Introduction
Sepsis is defined as a systemic inflammatory
response syndrome caused by infection, which induces an exacerbated
inflammatory response of the host immune system, mediated by
cytokine-induced immune cells, and causes changes in cellular
processes, including apoptosis (1). Globally, severe sepsis, septic shock
and the resulting organ failure are the most common causes of
mortality in intensive care units (ICUs), with mortality rates of
40–70%. Sepsis is a major cause of mortality, estimated to cause
~250,000 mortalities in the USA, annually. The survival rates of
patients with sepsis and infection in ICUs are poor. In Mexico, a
study of 21 hospitals, which included 1,039 children, reported an
incidence of sepsis in 16% of patients, and the most vulnerable age
group was 1–5 years (2). In
addition, another study conducted in 135 ICUs in Mexico reported
that sepsis was one of the most common causes of admission in 27.3%
of ICUs, with pneumonia being the primary cause of sepsis in the
majority (33%) of cases (3).
Septic shock was the leading cause of mortality in 30.4% of ICUs
analyzed in the study. In an ICU from Mexico City, sepsis and
septic shock were the initial diagnoses on admission (3,4).
Currently, it is not clear whether necrosis or
apoptosis have a predominant role in severe sepsis (4). The pathogenesis of multiple organ
dysfunction (MOD) and failure in patients with severe sepsis is
multifactorial. Sepsis triggers the secretion of pro-inflammatory
cytokines, activation of lymphocytes and other processes. This
syndrome has also been reported to cause immunosuppression by
decreasing the number of T and B lymphocytes as result of an
increase in apoptosis (5). A
previous study has demonstrated that apoptosis exacerbates sepsis,
and elevated expression of Bcl-2 apoptosis regulator (Bcl-2) or
administration of caspase inhibitors protects against lymphocyte
apoptosis and improves survival in a mouse model of sepsis
(6). Sepsis triggers apoptosis in
various organs leading to septic shock and MOD. It was previously
observed that the failure of these organs was associated with
increased expression of Fas cell surface death receptor (Fas),
therefore, apoptosis may be correlated with the severity of sepsis
(7). During sepsis, the immune
system is exposed to bacterial antigens, such as lipopolysaccharide
(LPS), which induces tissue damage. Thus, activated immune cell
death is a potential mechanism for the induction of T cell
apoptosis and the loss of immune function during sepsis. LPS
initiates a rapid immune response and leads to leukocyte
activation, coupled with the overproduction of pro-inflammatory
mediators, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1, IL-6, IL-12, interferon-γ, eicosanoids and free radicals of
nitric oxide, which are responsible for tissue damage that leads to
MOD. Subsequently, cytokine production switches from
pro-inflammatory to anti-inflammatory Th2 cytokines, including
IL-4, IL-5 and IL-10, and monocyte and dendritic cell apoptosis is
deactivated, which results in immunosuppression (8–10).
Apoptosis affects subpopulations of lymphocytes, including
CD8+ and CD4+ T cells, B cells and dendritic
cells. Furthermore, it has been observed that downregulation of the
immune response by regulatory T cells, and deactivation of the
immune response mediated by Th1 cells, contributes to
immunosuppression in sepsis (11,12).
Certain clinical and experimental observations have suggested that
apoptotic cell death has an important role in the pathogenesis and
development of MOD in patients with sepsis (4). The high mortality observed in
patients with severe sepsis and those exhibiting a systemic
response to infection may be caused by different levels of
apoptosis. Recent research concluded that TNF-α and Fas-ligand
(FasL) may participate in the induction of apoptosis during severe
sepsis (8). Fas expression has
been previously reported to be associated with decreased
inflammation and MOD, and increased expression of Fas-FasL was
reported to be associated with liver dysfunction, disseminated
intravascular coagulation and the development MOD in children with
severe sepsis (8). The mechanisms
of organ failure caused by sepsis remain unclear, but may be
associated with cell death, indicating that cellular function is
impaired. Organ damage during sepsis varies from marked and
accelerated apoptosis in intestinal epithelium and splenic
dendritic cells, to minimal levels of cell death in other organs
(4). In studies in rats,
macrophage apoptosis was increased following the induction of
sepsis, similar to the effect on monocytes (13). A previous study on patients
postmortem have indicated that the degree of apoptosis in
lymphocytes is associated with the severity of sepsis (14).
The YY1 transcription factor (YY1), also termed δ,
Nuclear Factor Erythroid 1 (NF-E1) or ubiquitous transcription
factor belonging to the GLI-Kruppel class of zinc finger proteins
(UCRBP), is a multifunctional ubiquitous transcription factor with
four zinc finger domains and two specific functions; to act as a
transcriptional repressor or activator. YY1 has been implicated in
various normal biological processes, including embryogenesis,
differentiation, cell replication and proliferation. It is reported
that YY1 binds to the silencing promoter region of Fas, which
decreases the expression of this death receptor, resulting in
resistance to apoptosis mediated by Fas-FasL. Thus, inhibition of
YY1 activity increases in Fas expression and sensitizes tumor cells
to Fas-mediated apoptosis (15–17).
MOD is a lethal complication in children with sepsis, and apoptosis
has an important role in its pathogenesis. Certain studies have
suggested an association between apoptosis and sepsis severity in
children (18–20) and the mechanisms involved may
include the Fas-FasL system. YY1 regulates the expression of Fas
and, therefore, mediates apoptosis via Fas-FasL in tumor cells.
However, at present, to the best of our knowledge, no studies have
demonstrated the role of YY1 in the pathogenesis of sepsis. The
present study assessed the correlation of Fas and YY1 expression
with apoptosis in peripheral blood cells, and with MOD in children
with sepsis.
Materials and methods
Patient information
The present study included a cohort of patients
(n=30) aged between 3 months and 16-years-old. All were diagnosed
with sepsis and admitted to the pediatric ICU of the General
Hospital from Medical Center La Raza, IMSS (Mexico City, Mexico).
The present study was approved by the National Commission for
Scientific Research and the Ethics Board of the Mexican Institute
for Social Security (IMSS R-2008-3502-13) and was performed in
accordance with the Declaration of Helsinki (21). A written informed consent was
obtained from each patient and all samples were obtained according
to National Commission for Scientific Research of the IMSS (CIS)
guidelines. The inclusion criteria were set and the following
clinical information was collected: Name; registration card no.;
sex; age; initial diagnosis and previous antimicrobial treatments.
All patients in the cohort were under 16-years-old, they were
admitted with different diagnoses and were diagnosed with sepsis
during their stay at the hospital. The sepsis diagnosis was treated
as the day 0 time point. A peripheral blood sample was obtained for
immunostaining and terminal deoxynucleotidyl transferase dUTP
nick-end labeling (TUNEL). Clinical evaluation was performed every
24 h to monitor sepsis, pediatric logistic organ dysfunction
(PELOD) and pediatric mortality index (PIM-2), until multiple organ
dysfunction developed or clinical improvement was observed.
Integral clinical evaluation was performed, and special attention
was paid to disease severity and any additional complications. The
severity of the disease was determined using PIM-2 and scoring was
performed with the first hour of arriving at the pediatric ICU. The
presence of MOD was determined using the PELOD scale. Each patient
was evaluated every 24 h during their ICU stay.
