Introduction
Diabetes mellitus, as a chronic endocrine metabolic
disease with a high incidence of complications, is a major health
risk worldwide (1). As a chronic
disease, micro- and macro-vascular complications frequently occur
in patients suffering from diabetes mellitus. In addition, complex
metabolic disorders involving lipids, carbohydrates and proteins
are frequently observed (2).
Patients with type 2 diabetes mellitus present with a more
aggressive course of disease, and an increased risk for early
hypertension and nephropathy compared with patients with type 1
diabetes mellitus. A combination of increased blood glucose levels
and organ damage caused by insulin secretion deficiency can result
in various pathological complications, including nephropathy
(3). Diabetic nephropathy has been
demonstrated to be caused by chronic inflammation (4). Activation of nuclear factor-κB
(NF-κB) was demonstrated to be involved in the pathogenesis of
diabetic nephropathy (5).
Standard therapies for diabetes focus on blood
glucose regulation and often fail to control the associated
complications (6). Certain widely
used medications for diabetic management have demonstrated
undesirable adverse effects, making further searching for safer and
more efficacious treatments of diabetes mellitus urgently
required.
Herbal medicines have demonstrated anti-diabetic
activities and the ability to reduce diabetic complications
(7). For example, Cordyceps
militaris polysaccharide-enriched fraction was revealed to have
a hypoglycemic effect on diabetic rats (8). Paecilomyces tenuipes, a
well-known Chinese medicinal entomopathogenic fungus, has a long
history of medicinal use in Asia (9). The anti-depressant, anti-tumor and
immunomodulatory activities of P. tenuipes have been
confirmed in previous studies (10,11).
Furthermore, P. tenuipes has been demonstrated to improve
lipid profiles and lipid peroxidation (12). An improved mutant strain named
P. tenuipes N45, with an elevated growth rate and
polysaccharide content, was obtained via chemical mutagenesis in
the present study. The strain has been successfully preserved in
the China Center for Type Culture Collection (CCTCC; no. M2011145).
P. tenuipes N45 has previously been demonstrated to have
anti-hyperglycemic, anti-hyperlipidemic and anti-oxidant effects in
alloxan-induced diabetic rats (13). Diabetes mellitus caused by alloxan,
which destroys the β cells of the pancreas, is pathologically
similar to type 1 diabetes mellitus (14,15).
It was therefore hypothesized that P. tenuipes N45 may
display similar beneficial effects on type 2 diabetes mellitus.
The present study aimed to investigate the
hypoglycemic, hypolipidemic and anti-inflammatory activities of
P. tenuipes N45 aqueous extract (PTNE) in a high-fat diet
and streptozotocin (STZ)-induced rat model. Following 4 weeks of
treatment with PTNE, several indexes associated with oxidation
resistance, hypoglycemic, hypolipidemic and anti-nephropathy
activities were measured. These data supported the potential use of
PTNE as an adjuvant therapy for type 2 diabetes mellitus.
Materials and methods
Submerged fermentation of P. tenuipes
N45
Spores collected from cultured P. tenuipes Pt
196 (RCEF 4339; Anhui Agricultural University, Anhui, China) were
washed twice with phosphate buffered saline (PBS) and adjusted to
1×107/ml. The spores were incubated in 1 mg/ml
nitrosoguanidine PBS solution at 25°C for 12 min and then cultured
in solid potato dextrose agar medium 96-well plates. For the
selection of mutants, individual strains were cultured in 250 ml
flasks. By comparing the dry mycelium weight and contents of
effective components (adenosine and polysaccharide) of three
consecutive cultivated generations, P. tenuipes N45 (CCTCC
no. M2011145) was obtained as the optimal strain. P.
tenuipes N45 was cultured in a rotary shaker incubator (10 L,
Biostat B; Germany) at 150 rpm for 5 days, and the temperature was
maintained at 26°C. The culture medium contained following
ingredients: 40 g/l glucose, 10 g/l peptone and 10 g/l yeast
extract powder. Mycelium pellets were harvested and lyophilized for
further usage.
Crude extract preparation
PTNE was prepared as follows: 100 g mycelial powder
was extracted twice in 5 L double distilled water at 80°C for 3 h.
Following centrifugation at 3,500 × g for 10 min, the
supernatant was evaporated under reduced pressure at 55°C in a
rotary evaporator. The extract was subsequently freeze-dried for
further experiments.
Diet/STZ-induced diabetic rat model
and drug administration procedure
The following experimental protocol was approved by
the Institution Animal Ethics Committee of Jilin University
(Changchun, China). A total of 60 Sprague-Dawley male rats (SCXK
(JI)-2011-0003) (purchased from Norman Bethune University of
Medical Science Jilin University, Jilin, China) were maintained on
a 12-h light/dark cycle (lights on 07:00-19:00) at 23±1°C with
water and food available ad libitum.
