell viability was
determined by the optical density ratio of treated cells over the
untreated control.
Cell cycle assay
WERI-Rb1 cells were plated at a high cell density
(7.5×105 cells/ml) or low cell density (1×105
cells/ml) and treated with TMP (200 µM) or a vehicle control. Cells
were collected after 24 h of treatment and washed with ice-cold PBS
and then fixed with 75% ice-cold ethanol at 4°C overnight. Before
analysis, the cells were washed twice with PBS and incubated in a
PI staining solution (0.05 mg/ml PI, 1 mM EDTA, 0.1% Triton-X-100™
and 1 mg/ml RNase A) for 30 min at 37°C. Subsequently, fluorescent
cells were analyzed using a BD FACSort™ flow cytometer (BD
Biosciences, San Jose, CA, USA). The data were analyzed with Flow
Jo software (Tree Star, Inc., Ashland, OR, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) assay
Total RNA was isolated with TRIzol Reagent and
dissolved in RNase-free water. Total RNA (1 µg) was subjected to
reverse transcription using a PrimeScript™ RT Reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China) following the
manufacturer's protocol.
Semi-quantitative PCR was performed to measure the
expression of CXCR4 in WERI-Rb1 cells and HeLa cells under
normal growth conditions using an automated thermocycler (Biometra
GmbH, Göttingen, Germany). The PCR program was as follows:
Pre-denaturation at 94°C for 5 min; and 30 cycles of denaturation
at 94°C for 1 min, annealing at 60°C, and extension at 72°C for 1
min. PCR products were separated by 2% agarose gel electrophoresis,
and the band intensities on the resulting gels were determined by
Scion Image software (Scion Image Corporation, Fredrick, MD, USA).
β-actin gene expression was examined as an internal control.
Quantitative PCR was employed to compare the expression of
CXCR4 in WERI-Rb1 cells treated with TMP (200 µM) or a
vehicle control using the SYBR Green system (Takara Biotechnology
Co., Ltd.), using the aforementioned thermocycling conditions. The
quantity of target gene mRNA relative to that of the internal
control gene, β-actin, was calculated using the
2−ΔΔCT method (19).
The following primer pairs were used: CXCR4,
5′-CTTATCCTGCCTGGTATTGTC-3′ and 5′-CAATGTAGTAAGGCAGCCAAC-3′; and
for β-actin, 5′-CACCACACCTTCTACAATGAG-3′ and
5′-TAGCACAGCCTGGATAGCAAC-3′. The data were analyzed in
triplicate.
Western blot assay
WERI-Rb1 cells were seeded in 60 mm dishes at
different cell densities (1×105 or 7.5×105
cells/ml) and treated with TMP (200 µM) or a vehicle control for
varying durations (12 or 24 h). After treatment, the cells were
washed with PBS and collected in radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China).
According to the bicinchoninic acid method for protein
quantification, equal amounts of protein (30 µg/well) were
separated on an 8% SDS-polyacrylamide gel by electrophoresis and
electrophoretically transferred to a PVDF membrane (EMD Millipore,
Billerica, MA, USA) at 250 mA for 1.5 h. The membrane was blocked
with a solution of 5% dried fat-free milk in TBS-Tween (TBS
containing 0.1% Tween-20) for 2 h, and then membrane-bound proteins
were probed with primary antibodies against CXCR4 (1:500 dilution)
and GAPDH (1:1,000 dilution) at 4°C overnight. Subsequently, the
membrane was washed 3 times with TBST for 5 min each time, followed
by incubation with HRP-conjugated anti-rabbit IgG (1:10,000) for 1
h at room temperature. GAPDH served as an internal control.
Finally, protein bands were detected with enhanced
chemiluminescence (EMD Millipore). Densitometric analysis of the
bands compared with the density of CXCR4 and GAPDH was performed
using ImageJ software (imagej.nih.gov).
Immunohistofluorescence assay
Human retinoblastoma tissues were originally fixed
with 10% formalin and then embedded in optimal cutting temperature
compound. Retinoblastoma tissue sections of 6 µm were used. Human
retinoblastoma tissue, untreated WERI-Rb1 cells and primary
cultured neurocytes were fixed with ice-cold 100% methanol for 15
min and then blocked with 10% normal goat serum (Cell Signaling
Technology, Inc., Danvers, MA, USA) for 30 min. Subsequently, the
human retinoblastoma tissue and untreated WERI-Rb1 cells were
incubated overnight at 4°C with primary antibody against CXCR4
(1:500 dilution), and primary cultured neurocytes were incubated
with primary antibodies against CXCR4 (1:500 dilution) and MAP-2
(1:100 dilution). Alexa Fluor 555 anti-rabbit lgG and Alexa Fluor
488 anti-mouse lgG were used as secondary antibodies (1:500
dilution) for a further 1 h at room temperature, and nuclei were
stained with DAPI.
