Introduction
Cell surface-associated mucin 1 (MUC1) is a
transmembrane protein consisting of an extracellular domain
containing 20–125 tandem repeat arrays, followed by a transmembrane
domain and a short cytoplasmic tail. MUC1 is overexpressed and
aberrantly glycosylated in carcinoma, including 90% of breast
cancer cases (1,2). Its broad distribution on primary
tumors and metastasis renders it an attractive vaccine target
(3,4). Studies have suggested that antibodies
targeting the MUC1-N-terminal domain may be instrumental in
preventing metastasis, counteracting immune suppression and
effecting antibody-dependent cell-mediated cytotoxicity (5,6). The
association between elevated levels of anti-MUC1 antibody and
improved survival rates has been observed in patients with
early-stage breast cancer and other types of cancer (6,7). The
initial evaluation of MUC1 peptide/glycopeptides has found them to
be immunogenic and safe, however, their antitumor responses are
limited (8). Adjuvant vaccination
with MUC1 glycopeptide polyvalent vaccines, which induce a marked
humoral response, may prevent the recurrence of disease in patients
with early breast cancer, which suggests MUC1 immunotherapy is
beneficial (9). Immunotherapy,
which relies on humoral responses to achieve its clinical effect,
is most effective in patients with a low tumor burden (minimal
residual disease) (10). The
marked immune response and immunologic memory, which are associated
with active immunotherapy, administered in an adjuvant setting,
enable constant surveillance to protect patients with cancer from
recurrence of disease, although it is of limited use in advanced
metastatic disease. However, the internalization of anti-MUC1
antibodies by tumor cells provides a route for the development of
therapeutics based on this antibody (11,12).
This allows for other therapeutic approaches, including the
delivery of toxic compounds into tumor cells, for example, antibody
drug conjugates or immunotoxins (13,14).
In our previous study (15), circulating anti-MUC1 antibody (IgG)
was detected using an indirect enzyme-linked immunosorbent assay
(I-ELISA) with a recombinant MUC1 protein containing six tandem
repeat sequences of MUC1. A significant negative correlation was
found between the serum levels of MUC1 antigen (cancer antigen
15-3; CA15-3) and that of anti-MUC1 antibody in patients with
malignant tumors at advanced stages. According to this result, the
present study hypothesized that circulating anti-MUC1 antibodies
were able to bind to MUC1 dispersed into the blood in stage IV
breast cancer. To clarify the binding of serum anti-MUC1 antibody
to the MUC1 antigen in stage IV breast cancer, the present study
analyzed serum samples collected from 61 patients with stage IV
breast cancer and 64 patients with early-stage breast cancer, and
performed comparative analysis between stage IV and early-stage
breast cancer.
Materials and methods
Specimens
Between January 2012 and February 2014, 61 serum
samples were obtained from 61 patients with newly diagnosed stage
IV breast cancer (31–67 years old; median 43 years) at the Second
Hospital of Jilin University (Changchun, China). In addition, 64
serum samples were obtained from 64 patients with early-stage
breast cancer (stages I, II and III; 35–65 years old; median 45
years) at the same hospital. Venous blood samples (5 ml) were
collected from patients and centrifuged at 225 × g and −4°C for 10
min. Subsequently, serum was separated immediately following
centrifugation and stored at −40 °C until analysis. All patients
included were post-primary treatment. The 61 patients with stage IV
breast cancer included 51 with infiltrating duct carcinoma, 4 with
medullary carcinoma and 6 with carcinoma simplex. All cases were
cases of recurrent disease, including 24 with regional lymph node
recurrence combined with distant metastasis and 37 with distant
metastasis only. The distant metastasis involved one or several
locations. Patients with distant metastasis included 16 with
hepatic metastasis, 30 with pulmonary metastasis, 30 with osseous
metastasis, 4 with brain metastasis, 14 with distant lymph node
metastasis (supraclavicular nodes of opposite side or lymph nodes
of neck), 10 with pleural metastasis and 12 with peritoneal
metastasis. The 64 patients with early-stage breast cancer
consisted of 57 with infiltrating duct carcinoma, 3 with medullary
carcinoma and 4 with carcinoma simplex; the disease staging was as
follows: Stage I (n=10); stage II (n=38); and stage III (n=16). The
clinical stages of breast cancer were according to the 7th edition
of the American Joint Committee on Cancer staging system (15). The study was approved by the
Ethical Committee of Jilin University. Written informed consent was
obtained from patients or their families.