MOD was defined as the presence of dysfunction in
two or more organs or systems, including the cardiovascular,
respiratory, neurological, hematological, renal, gastrointestinal
and hepatic systems. Complete blood counts, urinalysis, polyculture
and chest radiographs were performed. If a serologic sample was not
available, one was collected in the pediatric ICU. Furthermore,
additional studies were performed according to severity of the
disease or clinical conditions. Blood samples (5 ml) were collected
from a peripheral vein in an anticoagulant tube and were sent to
the research laboratory for analysis within 30–120 min following
sample collection. Apoptosis in peripheral blood cells was measured
using TUNEL analysis, and Fas and YY1 expression were determined
via immunocytochemistry. A total of 10 peripheral blood samples
were taken from patients who attended the clinical laboratory for
pre-operative studies for use as control samples. The control
patients did not have hematologic disease, infection or cancer, and
informed consent was obtained for the use of the samples.
Purification of peripheral blood
mononuclear cells (PBMCs)
Peripheral blood was diluted 1:1 with saline
solution (SS; 0.9% NaCl) and corresponding volume of sterile
Lymphoprep™ (Cosmo Bio USA, Inc., Carlsbad, CA, USA) was used. The
mixture was centrifuged at 355 × g/room temperature for 30 min.
Subsequently, the intermediate layer formed was separated, which
corresponds to mononuclear cells, and then washed with an equal
amount of SS and centrifuged for 20 min at 355 × g/room
temperature. Finally, the supernatant was decanted and the pellet
was resuspended in 1 ml of SS ready for counting.
Preparation of cells slides using
Cytospin
Following purification, PBMCs were counted using
trypan blue. After viable cells were counted, the concentration was
adjusted to 5×104 cells/µl when preparing cell slides.
Each slide had 200 µl of cell suspension added and was placed in a
Cytospin centrifuge (Cytospin™ 4; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Subsequently, cells slides were dried at room
temperature and were fixed with 3% formaldehyde at 4°C for 20 min
and stored for subsequent immunostaining.
Detection of apoptosis by TUNEL
assay
DNA fragmentation was determined by in situ
TUNEL assay on cells from patients with sepsis. Briefly, the cell
slides were treated with sodium citrate solution (pH=6; 0.01 M) for
20 min at 90°C in a water bath. Activity of endogenous peroxidase
was removed with hydrogen peroxide in 3% methanol for 25 min. The
cell slides were blocked with swine normal serum (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) at 2% for 2 h at room
temperature. Subsequently, 50 µl enzyme terminal deoxynucleotidyl
transferase reaction mixture (TUNEL Label mix, Roche Diagnostics,
Basel, Switzerland), was added to slides and incubated for 30 min
in a humidity chamber in the dark at 37°C, slides were washed for 5
times for 5 min with PBS. Anti-fluorescein antibody conjugated
horseradish peroxidase (20 µl; TUNEL POD Detection system, Roche
Diagnostics), was added and incubated at 37°C for 30 min in
darkness. Following washing, color development was carried out with
the addition of diaminobenzidine (DAB) Under a light microscope at
×20 magnification. The samples were counter-stained with
hematoxylin, dehydrated and embedded in resin for subsequent
analysis under a light microscope. To avoid inter-assay variation,
the TUNEL reaction was performed simultaneously on all slides, with
the same stock and enzymatic reaction development with the DAB
chromogen was performed at the same time. A semi-quantitative
assessment of cells staining was conducted by an expert
pathologist, who was blinded to the samples group. The stained
slides were verified by a second expert to ensure consistency in
the scoring. Apoptosis was considered high with >30% of cells
positive for apoptosis and low with <30% of positive cells
analyzed for each slide in three different areas. Data are
presented as positively stained target cells per 100 cells (range
0–100% positive), per region in the slide (4 regions per
slide).
Immunocytochemistry
The sample slides from Cytospin were analyzed by
immunocytochemistry as follows. Briefly, antigen retrieval was
performed with sodium citrate (pH 6.0; 0.01 M) for 20 min at 90°C
in a water bath, followed by three 5 min washes with 1X PBS.
Endogenous peroxidase activity was removed with 3% hydrogen
peroxide for two 25 min, and the slides were blocked with swine
normal serum at 2% at room temperature for 2 h. Slides were
incubated overnight at room temperature in humidity chambers with
rabbit anti-YY1 (1:250; catalog no. sc-281), rabbit anti-Fas
(1:250; catalog no. sc-1023) or rabbit anti-FasL (1:100; catalog
no. sc-6237; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
antibodies, with their respective isotype controls. After washing,
the cell slides were incubated with secondary antibody conjugated
to biotin (Universal LSAB kit; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) for 30 min in a humid chamber. Then
conjugated streptavidin horseradish peroxidase (Universal LSAB
kit), was added for 30 min. Finally, development was performed by
adding DAB substrate (liquid DAB, Dako; Agilent Technologies, Inc.)
and the reaction was stopped in tap water. Slides were stained with
hematoxylin, were dehydrated and covered with resin and left to dry
at room temperature for further light microscopic analysis (Olympus
BX-40; Olympus Corporation, Tokyo, Japan). In order to reduce
variations among experiments, the reaction was performed for each
marker at the same time in all groups. High YY1 expression was
defined as ≥10% of cells positive for YY1.
Data processing
The database was produced and information was
processed using the statistical analysis program GraphPad Prism
(GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as
the mean ± standard deviation for the number of separate
experiments indicated in each case. One-way analysis of variance
followed by Tukey's post hoc test was used to compare variance
within and among different groups. When necessary, unpaired
Student's t-test was used for comparison between two groups.
Spearman's correlation analysis was performed for correlation
testing. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient clinical characteristics
The current study included 30 patients diagnosed
with sepsis. Clinical data are presented in Table I. For the purposes of the study,
the patients were divided into two groups. Group 1 consisted of
patients with sepsis and septic shock (n=19), and group 2 consisted
of septic patients with MOD (n=11). The gender distribution was 14
females (47%) and 16 males (53%). The average age of group 1 and
group 2 patients was 51 months (range, 3–108 months) and 30.7
months (range, 2–180 months), respectively. The PELOD scores were
as follows: 0–10 points in 7 patients (mortality prediction
0.0–20%; no mortalities); 11–20 points in 9 patients (mortality
prediction 1.3–1.7%; no mortalities); 21–30 points in 8 patients
(mortality prediction 20.8–32.3%; 5 mortalities); and >30 points
in 6 patients (mortality prediction 87.7–90%; 5 mortalities). A
PIM-2 of 0.80–15.40% applied to 20 patients and no mortalities were
observed in this group, however, a PIM-2 of >16% was observed
for 10 patients and none of these patients survived. A total of 10
patients in group 2 had PIM-2s >16%. In group 1, one patient had
a PIM-2 of 20% (data not shown). A total of 17 of 30 patients
developed sepsis following surgery (56.6%), as indicated in the
Table I. Afflictions associated
with sepsis and MOD are presented in Table II. Sepsis etiology is presented in
Table III and blood culture
results are presented in Table
IV. The mean hospitalization period was 9.5 days with a maximum
of 23 days and a minimum of 2. The total lethality was 33.3%, with
83.3% in group 2 and 0.0% in group 1.
 | Table I.Age and characteristics of patients
with sepsis. |
Table I.