H2O2-induced
damage and MTT viability assays
Primary cultured rat retinal neurons were seeded in
a 24-well plate in 500 µl complete medium and then treated with TMP
(200 µM) or a vehicle control for 48 h at 37°C. After the TMP
treatment, the cells were treated with H2O2
(600 µM) for 15 min at 37°C. Subsequently, they were incubated in
H2O2-free medium with TMP or a vehicle
control at 37°C for 2.5 h. After 0, 24 or 48 h, 50 µl MTT was add
to each well, the plate was incubated for 4 h at 37°C and 500 µl
DMSO was added to each well. Absorbance was measured at 490 nm
using a fluorescence plate reader (Power Wave XS). Cell viability
was determined according to the optical density ratio of a treated
culture over an untreated control.
Statistical analysis
All experiments were performed at least three times
in vitro. Data are expressed as the mean ± standard error.
Differences between mean values were evaluated with two-tailed
Student's t-test (for 2 groups). All calculations and statistical
tests were performed using Excel 2003 (Microsoft Corporation,
Redmond, WA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
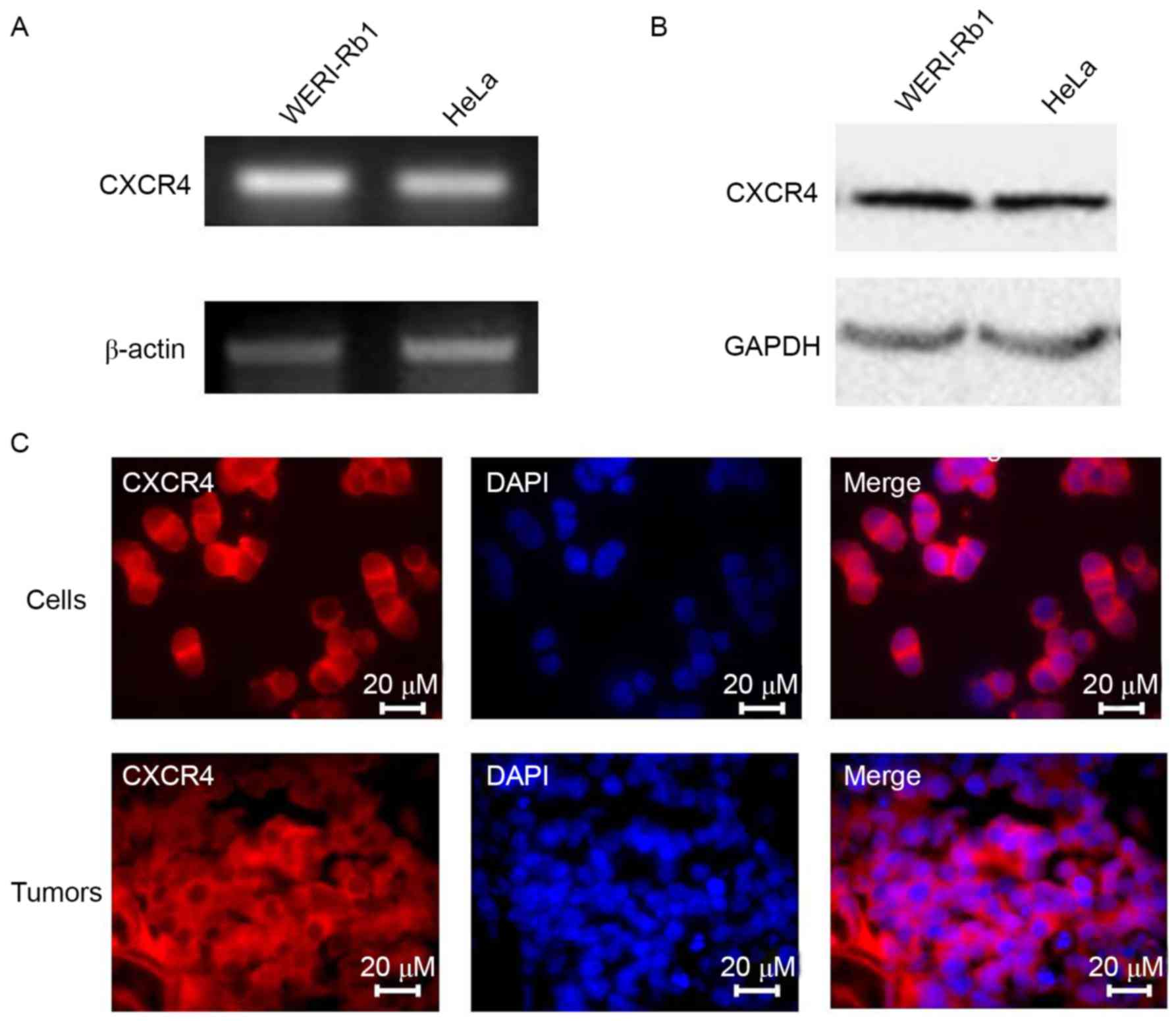
CXCR4 is expressed in WERI-Rb1 cells
and retinoblastoma tissue
As CXCR4 has important roles in cancer development
and progression (15), CXCR4
expression in WERI-Rb1 cells and in HeLa cells was determined as a
positive control. Total RNA and whole-cell lysates were obtained
for semi-quantitative PCR and western blot analyses. As
demonstrated in Fig. 1A and B,
CXCR4 mRNA and protein expression was higher in WERI-Rb1 cells
compared with HeLa cells. Immunofluorescence staining confirmed the
expression of CXCR4 in the cytoplasm of WERI-Rb1 cells and
retinoblastoma tissue (Fig. 1C).