Rats were fed with standard laboratory diet for 2
weeks. Subsequently, 50 rats weighing 260–300 g were selected for
the present study. To induce diabetes, 50 rats were fed with a high
fat diet (68.8% standard chow, 20% sucrose, 10% lard, 0.2%
cholesterol and 1% salt mixture, purchased from the Lab Animal
Center of Jilin University, Jilin, China) for 8 weeks, followed
with intraperitoneal injection of 30 mg/kg STZ once per day for 3
days (16). Rats injected with
citrate buffer only were used as the control (n=10). During the
experiment, rats were fed with 5% glucose solution for 4 h
following STZ injection to prevent hypoglycemia. Rats with fasting
serum glucose levels between 11 and 26 mmol/l were placed in the
diabetic groups. The diet/STZ-induced diabetic rats were randomly
separated into five groups and injected with 10 ml/kg physiological
saline (model group; MD; n=10), 120 mg/kg metformin (Met; positive
control group; n=10; met purchased from Beijing Jingfeng Zhiyao
Co., Ltd., Beijing, China) and 0.04, 0.2 or 1.0 g/kg PTNE
(n=10/group) for 4 continuous weeks. Bodyweight was recorded for
the duration of the experiment, and fasting blood glucose was
monitored using a Glucose Assay kit (Nanjing KeyGen Biotech Co.
Ltd., Nanjing, China) on day 28 after PTNE treatment, following 18
h food deprivation.
Oral glucose tolerance test
(OGTT)
Following 4 weeks of treatment, an OGTT was
performed on the diabetic rats. Following 12 h fasting, rats were
orally treated with physiological saline, 120 mg/kg Met or 0.04,
0.2 or 1.0 g/kg PTNE. After 30 min, 2.0 g/kg glucose was
administered orally to each rat. Blood samples were collected at 0,
0.5, 1 and 2 h, and plasma glucose concentrations were measured.
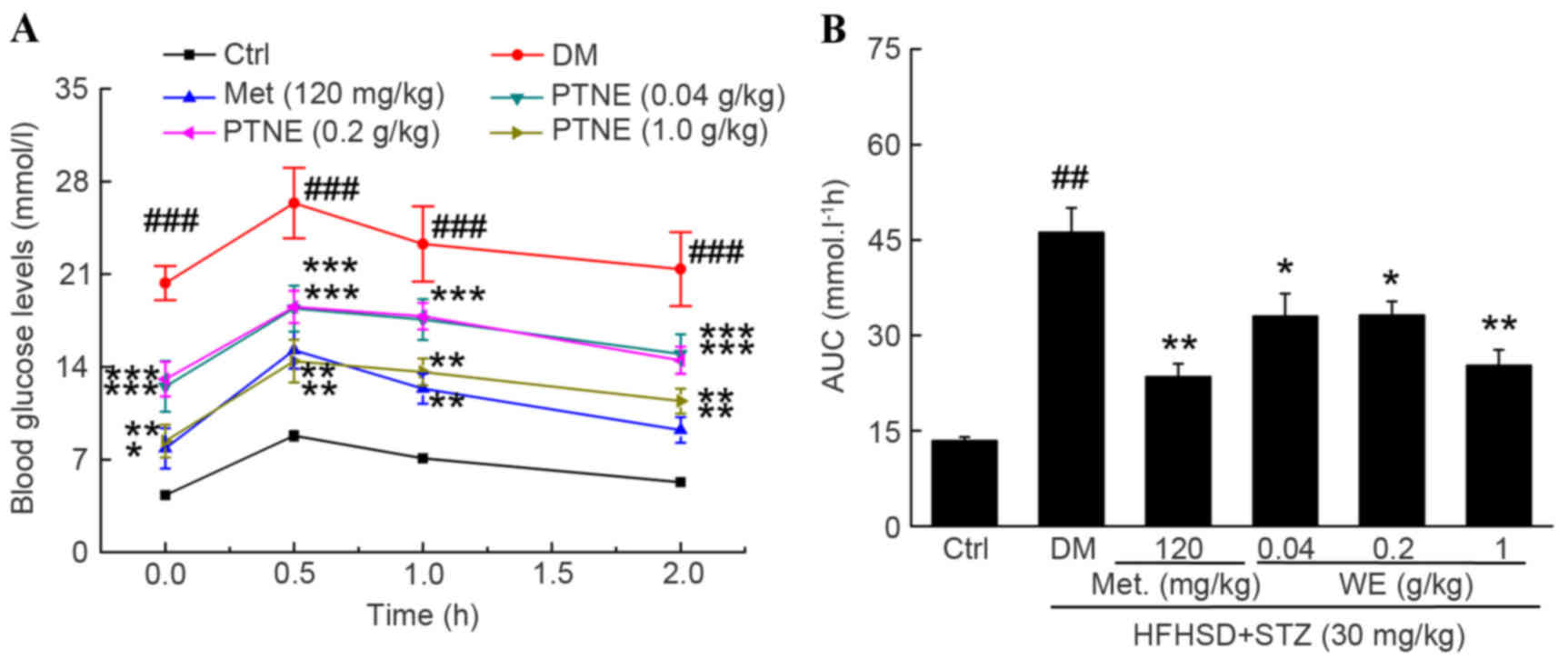
The area under the blood glucose curve (AUC) was calculated
according to the following equation (13): AUC = (basal glycemia + glycemia 0.5
h) ×0.25+(glycemia 0.5 h + glycemia 1 h) ×0.25 + (glycemia 1 h +
glycemia 2 h) ×0.5.
Biochemical index analysis
Prior to sacrifice, blood was sampled from the
hearts of all the rats, which were placed under anesthesia.
Superoxide dismutase (SOD; cat. no. A001-3), glutathione peroxidase
(GSH-Px; cat. no. A005), malondialdehyde (MDA; cat. no. A003-4),
low-density lipoprotein cholesterol (LDL-C; cat. no. A113-1), high
density lipoprotein cholesterol (HDL-C; cat. no. A113-2), total
cholesterol (TC; cat. no. A111-1), triglycerides (TG; cat. no.