Preparation of recombinant
protein
The recombinant 8R-MUCPT protein was prepared
according to a previously reported method (16). Briefly, a cDNA fragment, obtained
by polymerase chain reaction, containing the human MUC1 variable
number of tandem repeats (VNTR) encoding sequence with six tandem
repeats was subcloned into the prokaryotic expression vector pET26b
plasmid, in which there was an eight-arginine-encoding sequence at
the N-terminal insertion end. Subsequently, the pET26b-8R-MUCPT
plasmid was directly transformed into the BL21 Escherichia coli
(Beijing Dingguo Inc., Beijing, China) bacterial strain and
individual clones were grown in Luria-Bertani liquid medium with 4
µg/ml of chloramphenicol. Purification of the recombinant protein
with a polyhistidine-tail at the C-terminal insertion end was
performed using Ni-chromate affinity chromatography. Another
recombinant protein, 3aB, encoding 224 amino acids was used for the
ELISA inhibition test, which was also constructed in Department of
Molecular Biology, Institute of Basic Medical Sciences, Jilin
University.
Detection of anti-MUC1 IgG with
8R-MUCPT using I-ELISA
The serum anti-MUC1 IgG was detected using I-ELISA
(16). The 8R-MUCPT fusion protein
corresponding to the six VNTR region of the MUC1 protein core was
used as a catcher and coated onto the 96-well flat-bottom plates.
Individual results were calculated as the mean optical density (OD)
of two repeat sample wells; the cut-off value was 1.30, which was
defined as an anti-MUC1 IgG level equal to or higher than the mean
OD value of the total breast cancer samples.
Detection of MUC1 antigen using
ELISA
The CA15-3 antigen is defined as a glycoprotein,
which binds to two monoclonal antibodies, namely, DF3 and 115D8.
The DF3 antibody recognizes the VNTR of MUC1 (sequence DTRPAPGS),
and the 115D8 monoclonal antibody is the solid-phase capture
antibody, which binds to a peptide-carbohydrate epitope on the same
repeat (17). In the present
study, CA15-3 was measured using an ELISA kit (R&D Systems,
Inc., Minneapolis, MN, USA), and all steps were performed according
to the manufacturer's protocols. Briefly, 100 µl of the patient
sample, control and calibrator were dispensed in ligand-coated
tubes. Following the addition of 100 µl ligand-labeled monoclonal
antibody (mAb), the tubes were incubated at 37°C for 1 h.
Subsequently, 100 µl anti-ligand mAb was added, followed by
incubation for 1 h. The OD values were determined at 450 nm in an
ELISA autoreader (Labsystems Diagnostics Ltd., Vantaa, Finland).
The cut-off value was 36.0 U/ml.
Western blot analysis and inhibition
test with MUC1 VNTR antigen
According to the previously reported method
(18), purified recombinant
8R-MUCPT protein (10 µg) was resolved by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and then transferred
onto a nitrocellulose membrane (Advantec MFS, Inc., Dubulin, CA,
USA). The membranes were sectioned vertically into 4 sections of
the same size and were incubated in 5% milk/PBS (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) at 4°C overnight. The
following day, the membranes were preincubated with either mouse
anti-human MUC1 VNTR mAb (BD Pharmingen, Inc., San Diego, CA, USA)
in a 1:50 dilution or mouse anti-human monoclonal anti-hepatitis B
surface antigen antibody (Bioson Corporation, Beijing, China) in a
1:20 dilution at room temperature for 3 h. Following washing with
PBS, the membranes were incubated with serum positive for the
anti-MUC1 antibody, derived from an MUC1-positive patient with
stage IV breast cancer, in a 1:5 dilution at 4°C overnight, and
then washed and incubated with peroxidase-conjugated goat
anti-human IgG (Bioson Corporation) in a 1:200 dilution with 5%
milk/PBS at 25°C for 1 h. Following washing, 3.3′-diaminobenzidine
was added to the membranes for the coloration reaction.
ELISA inhibition test with MUC1 VNTR
antigen
Each serum sample was incubated, respectively, with
8R-MUCPT, 3aB, poly-arginine (poly-R, Sigma-Aldrich; Merck
Millipore) or poly-histidine (poly-H, Sigma-Aldrich; Merck
Millipore) at the same molar concentration of 8 mM, followed by the
detection of anti-MUC1 antibodies using I-ELISA with 8R-MUCPT as a
coating antigen. All samples were measured in duplicate. The
inhibition ratio of 8R-MUCPT in the binding reaction of anti-MUC1
IgG and MUC1 antigen was calculated as follows: Inhibition ratio
(%) = (OD value of serum sample incubated with 8R-MUCPT-OD value of
control serum or serum sample incubated without an inhibitor) ×100
/ OD value of control serum.