Therefore, it may be a potential therapeutic target for
retinoblastoma treatment.
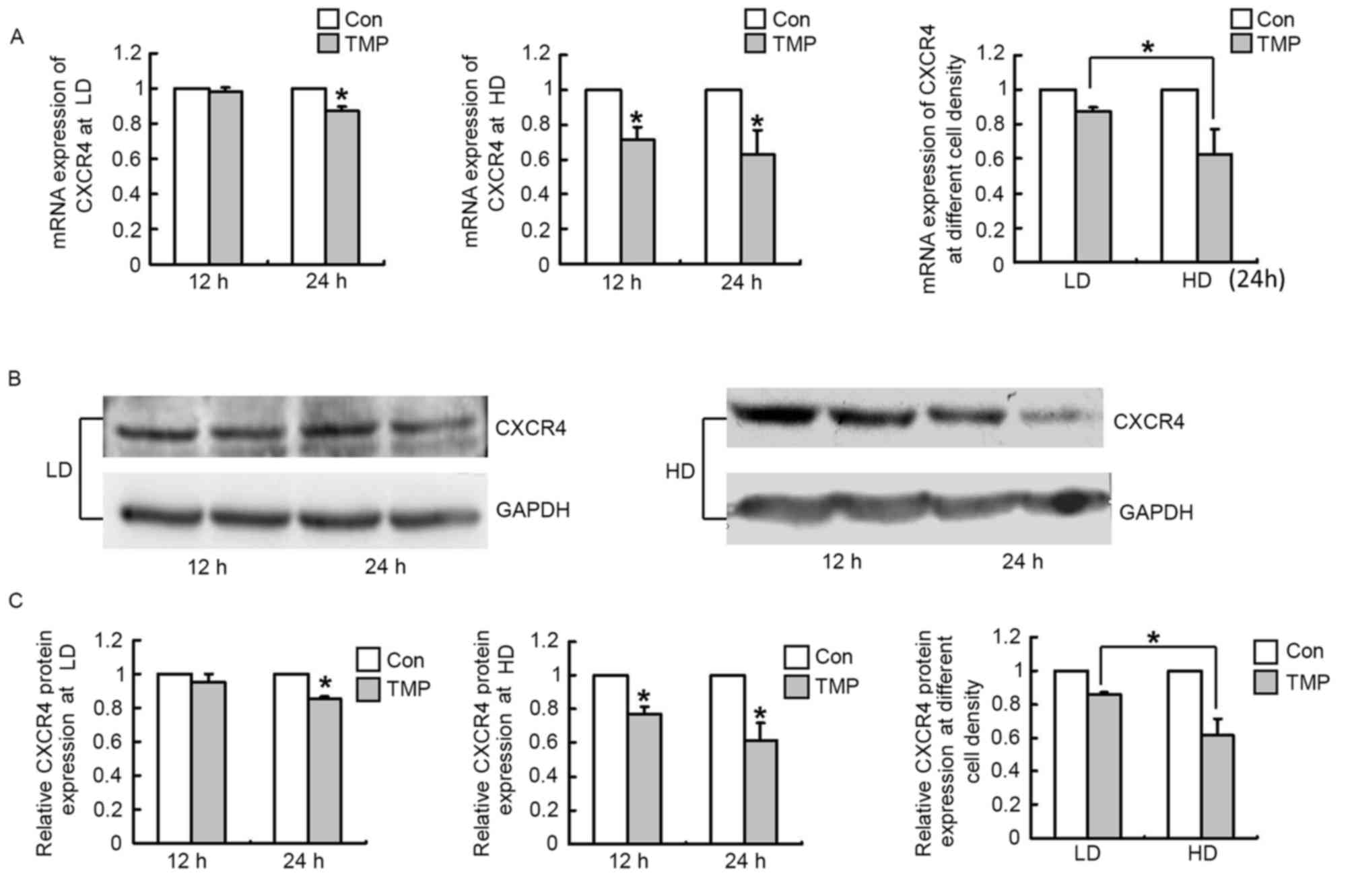
TMP-mediated downregulation of CXCR4
in WERI-Rb1 cells is dependent on cell density
It was previously reported that TMP suppresses the
growth of C6 glioma by reducing the expression of CXCR4 (14). To examine whether TMP downregulates
CXCR4 expression in WERI-Rb1 cells and to determine whether its
effect is associated with cell density, we measured the mRNA and
protein levels of CXCR4 after TMP treatment (12 or 24 h) in
WERI-Rb1 cells plated at a low density (1×105 cells/ml)
or high density (7.5×105 cells/ml). Fig. 2A demonstrated that the mRNA
expression of CXCR4 in WERI-Rb1 cells was not altered after TMP
treatment for 12 h at the low density; no significant difference
was observed between the test and control groups. At 24 h, CXCR4
expression was marginally downregulated by the TMP treatment,
exhibiting a 12.8% decrease (P<0.05). However, CXCR4 mRNA
expression was significantly decreased after TMP treatment for 12
and 24 h at the high cell density, exhibiting a 28.9 and 37.5%
decrease, respectively (P<0.05; Fig. 2A). Moreover, the decrease in CXCR4
mRNA expression at the high cell density was 2.93-fold greater than
that at the low cell density at the 24 h time point (P<0.05;
Fig. 2). The results of western
blotting were consistent with those of RT-qPCR (Fig. 2B). Relative quantification of CXCR4
expression revealed that there was also no significant difference
between the test and control groups after TMP treatment at the 12 h
time point at the low density, with a 14.3% decrease in CXCR4
expression at the 24 h time point (P<0.05; Fig. 2C). Similarly, its expression was
markedly decreased after TMP treatment for 12 and 24 h at the high
cell density, exhibiting a 22.9 and 38.7% decrease, respectively
(P<0.05; Fig. 2C), and the
decrease in its expression at the high cell density was 2.76-fold
greater than that at the low cell density at 24 h time point
(P<0.05; Fig. 2C). These
findings further confirm that TMP significantly inhibits CXCR4
expression in WERI-Rb1 cells and that its effect is sensitive to
cell density. Furthermore, TMP treatment downregulated CXCR4
expression in WERI-Rb1 cells in a time-dependent manner (Fig. 2). These results further support the