A110-1), blood urea nitrogen (BUN; cat. no. C013-2) and creatinine
(Cr; cat. no. C011-2) levels were analyzed using commercial kits
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). The levels of
insulin (cat. no. CK-E30620), interleukin 2 (IL-2; cat. no.
CK-E30648), interleukin 6 (IL-6; cat. no. CK-E30646), NF-κB (cat.
no. CK-E91672R) and tumor necrosis factor-β (TNF-β; cat. no.
CK-E30527) were measured using the enzyme-linked immune sorbent
assay method (Shanghai Yuan Ye Biotechnology Co. Ltd. Shanghai,
China).
Statistical analysis
All data were expressed as the mean ± standard error
of the mean. Statistical significance was determined by a one-way
analysis of variance followed by post-hoc multiple comparisons with
Dunn's test, using SPSS 16.0 software (SPSS, Inc., Chicago, IL,
USA). P≤0.05 was considered to indicate a statistically significant
difference.
Results
Hypoglycemic effects of PTNE in
diabetic rats
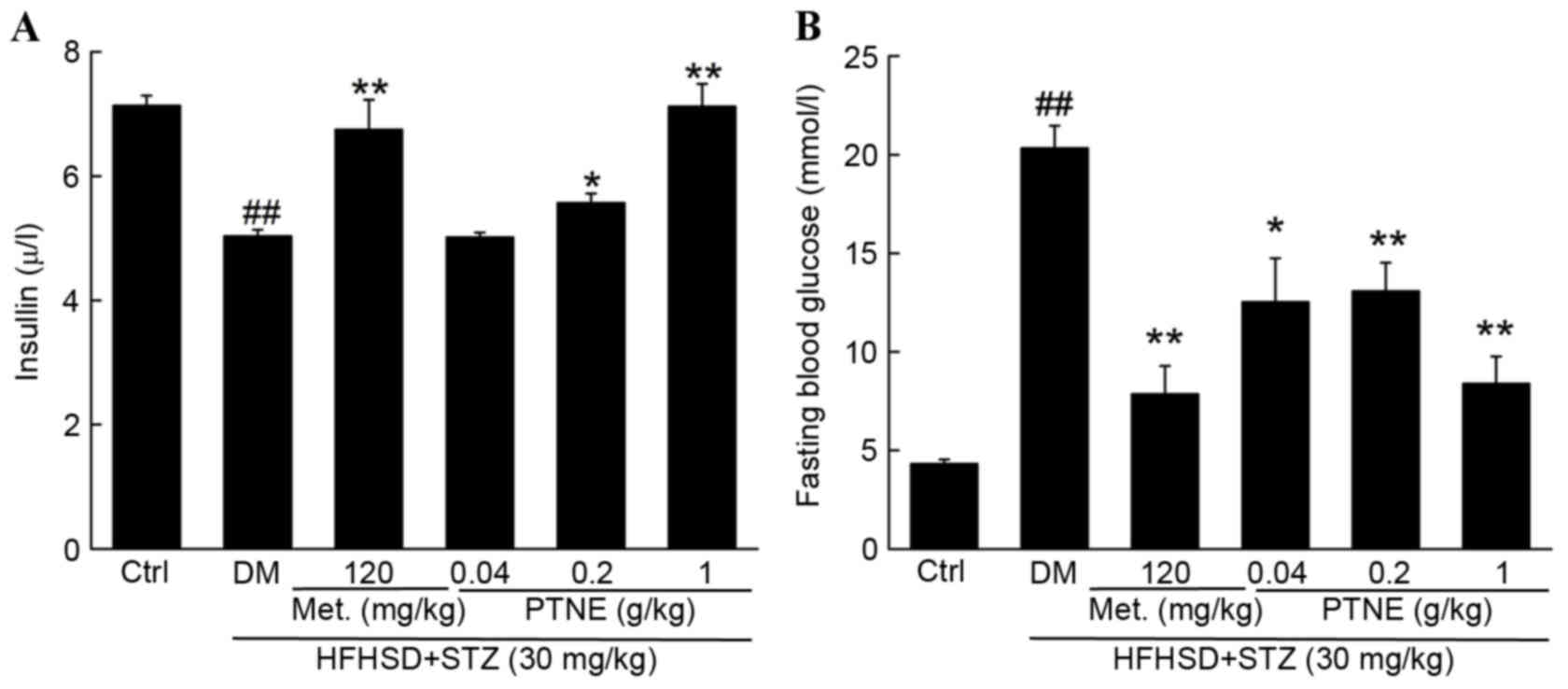
Changes in bodyweight, fasting blood glucose and
serum insulin levels were measured to investigate the hypoglycemic
effects of PTNE. Compared with the control group, decreased
bodyweight and serum insulin levels and increased fasting blood
glucose concentration were observed in diet/STZ-induced diabetic
rats (P<0.01; Table I). Normal
body weight, insulin and fasting blood glucose levels were
partially restored following 4 weeks of Met administration compared
with the model group (P<0.01; Fig.
1 and Table I). Similarly,
PTNE treatment improved the body weight of diabetic rats,
especially following 4 weeks of treatment (P<0.05; Table I). Compared with the model group, 1
g/kg PTNE treatment increased serum insulin levels by ~41.5%
(P<0.01; Fig. 1A). The fasting
blood glucose concentration in the model group was increased by
11.1 mmol/l compared with the control group (P<0.01; Fig. 1B) but 1 g/kg PTNE treatment
suppressed the fasting blood glucose levels by 58.7% compared with
the model group (P<0.01; Fig.