Age and characteristics of patients
with sepsis.
| A, Age of patients
with sepsis involved in the study |
|---|
|
|---|
| Value | Age (months) |
|---|
| Range | 3–180 |
| Mean | 41.44 |
|
| B, Characteristics
of patients with sepsis |
|
| Characteristic | Number of patients
(%) |
|
| Gender |
|
|
Female | 47.00 |
|
Male | 53.00 |
| Main site of
infection/septic origin |
|
|
Lung | 26.00 |
|
Heart | 10.00 |
| Central
nervous system |
6.60 |
| Surgical site |
|
|
Heart | 30.00 |
|
Abdomen | 23.30 |
|
Cranium |
3.30 |
| Septic stage |
|
| Severe
sepsis | 33.00 |
| Septic
shock | 33.00 |
|
Multiple organ
dysfunction | 40.00 |
| Outcome |
|
|
Survival | 66.60 |
 | Table II.Sepsis etiology in pediatric patients
with sepsis that also developed multiple organ dysfunction. |
Table II.
Sepsis etiology in pediatric patients
with sepsis that also developed multiple organ dysfunction.
| Etiological
factor | Number of
patients |
|---|
| Heart
post-surgery |
|
| Canal
atrioventricular correction | 2 |
|
Tetralogy of Fallot
correction | 1 |
|
Anomalous venous connection
correction | 1 |
| Post-infection |
|
|
Nosocomial pneumonia | 3 |
|
Abdominals sepsis | 1 |
|
Endocarditis | 1 |
|
Neuroinfections | 1 |
|
Ventriculitis | 1 |
 | Table III.Sepsis etiology in pediatric patients
without MOD. |
Table III.
Sepsis etiology in pediatric patients
without MOD.
| Etiological
factor | Number of
patients |
|---|
| Abdominal
sepsis | 1 |
| Laparoscopy
appendicitis complicated | 3 |
| Intestinal
occlusion post-surgery | 2 |
| Nosocomial
pneumonia | 5 |
| Endocarditis | 1 |
| Pericardial
effusion from chronic kidney disease | 1 |
| Craniotomy tumor
resection | 1 |
| Heart
post-surgery | 2 |
 | Table IV.Results of blood culture testing in
patients with sepsis involved in the study (n=30). |
Table IV.
Results of blood culture testing in
patients with sepsis involved in the study (n=30).
| Results of blood
culture | Number of
patients |
|---|
| Candida
albicans | 7 |
|
Staphylococcus coagulase
negative | 4 |
|
Klebsiella | 1 |
|
Pseudomona | 2 |
| H. faecalis | 1 |
| Negative | 15 |
Levels of apoptosis, and the
expression levels of Fas and YY1 in PBMCs from pediatric patients
with sepsis
The present study investigated the levels of
apoptosis in PBMCs from children with sepsis using an in
situ TUNEL assay. Fig. 1A
presents a representative micrograph of the TUNEL-stained cells in
patients with sepsis and healthy controls. The cells from patients
with sepsis demonstrate morphological changes with a scant
cytoplasm, a fragmented nucleolus (apoptotic bodies) and vacuoles,
compared with cells from healthy controls (Fig. 1A). Analysis of the percentage of
TUNEL-positive cells was performed as described in the Materials
and methods. Fig. 1A (bottom
panel) demonstrates a significant increase in the number of
apoptotic cells in patients with sepsis compared with the healthy
controls (P=0.0007). Additionally, the expression of Fas in PBMCs
from patients with sepsis and healthy controls was analyzed using
immunocytochemistry. Fig. 1B
presents representative micrographs of Fas expression and
demonstrates an increase in Fas expression in patients with sepsis
compared with the healthy controls. The expression of these
proteins was predominantly observed in the cytoplasm and the
membrane. Analysis of the percentage of Fas-positive cells was
performed as described in the Materials and methods. Fig. 1B (bottom panel) presents the
quantified expression of Fas. Notably, there was a significant
increase in the percentage of Fas-positive cells in patients with
sepsis compared with in the healthy controls (P=0.022). Normal IgG
was used as a negative control for immunostaining. Fig. 1C presents representative
micrographs of YY1 expression in patients with sepsis and healthy
controls, in which a modest increase in YY1 expression was observed
in patients with sepsis compared with healthy controls. YY1
expression was predominantly detected in the cytoplasm, however,
notably, YY1 expression was also observed in the nucleolus.
Additionally, cells from patients with sepsis exhibited a
morphology that was characteristic of infection, with vacuoles,
banded nuclei, relaxed chromatin and abundant cytoplasm. Fig. 1C (bottom panel) demonstrates that
the number of YY1-positive cells was significantly increased in
patients with sepsis compared with healthy controls (P=0.011). The
potential correlation of Fas and YY1 expression with levels of
apoptosis was also analyzed. Fig.
1D demonstrates that there was a positive correlation between
Fas expression and the level of apoptosis (r=0.5013, P=0.020).
These results demonstrate that apoptosis and the expression of Fas
are increased in patients with sepsis, and that the levels of
apoptosis are positively correlated with the expression of Fas.
This indicates that the apoptosis induced in patients with sepsis
may be mediated by Fas expression. By contrast, an inverse
correlation was detected between YY1 expression and apoptosis
levels (r=−0.4988, P=0.049; Fig.
1E). Together, these results suggest that YY1 may inhibit
apoptosis via regulation of Fas expression. Additionally, the
percentage of FasL-positive cells was significantly higher in
patients with sepsis compared with the healthy controls (P=0.033;
Fig. 1F).
