Introduction
Embryo implantation, a key part of early pregnancy,
is a complex reproductive process. Disruption of embryo
implantation may lead to a miscarriage. During implantation, the
endometrium undergoes morphological and physiological alterations
to become receptive to the embryo and permit its invasion (1). In addition to conceptus quality, the
establishment of uterine receptivity is necessary for successful
embryo implantation. It has been reported that worldwide ~10–15% of
couples experience infertility during their reproductive years,
which is primarily associated with implantation failure (2). Previous studies have identified
compromised reproduction in women with polycystic ovary syndrome
(PCOS), obesity and type 2 diabetes, which includes impaired oocyte
and embryo quality, lower fertilization rates, decreased embryo
implantation rates and elevated risk of spontaneous loss of
pregnancy following in vitro fertilization (3–7).
However, the underlying molecular mechanisms remain to be
elucidated.
Insulin is a protein hormone secreted by pancreatic
β cells that consist of A- and B- polypeptide chains, which are
linked by disulfide bonds. Insulin promotes the uptake and
utilization of glucose, and inhibits glycogenolysis and
gluconeogenesis. Therefore, it is extensively involved in the
metabolism of glucose, fat and protein (8). Hyperinsulinemia and insulin
resistance are established characteristics of women with PCOS,
obesity and type 2 diabetes (9,10).
Previous studies have explored the direct role and underlying
mechanisms of abnormal oocyte and embryo development and
hyperinsulinemia during lower embryo implantation in women with
PCOS, obesity and type 2 diabetes (11,12).
However, the importance of hyperinsulinemia and insulin resistance
in the maternal endometrium remains to be elucidated. The insulin
receptor has been previously demonstrated to exhibit significantly
altered expression patterns in the menstrual cycle (13). Insulin receptor expression was
observed to be present primarily in the secretory phase and
localized at the stromal cells whereas its expression was
downregulated from follicular to luteal phases (13). In addition, insulin regulated-mTOR
signaling is important for lipid and glucose metabolism in skeletal
muscle and liver (14–17), and a previous study revealed that
mTOR was essential for endometrial receptivity (18). These results demonstrated that
insulin may serve an important function in the endometria.
Although, a previous clinical study revealed the reduced expression
of markers for endometrial receptivity in PCOS patients (19), to the best of our knowledge there
is a current lack of evidence to confirm the abnormal expression of
endometrial receptivity markers resulting from hyperinsulinemia and
insulin resistance.
It is possible that hyperinsulinemia and insulin
resistance may be responsible for reduced endometrial receptivity
in hyperinsulinemic and insulin-resistant females. Therefore, the
aim of the present study was to generate a hyperinsulinemic and
insulin-resistant mouse model in order to determine whether
hyperinsulinemia may affect endometrial receptivity.
Materials and methods
Establishment of mouse model
A total of 100 Kunming female mice (weight, 22±1.8
g; age, 6 weeks) were used to generate this animal model. Ethical
approval for the use of animals in this study was obtained from
Chongqing Medical University (Chongqing, China). Care and handling
of these mice was conducted in accordance with the animal research
committee guidelines established by the Ethics Committee of the
Institute of Zoology, Chongqing Medical University. An insulin
intervention mouse model was established as previously described by
Ou et al (11). Mice were
randomly divided into control and insulin-treated groups
(n=50/group). The treatment protocol was composed of 2 subcutaneous
injections of saline for the control group or insulin for
insulin-treated group, in order to induce hyperinsulinemia. Human
recombinant insulin (Insulin glargine) was purchased from Sanofi
(Shanghai, China). For the insulin-treated group, 0.05 IU insulin
was injected until day 16, and from day 17 on it was gradually
increased until reaching 0.8 IU/day by day 23 (20,21)
(day 17, 0.2 IU; day 18, 0.35 IU; day 19, 0.5 IU; day 20, 0.65 IU;
day 21, 0.8 IU; day 22, 0.8 IU; day 23, 0.8 IU). All mice were then
mated with fertile males (female: male, 2:1) overnight and the
female mice were checked for vaginal plugs the following morning.
The day at which a positive vaginal plug was identified was
considered to be day 1 (D1) of pregnancy.