1B).
 | Table I.Bodyweight of rats in each group. |
Table I.
Bodyweight of rats in each group.
|
| Body weight
(g) |
|---|
|
|
|
|---|
| Group | Day 0 | Week 8 | 3-day STZ
injection | 2-week
treatment | 4-week
treatment |
|---|
| Control | 165±5.21 | 365.6±8.8 | 367.1±4.1 | 370.7±4.1 | 376.7±9.0 |
| DM | 168±6.8 | 369.7±4.8 | 314.6±18.9 |
292.7±9.3a |
297.1±10.3a |
| Met (120
mg/kg) | 170±7.9 | 369.9±5.9 | 319±10.1 |
323.7±8.5b |
333±4.1c |
| PTNE (g/kg) |
|
|
|
|
|
| 0.04 | 167±4.3 | 374.1±6.3 | 293.8±16.3 | 294.3±12.2 | 294.6±8.1 |
| 0.20 | 169.8±5.7 | 373.2±8.5 | 296.3±13.5 | 314.1±4.8 |
323.4±7.5b |
| 0.10 | 164±4.3 | 369.1±9.8 | 329.83±7.8 |
321.4±12.9b |
331.4±10.7b |
OGTT was used as a second diagnostic index to
further confirm the hypoglycemic activities of PTNE. A
significantly increased fasting blood glucose concentration was
observed in the model group from 0.5 to 2.0 h compared with the
control group (P<0.001; Fig.
2A) following oral glucose administration. Similar to Met, PTNE
treatment partially prevented the increase of blood glucose levels
at all chosen doses, but especially at 1.0 g/kg, compared with the
model group (P<0.01; Fig. 2A).
The calculated AUC values revealed an impaired glucose tolerance
state in the model group compared with the control group
(P<0.01; Fig. 2B), but 0.04,
0.2 and 1.0 g/kg PTNE and 120 mg/kg Met treatment all decreased the
AUC compared with the model group (P<0.05; Fig. 2B).
Hypolipidemic effects of PTNE in
diabetic rats
Compared with the control group, TC (Fig. 3A), TG (Fig. 3B) and LDL-C levels (Fig. 3C) were increased in the model group
(P<0.05), and HDL-C levels were decreased in the model group
compared with the control group (P<0.05, Fig. 3D). Treatment with 120 mg/kg Met
significantly reduced TC and LDL-C serum levels compared with the
model group (P<0.05), but failed to alter the levels of TG and
HDL-C compared with the model group. However, PTNE treatment
decreased TC, TG and LDL-C levels compared with the model group
(Fig. 3A-C) and reversed the
suppression of HDL-C levels in the serum of the model group
(P<0.05; Fig. 3D).
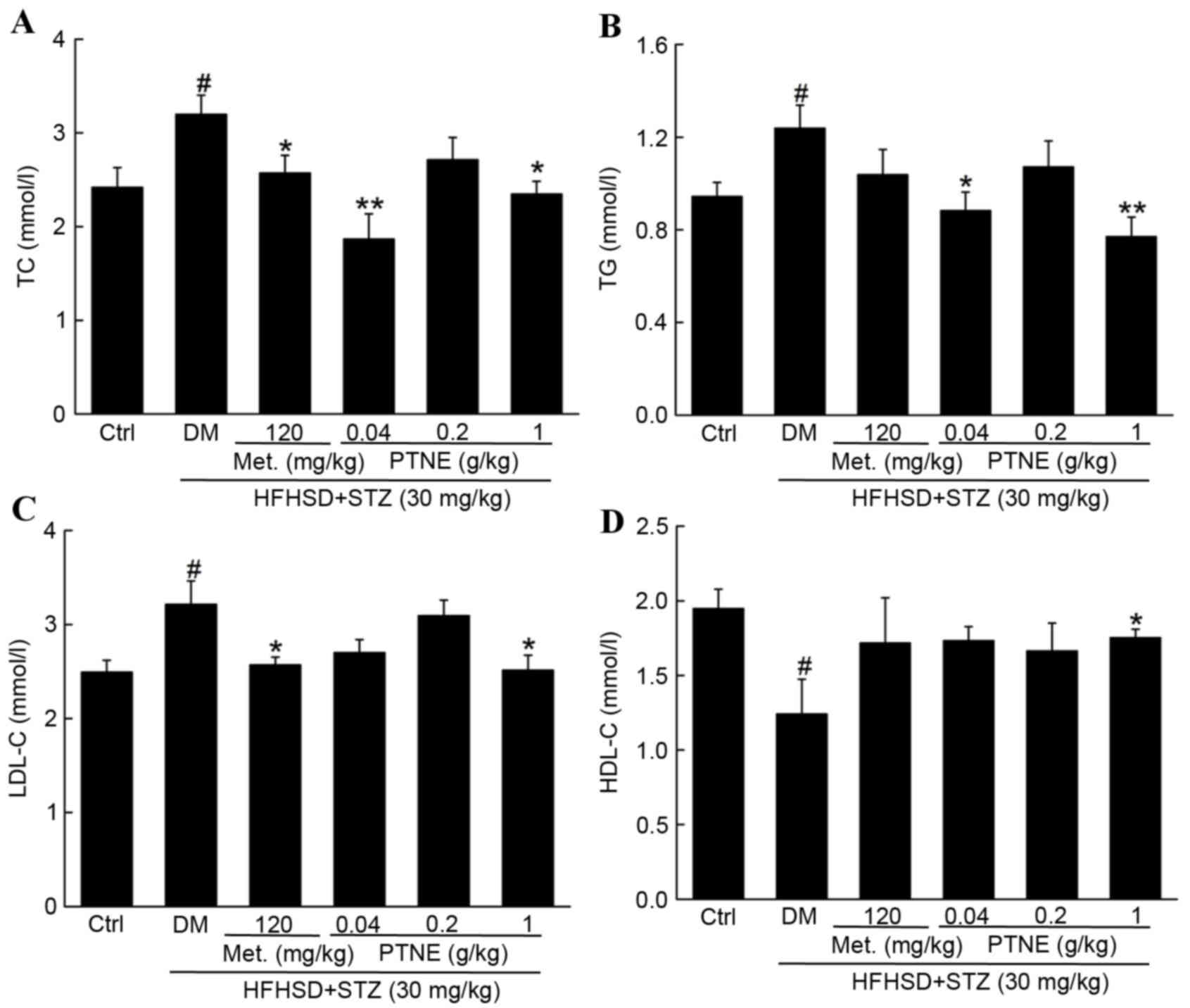 | Figure 3.Beneficial effects of Met and PTNE
treatment on (A) TC, (B) TG, (C) LDL-C and (D) HDL-C in DM rats.
