Introduction
β-thalassemia consists of a diverse group of
inherited blood disorders, which are characterized by abnormalities
in β-globin production (1). In
Southeast Asia, particularly in Thailand, the most common and
severe type of compound heterozygous β-thalassemia is
β0-thalassemia/hemoglobin E (HbE) disease (2). The β0-thalassaemia/HbE
phenotype is caused by co-inheritance of a β-thalassemia allele
from one parent and a structural HbE variant from the other parent.
HbE is induced following the substitution of a lysine for glutamic
acid at codon 26 of β-globin. As a result, b-globin is unstable
in vitro and is rapidly degraded, which leads to a
functional deficiency (3,4).
β0-thalassaemia/HbE is primarily
associated with a reduction in β chain synthesis, which leads to a
globin chain imbalance, ineffective erythropoiesis, oxidative
damage and shortened red blood cell survival (1,5). HbE
instability is a minor factor in the overall pathophysiology of
β0-thalassaemia/HbE. However, during intercurrent
complications, such as infection or febrile illnesses, it may
result in accelerated red cell hemolysis (3,6). In
β0-thalassemias, two major pathways are involved in
erythroid cell destruction; the first induces premature destruction
of erythroid precursors in the bone marrow and is known as
ineffective erythropoiesis (5,7),
while the second induces hemolysis through the destruction of
mature red blood cells (RBCs) containing unmatched α-globin
inclusions in the blood circulation (7). The loss of erythroid precursors
and/or mature RBCs leads to anemic conditions, thus promoting
increased production of erythropoietin (EPO) from the kidneys. The
increased level of EPO subsequently promotes increased erythroid
expansion and extramedullary erythropoiesis (8).
β0-thalassemia/HbE is classified as a
disease associated with extravascular hemolysis, as abnormal RBCs
are engulfed by the reticuloendothelial system, thus inducing
splenomegaly and hypersplenism (5,9). A
splenectomy is performed to reduce the risk of spleen-induced
extravascular hemolysis in patients exhibiting signs of
hypersplenism, which increases blood transfusion requirements
(9). A previous study reported
increased levels of serum cell-free Hb, which provides evidence of
intravascular hemolysis (10).
MicroRNAs (miRNAs/miR) are a class of short single-
stranded RNAs, ~20 to 25 nucleotides in length in the mature form,
which negatively regulate target genes at the post-transcriptional
level by degrading complementary mRNA or inhibiting its translation
(11,12). miRNAs are important molecules
involved in development, and cell proliferation, differentiation
and apoptosis (13). Previous
studies have demonstrated that miRNAs are highly abundant and
stable in the blood circulation (14,15).
Examinations of circulating miRNA profiles in the plasma or serum
obtained from normal healthy subjects have suggested that a number
of circulating miRNAs may be of blood cell origin (16). Circulating miRNAs are considered to
be a useful non-invasive biomarker for various diseases (17,18).
A previous study investigated the expression
patterns of 4 miRNAs (miR-451, miR-155, miR-223 and miR-221) during
normal eythropoiesis using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis (19). This previous study focused on
miR-451, an erythroid cell-specific miRNA (20,21),
which was revealed to be upregulated during erythroid maturation
(19). It has been demonstrated
that miR-451 promotes the maturation of committed erythroblasts,
and the hematopoietic transcription factor, GATA-binding factor 1,
directly regulates its expression (22). Functional studies using gain-and
loss-of-function approaches have demonstrated that miR-451 is
associated with human erythroid maturation (23). By contrast, cellular expression of
miR-155 in cultured erythroid cells markedly decreased during
maturation, and was 200-fold lower in mature RBCs (19,24).
miR-155 has been previously characterized as a
multifunctional miRNA that serves an important role in pathological
processes associated with a number of diseases, including cancer,
inflammation, immunity and cardiovascular diseases (25). In addition, overexpression of
miR-155 as an oncogenic miRNA in hematological malignancies and
solid tumors has been reported (26,27).
In the present study, the authors hypothesized that
the levels of erythroid specific miRNAs would increase in the
plasma of patients with intravascular hemolysis, particularly in
severe cases. miR-451 and miRNA-155 levels were analyzed in plasma
samples from patients with β0-thalassemia/HbE and normal
control subjects, using a rapid and sensitive quantitative RT-qPCR
assay.