Detection of serum insulin,
progesterone (P4), estradiol (E2) levels and plasma glucose
levels
Blood samples (~1 ml) were collected from the eye
socket on insulin treated day 23 and incubated at room temperature
for 3 h to obtain the serum. The serum levels of insulin, P4 and E2
were detected using enzyme-linked immunosorbent assay kits (YH1332,
YH4016 and YH3997; Shanghai Yan Hui Biological Technology, Co.,
Ltd., Shanghai, China) according to the manufacturer's protocol.
Absorbance was read at 450 nm. An ACCU-CHEK Advantage blood glucose
meter (Roche Applied Science, Mannheim, Germany) was used to
determine the glucose concentrations in the blood, which were taken
from the cut tail tip. Homeostasis model assessment (HOMA) were
calculated as: Plasma glucose level×serum insulin level/22.5.
Quantification of implantation sites
and collection of endometrial tissue
In order to examine attachment and implantation,
pregnant dams were sacrificed by cervical dislocation on D5 and
following Trypan blue (10 mg/ml) injection into the tail veins
(22). The number of implantation
sites was identified by distinct blue bands. In addition,
endometrial tissues at D4-D6 were collected and squeezed from the
uterine horns by the use of fine forceps under an anatomical
microscope (23). The isolated
endometrial tissues were immediately stored in liquid nitrogen.
Reverse transcription-quantitative
polymerase chain reaction
TRIzol reagent (Takara Biotechnology Co., Ltd.,
Dalian, China) was used for total RNA extraction from 50 mg
endometrium obtained from D4-D6, according to the manufacturer's
protocol. RNA concentration and purity was determined by
spectrophotometric determination of OD260/280 ratio.
Total RNA (0.5 mg) was used for cDNA synthesis in a 10 ml reaction
system using the PrimeScript RT reagent kits (Takara Biotechnology
Co., Ltd.). The specific primers for estrogen receptor (Esr) 1,
Esr2, progesterone receptor (Pgr), homeobox A10 (Hoxa10) and
β-actin are presented in Table I.
qPCR was performed using the CFX96 Real-Time PCR Detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and SYBR Premix Ex
Taq (Takara Biotechnology Co., Ltd.). The reaction mixture
consisted of 12.5 µl SYBR Premix Ex Taq (2X), 0.5 µl of each
specific primer (10 µM) and 2 µl cDNA. Water was then added to a
final volume of 25 µl. Following initial denaturation at 95°C for
30 sec, PCR was performed for 40 cycles according to the following
parameters: Denaturation at 95°C for 10 sec; annealing and
extension at 60°C for 30 sec. Experiments were performed in
triplicate for each sample. Gene expression was obtained by
normalizing cDNA quantity to that of β-actin and calculated using
the 2−ΔΔCq method (24).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| Esr1 |
CACCAGATCCAAGGGAA |
CGGCGTTGAACTCGTAG |
| Esr2 |
GACTGTAGAACGGTGTGGTCATCAA |
CTGTGAGGTAGGAATGCGAAAC |
| Pgr |
GCCTATACCGATCTCCCTG |
TTCCCTATGAGTGGCTTCTAC |
| Hoxa10 |
AACGCTGCCCTTACACGA |
GTGGACGCTACGGCTGAT |
| β-actin |
CCTGAGGCTCTTTTCCAGCC |
TAGAGGTCTTTACGGATGTCAACGT |
Western blot analysis
Proteins were extracted from 200 mg endometrium
obtained from D4-D6 mice. Endometrial tissues were lysed using RIPA
lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China).
The concentration of protein was determined using a bicinchoninic
acid protein assay kit (Beyotime Institute of Biotechnology).