hypothesis that CXCR4 may be involved in mediating the effect of
TMP on retinoblastoma and that TMP-mediated downregulation of CXCR4
may be associated with cell density.
Effects of TMP on inhibiting
proliferation and the cell cycle of WERI-Rb1 cells are dependent on
cell density
Considering that the TMP-mediated downregulation of
CXCR4 in WERI-Rb1 cells depends on cell density and that CXCR4
expression is closely associated with to the proliferation of
cancer cells, WERI-Rb1 cells were seeded at different cell
densities (105 or 7.5×105 cells/ml) to
determine the bioactivity of TMP on WERI-Rb1 cell growth and
whether the inhibitory effect is associated with cell density. AMD
3100, a CXCR4 antagonist, was used as a positive control. As
presented in Fig. 3A, TMP (200 µM)
reduced WERI-Rb1 cell viability at after 24 h of treatment, and
this inhibition was stronger at the high cell density than at the
low cell density, with inhibition rates of 10 and 27%, respectively
(P<0.05). AMD3100-treated WERI-Rb1 cells also exhibited reduced
viability in a density-dependent manner, with inhibition rates of 8
and 24% at the low cell density and at the high cell density,
respectively (P<0.05; Fig. 3B).
The inhibition of cell growth may be a result of cell cycle arrest.
To determine whether the inhibitory effects of TMP on WERI-Rb1 cell
proliferation at different cell densities involves cell cycle
alterations, the cell cycle profiles of WERI-Rb1 cells at different
cell densities were examined by flow cytometry. The cell cycle was
analyzed after treatment with TMP for 24 h, and the results
demonstrated that at a low cell density (1×105
cells/ml), there were no significant differences in the cell cycle
profiles of WERI-Rb1 cells between the test and control groups
(Fig. 3C). However, TMP treatment
was capable of inducing G1 phase arrest (62.83±7.53%) in the
high-density cells (7.5×105 cells/ml) compared with
control cells (55.52±6.86%; P<0.05; Fig. 3D). Therefore, the results
demonstrated that the inhibition of WERI-Rb1 cell proliferation by
TMP was greater at the high cell density than at the low density.
Furthermore, its inhibitory effect on cell proliferation was
induced by other factors besides the arrest of the cell cycle at
the G1 phase and cell cycle inhibition.