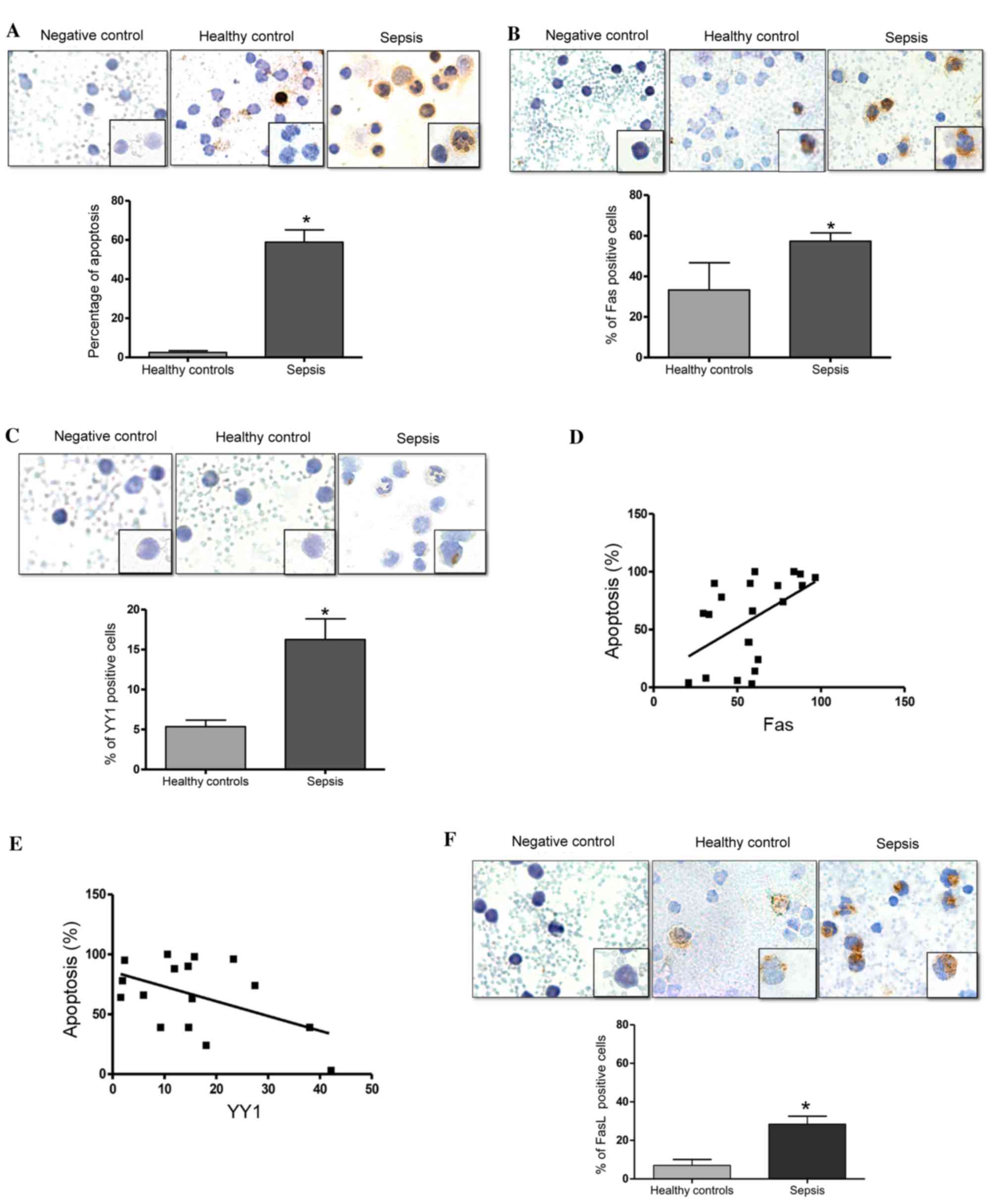 | Figure 1.Septic pediatric patients exhibit
increased levels of apoptosis. (A) Apoptosis was evaluated using an
in situ TUNEL assay in PBMCs from pediatrics patients with
sepsis. Upper panels: Negative control assay with no enzyme,
healthy control PBMCs and PBMCs from pediatric patients with
sepsis. Magnification at ×40, and ×100 in lower right square.
Bottom panel: Quantification of TUNEL-positive cells (%) in healthy
controls and patients with sepsis (*P=0.0007 vs. healthy controls;
Student's t test). (B) Fas expression was determined by
immunocytochemistry on PBMCs from pediatric patients with sepsis.
Upper panels: Isotype control, healthy control and patient with
sepsis. Magnification at ×40, and ×100 in lower right square.
Bottom panel: Quantification of Fas-positive cells (%) in healthy
controls and patients with sepsis (*P=0.022 vs. healthy controls;
Student's t test). (C) YY1 expression was determined by
immunohistochemistry in PBMCs from pediatric patients with sepsis.
Upper panels: Isotype control, healthy control and patient with
sepsis. Magnification at ×40, and ×100 in lower right square.
Bottom panel: Quantification of YY1-positive cells (%) in healthy
controls and patients with sepsis (*P<0.05 vs. healthy controls;
Student's t test). Correlation analysis between apoptosis and (D)
Fas and (E) YY1 expression in PBMCs from pediatric patients with
sepsis. (*P<0.05; r=0.5013 and 0.4003, respectively; Spearman's
correlation analysis). (F) FasL expression was determined by
immunocytochemistry in PBMCs from pediatric patients with sepsis.
Upper panel: Isotype control, healthy control and patient with
sepsis. Magnification. ×40, and ×100 on lower right square. Bottom
panel: Quantification of FasL-positive cells (%) in healthy
controls and patients with sepsis (*P=0.033 vs. healthy controls;
Student's t test). Data are presented as the mean ± standard
deviation of three independent experiments. TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling;
PBMCs, peripheral blood mononuclear cells; Fas, Fas cell surface
death receptor; YY1, YY1 transcription factor; FasL, Fas
ligand. |
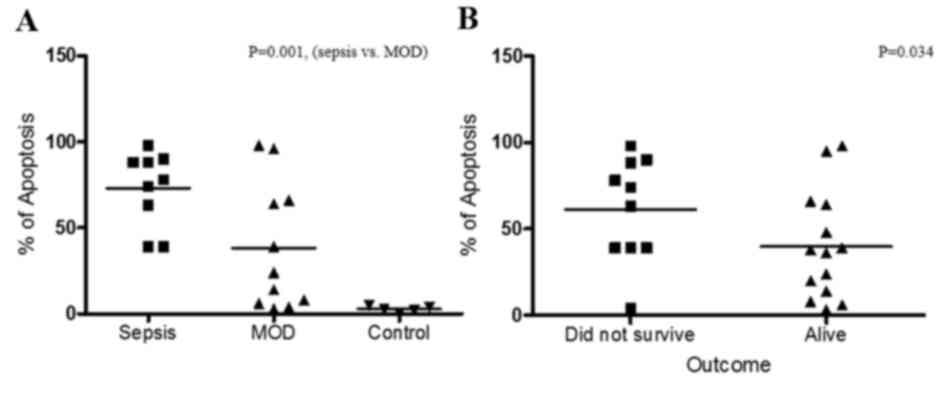
Apoptosis of PBMCs has a direct
association with sepsis
To define the potential role of apoptosis in the
pathogenesis of sepsis, comparative analyses of apoptosis and the
different clinical stages of the patients was performed. As
presented in Fig. 2A, comparing
apoptosis between patients with sepsis only (group 1) and those
with sepsis and MOD (group 2) demonstrated that patients with
sepsis exhibited increased levels of apoptosis (P=0.001) compared
with patients with MOD. In addition, the association between the
outcome of the patient and apoptosis was analyzed (Fig. 2B). The results demonstrated that
there is an inverse association between the patient outcome and
apoptosis, as the level of apoptosis was higher in patients that
did not survive compared with those that did survive (P=0.034).
Positive correlation between Fas
expression and PELOD index in patients with sepsis
The PELOD index is useful in establishing prognosis
during sepsis. In the current study, the correlation of the PELOD
index with apoptosis was analyzed. Interestingly, a direct positive
correlation between the level of apoptosis and the PELOD index was
detected (P=0.01, r=0.413; Fig.
3A). The study also analyzed the correlation of Fas and YY1
expression with the PELOD index. Fig.
3B demonstrates that there was a positive correlation between
Fas expression and PELOD (P=0.026, r=0.24). By contrast, the
expression of YY1 and the PELOD index were inversely correlated
(P=0.21, r=−0.29; Fig. 3C). In
addition, the association of the expression of Fas and YY1, and
patient outcome was analyzed. The results demonstrated that there
was no correlation between the expression of Fas and a fatal
outcome. Similarly, no correlation between YY1 expression and
patient outcome was detected (data not shown).