Proteins were boiled in 5X SDS sample loading buffer for a minimum
of 10 min and subsequently stored at −80°C prior to use. 50 µg
proteins were separated on 10% SDS-PAGE gels. Proteins were then
transferred onto polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc.). Membranes were subsequently blocked with 5%
non-fat milk at room temperature, before they were incubated with
rabbit monoclonal anti-Hoxa10 (SC17159; 1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), mouse monoclonal anti-Esr1
(ab66102; 1:1,000; Abcam, Cambridge, UK), mouse monoclonal anti-Pgr
(ab2765; 1:500; Abcam), mouse monoclonal anti-Esr2 (ab288; 1:1,000;
Abcam), rabbit monoclonal anti-mechanistic target of rapamycin
(mTOR; 2983; 1:500; Cell Signaling Technology, Inc., Danvers, MA,
USA), rabbit monoclonal anti-phosphorylated (p)-mTOR (5536; 1:500;
Cell Signaling Technology, Inc.), rabbit monoclonal anti-ribosomal
protein S6 kinase β-1 (p70S6K; 2708; 1:1,000; Cell Signaling
Technology, Inc.), rabbit monoclonal anti-p-p70S6K (9234; 1:500;
Cell Signaling Technology, Inc.) and mouse monoclonal anti-β-actin
(A5441; 1:1,500; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
overnight at 4°C. The membranes were washed 3 times with
Tris-buffered saline containing Tween-20 (0.2%), and then incubated
for 1 h on room temperature with a horseradish peroxidase
(HRP)-conjugated secondary antibody (TA130003 and TA130023;
1:1,000; OriGene Technologies, Inc., Beijing, China). The positive
bands were detected using HRP-enhanced chemiluminescence reagents
(WBKLS0500; Merck KGaA) and quantified by densitometry analysis
using Quantity One software version 4.6.2 (Bio-Rad Laboratories,
Inc.). Protein expression was normalized to β-actin.
Hematoxylin and eosin (H&E)
staining
H&E staining was performed according to the
manufacturer's protocol (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). Briefly, sections (4 µm in thickness)
of uterine tissue sacrificed on D5 were deparaffinized in xylene
and rehydrated in decreasing concentrations of ethanol.
Subsequently, the sections were stained with hematoxylin (0.45%)
for 3 min and with eosin (1%) for 30 sec at room temperature,
followed by dehydration until they were cleared. Finally, the
tissue sections were mounted with neutral gum.
Immunohistochemistry
Mice uteri at D5 were fixed in 4% paraformaldehyde
and embedded in paraffin. The tissue was then cut to 5 µm sections.
Immunohistochemistry was performed using DAB color reagent kit
(SP-9000; Zhongshan Biosciences Inc. China) according to the
manufacturer's protocols. The sections were examined and imaged
under a microscope (BX43, Olympus Corporation, Tokyo, Japan).
Non-immune goat serum (Zhongshan Biosciences Inc. China) was used
instead of the primary antibody as a negative control.
Statistical analysis
All experiments were repeated at least three times.
The data were analyzed using SPSS software (version, 16.0; SPSS
Inc., Chicago, IL, USA). The Student's t-test was used to determine
the differences in serum insulin, P4 and E2 levels, mRNA and
protein expression levels and the number of implantation sites
between the control and insulin-treated groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Levels of insulin, E2 and P4 in
serum
In order to determine the effects of maternal
insulin resistance on embryo implantation, a mouse model of
hyperinsulinemia was established through exogenous insulin
injection (11,21). The effects of chronic treatment
with insulin on mouse weight, and the serum levels of insulin, E2
and P4 are presented in Table II.
In addition, serum glucose levels and the HOMA values were
significantly higher in the insulin-treated group. The results
demonstrated that the insulin-treated group developed chronic
insulin resistance and hyperinsulinemia.
 | Table II.Effect of chronic insulin treatment
on maternal weight, metabolic parameters and serum hormone
levels. |
Table II.
Effect of chronic insulin treatment
on maternal weight, metabolic parameters and serum hormone
levels.
|
| Group |
|---|
|
|
|
|---|
| Parameter | Control |
Insulin-treated |
|---|
| Number of mice | 50 | 50 |
| Body weight
(g) | 38.7±2.30 |
34.46±1.21a |
| Plasma glucose
(mM/l) | 7.14±1.05 |
9.62±0.50a |
| Serum insulin
(mU/l) | 16.57±0.74 |
26.77±1.17a |
| HOMA (glucose ×
insulin/22.5) | 5.25±0.71 |
11.43±0.22a |
| E2 (pg/ml) | 57.15±4.40 |
32.52±1.76a |
| P4 (pg/ml) | 6.51±0.24 |
9.56±0.28a |
Number of implantation sites on
D5
A previous study demonstrated that endometrial
receptivity is maintained on day 5 of pregnancy in mice (25). In order to determine the potential
role of hyperinsulinemia on endometrium receptivity, the present
study recorded the number of embryo implantation sites on D5 by
counting the distinctive blue bands on the mice uteri as a result
of Trypan blue staining. No significant difference between the
number of implantation sites in the insulin-treated group was
observed when compared with the control group (Fig. 1A). In addition, no alterations in
the morphology of the uteri between the two groups were observed
(Fig. 1B).