Data are expressed as the mean ± standard error of the mean (n=10).
#P<0.05 vs.ctrl; *P<0.05 and **P<0.01 vs. DM
group. Met, metformin; PTNE, Paecilomyces tenuipes N45 water
extracts; TC, total cholesterol; Ctrl, control; DM,
diet/STZ-induced diabetic rat model; Met, metformin; PTNE,
Paecilomyces tenuipes N45 water extracts; HFHSD, high fat
and high sucrose diet; STZ, streptozotocin; TG, triglycerides;
LDL-C, low-density lipoprotein cholesterol; HDL-C, low-density
lipoprotein cholesterol. |
Anti-oxidative effects of PTNE in
diabetic rats
Nutritional factors, including antioxidants,
influence the management of diabetes mellitus and its complications
(17). An imbalance between
oxidative stress and antioxidative defense in diabetics accelerates
diabetic complications (18). SOD
and GSH-Px activity levels were decreased in the model group
compared with the control group (P<0.05; Table II), and overproduction of MDA
serum levels were increased in the model group compared with the
control group (P<0.05; Table
II). Similar to Met treatment, PTNE partially restored SOD,
GSH-Px and MDA serum levels, especially at the dose of 1 g/kg
(P<0.05; Table II).
 | Table II.Met and PTNE treatment increased SOD
and GSH-Px activity, and reduced MDA serum levels of DM rats. |
Table II.
Met and PTNE treatment increased SOD
and GSH-Px activity, and reduced MDA serum levels of DM rats.
| Group | SOD (µ/ml) | GSH-Px
(µmol/l) | MDA (nmol/ml) |
|---|
| Control | 368.4±30.4 | 246.9±17.7 | 6.2±1.5 |
| DM |
196.3±18.5b |
176.1±22.3a |
23.5±4.9b |
| Met (120
mg/kg) |
316.5±30.9d |
254.5±27.1c |
9.7±3.1c |
| PTNE (g/kg) |
|
|
|
| 0.04 | 256.1±24.6 |
241.6±15.5c | 25.3±3.7 |
| 0.20 |
280.9±13.4c | 202.6±17.2 | 22.2±4.6 |
| 0.10 |
264.5±17.5c | 218.1±10.8 |
11.7±1.7c |
Anti-nephropathic effects of PTNE in
diabetic rats
BUN and Crare recognized as sensitive indexes for
kidney injury (19) and BUN and Cr
levels were significantly increased in the model group compared
with the control group (P<0.05; Fig. 4A and B). Treatment with Met and
PTNE for 4 weeks significantly attenuated this increase in BUN
(P<0.05; Fig. 4A) and Cr
(P<0.05; Fig. 4B). Severe
inflammation occurred in the model group and was characterized by
the release of multiple inflammatory factors, including increased
serum levels of IL-2 (P<0.05; Fig.
4C), IL-6 (P<0.05; Fig. 4D)
and TNF-β (P<0.05; Fig. 4E)
compared with the control group. Met and PTNE treatment
significantly suppressed these indexes, with IL-2 (Fig. 4C) IL-6 (Fig. 4D) and TNF-β (Fig. 4E) serum levels significantly
decreased compared with the model group (P<0.05). Notably, 120
mg/kg Met and 1.0 g/kg PTNE treatment decreased NF-κB serum levels
by 20.5 and 17.8%, respectively, compared with the model group
(P<0.05; Fig. 4F).