Detection of MUC1 IgG affinity by urea
degradation combining ELISA
8R-MUCPT protein (10 µg/ml) in 0.05 mol/l carbonate
buffer (pH 9.6) was coated onto 96-well flat-bottom plates at 4°C
for 17 h. The plates were washed with PBS three times, blocked with
5% milk/PBS at 37°C for 2 h, and then incubated with sera from the
patients in a 1:40 dilution with 10% milk/PBS at room temperature
for 30 min. Following washing twice with 0.05% Tween-20/PBS
(Sigma-Aldrich; Merck Millipore), two plate wells were incubated
with 8 mol/l urea solution (Sigma-Aldrich; Merck Millipore) and
another two wells with PBS at room temperature for 10 min. The
plates were washed three times with 0.05% Tween-20/PBS, and
incubated with peroxidase-conjugated goat anti-human IgG in a 1:
250 dilution with 5% milk/PBS at room temperature for 1 h. The
reaction was terminated by adding O-phenylenediamine
dihydrochloride following washing three times with 0.05%
Tween-20/PBS. The OD value was determined at A492 in an ELISA
autoreader (Labsystems Diagnostics Ltd., Vantaa, Finland). All
samples were measured in four wells. The avidity index (AI) was
calculated as follows: AI = (OD with urea / OD without urea)
×100.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The percentages among the
different groups were compared using the χ2 test.
Differences between clinical stages were analyzed using a
Kruskal-Wallis test. Correlations between two experimental groups
were evaluated using linear regression analysis. An unpaired
two-sample t-test was used to compare between values of two groups.
P<0.05 determined in the two-tailed test was considered to
indicate a statistically significant difference.
Results
I-ELISA results of circulating
anti-MUC1 IgG and Ca15-3 antigen
As shown in Table
I, the serum level and positive rate of anti-MUC1 IgG were
marginally elevated in patients with stage II breast cancer,
compared with those with stage I breast cancer, but were marginally
decreased in stage III breast cancer. Elevated levels were found in
the stage IV patients. The patients with stages II, III and IV
breast cancer showed a gradual increase in the level of Ca15-3
antigen and rate of positivity, compared with those with stage I
disease. The OD value and positivity of anti-MUC1 IgG in the
patients with stage IV cancer with regional lymph node recurrence
and distant metastasis were marginally higher, compared with those
in patients with distant metastasis only. By contrast, the serum
level and positivity of Ca15-3 in patients with regional lymph node
recurrence with distant metastasis were decreased, compared with
those in patients with distant metastasis only, however, no
statistically significant correlations were found among the groups
(P>0.05).
 | Table I.Expression of anti-MUC1 IgG and Ca15-3
antigen in serum samples of patients with breast cancer. |
Table I.
Expression of anti-MUC1 IgG and Ca15-3
antigen in serum samples of patients with breast cancer.