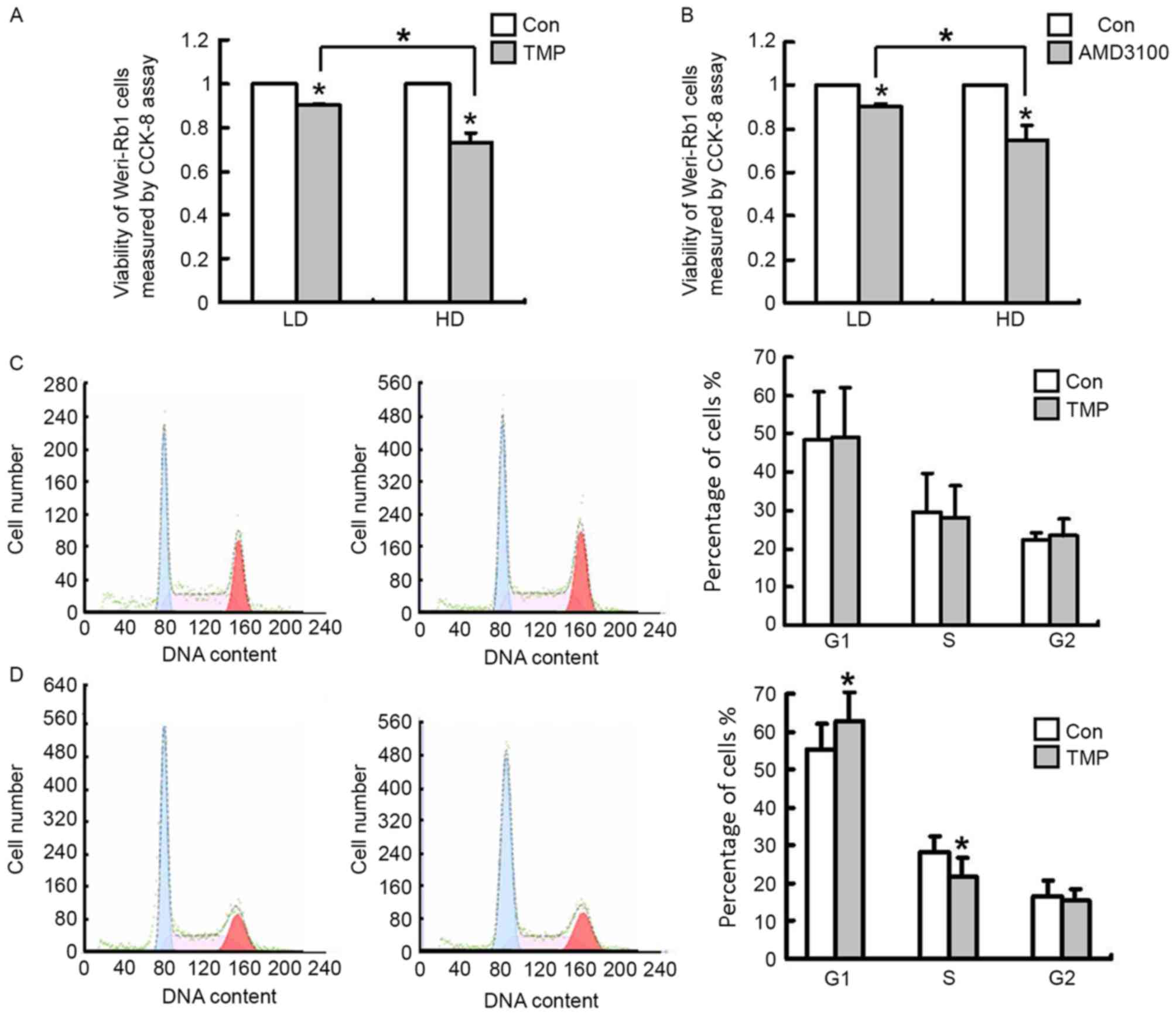 | Figure 3.TMP (200 µM) reduces cell viability
and affects the cell cycle of WERI-Rb1 cells. (A) Viability of HD
WERI-Rb1 cells (7.5×105 cells/ml) is reduced by TMP
treatment. By contrast, the inhibitory effect of TMP is less potent
at LD (1×105 cells/ml). (B) The inhibitory effect of
AMD3100 (10 ng/ml), a CXCR4 antagonist, on WERI-Rb1 cells is also
sensitive to cell density. Cell viability was analyzed by the CCK-8
assay, and the data are presented as the survival rate relative to
Con group. (C) TMP (200 µM for 24 h) does not alter the cell cycle
profile of LD WERI-Rb1 cells. (D) WERI-Rb1 cells arrested at the
G1phase fail to enter into the S and G2/M phases compared with
controls when they are cultured at HD. All results were confirmed
in three independent experiments (*P<0.05 vs. Con, or comparison
indicated by brackets). CCK-8, Cell Counting Kit-8; Con, control;
TMP, tetramethylpyrazine; LD, low cell density; HD, high cell
density. |
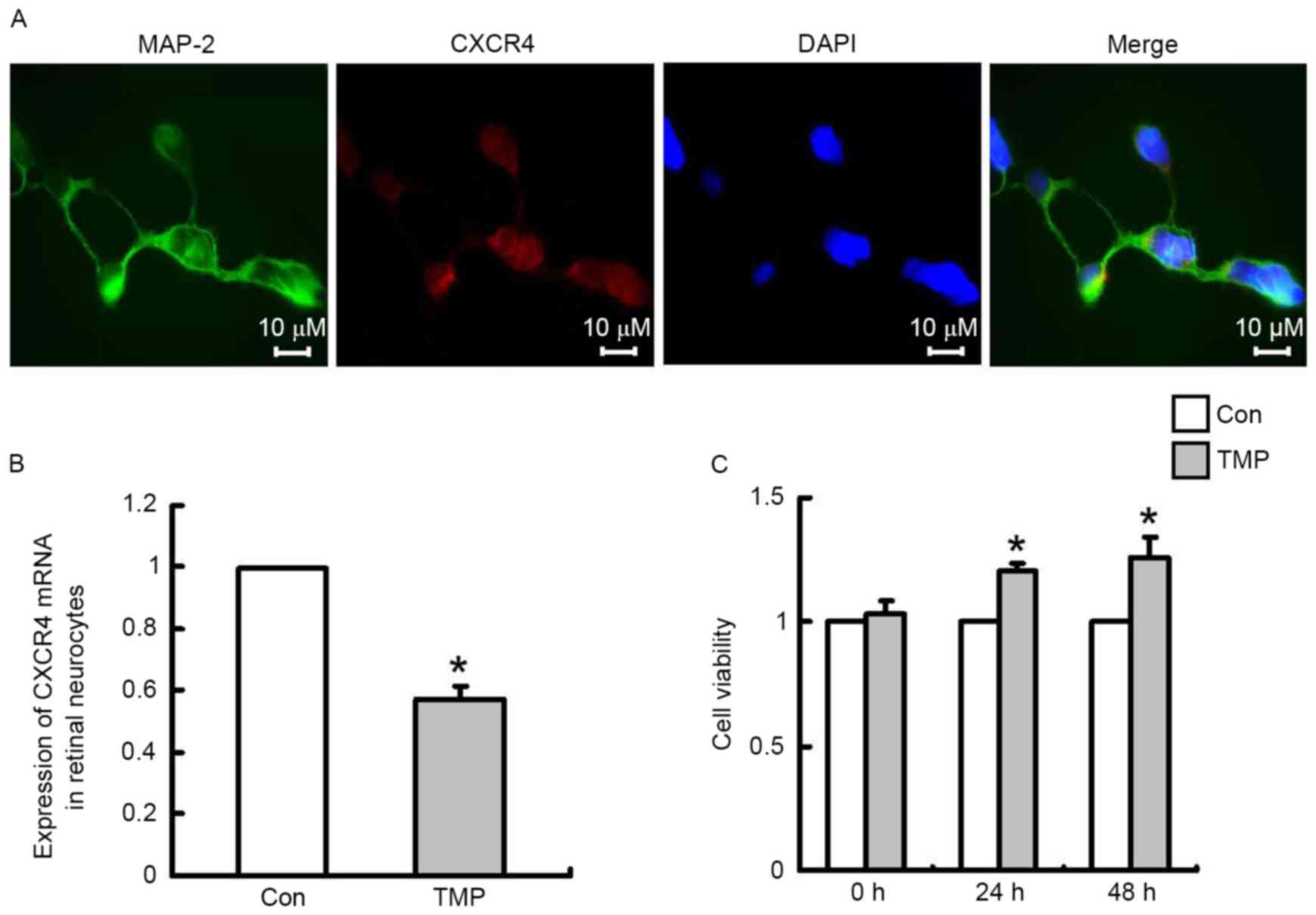
TMP downregulates the expression of
CXCR4 in primary rat retinal neurocytes and protects these cells
from H2O2-induced damage
Retinoblastoma is a malignant intraocular tumor that
arises in the retina, and development of this type of tumor can
cause injury to retinal neurons. Our previous studies have verified
that TMP protects rat cerebral neurons by downregulating CXCR4