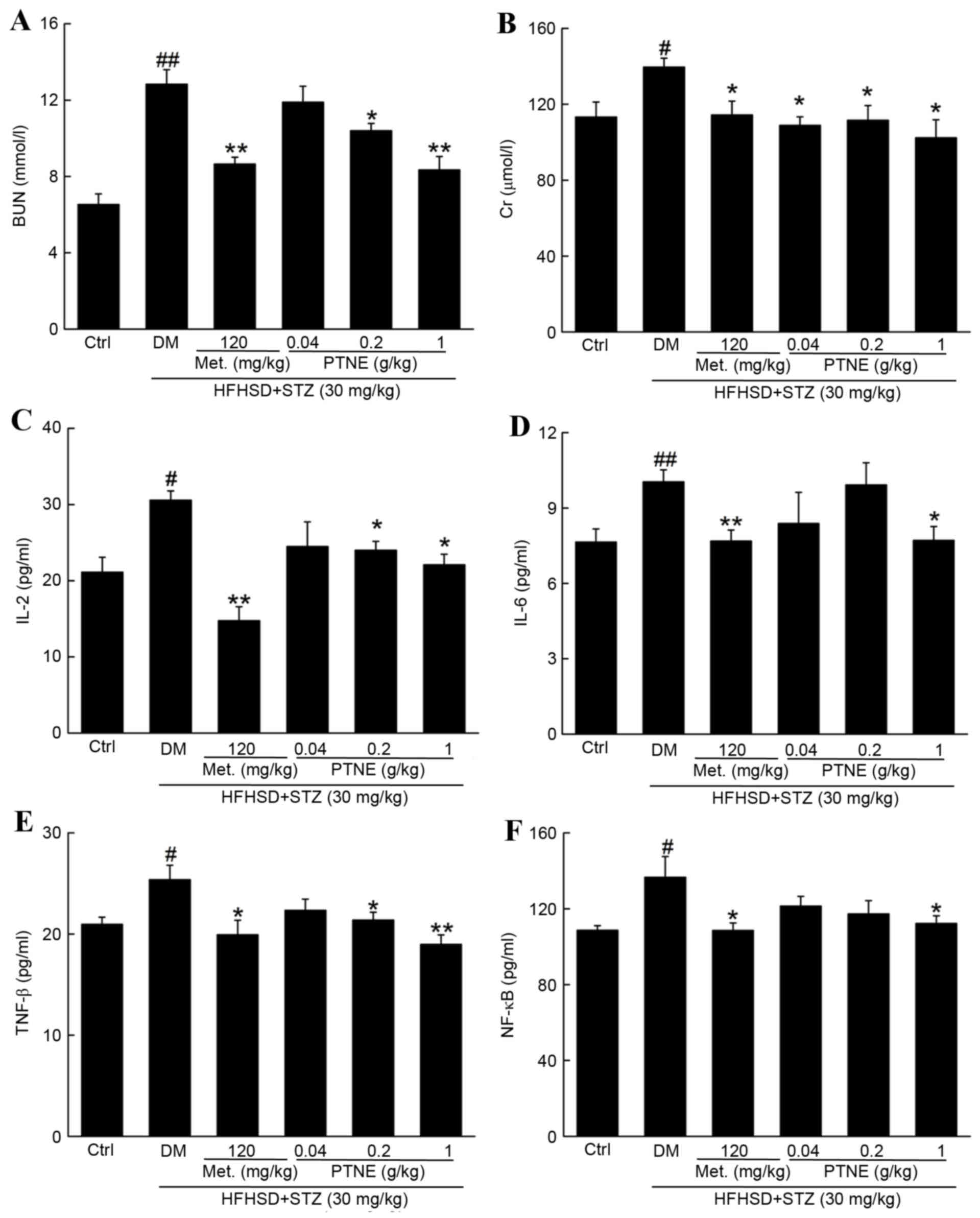 | Figure 4.Anti-nephropathic effects of PTNE
treatment in DM rats. The serum levels of (A) BUN, (B) Cr, (C)
IL-2, (D) IL-6, (E) TNF-β and (F) NF-κB were determined via ELISA.
Data are expressed as the mean ± standard error of the mean (n=10).
#P<0.05 and ##P<0.01 vs. ctrl;
*P<0.05 and **P<0.01 vs. DM group. PTNE, Paecilomyces
tenuipes N45 water extracts; DM, diet/STZ-induced diabetic rat
model; BUN, blood urea nitrogen; Ctrl, control; DM,
diet/STZ-induced diabetic rat model; Met, metformin; PTNE,
Paecilomyces tenuipes N45 water extracts; HFHSD, high fat
and high sucrose diet; STZ, streptozotocin; Cr, creatinine; IL,
interleukin; TNF-β: tumor necrosis factor-β; NF-κB: nuclear
factor-κB. |
Discussion
The rat model of diabetes, induced by a high-fat
diet and a low dose of STZ, displays similar metabolic features to
those of human type 2 diabetes mellitus (20), which is a complex and heterogeneous
disorder associated with a progressive decline in insulin
action.
Previous research has focused on the use of
traditional folk medicines produced from natural plants, which has
resulted in significant developments in functional food,
nutraceuticals and pharmaceuticals (21,22).
In contrast to the majority of treatments used to manage diabetes,
as a crude fungus, P. tenuipes contains a complex mixture of
bioactive components, which have multiple molecular targets. This
multipronged targeting approach may reduce hyperglycemia,
inflammation and oxidative stress in a more natural way, and may
result in fewer adverse side effects. Based on the findings of the
present study and its toxicological profile (23), P. tenuipes N45 has been
demonstrated to be both safe and effective.
As an agent for inducing diabetes, STZ causes β-cell
injury in the pancreas (24). The
pancreas is responsible for regulating serum glucose concentration.
The anti-hyperglycemic activity of natural products usually occurs
due to the restoration of pancreatic function via the enhancement
of insulin secretion (7). In the
present study, PTNE increased serum insulin levels and demonstrated
positive activity on OGTT, which is an important index for
evaluating islet function (25).