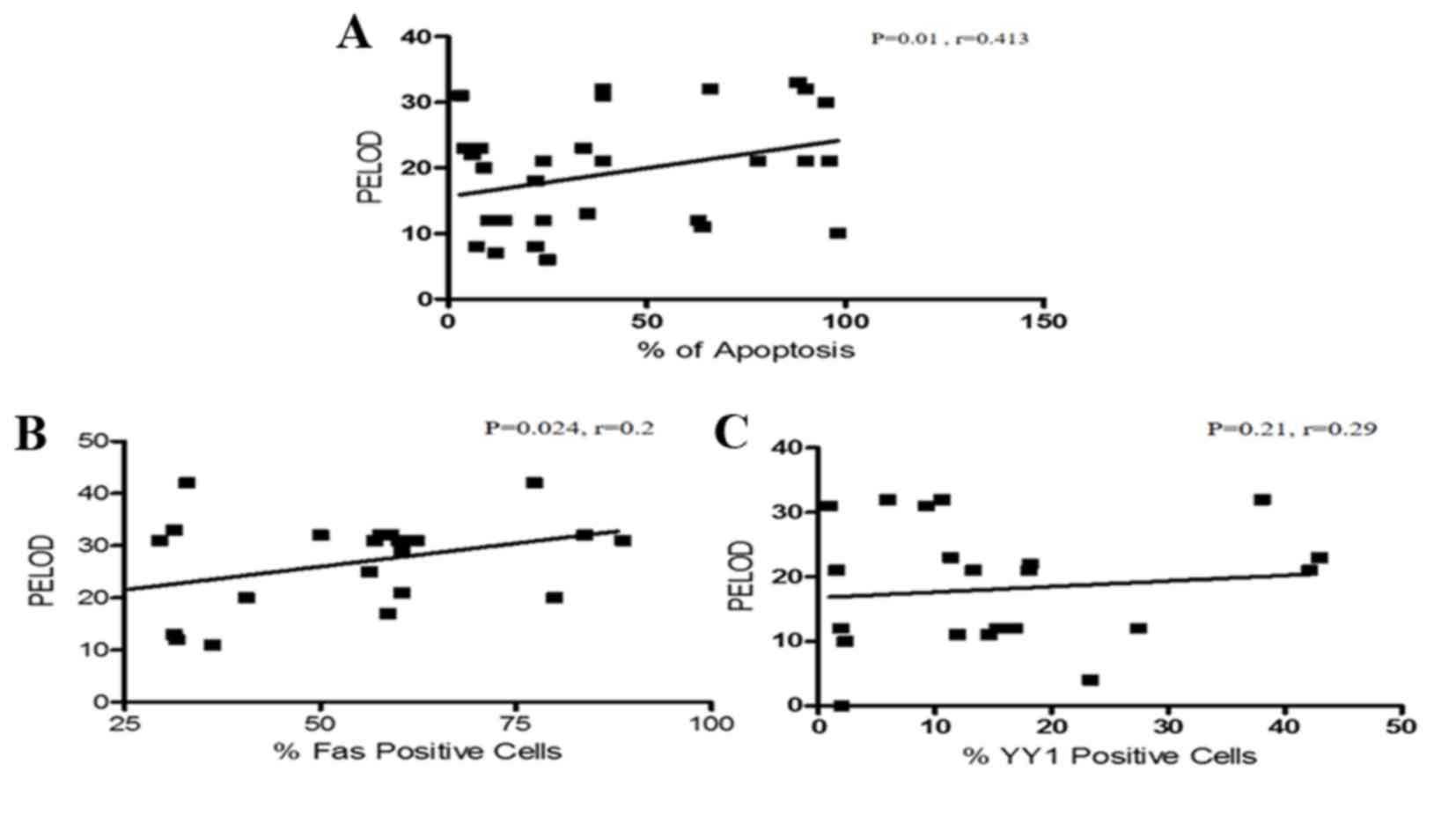 | Figure 3.Correlation analysis between the
PELOD index and apoptosis, Fas expression and YY1 expression. (A)
Correlation analysis between apoptosis levels and PELOD index in
PBMCs from pediatric patients with sepsis. There was a positive
correlation between the PELOD index and apoptosis. (Spearman test,
P=0.01, r=0.413). (B) Correlation analysis of Fas expression and
PELOD index in PBMCs derived from pediatric patients with sepsis.
There was a positive correlation between the PELOD index and Fas
expression (Spearman test, P=0.024, r=0.2). (C) Correlation
analysis between YY1 expression and PELOD Index in PBMCs derived
from pediatric patients with sepsis. There is no significant
correlation between YY1 expression and the PELOD index. (Spearman
test, P=0.21, r=0.29). PELOD, pediatric logistic organ dysfunction;
PBMC, peripheral blood mononuclear cell; Fas, Fas cell surface
death receptor; YY1, YY1 transcription factor. |
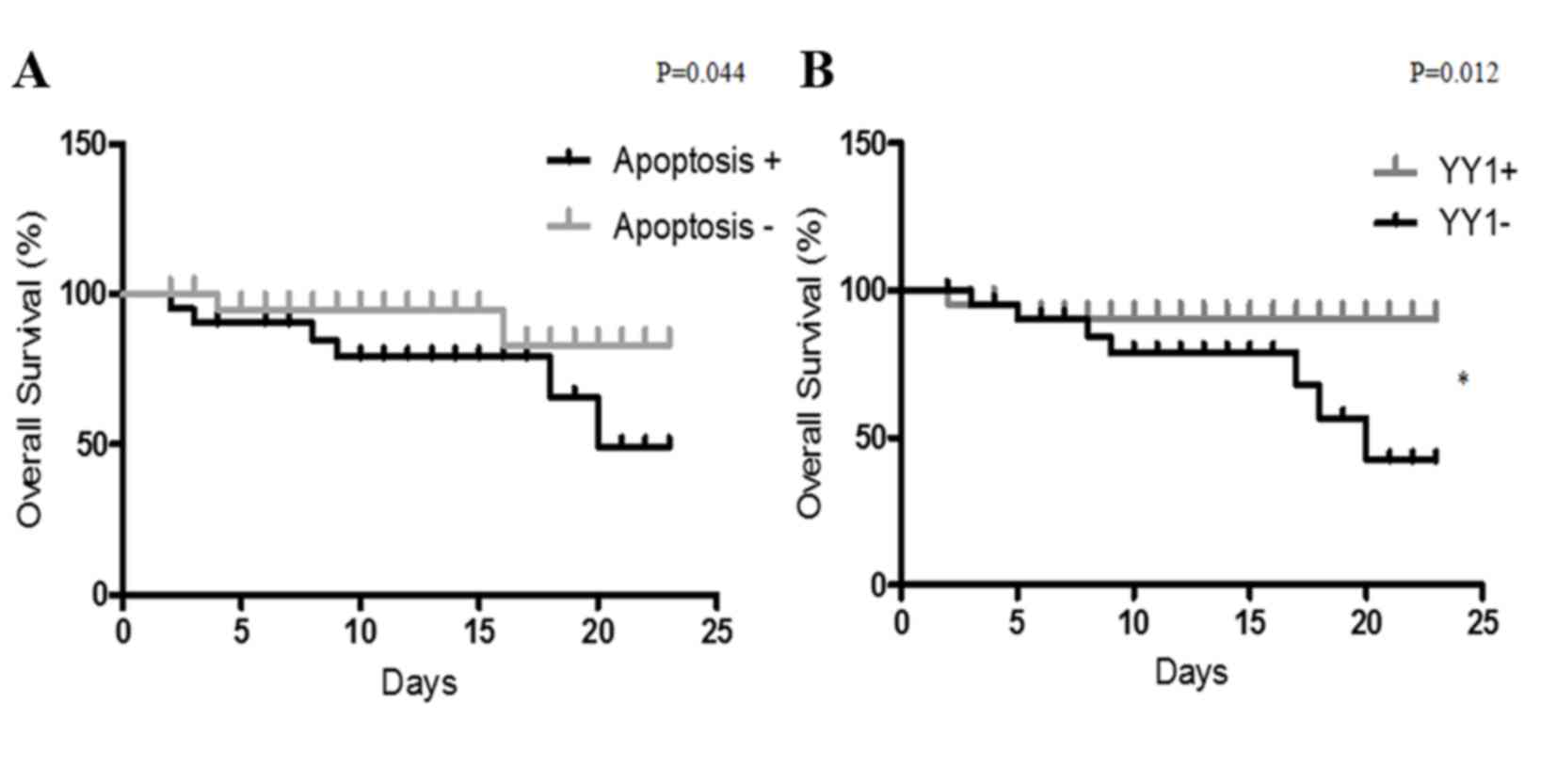
YY1 expression as a biomarker of
outcome in patients with sepsis
The present study analyzed whether YY1 expression
and apoptosis levels may predict the clinical outcome of patients
with sepsis. The cohort was analyzed for high/low YY1 expression
and apoptosis. High YY1 expression was defined as 10% of cells
positive for YY1 and apoptosis was considered high with >30% of
cells positive for apoptosis. As presented in Fig. 4, the clinical outcomes of patients
with sepsis were compared with YY1 expression and apoptosis by
Kaplan-Meier analysis. The follow-up (23 days) overall survival
scores were 83 and 49% for patients with low apoptosis and high
apoptosis, respectively (Fig. 4A).