expression (14). To confirm that
this extract has protective effects on retinal neurons, the
expression of CXCR4 in primary rat retinal neurocytes was measured
in vitro. Dual immunofluorescence staining for MAP-2 and
CXCR4 revealed that CXCR4 was expressed in retinal neurons. MAP-2
was used as a marker of neuronal cells (Fig. 4A). After TMP treatment for 24 h,
RT-qPCR was performed to determine CXCR4 mRNA expression in primary
retinal neurocytes. As demonstrated in Fig. 4B, TMP significantly inhibited CXCR4
mRNA expression in these cells compared with the control cells
(P<0.05), with an inhibition rate of 42.9%. Subsequently,
retinal neurocytes were pretreated with TMP or a vehicle control
for 48 h and then the cells were exposed to
H2O2 (600 µM) for 15 min. Subsequently, the
cells were incubated in an H2O2-free medium
with TMP or a vehicle control at 37°C. After 0, 24 or 48 h, an MTT
assay was performed to measure cell viability. As demonstrated in
Fig. 4C, neurons in the
experimental group exhibited increased cell viability compared with
the control group, with a viable cell rate increase to 120% (24 h)
or 126% (48 h) relative to the control (P<0.05); at 0 h no
significant difference was observed between the two groups.
Additionally, the effect occurred in a time-dependent manner.
Therefore, the results indicated that TMP possesses an
anti-retinoblastoma function and exerts a protective effect on
neurons.