|
|
| Optical density
(positive rate, %) |
|---|
|
|
|
|
|---|
| Stage | n | Anti-MUC1 IgG | Ca15-3 antigen |
|---|
| I | 10 | 1.32±0.18
(30.00) | 20.35±1.29
(10.00) |
| II | 38 | 1.33±0.24
(36.84) | 24.34±7.98
(15.79) |
| III | 16 | 1.14±0.24
(18.75) | 30.79±7.06
(31.25) |
| IV | 61 | 1.32±0.38
(44.26) | 32.80±8.50
(45.90) |
| Regional lymph node
recurrence with distant metastasis | 24 | 1.40±0.26
(50.00) | 32.05±7.92
(41.67) |
| Distant metastasis
only | 37 | 1.27±0.32
(40.54) | 33.29±6.96
(48.65) |
| Total | 125 | 1.30±0.27
(37.60) | 28.97±8.90
(32.00) |
Association between circulating
anti-MUC1 IgG and the Ca15-3 antigen in stage IV breast cancer
No significant correlation was found between the
level of circulating anti-MUC1 IgG and serum expression of CA15-3
in patients with stage IV breast cancer. However, a significant
negative correlation was observed when positive serum level of
CA15-3 antigen and/or anti-MUC1 IgG were selected (r=−0.417;
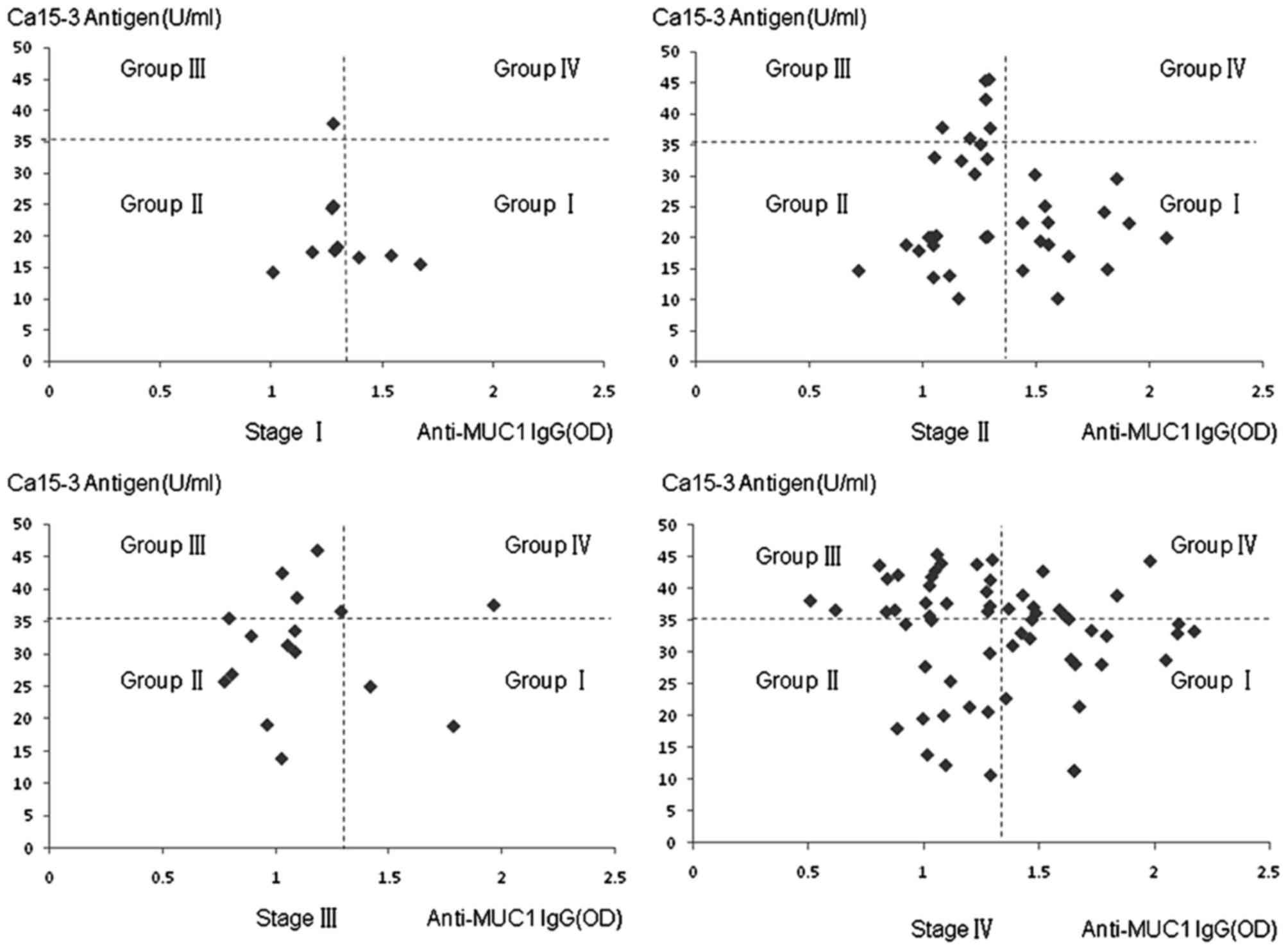
P=0.0044; Fig. 1), which suggested
the formation of an MUC1 circulating immune complex (MUC1-CIC) or
the binding of anti-MUC1 IgG with CA15-3 antigen in stage IV breast
cancer. The same was observed in stage II breast cancer, in which
there was a negative correlation when positive CA15-3 antigen
and/or anti-MUC1 IgG were selected (r=−0.630; P=0.0029; Fig. 1). For stages I and III, no
significant correlations were found due to fewer samples. However,
if all positive serum levels of CA15-3 antigen and/or anti-MUC1 IgG
were selected between stages I and III, omitting stage IV, a
negative correlation was observed (r=−0.605; P<0.001; Fig. 1).