Additionally, the follow-up overall survival scores were 90 and 42%
for patients with high YY1 and low YY1 patients, respectively
(P=0.012 Fig. 4B). The results
demonstrated that high apoptosis and low YY1 expression are
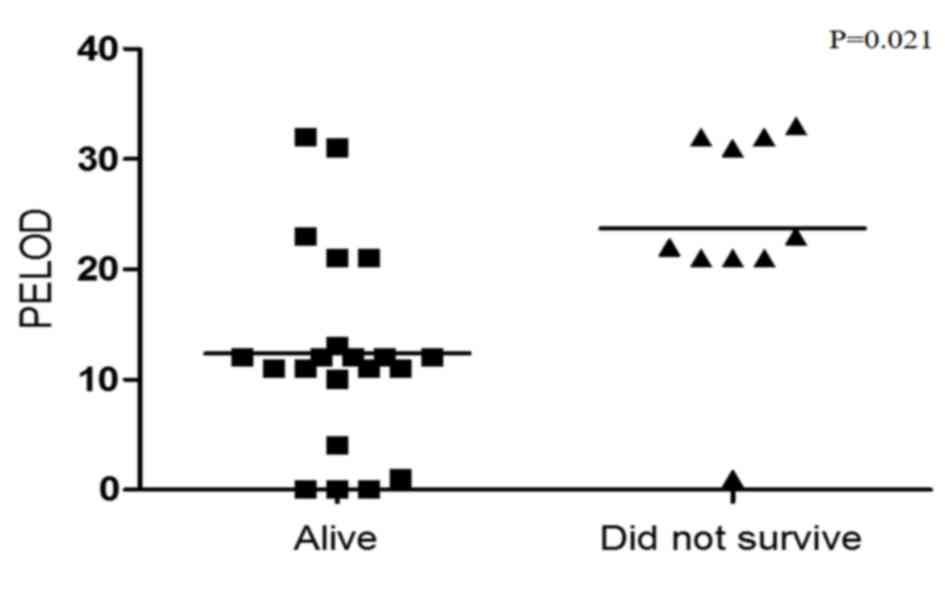
indicators of poor overall survival in patients with sepsis. In
addition, survival was compared with PELOD index. Fig. 5 revealed that a low PELOD index was
associated with increased survival (P=0.021).
Discussion
Apoptosis is a cell self-destruction mechanism that
has an important role in tissue homeostasis, embryonic development
and control of the immune response (22,23).
However, when apoptosis is activated at an inappropriate time, it
can cause the death of important cells in the immune system
(24,25). Lymphocyte apoptosis is important
for the function of the immune response (26), as it is involved in the positive
and negative selection of B and T cells (27). Apoptosis also regulates the
intensity and duration of the immune response, as activated
lymphocytes undergo apoptosis following an immune response to limit
inflammation (28). However, when
apoptosis malfunctions, the results can be serious and can be
damaging for the organism.
Apoptosis is also an important mechanism in the
pathogenesis of MOD in sepsis. It has been previously reported that
apoptosis of neutrophils and lymphocytes is an early event during
sepsis and MOD. The half-life of a circulating neutrophil is 6–20
h, and they can survive >48 h at sites of inflammation (29). It is known that there are high
numbers of apoptotic lymphocytes in specific organs or in the
periphery of patients with sepsis that subsequently developed MOD.
The majority of children that die from severe sepsis and MOD do not
fully eradicate the infection (30,31).
This indicates that the cellular components of the immune response
may be compromised in patients with MOD (32). A previous study has demonstrated
that lymphocyte apoptosis is associated with the development of
severe sepsis and MOD, as in an experimental model of sepsis the
inhibition of apoptosis reduced bacteremia and increased survival
(33). Lymphocyte apoptosis may be
altered by steroids, cytokines, including TNF-α, IL-1 and IL-6,
oxygen free radicals, nitric oxide and T cells expressing FasL
(34). There is an association
between apoptosis in peripheral cells, or other tissue-specific
cell types, with the severity of sepsis and the development of MOD,
and this apoptosis is mediated by Fas/FasL expression, however, the
potential mechanisms that regulate the expression of Fas/FasL are
not fully established (35).
The current study analyzed apoptosis levels, and the
expression of Fas and YY1, in PBMCs from children with sepsis, and
investigated associations with clinical data (including one of the
most serious complications of sepsis, MOD). To analyze apoptosis
levels, a control group of healthy individuals were compared with
the patients with sepsis. The results demonstrated a significant
increase in PBMC apoptosis levels in patients with sepsis compared
with the controls. In addition to TUNEL staining, morphological
changes were observed in the cells from patients with sepsis,
including changes to the cytoplasm, vacuoles and nuclei. A previous
study has demonstrated the association between apoptosis mediated
by Fas/FasL expression, and the severity of sepsis and MOD
(35), however, the potential
mechanisms of Fas regulation in this context remain unknown.
Previous studies by our group and others have demonstrated that the
transcription factor YY1 negatively regulates Fas expression, and
inhibition of this transcription factor leads to increased
expression of Fas, which results in an increased susceptibility to
apoptosis in tumor cells (36,37).
The results of the present study demonstrated that there was
significant overexpression of Fas and FasL in PBMCs from patients
with sepsis compared with healthy controls (Fig. 1B and F). A previous study has
reported that Fas and FasL expression is tissue specific (35). However, the current study is the
first, to the best of our knowledge, to analyze Fas expression in
PBMCs from children with sepsis. Additionally, tissue-specific
apoptosis was previously demonstrated to be correlated with Fas
expression in PBMCs (4).