Materials and methods
Subjects
The present study was conducted with the approval of
the Ethics Committee from the Mahidol University Institutional
Review Board (Mahidol University, Nakhon Pathom, Thailand) and
informed consent was obtained from each participant (age 18–50
years) enrolled at the Nakhon Pathom Hospital (Nakhon Pathom,
Thailand) between April 2010 and May 2011. A total of 6 ml
EDTA-preserved blood from 23 patients with
β0-thalassemia/HbE (10 mild and 13 severe cases) and 16
(normal control subjects) were collected. There were 12 females and
11 males for the β0-thalassemia/HbE cohort and 10
females and 6 males for normal control subjects. The normal control
subjects were screened to be free of thalassemia using red blood
cell (RBC) indices, hemoglobin typing, multiplex gap-PCR and
reverse dot blot hybridization. Standard methods, including
complete blood count (CBC), Hb typing and reverse dot blot
hybridization were used for diagnosis of
β0-thalassemia/HbE (28). CBCs and RBC indices of the control
subjects and β0-thalassemia/HbE patients were determined
using an automated cell counter (ADVIA 210; Bayer, Pittsburgh, PA,
USA). Hb typing was performed using an automatic high performance
liquid chromatography system following manufacturer's instructions
(VARIANT II™ Hemoglobin Testing systems; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The double-heterozygous state for
β0-thalassemia and HbE alleles in all patients was
confirmed by reverse dot blot hybridization according to the
previously described protocol (28). Briefly, the β-globin gene was
amplified using four biotinylated primers named China 1, China 2,
China 3, China 4, (Bio-Synthesis, Lewisville, TX, USA). The
sequences of the modified primers are as follows: China 1,
5′-biotin GTA CGG CTG TCA TCA CTT AGA CCT CA-3′; China 2,
5′-biotinTGC AGC TTG TCA CAG TGC AGC TCA CT-3′; China 3, 5′-biotin
GTG TAC ACA TAT TGA CCA AA-3′; China 4, 5′-biotinAGC ACA CAG ACC
AGC ACG TT-3′ (28). Amplified DNA
products were hybridized with immobilizing allele-specific
oligonucleotide probes on a nylon membrane (Biodyne C; Pall Life
Sciences, Port Washington, NY, USA) at 45°C for 30 min. Detection
of the hybridization reaction was performed by conjugation of
streptavidin-alkaline phosphatase (Roche Applied Science, Penzberg,
Germany). The severity grading of β0-thalassemia/HbE
patients was performed based on six parameters including:
Hemoglobin level, age at disease presentation, age at receiving
first blood transfusion, requirement for transfusion, spleen size,
and growth and development, following the standard protocol as
described previously (29).
Plasma RNA preparation
A total of 6 ml EDTA-preserved blood samples were
centrifuged at 650 × g for 20 min at room temperature. Plasma was
separated from the samples and was filtered through a 0.45 µM
Minisart® syringe filter membrane (Merck Millipore,
Darmstadt, Germany). A total of 200 µl plasma was diluted with 50
µl RNase free water and 1 µl glycogen (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) prior to homogenization
with 750 µl Isogen-LS reagent (Nippon Gene Co., Ltd., Tokyo, Japan)
by vortexing and pipetting. Synthetic cel-miR-39 RNA (1 fmol;
Qiagen GmbH, Hilden, Germany) was spiked into the denatured sample
solution. Chloroform (200 µl) was subsequently added, and the
suspension was shaken vigorously. The samples were then centrifuged
at 18,000 g, 4°C for 15 min, and the RNA in the aqueous phase was
precipitated by adding 400 µl isopropanol for 10 min at room
temperature. RNA pellets were washed with 1 ml ethanol (70%) and
resolubilized in 15 µl RNase-free water.