The beneficial effect on pancreatic function may be an important
contributor to the hypoglycemic effect of PTNE.
Altered lipid metabolism, including changes in TG,
TC, HDL-C and LDL-C levels, are observed in type 2 diabetes
mellitus (26). This is a risk
factor for coronary heart disease (27). PTNE may be beneficial in preventing
coronary heart disease and atherosclerosis via the regulation of
lipid metabolism. During development of diabetes mellitus, insulin
resistance is responsible for the release of adipocytokines and
relaxation of the afferent arteriole. The risk of the development
of atherosclerosis in diabetes mellitus can be reduced by elevating
HDL-C levels (28). On the other
hand, diabetes-associated dyslipidemia results in lipid
accumulation in the kidney, which leads to insulin resistance,
inflammation and oxidative stress (29). The antilipemic effects of PTNE may
be involved in its anti-diabetic and anti-nephropathic
activities.
Diabetic nephropathy represents a risk factor for
mortality, including cardiovascular mortality (30). Suppressed BUN and creatinine
levels, which are important markers of kidney damage, were observed
in PTNE-treated diabetic rats. It has previously been reported that
chronic inflammation during the development of diabetes contributes
to nephropathy (31).
Interleukins, especially IL-2 and IL-6, are involved in glomerular
damage (32). Furthermore, high
glucose levels initiate an inflammatory response characterized by
the activation of the pro-inflammatory NF-κB pathway, which
promotes the expression and activity of its downstream inflammatory
mediators (33). Based on the
results of the present study, the renal protective activity of PTNE
against diabetic nephropathy has been confirmed.
Hyperglycemia leads to accumulation of ROS, which in
turn mediate various metabolic defects associated with the diabetic
state (29). An imbalance between
oxidative stress and antioxidative defense in diabetes results in
cell and tissue damages and accelerate diabetic complications
(18). SOD and GSH-Px are regarded
as the primary defense systems against ROS generation (34). MDA is a major product of lipid
peroxidation, and low levels of MDA suggest reduced lipid
peroxidation and weaker oxidant stress (32). Anti-oxidant agents are emerging as
potential agents for preventing pancreatic β cell destruction
(28). Therefore, the
antioxidative effects of PTNE may be another important contributor
to its modulation of blood glucose and lipid metabolism and
inflammation.
In summary, in diet/STZ-induced diabetic rats, the
hypolipidemic, hypoglycemic and anti-diabetic nephritic effects of
PTNE have been confirmed. Previous data suggest that all these
effects may be associated with to the modulation of oxidative
damages by PTNE. The findings of the present study support the use
of PTNE as a pharmaceutical agent for the management of diabetes
and diabetic complications.
Acknowledgements
The present study was supported by the Science and
Technology Key Project in Jilin Province of China (grant nos.
20140311072YY, 20150203002NY, 20160520036JH and 20160204029YY).
References
|
1
|
Reagan LP: Diabetes as a chronic metabolic
stressor: Causes, consequences and clinical complications. Exp
Neurol. 233:68–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kerner W and Bruckel J: German Diabetes
Association: Definition, classification and diagnosis of diabetes
mellitus. Exp Clin Endocrinol Diabetes. 122:384–386. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winkler G, Hidvegi T, Vandorfi G, Balogh S
and Jermendy G: Risk-stratified screening for type 2 diabetes in
adult subjects: Results from hungary. Diabetologia. 54:S119.
2011.
|
|
4
|
Tuttle KR: Linking metabolism and
immunology: Diabetic nephropathy is an inflammatory. J Am Soc
Nephrol. 16:1537–1538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fornoni A, Ijaz A, Tejada T and Lenz O:
Role of inflammation in diabetic nephropathy. Curr Diabetes Rev.
4:10–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kania DS, Gonzalvo JD and Weber ZA:
Saxagliptin: A clinical review in the treatment of type 2 diabetes
mellitus. Clin Ther. 33:1005–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malviya N, Jain S and Malviya S:
Antidiabetic potential of medicinal plants. Acta Pol Pharm.
67:113–118. 2010.PubMed/NCBI
|
|
8
|
Zhang G, Huang Y, Bian Y, Wong JH, Ng TB
and Wang H: Hypoglycemic activity of the fungi Cordyceps militaris,
Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens
in streptozotocin-induced diabetic rats. Appl Microbiol Biotechnol.
72:1152–1156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukatsu T, Sato H and Kuriyama H:
Isolation, inoculation to insect host, and molecular phylogeny of
an entomogenous fungus Paecilomyces tenuipes. J Invertebr Pathol.
70:203–208. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin YY, Ming L, Zheng LF, Kan HW, Li CR
and Li WP: Bioactive compounds from Paecilomyces tenuipes
regulating the function of the hypothalamo-hypophyseal system axis
in chronic unpredictable stress rats. Chin Med J (Engl).
120:1088–1092. 2007.PubMed/NCBI
|
|
11
|
Lee DH, Park T and Kim HW: Induction of
apoptosis by disturbing mitochondrial-membrane potential and
cleaving PARP in Jurkat T cells through treatment with
acetoxyscirpenol mycotoxins. Biol Pharm Bull. 29:648–654. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SM, Park NS, Jin BR, Kang HS, Jung JH
and Park E: Effects of Paecilomyces tenuipes cultivated in egg yolk
on lipid metabolism in rats on a high fat-cholesterol diet. J Med
Food. 9:214–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du L, Liu C, Teng M, Meng Q, Lu J, Zhou Y,
Liu Y, Cheng Y, Wang D and Teng L: Antidiabetic activities of
Paecilomyces tenuipes N45 extract in alloxan-induced diabetic mice.