Endometrium receptivity is impaired by
maternal hyperinsulinemia
Although no significant difference was evident
between the two groups when examined visually, it was unclear
whether endometrium receptivity was altered at the microscopic
scale. In order to investigate this further, morphological
examination of uterine cross-sections was performed at D5, and no
marked difference between the control and insulin-treated groups
was observed (Fig. 2). During the
implantation window, the process of embryo implantation is divided
into the following three steps: Pre-implantation on day 4,
peri-implantation on day 5 and post-implantation on day 6 of
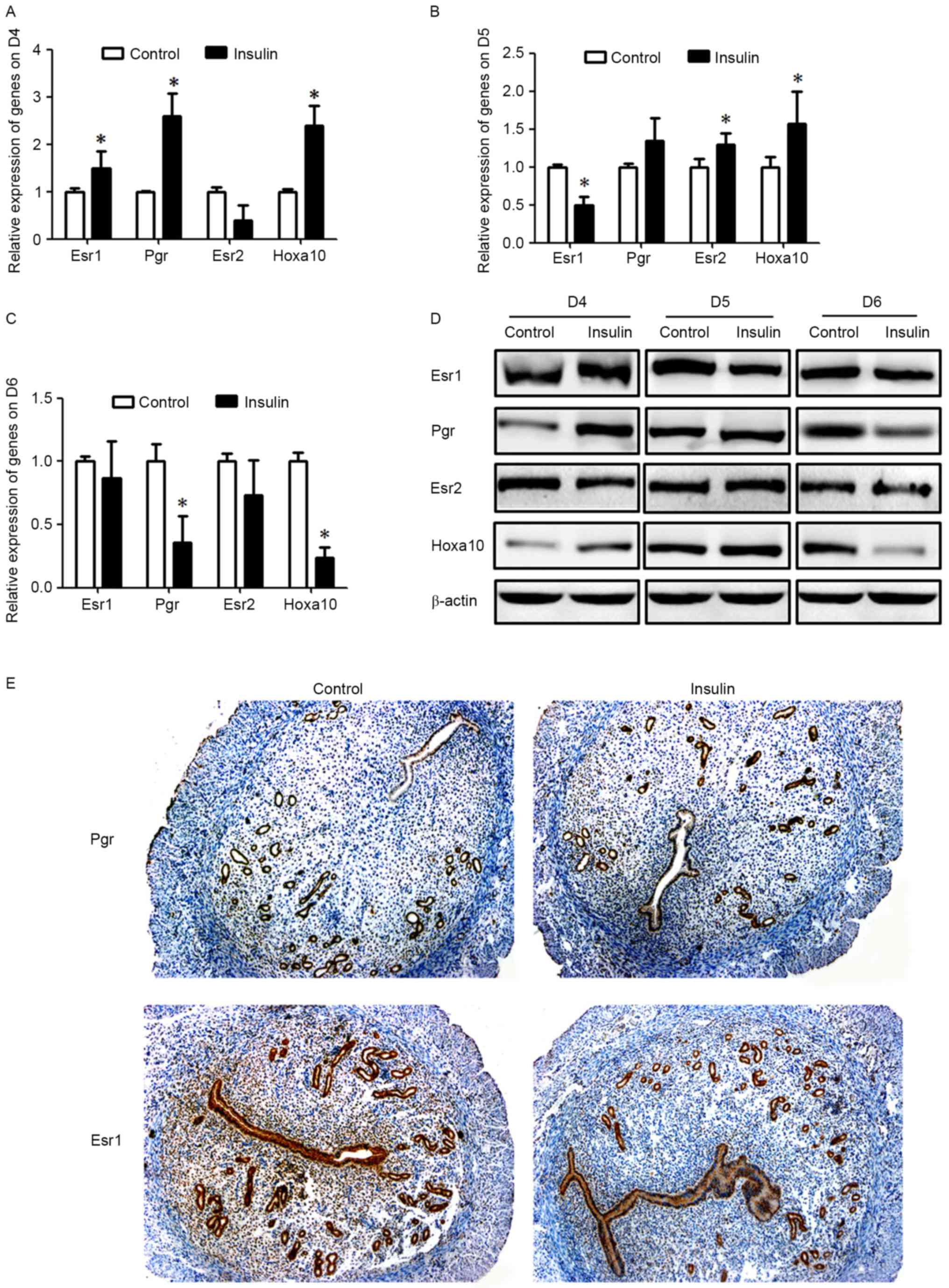
gestation in mice (26,27). The expression levels of molecules
associated with endometrial receptivity, such as Esr1 and Pgr may
vary, depending on their functions during the different steps of
the embryo implantation process. The present study determined that
expression levels of Esr1, Pgr and Hoxa10 were significantly
upregulated (P<0.05, Fig. 3)
and Esr2 levels were downregulated in the insulin-treated group
when compared with the control group on D4 (P<0.05, Fig. 3). However, no obvious differences
in Pgr expression levels were observed during peri-implantation on
D5 (Fig. 3). Unlike Pgr, Esr1,
Esr2 and Hoxa10 were significantly imbalanced in mice with
hyperinsulinemia (P<0.05; Fig. 3B,
D and E). In addition, a marked reduction in the levels of
these molecules was recorded during post-implantation on D6 of
gestation in mice (Fig. 3C and D).
Therefore, it is possible that the establishment process of
endometrium receptivity was altered by maternal hyperinsulinemia,
even though the embryo was able to implant into endometrium.
mTOR may be responsible for altered
endometrial receptivity by maternal hyperinsulinemia
A previous study revealed that mTOR was essential
for endometrial receptivity (18),
and previous studies have demonstrated that insulin could serve its
roles though mTOR (28,29). Therefore, the present study
investigated mTOR signaling in the endometrium following insulin
treatment. During the implantation window, the vital time for
endometrial receptivity, western blotting of endometrial tissues
revealed a significant increase in endometrial p-mTOR and p-p70S6K
protein expression levels in the insulin-treated group when