As presented in Fig.
1, for each stage of breast cancer, the OD value of the serum
sample was divided into four groups (I, II, III and IV) by cut-off
lines. In group I, positive anti-MUC1 IgG was detected together
with negative CA15-3 antigen. Negative anti-MUC1 IgG and negative
CA15-3 antigen was present in group II. In group III, negative
anti-MUC1 IgG and positive CA15-3 antigen were found. In group IV,
positive anti-MUC1 IgG and positive CA15-3 were shown. In the stage
IV cancer samples, an increased number (8/61) were in group IV,
compared with samples in other stages of disease (1/64;
χ2=4.629; P=0.031). Fewer samples were positive for
CA15-3 (12/64) in early-stage breast cancer, compared with stage IV
breast cancer (28/61; χ2=10.58; P=0.0011). The above
results suggested that circulating anti-MUC1 antibody was able to
bind serum MUC1 antigen and form MUC1-CIC in stage IV and
early-stage breast cancer, however, the compatibility of anti-MUC1
IgG and MUC1 antigen in stage IV breast cancer may have been
lower.
Western blot analysis and inhibition
test with the MUC1 VNTR antigen
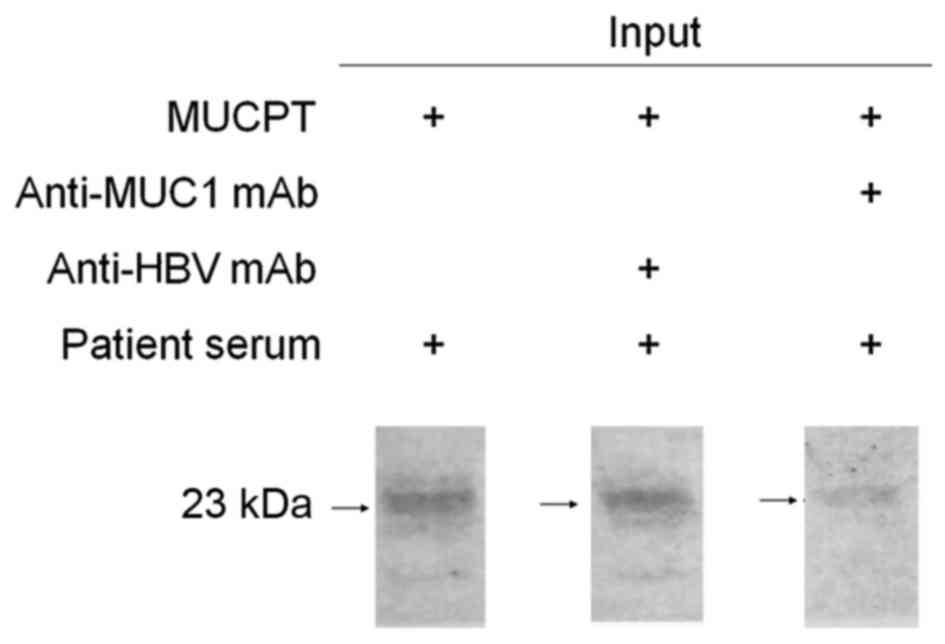
The results of the western blot analysis and
inhibition test showed that 8R-MUCPT was recognized by natural
anti-MUC1 antibody in the serum, and this was inhibited by
anti-MUC1 VNTR mAb when at a sufficient quantity to neutralize the
VNTR region on 8R-MUCPT (Fig. 2).
This demonstrated that circulating anti-MUC1 antibody was able to
bind to the MUC1 antigen in stage IV breast cancer.