Similarly, the correlation analysis of the present study
demonstrated that PBMC apoptosis was positively correlated with Fas
expression. The present study has particular importance as, to the
best of our knowledge, it is the first to investigate the
expression of YY1 in PBMCs from patients with sepsis, and to
investigate its correlation with Fas expression and apoptosis. The
results highlighted that YY1 was predominantly expressed in the
nuclei of PBMCs (Fig. 1C). The
increased expression of YY1 detected was modest and other studies
have reported contradictory results (38), demonstrating that activated T cells
have high YY1 expression. Sepsis is induced by the activation of T
lymphocytes following infection, and high levels of YY1 are
expressed in PBMCs from patients with sepsis. However, sepsis is
initially a pro-inflammatory process that progresses into an
anti-inflammatory state, which is predominantly mediated by
apoptosis. However, it is important to consider that all of these
stages may occur simultaneously (39). We hypothesize that this phenomenon
induces downregulation of YY1 and contributes to the induction of
apoptosis in PBMCs cells, leading to an immunosuppressive state
that triggers severe sepsis, septic shock and MOD. The current
study demonstrated that apoptosis was inversely correlated with YY1
expression (Fig. 1E). These
results indicated that YY1 expression may have an anti-apoptotic
effect, partially by downregulation of the expression pro-apoptotic
proteins, including Fas. However, the results did not indicate that
there was an inverse correlation between Fas and YY1 expression
(data not shown). This was potentially due to the low levels of YY1
expression in the PBMCs from the patients with sepsis and, it was
therefore difficult to establish a clear negative correlation with
the levels of Fas expression.
Previous studies have demonstrated that YY1
expression in hematological malignances, including lymphoma and
leukemia, is associated with patient outcome (40,41),
suggesting that YY1 expression has an important role in these
diseases and, consistent with the results of the present study, can
potentially be used as a prognostic biomarker to predict outcome in
pediatric patients.
The results of the present study demonstrated that
pediatric patients with sepsis have higher PBMC apoptosis compared
with septic patients that developed MOD (Fig. 2A). These findings are not
consistent with a previous report, which observed that apoptosis
was positively correlated with the development of MOD (42). However, the results of the present
study indicated that PBMCs exhibited high apoptosis in the early
stages of sepsis, which diminished following MOD development. These
results were corroborated by analyzing the association between
apoptosis levels and patient outcome (Fig. 2B). The results demonstrated that
patients with high apoptosis levels have a poorer survival outcome
compared with patients with low apoptosis levels. This indicated
that although apoptosis is involved in immunosuppression of these
patients, it may also have a compensatory role and, therefore,
prevent progression to MOD and promote survival.
As discussed, MOD is the most common cause of death
in the pediatric ICU. Determination of the severity of this
syndrome may be useful for predicting disease progression. The
PELOD index is currently used to assess the severity of MOD in
children with sepsis (43). The
current study analyzed the association of the PELOD index with the
outcome of patients, and demonstrated that mortality is clearly
increased in patients with a greater PELOD index (up to 20), which
is consistent with previous reports (43,44)
(Fig. 5). As expected, there was a
direct positive correlation between the PELOD index and apoptosis
levels in PBMCs (Fig. 3A). These
results indicate that apoptosis is not a protective factor in these
patients. Apoptosis may be useful in clinical evaluation as a
potential prognostic marker. To the best of our knowledge, the
current study is the first to demonstrate a positive correlation
between the PELOD index and apoptosis in PBMCs from pediatric
patients with sepsis. A positive correlation between the PELOD
index and Fas expression (Fig.
3B), and an inverse correlation between the PELOD index and YY1
expression (Fig. 3C) were also
observed. These results indicate that Fas may mediate apoptosis
induction and is correlated with a high PELOD index, and YY1 has a
negative role in the regulation of Fas expression and reduces
apoptosis to improve the prognosis of patients with sepsis.
Finally, the results demonstrated that apoptosis is a prognostic
factor for poor outcomes in pediatric patients with sepsis
(Fig. 4A).
To the best of our knowledge, the present study is
the first report to investigate YY1 expression in PBMC from
patients with sepsis. We hypothesize that YY1 expression and
apoptosis levels may be used as markers, in combination with
others, to predict the outcome of patients with sepsis.
Further studies are required to determine the
mechanisms that underlie the association of YY1 expression with
clinical outcome. In addition, determining cytokine secretion, and
its association with the expression of YY1 may be important, as
will determining the prognostic index of YY1 and the anti-apoptotic
Bcl-2 protein in pediatric patients with sepsis.
Acknowledgements
The present study was partially supported by the
Health Research Fundings (grant MIV FIS/IMSS/PROT/G09/759) from the
IMSS. The authors acknowledge the participation of Brenda Viridiana
González Revilla, Vanessa Retana Mayen, Miriam Hernández Atenógenes
and Juan Carlos Vázquez Islas, (Immunology & Infections
Diseases Medical Research Unit, Medical Center La Raza IMSS), for
their participation in the realization of this work and to Mario
Morales-Martinez (UIMEO National Medical Center Siglo XXI, IMSS) in
the preparation of the manuscript.
References
|
1
|
Hotchkiss RS and Nicholson DW: Apoptosis
and caspases regulate death and inflammation in sepsis. Nat Rev
Immunol. 6:813–822. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rayo AC, Hernández GL, Huerta GL, Ortiz
AV, Reyes RM and Gómez RG: Mecanismos patogénicos en sepsis y
choque séptico. Med Interna Mex. 24:38–42. 2008.
|
|
3
|
Carrillo-Esper R, Carrillo-Cordova JR and
Carrillo-Cordova LD: Epidemiological study of sepsis in Mexican
intensive care units. Cir Cir. 77:279–385. 2009.(In Spanish).
PubMed/NCBI
|
|
4
|
Hotchkiss RS, Swanson PE, Freeman BD,
Tinsley KW, Cobb JP, Matuschak GM, Buchman TG and Karl IE:
Apoptotic cell death in patients with sepsis, shock, and multiple
organ dysfunction. Crit Care Med. 27:1230–1251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hotchkiss RS, Swanson PE, Cobb JP,
Jacobson A, Buchman TG and Karl IE: Apoptosis in lymphoid and
parenchymal cells during sepsis: Findings in normal and T- and
B-cell-deficient mice. Crit Care Med. 25:1298–1307. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotchkiss RS, Swanson PE, Knudson CM,
Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ
and Karl IE: Overexpression of Bcl-2 in transgenic mice decreases
apoptosis and improves survival in sepsis. J Immunol.
162:4148–4156. 1999.PubMed/NCBI
|
|
7
|
Chung CS, Song GY, Moldawer LL, Chaudry IH
and Ayala A: Neither fas ligand nor endotoxin is responsible for
inducible peritoneal phagocyte apoptosis during sepsis/peritonitis.
J Surg Res. 91:147–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hotchkiss RS, Osmon SB, Chang KC, Wagner
TH, Coopersmith CM and Karl IE: Accelerated lymphocyte death in
sepsis occurs by both the death receptor and mitochondrial
pathways. J Immunol. 174:5110–5118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niu J, Azfer A and Kolattukudy PE:
Protection against lipopolysaccharide-induced myocardial
dysfunction in mice by cardiac-specific expression of soluble Fas.
J Mol Cell Cardiol. 44:160–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren Y, Xie Y, Jiang G, Fan J, Yeung J, Li
W, Tam PK and Savill J: Apoptotic cells protect mice against
lipopolysaccharide-induced shock. J Immunol. 180:4978–4985. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gautier EL, Huby T, Saint-Charles F,
Ouzilleau B, Chapman MJ and Lesnik P: Enhanced dendritic cell
survival attenuates lipopolysaccharide-induced immunosuppression
and increases resistance to lethal endotoxic shock. J Immunol.