compared with the control group (P<0.05; Fig. 4). No marked difference in the
levels of total mTOR and p70S6K protein expression levels was
identified in the insulin-treated group when compared with the
control group (Fig. 4).
Discussion
Previous clinical studies have identified
compromised reproduction in women with PCOS, obesity and type 2
diabetes (30,31). Hyperinsulinemia and insulin
resistance are established characteristics of women with PCOS,
obesity and type 2 diabetes (32,33).
Previous studies have investigated lower embryo implantation rates
in females with these conditions, and an association between
abnormal oocyte and embryo development and hyperinsulinemia
(11,12). However, the importance of
hyperinsulinemia and insulin resistance on maternal endometrium
remains to be elucidated. The present study determined that mice
endometrium receptivity in early pregnancy was affected by maternal
hyperinsulinemia.
During early pregnancy, the establishment of uterine
receptivity is necessary for successful embryo implantation.
Uterine receptivity is established and maintained through a series
of specific cellular and molecular events (34). However, in the event that the genes
associated with the establishment of uterine receptivity are
expressed abnormally, embryo implantation may be affected directly
and lead to spontaneous abortion (35). In the animal model developed in the
present study, the number of implantation sites at D5 was not
significantly different following treatment with insulin. These
results may be due to the short duration of insulin treatment.
Females with PCOS, obesity and type 2 diabetes are exposed to
hyperinsulinemia and insulin resistance for a long period of time.
The present study determined that although the embryo was able to
implant into endometrium, the genes associated with uterine
receptivity, including Esr1, Pgr, Hoxa10 and Esr2 were dysregulated
in mice with maternal hyperinsulinemia. Therefore, it is possible
that the process of endometrium receptivity establishment was
altered by maternal hyperinsulinemia. Although the present study
did not investigate the effect of hyperinsulinemia on endometrium
function following embryo implantation into the uterus, a previous
study determined that abnormal endometrium receptivity may increase
the risk of abnormal decidualization and placentation in the later
stages of pregnancy and lead to a miscarriage (34).