ELISA inhibition test with the MUC1
VNTR antigen
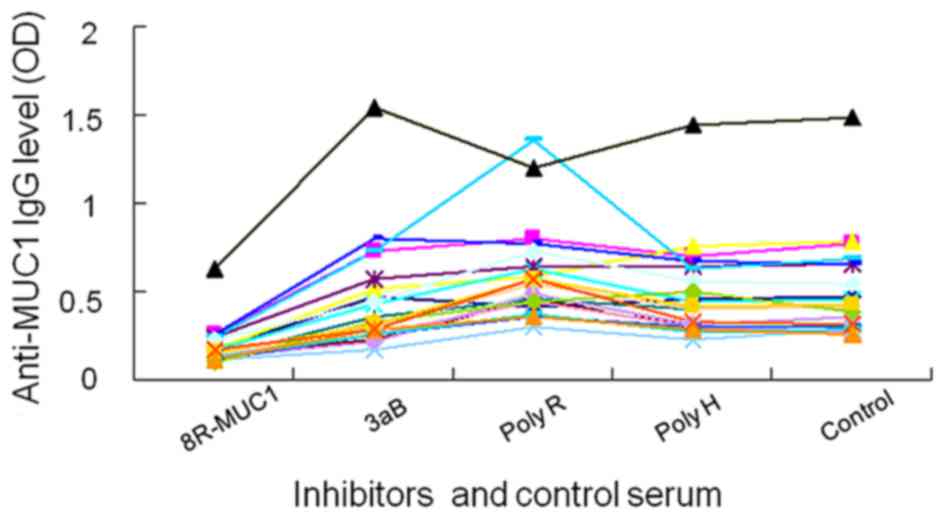
The results of the ELISA inhibition test showed that
8R-MUCPT efficiently neutralized the natural anti-MUC1 antibody in
the serum, whereas the recombinant proteins 3aB, poly-R and poly-H
did not have this effect. This result confirmed that the MUC1
antigen or tandem repeat sequence of MUC1 reacted with anti-MUC1
antibody in the sera of patients with stage IV and early-stage
breast cancer (Fig. 3).
A total of 11 serum samples from IV stage breast
cancer and 11 samples from early-stage breast cancer were assessed
in the inhibition test. The inhibition ratios of 8R-MUCPT in the
binding of anti-MUC1 IgG and the MUC1 antigen were all >45%,
with no statistically significant difference in the between the two
groups (t-test, P=0.778). This result suggested that there was no
significant difference in the affinity of MUC1-IgG between serum
samples from stage IV and early-stage IV breast cancer, as MUC1-IgG
affinity affects the inhibition ratio of 8R-MUCPT in the binding
reaction in the assay.
Detection of the affinity of MUC1 IgG
by urea degradation combining ELISA
The results of the urea degradation combining ELISA
showed that the AIs of anti-MUC1 IgG in stage IV and early-stage
breast cancer were 47.13±11.45 and 47.53±16.10%, respectively, with
no significant difference between the two groups (t-test,
P=0.873).
Discussion
Previous studies in patients with early-stage breast
cancer and other malignant tumors have found that there is a
negative correlation between circulating MUC1 antigen and anti-MUC1
antibody, which indicates the formation of MUC1-CIC or the binding
of anti-MUC1 antibody to MUC1 antigen in serum (19–21).
In early-stage breast cancer, higher levels of MUC1-CIC are found,
compared with levels at advanced stages of the disease. The levels
of MUC1 are low, and marginally higher values of free anti-MUC1
antibodies are found. By contrast, patients with stage IV breast
cancer present with low MUC1-CIC, but increased anti-MUC1 antibody
and MUC1 antigen positivity, with high positive rates of anti-MUC1
antibody and MUC1 antigen in sera (6,19,22).
This indicates that anti-MUC1 antibodies are of low affinity. In
the present study, the number of serum samples detected and
analyzed from patients with stage IV breast cancer was higher,
compared with that in a previous study (19), and were compared with the data from
early-stage breast cancer. The results showed that there was
formation of MUC1-CIC or binding of anti-MUC1 antibody to MUC1
antigen in serum samples from stage IV and early-stage breast
cancer. According to the previous study (19), at least one in six patients with
stage IV breast cancer showed higher serum levels of MUC1, and
increased free IgM, IgG and MUC1-CIC. In addition, Nakamura et
al (18) reported that 27
patients with stage III or IV colorectal cancer were detected with
serum MUC1 antigen or anti-MUC1 antibody, and 2 of these patients
were positive for serum MUC1 antigen and increased anti-MUC1
antibodies, including one with values marginally above the
cut-off.