180:6941–6946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Unsinger J, Herndon JM, Davis CG, Muenzer
JT, Hotchkiss RS and Ferguson TA: The role of TCR engagement and
activation-induced cell death in sepsis-induced T cell apoptosis. J
Immunol. 177:7968–7973. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ayala A, Urbanich MA, Herdon CD and
Chaudry IH: Is sepsis-induced apoptosis associated with macrophage
dysfunction? J Trauma. 40:568–574. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharshar T, Gray F, Poron F, Raphael JC,
Gajdos P and Annane D: Multifocal necrotizing leukoencephalopathy
in septic shock. Crit Care Med. 30:2371–2375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gordon S, Akopyan G, Garban H and Bonavida
B: Transcription factor YY1: Structure, function, and therapeutic
implications in cancer biology. Oncogene. 25:1125–1142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huerta-Yepez S, Vega M, Garban H and
Bonavida B: Involvement of the TNF-alpha autocrine-paracrine loop,
via NF-kappaB and YY1, in the regulation of tumor cell resistance
to Fas-induced apoptosis. Clin Immunol. 120:297–309. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garbán HJ and Bonavida B: Nitric oxide
inhibits the transcription repressor Yin-Yang 1 binding activity at
the silencer region of the Fas promoter: A pivotal role for nitric
oxide in the up-regulation of Fas gene expression in human tumor
cells. J Immunol. 167:75–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Freitas I, Fernández-Somoza M,
Essenfeld-Sekler E and Cardier JE: Serum levels of the
apoptosis-associated molecules, tumor necrosis factor-alpha/tumor
necrosis factor type-I receptor and Fas/FasL, in sepsis. Chest.
125:2238–2246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hotchkiss RS, Tinsley KW and Karl IE: Role
of apoptotic cell death in sepsis. Scand J Infect Dis. 35:585–592.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adrie C, Bachelet M, Vayssier-Taussat M,
Russo-Marie F, Bouchaert I, Adib-Conquy M, Cavaillon JM, Pinsky MR,
Dhainaut JF and Polla BS: Mitochondrial membrane potential and
apoptosis peripheral blood monocytes in severe human sepsis. Am J
Respir Crit Care Med. 164:389–395. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
World Medical Association: World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holmström TH and Eriksson JE:
Phosphorylation-based signaling in Fas receptor-mediated apoptosis.
Crit Rev Immunol. 20:121–152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rathmell JC and Thompson CB: Pathways of
apoptosis in lymphocyte development, homeostasis, and disease.
Cell. 109:(Suppl). S97–S107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fadeel B and Orrenius S: Apoptosis: A
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wyllie AH, Morris RG, Smith AL and Dunlop
D: Chromatin cleavage in apoptosis: Association with condensed
chromatin morphology and dependence on macromolecular synthesis. J
Pathol. 142:67–77. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Porter BO and Malek TR: Prostaglandin E2
inhibits T cell activation-induced apoptosis and Fas-mediated
cellular cytotoxicity by blockade of Fas-ligand induction. Eur J
Immunol. 29:2360–2365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCarthy NJ, Smith CA and Williams GT:
Apoptosis in the development of the immune system: Growth factors,
clonal selection and bcl-2. Cancer Metastasis Rev. 11:157–178.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Newton K and Strasser A: Cell death
control in lymphocytes. Adv Immunol. 76:179–226. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toledano BJ, Bastien Y, Noya F and Mazer
B: Characterization of B lymphocytes rescued from apoptosis by
platelet-activating factor. Cell Immunol. 191:60–68. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ashare A, Monick MM, Powers LS, Yarovinsky
T and Hunninghake GW: Severe bacteremia results in a loss of
hepatic bacterial clearance. Am J Respir Crit Care Med.
173:644–652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stearns-Kurosawa DJ, Osuchowski MF,
Valentine C, Kurosawa S and Remick DG: The pathogenesis of sepsis.
Annu Rev Pathol. 6:19–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hotchkiss RS, Tinsley KW, Swanson PE,
Schmieg RE Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP,
Buchman TG and Karl IE: Sepsis-induced apoptosis causes progressive
profound depletion of B and CD4+ T lymphocytes in humans. J
Immunol. 166:6952–6963. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hotchkiss RS, Chang KC, Swanson PE,
Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C,
Grimm E, et al: Caspase inhibitors improve survival in sepsis: A
critical role of the lymphocyte. Nat Immunol. 1:496–501. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roth E and Pircher H: IFN-gamma promotes
Fas ligand- and perforin-mediated liver cell destruction by
cytotoxic CD8 T cells. J Immunol. 172:1588–1594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doughty L, Clark RS, Kaplan SS, Sasser H
and Carcillo J: sFas and sFas ligand and pediatric sepsis-induced
multiple organ failure syndrome. Pediatr Res. 52:922–927. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vega MI, Jazirehi AR, Huerta-Yepez S and
Bonavida B: Rituximab-induced inhibition of YY1 and Bcl-xL
expression in Ramos non-Hodgkin's lymphoma cell line via inhibition
of NF-kappa B activity: Role of YY1 and Bcl-xL in Fas resistance
and chemoresistance, respectively. J Immunol. 175:2174–2183. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krippner-Heidenreich A, Walsemann G,
Beyrouthy MJ, Speckgens S, Kraft R, Thole H, Talanian RV, Hurt MM
and Lüscher B: Caspase-dependent regulation and subcellular
redistribution of the transcriptional modulator YY1 during
apoptosis. Mol Cell Biol. 25:3704–3714. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo J, Casolaro V, Seto E, Yang WM, Chang
C, Seminario MC, Keen J and Georas SN: Yin-Yang 1 Activates
Interleukin-4 Gene Expression in T Cells. J Biol Chem.
276:48871–48878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Denlinger LC and Proctor RA: Potential
risk factors for developing apoptosis during septic shock. Crit
Care Med. 28:2133–2134. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sakhinia E, Glennie C, Hoyland JA, Menasce
LP, Brady G, Miller C, Radford JA and Byers RJ: Clinical
quantitation of diagnostic and predictive gene expression levels in
follicular and diffuse large B-cell lymphoma by RT-PCR gene
expression profiling. Blood. 109:3922–3928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Krammer PH: CD95(APO-1/Fas)-mediated
apoptosis: Live and let die. Adv Immunol. 71:163–210. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hofer S, Brenner T, Bopp C, Steppan J,
Lichtenstern C, Weitz J, Bruckner T, Martin E, Hoffmann U and
Weigand MA: Cell death serum biomarkers are early predictors for
survival in severe septic patients with hepatic dysfunction. Crit
Care. 13:R932009. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Leteurtre S, Martinot A, Duhamel A, Proulx
F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J,
Hubert P, et al: Validation of the paediatric logistic organ
dysfunction (PELOD) score: Prospective, observational, multicentre
study. Lancet. 362:192–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Proulx F, Gauthier M, Nadeau D, Lacroix J
and Farrell CA: Timing and predictors of death in pediatric
patients with multiple organ system failure. Crit Care Med.
22:1025–1031. 1994. View Article : Google Scholar : PubMed/NCBI
|