Discussion
A growing body of evidence suggests that TMP
possesses a potent inhibitory effect on cancer, and the relevant
molecular mechanisms involved have been reported to include
decreased DNA synthesis in cells via inhibition of mitosis and
reduced of the expression of resistant genes (2). The present study demonstrated that
TMP downregulated the expression of CXCR4 at the RNA and protein
levels in WERI-Rb1 cells, and that it reduced WERI-Rb1 cell
viability. Notably, the effect of TMP on WERI-Rb1 cells was
sensitive to cell density. At a high cell density, the expression
of CXCR4 was reduced dramatically, and cell viability was strongly
inhibited by TMP treatment. However, the effect of TMP was minimal
at a low cell density. In addition, TMP downregulated the
expression of CXCR4 in rat retinal neurocytes and protected them
from H2O2-induced damage in vitro.
Previous studies have indicated that CXCR4 has
important roles in tumor progression (15–17).
For example, treatment with AMD3100 (a CXCR4 antagonist) or
chemotherapy combined with AMD3100 has been shown to induce
caspase-3-mediated apoptosis and to inhibit glioma growth in
vivo (20). A high level of
CXCR4 expression promotes tumor proliferation, angiogenesis,
migration and metastasis (21). It
has been demonstrated that the expression of CXCR4 in WERI-Rb1
cells was also dependent on cell density, as expression in
high-density cells was higher than that in low-density cells
(unpublished data). Notably, TMP significantly downregulated
CXCR4 expression in high-density WERI-Rb1 cells, however the
effect was not as potent in cells cultured at low density. Based on
these evidences, we hypothesize that TMP possesses a strong
anti-retinoblastoma effect when a tumor is actively proliferating,
thus may be of therapeutic value to supplement chemotherapy to
inhibit tumor growth and metastasis. Elucidation of the mechanism
of the TMP-mediated downregulation of CXCR4 in high-density
cells requires further investigation.
CXCR4 is closely associated with the cell cycle
(22,23), and its downregulation results in
reductions in the expression of certain cell cycle-associated
proteins, including cyclin D1, which is a subtype of cyclin D that
affects the G1/S phase control point in the cell cycle (24,25).
Accordingly, the cell cycle profile data in the current study
demonstrated that TMP treatment resulted in arrest of WERI-Rb1
cells in the G1 phase when the cells were cultured to a high
density. These cell cycle data are similar to the results of our
previous study (18), revealing
that TMP only affects the cell cycle of glioma C6 cells at 100%
confluency. However, the different cell type the previous study
produced differing results, with TMP inducing arrest in the S phase
in C6 cells, and significantly reducing the G1 and G2 populations
compared with control cells (18).
Therefore, TMP may reduce CXCR4 expression, and subsequently arrest
the cell cycle at the G1 phase, eventually inhibiting tumor cell
proliferation in retinoblastoma. Additionally, in the current
study, the cell cycle of WERI-Rb1 cells was not altered after TMP
treatment for 24 h at a low cell density, with only a minor
inhibitory effect on cell proliferation. These data suggest that
the cell cycle inhibition may a factor that affect retinoblastoma
cell proliferation.
Furthermore, Chuanxiong has been used in the
clinical setting >2,000 years, and its bioactive extract, TMP,
is also an effective medicine currently used in the treatment of
neural diseases in China (10–13).
Our previous study has demonstrated that CXCR4 is a target gene of
TMP in neural protection (14).
The results of the present study demonstrated that CXCR4 was
expressed in retinal MAP-2-positive cells in vitro, and TMP
significantly downregulated CXCR4 expression in retinal
neurocytes and increased cell viability. Therefore, TMP not only
inhibits retinoblastoma cell growth, it also protects retinal
neurocytes.
In conclusion, the findings of the present study
suggest that the TMP-mediated CXCR4 pathway may be sensitive
to cell density. TMP could be a potentially effective and safe
therapeutic agent to supplement chemotherapy during retinoblastoma
treatment. The current study will extend the application of TMP
treatment in clinical therapy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation (project no. 81370987).
References
|
1
|
Meel R, Radhakrishnan V and Bakhshi S:
Current therapy and recent advances in the management of
retinoblastoma. Indian J Med Paediatr Oncol. 33:80–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Liu X, Zuo T, Liu Y and Zhang JH:
Tetramethylpyrazine reverses multidrug resistance in breast cancer
cells through regulating the expression and function of
P-glycoprotein. Med Oncol. 29:534–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang XB, Wang SS, Zhang QF, Liu M, Li HL,
Liu Y, Wang JN, Zheng F, Guo LY and Xiang JZ: Inhibition of
tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug
resistant human hepatocellular carcinoma cells. Oncol Rep.
23:211–215. 2010.PubMed/NCBI
|
|
4
|
Yin J, Yu C, Yang Z, He JL, Chen WJ, Liu
HZ, Li WM, Liu HT and Wang YX: Tetramethylpyrazine inhibits
migration of SKOV3 human ovarian carcinoma cells and decreases the
expression of interleukin-8 via the ERK1/2, p38 and AP-1 signaling
pathways. Oncol Rep. 26:671–679. 2011.PubMed/NCBI
|
|
5
|
Cao J, Miao Q, Miao S, Bi L, Zhang S, Yang
Q, Zhou X, Zhang M, Xie Y, Zhang J and Wang S: Tetramethylpyrazine
(TMP) exerts antitumor effects by inducing apoptosis and autophagy
in hepatocellular carcinoma. Int Immunopharmacol. 26:212–220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang XJ, Xu YH, Yang GC, Chen HX and Zhang
P: Tetramethylpyrazine inhibits the proliferation of acute
lymphocytic leukemia cell lines via decrease in GSK-3β. Oncol Rep.