In order to confirm the interaction between the
serum anti-MUC1 antibody and MUC1 antigen in stage IV breast
cancer, and to examine differences in IgG affinity between serum
samples from stage IV and early-stage breast cancer, the present
study performed western blot analysis, an inhibition test and an
ELISA inhibition test. The results of the western blot analysis and
inhibition test showed that serum anti-MUC1 antibody in stage IV
breast cancer was bound to MUC1 antigen. In the ELISA inhibition
test, the inhibitors respectively represented an unpurified
component of 8R-MUCPT protein, synthetic peptides of 8 lysine and a
poly-hist tail, which were designed to prevent the effect of
irrelevant antibodies in detecting anti-MUC1 antibody. The results
showed that only 8R-MUCPT inhibited the reaction, which confirmed
that the serum MUC1 antigen or tandem repeat sequence of MUC1
reacted with anti-MUC1 antibody in the serum of patients with stage
IV and early-stage breast cancer. A total of 11 serum samples from
IV stage breast cancer and 11 from early-stage breast cancer were
assessed. No statistically significant difference was found between
the two groups (t-test, P=0.778). MUC1-IgG affinity can affect the
binding of MUC1 antigen with anti-MUC1 antibody, thus affecting the
inhibition ratio. The results of the present study suggested that
there was no significant difference in MUC1-IgG affinity between
stage IV and early-stage breast cancer.
To further examine the difference in IgG affinity
between serum samples from stage IV and early-stage breast cancer,
all serum samples selected were analyzed using a urea degradation
combining ELISA. This assay is a common method to detect the
affinity of an antibody (23,24)
and also enables the examination of a larger sample size. The
results showed no differences between stage IV and early-stage
breast cancer in the affinity of anti-MUC1 IgG (t-test,
P=0.873).
Usually, in addition to the affinity of an antibody,
the ratio between the antigen and antibody, the concentration of
the antigen and/or antibody, and other factors can have an effect
on the binding of antigen and antibody or the formation of the
antigen-antibody complex. In stage IV breast cancer, there was
increased serum MUC1 antigen (25), therefore, the concentration of MUC1
antigen requires consideration if binding is decreased or MUC1-CIC
is low.
In conclusion, circulating anti-MUC1 antibody was
found to bind serum MUC1 antigen in stage IV breast cancer,
however, the compatibility of anti-MUC1 antibody and MUC1 antigen
may be low. No significant difference was found in the affinity of
anti-MUC1 antibody between stage IV breast cancer and early-stage
breast cancer. In the future, the low compatibility of anti-MUC1
antibody and MUC1 antigen in stage IV breast cancer requires
further investigation.
Acknowledgements
This study was supported by the Research Foundation
of the Science and Technology Department of Jilin Province, China
(grant no. 20120714).
References
|
1
|
Sinn BV, von Minckwitz G, Denkert C,
Eidtmann H, Darb-Esfahani S, Tesch H, Kronenwett R, Hoffmann G,
Belau A, Thommsen C, et al: Evaluation of Mucin-1 protein and mRNA
expression as prognostic and predictive markers after neoadjuvant
chemotherapy for breast cancer. Ann Oncol. 24:2316–2324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haddon L and Hugh J: MUC1-mediated
motility in breast cancer: A review highlighting the role of the
MUC1/ICAM-1/Src signaling triad. Clin Exp Metastasis. 32:393–403.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmon L, Avivi I, Kovjazin R, Zuckerman
T, Dray L, Gatt ME, Or R and Shapira MY: Phase I/II study exploring
ImMucin, a pan-major histocompatibility complex, anti-MUC1 signal
peptide vaccine, in multiple myeloma patients. Br J Haematol.
169:44–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kovjazin R, Horn G, Smorodinsky NI,
Shapira MY and Carmon L: Cell surface-associated anti-MUC1-derived
signal peptide antibodies: implications for cancer diagnostics and
therapy. PLoS One. 9:e854002014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohit E, Hashemi A and Allahyari M: Breast
cancer immunotherapy: Monoclonal antibodies and peptide-based
vaccines. Expert Rev Clin Immunol. 10:927–961. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
von Mensdorff-Pouilly S, Verstraeten AA,
Kenemans P, Snijdewint FG, Kok A, Van Kamp GJ, Paul MA, Van Diest
PJ, Meijer S and Hilgers J: Survival in early breast cancer
patients is favorably influenced by a humoral immune response to
polymorphic epithelial mucin. J Clin Oncol. 18:574–583. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamanaka Y, Suehiro Y, Fukui M, Shikichi
K, Imai K and Hinoda Y: Circulating anti-MUC1 IgG antibodies as a
favorable prognostic factor for pancreatic cancer. Int J Cancer.