mTOR is a member of the phosphatidylinositol
3-kinase-associated kinase superfamily. It is a core component of
raptor-mTOR (mTORC1) and rictor-mTOR (mTORC2) complexes that
control various cellular processes. mTORC1 and mTORC2 regulate
several elements downstream of the type I insulin-like growth
factor receptor and insulin receptor (36). mTOR serves a crucial role in
mammalian growth control as an important molecule of signal
transduction, including cell proliferation, growth, differentiation
and apoptosis (37–40). Insulin could serve its roles though
mTOR (28,29). Neil et al determined that
insulin may induce activation of mTOR signaling in glioblastoma
cells (41). Fritzen et al
observed that mTOR signaling via unc-51 like autophagy activating
kinase 1 may mediate the autophagy-inhibiting effect of insulin in
human skeletal muscle (42). In
addition, previous studies have determined that mTOR was essential
for early mouse embryo growth and proliferation of embryonic stem
cells (43–45). Furthermore, the importance of mTOR
in uterine tissues has been verified in various studies (46–48).
A previous study demonstrated that endometrial receptivity was
compromised by the intrauterine injection with rapamycin (18), which is an inhibitor of mTOR. In
the present study, a significant increase in endometrial p-mTOR and
p-p70S6K protein expression was detected in the insulin-treated
group. Therefore, mTOR signaling may contribute to impaired
endometrium receptivity during maternal hyperinsulinemia and
insulin resistance. However, future intervention studies are
required in order for these results to be confirmed.
In conclusion, the present study determined that the
effect of maternal hyperinsulinemia on endometrium receptivity may
be important for embryo implantation. Although an embryo may
implant into endometrium, mice endometrium receptivity in early
pregnancy may be affected by maternal hyperinsulinemia. In
addition, mTOR signaling may be involved in this process. The
underlying molecular mechanisms involving mTOR require further
investigation. The present study provided vital preliminary data
for future investigation of compromised reproduction in women with
hyperinsulinemia.
Acknowledgements
The present study was supported by the National
Natural Science foundation of China (grant no. 81300486 and
81300678), the Natural Science Foundation of Chongqing (grant no.
cstc2015jcyjA10013), the Scientific Research Program of Science and
the Technology Commission of Yuzhong District of Chongqing (grant
no. 20150104).
References
|
1
|
Nagashima T, Li Q, Clementi C, Lydon JP,
DeMayo FJ and Matzuk MM: BMPR2 is required for postimplantation
uterine function and pregnancy maintenance. J Clin Invest.
123:2539–2550. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mori M, Kitazume M, Ose R, Kurokawa J,
Koga K, Osuga Y, Arai S and Miyazaki T: Death effector
domain-containing protein (DEDD) is required for uterine
decidualization during early pregnancy in mice. J Clin Invest.
121:318–327. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dale PO, Tanbo T, Haug E and Abyholm T:
The impact of insulin resistance on the outcome of ovulation
induction with low-dose follicle stimulating hormone in women with
polycystic ovary syndrome. Hum Reprod. 13:567–570. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Svendsen PF, Nilas L, Nørgaard K, Jensen
JE and Madsbad S: Obesity, body composition and metabolic
disturbances in polycystic ovary syndrome. Hum Reprod.
23:2113–2121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Igosheva N, Abramov AY, Poston L, Eckert
JJ, Fleming TP, Duchen MR and McConnell J: Maternal diet-induced
obesity alters mitochondrial activity and redox status in mouse
oocytes and zygotes. PLoS One. 5:e100742010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lintsen AM, Pasker-de Jong PC, de Boer EJ,
Burger CW, Jansen CA, Braat DD and van Leeuwen FE: Effects of
subfertility cause, smoking and body weight on the success rate of
IVF. Hum Reprod. 20:1867–1875. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cano F, García-Velasco JA, Millet A,
Remohí J, Simón C and Pellicer A: Oocyte quality in polycystic
ovaries revisited: Identification of a particular subgroup of
women. J Assist Reprod Genet. 14:254–261. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steiner DF, Park SY, Støy J, Philipson LH
and Bell GI: A brief perspective on insulin production. Diabetes
Obes Metab. 11:(Suppl 4). 189–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Homburg R: Should patients with polycystic
ovarian syndrome be treated with metformin? A note of cautious
optimism. Hum Reprod. 17:853–856. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasumov T, Solomon TP, Hwang C, Huang H,
Haus JM, Zhang R and Kirwan JP: Improved insulin sensitivity after
exercise training is linked to reduced plasma C14: 0 ceramide in
obesity and type 2 diabetes. Obesity (Silver Spring). 23:1414–1421.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ou XH, Li S, Wang ZB, Li M, Quan S, Xing
F, Guo L, Chao SB, Chen Z, Liang XW, et al: Maternal insulin
resistance causes oxidative stress and mitochondrial dysfunction in
mouse oocytes. Hum Reprod. 27:2130–2145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schulte MM, Tsai JH and Moley KH: Obesity
and PCOS: The effect of metabolic derangements on endometrial
receptivity at the time of implantation. Reprod Sci. 22:6–141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mioni R, Mozzanega B, Granzotto M,
Pierobon A, Zuliani L, Maffei P, Blandamura S, Grassi S, Sicolo N
and Vettor R: Insulin receptor and glucose transporters mRNA
expression throughout the menstrual cycle in human endometrium: A
physiological and cyclical condition of tissue insulin resistance.