33:2368–2374. 2015.PubMed/NCBI
|
|
7
|
Jiang Y, Liu C, Chen W, Wang H, Wang C and
Lin N: Tetramethylpyrazine enhances vascularization and prevents
osteonecrosis in steroid-treated rats. Biomed Res Int.
2015:3158502015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Deng M and Zhou S:
Tetramethylpyrazine inhibits hypoxia-induced pulmonary vascular
leakage in rats via the ROS-HIF-VEGF pathway. Pharmacology.
87:265–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai X, Chen Z, Pan X, Xia L, Chen P, Yang
Y, Hu H, Zhang J, Li K, Ge J, et al: Inhibition of angiogenesis,
fibrosis and thrombosis by tetramethylpyrazine: Mechanisms
contributing to the SDF-1/CXCR4 axis. PLoS One. 9:e881762014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You JM, Zhang ZG, Liu C and Ji YX:
Ischemic stroke and the regulation of syndrome of traditional
Chinese medicine compound efficacy TMP combined. Chin Archives
Tradit Chin Med. 28:2666–2668. 2010.
|
|
11
|
Tang ZY, Wang SL and Lin Y: Progress in
protective effects of tetramethylpyrazine on diabetes complications
in nervous system and possible mechanisms. Chin J Pharmacol
Toxicol. 25:114–118. 2011.
|
|
12
|
Chen Y and Liu M: Systemic evaluation of
security of ligustrazine for treatment of cerebral infarction. Chin
J Clin Rehab. 8:1299–1301. 2004.
|
|
13
|
Yang XG and Jiang C: Ligustrazine as a
salvage agent for patients with relapsed or refractory
non-Hodgkin's lymphoma. Chin Med J (Engl). 123:3206–3211.
2010.PubMed/NCBI
|
|
14
|
Chen Z, Pan X, Georgakilas AG, Chen P, Hu
H, Yang Y, Tian S, Xia L, Zhang J, Cai X, et al:
Tetramethylpyrazine (TMP) protects cerebral neurocytes and inhibits
glioma by down regulating chemokine receptor CXCR4 expression.
Cancer Lett. 336:281–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Furusato B, Mohamed A, Uhlén M and Rhim
JS: CXCR4 and cancer. Pathol Int. 60:497–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X and Chen X: Study on the effects of
tetramethylpyrazine on tumor cells: Survey and prospects. Zhongguo
Zhong Yao Za Zhi. 28:295–298. 2003.(In Chinese). PubMed/NCBI
|
|
17
|
Muller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu K, Chen Z, Pan X, Yang Y, Tian S, Zhang
J, Ge J, Ambati B and Zhuang J: Tetramethylpyrazine-mediated
suppression of C6 gliomas involves inhibition of chemokine receptor
CXCR4 expression. Oncol Rep. 28:955–960. 2012.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schulte A, Günther HS, Phillips HS,
Kemming D, Martens T, Kharbanda S, Soriano RH, Modrusan Z, Zapf S,
Westphal M and Lamszus K: A distinct subset of glioma cell lines
with stem cell-like properties reflects the transcriptional
phenotype of glioblastomas and overexpresses CXCR4 as therapeutic
target. Glia. 59:590–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Darash-Yahana M, Pikarsky E, Abramovitch
R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E
and Peled A: Role of high expression levels of CXCR4 in tumor
growth, vascularization, and metastasis. FASEB J. 18:1240–1242.
2004.PubMed/NCBI
|
|
22
|
Mo W, Chen J, Patel A, Zhang L, Chau V, Li
Y, Cho W, Lim K, Xu J, Lazar AJ, et al: CXCR4/CXCL12 mediate
autocrine cell-cycle progression in NF1-associated malignant
peripheral nerve sheath tumors. Cell. 152:1077–1090. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang P, Wang G, Huo H, Li Q, Zhao Y and
Liu Y: SDF-1/CXCR4 signaling up-regulates survivin to regulate
human sacral chondrosarcoma cell cycle and epithelial-mesenchymal
transition via ERK and PI3K/AKT pathway. Med Oncol. 32:3772015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu T, Wu Y, Huang Y, Yan C, Liu Y, Wang Z,
Wang X, Wen Y, Wang C and Li L: RNAi targeting CXCR4 inhibits tumor
growth through inducing cell cycle arrest and apoptosis. Mol Ther.
20:398–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji AJ, Liu SL, Ju WZ and Huang XE:
Anti-proliferation effects and molecular mechanisms of action of
tetramethypyrazine on human SGC-7901 gastric carcinoma cells. Asian
Pac J Cancer Prev. 15:3581–3586. 2014. View Article : Google Scholar : PubMed/NCBI
|