Mol Med Rep. 13:1701–1708. 2016.PubMed/NCBI
|
|
14
|
Balamurugan K, Nishanthini A and Mohan VR:
Antidiabetic and antihyperlipidaemic activity of ethanol extract of
Melastoma malabathricum Linn. leaf in alloxan induced diabetic
rats. Asian Pac J Trop Biomed. 4:(Suppl 1). S442–S448. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakahara Y, Ozaki K, Sano T, Kodama Y and
Matsuura T: Assessment of alloxan-induced diabetic rats as a
periodontal disease model using a selective cyclooxygenase (COX)-2
inhibitor. J Toxicol Pathol. 27:123–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bajaj M, Suraamornkul S, Pratipanawatr T,
Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y and
DeFronzo RA: Pioglitazone reduces hepatic fat content and augments
splanchnic glucose uptake in patients with type 2 diabetes.
Diabetes. 52:1364–1370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JH, Park NS, Lee SM and Park E:
Effect of dongchunghacho rice on blood glucose level, lipid
profile, and antioxidant metabolism in streptozotocin-induced
diabetic rats. Food Sci Biotechnol. 20:9332011. View Article : Google Scholar
|
|
18
|
Luo Q, Cai YZ, Yan J, Sun M and Corke H:
Hypoglycemic and hypolipidemic effects and antioxidant activity of
fruit extracts from Lycium barbarum. Life Sci. 76:137–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu S, Wang J, Zhang Y, Li V, Kong J, He J
and Li XM: Unpredictable chronic mild stress induces anxiety and
depression-like behaviors and inactivates AMP-activated protein
kinase in mice. Brain Res. 1576:81–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: A model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu W, Zhou Q, Yin JJ, Yao Y and Zhang JL:
Anti-diabetic effects of polysaccharides from Talinum triangulare
in streptozotocin (STZ)-induced type 2 diabetic male mice. Int J
Biol Macromol. 72:575–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He J and Li YL: Ginsenoside Rg1
downregulates the shear stress induced MCP-1 expression by
inhibiting MAPK signaling pathway. Am J Chin Med. 43:305–317. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du L, Liu Y, Liu C, Meng Q, Song J, Wang
D, Lu J and Teng L, Zhou Y and Teng L: Acute and subchronic
toxicity studies on safety assessment of Paecilomyces tenuipes N45
extracts. Comb Chem High Throughput Screen. 18:809–818. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei L, Lu Y, He S, Jin X, Zeng L, Zhang S,
Chen Y, Tian B, Mai G, Yang G, et al: Induction of diabetes with
signs of autoimmunity in primates by the injection of
multiple-low-dose streptozotocin. Biochem Biophys Res Commun.
412:373–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buchanan TA, Xiang AH, Peters RK, Kjos SL,
Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN and
Azen SP: Preservation of pancreatic beta-cell function and
prevention of type 2 diabetes by pharmacological treatment of
insulin resistance in high-risk hispanic women. Diabetes.
51:2796–2803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arvind K, Pradeepa R, Deepa R and Mohan V:
Diabetes & coronary artery disease. Indian J Med Res.
116:163–176. 2002.PubMed/NCBI
|
|
27
|
Sakatani T, Shirayama T, Suzaki Y,
Yamamoto T, Mani H, Kawasaki T, Sugihara H and Matsubara H: The
association between cholesterol and mortality in heart failure.
Comparison between patients with and without coronary artery
disease. Int Heart J. 46:619–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vasan S, Foiles P and Founds H:
Therapeutic potential of breakers of advanced glycation end
product-protein crosslinks. Arch Biochem Biophys. 419:89–96. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rolo AP and Palmeira CM: Diabetes and
mitochondrial function: Role of hyperglycemia and oxidative stress.
Toxicol Appl Pharm. 212:167–178. 2006. View Article : Google Scholar
|
|
30
|
Targher G, Zoppini G, Chonchol M, Negri C,
Stoico V, Perrone F, Muggeo M and Bonora E: Glomerular filtration
rate, albuminuria and risk of cardiovascular and all-cause
mortality in type 2 diabetic individuals. Nutr Metab Cardiovas Dis.
21:294–301. 2011.
|
|
31
|
Chow FY, Nikolic-Paterson DJ, Atkins RC
and Tesch GH: Macrophages in streptozotocin-induced diabetic
nephropathy: Potential role in renal fibrosis. Nephrol Dial
Transplant. 19:2987–2996. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Padmavathi R, Senthilnathan P, Chodon D
and Sakthisekaran D: Therapeutic effect of paclitaxel and propolis
on lipid peroxidation and antioxidant system in 7,12 dimethyl
benz(a)anthracene-induced breast cancer in female Sprague Dawley
rats. Life Sci. 78:2820–2825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C,
Li J, Feng Z, Yang S, Li X and Liang G: Inhibition of high
glucose-induced inflammatory response and macrophage infiltration
by a novel curcumin derivative prevents renal injury in diabetic
rats. Br J Pharmacol. 166:1169–1182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JO, Kim KS, Lee GD and Kwon JH:
Antihyperglycemic and antioxidative effects of new herbal formula
in streptozotocin-induced diabetic rats. J Med Food. 12:728–735.
2009. View Article : Google Scholar : PubMed/NCBI
|