Gynecol Endocrinol. 28:1014–1018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williamson DL, Dungan CM, Mahmoud AM, Mey
JT, Blackburn BK and Haus JM: Aberrant REDD1-mTORC1 responses to
insulin in skeletal muscle from Type 2 diabetics. Am J Physiol
Regul Integr Comp Physiol. 309:R855–R863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caron A, Richard D and Laplante M: The
Roles of mTOR complexes in lipid metabolism. Annu Rev Nutr.
35:321–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharawy MH, El-Awady MS, Megahed N and
Gameil NM: Attenuation of insulin resistance in rats by agmatine:
Role of SREBP-1c, mTOR and GLUT-2. Naunyn Schmiedebergs Arch
Pharmacol. 389:45–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y,
Chen S, Zhu J, Sheng H and Guo F: Effects of individual
branched-chain amino acids deprivation on insulin sensitivity and
glucose metabolism in mice. Metabolism. 63:841–850. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, He J, Ding Y, Zeng L, Gao R, Cheng
S, Liu X and Wang Y: The role of MTOR in mouse uterus during embryo
implantation. Reproduction. 138:351–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang A, Ji L, Shang W, Li M, Chen L, White
RE and Han G: Expression of GPR30, ERα and ERβ in endometrium
during window of implantation in patients with polycystic ovary
syndrome: A pilot study. Gynecol Endocrinol. 27:251–255. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poretsky L, Clemons J and Bogovich K:
Hyperinsulinemia and human chorionic gonadotropin synergistically
promote the growth of ovarian follicular cysts in rats. Metabolism.
41:903–910. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lima MH, Souza LC, Caperuto LC, Bevilacqua
E, Gasparetti AL, Zanuto R, Saad MJ and Carvalho CR: Up-regulation
of the phosphatidylinositol 3-kinase/protein kinase B pathway in
the ovary of rats by chronic treatment with hCG and insulin. J
Endocrinol. 190:451–459. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paria BC, Huet-Hudson YM and Dey SK:
Blastocyst's state of activity determines the ‘window’ of
implantation in the receptive mouse uterus. Proc Natl Acad Sci USA.
90:10159–10162. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan Y, Tan D, He M, Gu M, Wang Z, Zeng G
and Duan E: A model for implantation: Coculture of blastocysts and
uterine endometrium in mice. Biol Reprod. 72:556–561. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zorn TM, Soto-Suazo M, Pellegrini CR,
Oliveira JG and Stumpf WE: Estradiol receptor binding to the
epithelium of uterine lumen and glands: Region- and time-related
changes during preimplantation and periimplantation periods studied
by autoradiography. Histochem Cell Biol. 120:1–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu G, Zhang X, Lin H, Wang H, Li Q, Ni J
and Zhu C: Effects of E-cadherin on mouse embryo implantation and
expression of matrix metalloproteinase-2 and −9. Biochem Biophys
Res Commun. 343:832–838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan SY: Effects of prostaglandin E2 and
F2 alpha on peri-implantation development of mouse embryos in
vitro. Prostaglandins. 42:321–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palaniappan M, Menon B and Menon KM:
Stimulatory effect of insulin on theca-interstitial cell
proliferation and cell cycle regulatory proteins through MTORC1
dependent pathway. Mol Cell Endocrinol. 366:81–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Miard S, Laplante M, Sonenberg N and
Picard F: Insulin stimulates IGFBP-2 expression in 3T3-L1
adipocytes through the PI3K/mTOR pathway. Mol Cell Endocrinol.