103:97–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reddish M, MacLean GD, Koganty RR,
Kan-Mitchell J, Jones V, Mitchell MS and Longenecker BM: Anti-MUC1
class I restricted CTLs in metastatic breast cancer patients
immunized with a synthetic MUC1 peptide. Int J Cancer. 76:817–823.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang CK, Katsara M and Apostolopoulos V:
Strategies used for MUC1 immunotherapy: Human clinical studies.
Expert Rev Vaccines. 7:963–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von Mensdorff-Pouilly S, Moreno M and
Verheijen RH: Natural and induced humoral responses to MUC1.
Cancers (Basel). 3:3073–3103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pericleous LM, Richards J, Epenetos AA,
Courtenay-Luck N and Deonarain MP: Characterisation and
internalisation of recombinant humanized HMFG-1 antibodies against
MUC1. Br J Cancer. 93:1257–1266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thie H, Toleikis L, Li J, von Wasielewski
R, Bastert G, Schirrmann T, Esteves IT, Behrens CK, Fournes B,
Fournier N, et al: Rise and fall of an anti-MUC1 specific antibody.
PLos One. 6:e159212011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pastan I, Hassan R, FitzGerald DJ and
Kreitman RJ: Immunotoxin treatment of cancer. Annu Rev Med.
58:221–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teicher BA: Antibody-drug conjugate
targets. Curr Cancer Drug Targets. 9:982–1004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge SB and Compton CC: The American joint
committee on cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Y and Wang L, Zhang P, Wei H, Gao R,
Liu X, Yu Y and Wang L: Detection of circulating anti-mucin 1
(MUC1) antibodies in breast tumor patients by indirect
enzyme-linked immunosorbent assay using a recombinant MUC1 protein
containing six tandem repeats and expressed in Escherichia coli.
Clin Vaccine Immunol. 17:1903–1908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klee GG and Schreiber WE: MUC1
gene-derived glycoprotein assays for monitoring breast cancer (CA
15-3, CA 27.29, BR): Are they measuring the same antigen? Arch
Pathol Lab Med. 128:1131–1135. 2004.PubMed/NCBI
|
|
18
|
Nakamura H, Hinoda Y, Nakagawa N,
Makiguchi Y, Itoh F, Endo T and Imai K: Detection of circulating
anti-MUC1 mucin core protein antibodies in patients with colorectal
cancer. J Gastroenterol. 33:354–361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Croce MV, Isla-Larrain MT, Demichelis SO,
Gori JR, Price MR and Segal-Eiras A: Tissue and serum MUC1 mucin
detection in breast cancer patients. Breast Cancer Res Trea.
81:195–207. 2003. View Article : Google Scholar
|
|
20
|
Richards ER, Devine PL, Quin RJ, Fontenot
JD, Ward BG and McGuckin MA: Antibodies reactive with the protein
core of MUC1 mucin are present in ovarian cancer patients and
healthy women. Cancer Immunol Immunother. 46:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Treon SP, Maimonis P, Bua D, Young G, Raje
N, Mollick J, Chauhan D, Tai YT, Hideshima T, Shima Y, et al:
Elevated soluble MUC1 levels and decreased anti-MUC1 antibody
levels in patients with multiple myeloma. Blood. 96:3147–3153.
2000.PubMed/NCBI
|
|
22
|
von Mensdorff-Pouilly S, Gourevitch MM,
Kenemans P, Verstraeten AA, Litvinov SV, van Kamp GJ, Meijer S,
Vermorken J and Hilgers J: Humoral immune response to polymorphic
epithelial mucin (MUC1) in patients with benign and malignant
breast tumors. Eur J Cancer. 32:1325–1331. 1996. View Article : Google Scholar
|
|
23
|
Revello MG, Gorini G and Gerna G: Clinical
evaluation of a chemiluminescence immunoassay for determination of
immunoglobulin g avidity to human cytomegalovirus. Clin Diagn Lab
Immunol. 11:801–805. 2004.PubMed/NCBI
|
|
24
|
Schoenardie ER, Scaini CJ, Avila LF,
Sperotto RL, Borsuk S, Felicetti CD, Pepe M and Berne ME:
Determination of IgG avidity in BALB/c mice experimentally infected
with Toxocara canis. Rev Bras Parasitol Vet. 23:403–406. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moazzezy N, Farahany TZ, Oloomi M and
Bouzari S: Relationship between preoperative serum CA 15–3 and CEA
levels and clinicopathological parameters in breast cancer. Asian
Pac J Cancer Prev. 15:1685–1688. 2014. View Article : Google Scholar : PubMed/NCBI
|