358:63–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Catalano PM and Shankar K: Obesity and
pregnancy: Mechanisms of short term and long term adverse
consequences for mother and child. BMJ. 356:j12017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moran L and Teede H: Metabolic features of
the reproductive phenotypes of polycystic ovary syndrome. Hum
Reprod Update. 15:477–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diamanti-Kandarakis E and Dunaif A:
Insulin resistance and the polycystic ovary syndrome revisited: An
update on mechanisms and implications. Endocr Rev. 33:981–1030.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Templeman NM, Skovsø S, Page MM, Lim GE
and Johnson JD: A causal role for hyperinsulinemia in obesity. J
Endocrinol. 232:R173–R183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H and Dey SK: Roadmap to embryo
implantation: Clues from mouse models. Nat Rev Genet. 7:185–199.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quenby S, Anim-Somuah M, Kalumbi C,
Farquharson R and Aplin JD: Different types of recurrent
miscarriage are associated with varying patterns of adhesion
molecule expression in endometrium. Reprod Biomed Online.
14:224–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sabatini DM: mTOR and cancer: Insights
into a complex relationship. Nat Rev Cancer. 6:729–734. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vahidnezhad H, Youssefian L and Uitto J:
Molecular genetics of the PI3K-AKT-mTOR pathway in genodermatoses:
Diagnostic implications and treatment opportunities. J Invest
Dermatol. 136:15–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wataya-Kaneda M: Mammalian target of
rapamycin and tuberous sclerosis complex. J Dermatol Sci.
79:93–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Somers MJ and Paul E: Safety
considerations of mammalian target of rapamycin inhibitors in
tuberous sclerosis complex and renal transplantation. J Clin
Pharmacol. 55:368–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huo F, Zhang C, He H and Wang Y:
MicroRNA-144-3p inhibits proliferation and induces apoptosis of
human salivary adenoid carcinoma cells via targeting of mTOR.
Biotechnol Lett. 38:409–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Neil J, Shannon C, Mohan A, Laurent D,
Murali R and Jhanwar-Uniyal M: ATP-site binding inhibitor
effectively targets mTORC1 and mTORC2 complexes in glioblastoma.
Int J Oncol. 48:1045–1052. 2016.PubMed/NCBI
|
|
42
|
Fritzen AM, Madsen AB, Kleinert M, Treebak
JT, Lundsgaard AM, Jensen TE, Richter EA, Wojtaszewski J, Kiens B
and Frøsig C: Regulation of autophagy in human skeletal muscle:
Effects of exercise, exercise training and insulin stimulation. J
Physiol. 594:745–761. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Martin PM and Sutherland AE: Exogenous
amino acids regulate trophectoderm differentiation in the mouse
blastocyst through an mTOR-dependent pathway. Dev Biol.
240:182–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gangloff YG, Mueller M, Dann SG, Svoboda
P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G and
Kozma SC: Disruption of the mouse mTOR gene leads to early
postimplantation lethality and prohibits embryonic stem cell
development. Mol Cell Biol. 24:9508–9516. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim J, Song G, Wu G, Gao H, Johnson GA and
Bazer FW: Arginine, leucine, and glutamine stimulate proliferation
of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1
signal transduction pathway. Biol Reprod. 88:1132013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wollenhaupt K, Brüssow KP, Albrecht D and
Tomek W: The Akt/mTor signaling cascade is modified during
placentation in the porcine uterine tissue. Reprod Biol.
13:184–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mahdi H, Xiu J, Reddy SK and DeBernardo R:
Alteration in PI3K/mTOR, MAPK pathways and Her2
expression/amplification is more frequent in uterine serous
carcinoma than ovarian serous carcinoma. J Surg Oncol. 112:188–194.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guntas G, Hallett RA, Zimmerman SP,
Williams T, Yumerefendi H, Bear JE and Kuhlman B: Engineering an
improved light-induced dimer (iLID) for controlling the
localization and activity of signaling proteins. Proc Natl Acad Sci
USA. 112:112–117. 2015. View Article : Google Scholar : PubMed/NCBI
|