RNA preparation from packed red
cells
The packed red cells were separated from the same
cohort, including 9 normal subjects, 9 mild and 9 severe
β0-thalassemia/HbE patients. Following separating plasma
from whole blood samples by centrifugation at 650 × g for 20 min at
room temperature, the remaining fraction was further used to
separate packed red cells by density gradient centrifugation using
Lymphoprep (density 1.077±0.001 g/ml; Alere Technologies GmbH,
Jena, Germany) to eliminate mononuclear cells. A total of 100 µl of
packed red cells were separated and homogenized with 750 µl
Isogen-LS reagent (Nippon Gene Co., Ltd.). A total of 200 µl
chloroform was added, and the suspension was shaken vigorously. The
homogenized samples were centrifuged at 18,000 g for 15 min before
RNA in the aqueous phase was separated into a fresh tube and
precipitated by incubating in 400 µl isopropanol for 10 min at room
temperature. RNA pellets were washed with 1 ml ethanol (70%) and
resolubilized in 15 µl RNase-free water.
RT-qPCR analysis
A fixed volume of 5 µl eluted RNA solution was
reverse transcribed to cDNA using looped specific primers for
cel-miR-39, human miR-451, human miR-155 and let-7a obtained from
TaqMan MicroRNA assay kits (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA; cat. nos. 478293_mir,
478107_mir, 477927_mir, 478575_mir, respectively), and a
TaqMan® MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. For detection of the miRNAs, qPCR
analysis was performed using TaqMan MicroRNA assay kits and
Universal PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.; cat. no. 4304437). PCR was performed using the
Takara Thermal Cycler Dice (Takara Bio, Inc., Otsu, Japan). Each
reaction was performed in duplicate. For RT-qPCR analysis of plasma
miR-451 and miR-155 expression, the expression of a known quantity
of spiked cel-miR-39 was used for normalization (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The quantification
cycle (Cq) of miR-451 and miR-155 was used to calculate
the ∆Cq value using the following formula:
∆Cq=Cq (cel-miR-39) - Cq (miRNA of
interest). Therefore, a higher ∆Cq value indicated
a higher expression level of the miRNA of interest. For the
analysis of cellular miR-451 in packed red cells, the expression of
let-7a was used for normalization. The ∆Cq value of
miR-451 was calculated from the Cq of the miRNAs by
subtracting miR-451 from the average Cq of let-7a.
Statistical analysis
The association between plasma miR-451, miR-155
levels and disease severity was analyzed by compared the levels of
each miRNA between the mild and severe group using the Student's
t-test. Mann-Whitney U tests were used to evaluate differences
between two groups. Receiver operating characteristic (ROC) curves
were plotted to evaluate the diagnostic accuracy of each miRNA. The
sensitivity and specificity of the cut-off values were defined as
the maximized area under the ROC curve (AUC) with a 95% confidence
interval value. The correlation between miR-451 or miR-155 and
clinical laboratory parameters was evaluated using the Pearson's
correlation test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using the GraphPad Prism software (version, 5.0; GraphPad
Software, Inc., La Jolla, CA, USA).
Results
Clinical data of patients with
β0-thalassemia/HbE
The clinical data of the 16 control subjects and 23
patients with β0-thalassemia/HbE are shown in Table I. A total of 11 male and 12 female
patients with β0-thalassemia/HbE, and 5 male and 11
female control subjects were employed in the current study. Out of
the 23 patients with β0-thalassemia/HbE, a total of 10
were splenectomized. Patients with β0-thalassemia/HbE
exhibited significantly lower RBC counts, Hb level, hematocrit
(Hct), mean corpuscular volumes (MCV), mean corpuscular Hb (MCH)
and mean corpuscular Hb concentrations (MCHC) (all P<0.05),
however, they exhibited significantly higher reticulocyte counts
(P<0.05), when compared with the normal control subjects
(Table I). Furthermore, severe
β0-thalassemia/HbE patients exhibited significantly
higher reticulocyte and platelet counts, and significantly lower
RBC counts, Hb, MCV and MCHC levels (all P<0.05) when compared
with mild β0-thalassemia/HbE patients (Table I).
 | Table I.Hematological and clinical parameters
of patients with β0-thalassemia/HbE and normal control
subjects. |
Table I.
Hematological and clinical parameters
of patients with β0-thalassemia/HbE and normal control
subjects.
| Clinical
parameters | Control (n=16) | Mild (n=10) | Severe (n=13) |
|---|
| Gender
(male/female) | 5/11 | 4/6 | 7/6 |
| RBC count
(1×109 cells/ml) |
4.42±0.19a |
4.19±0.32b | 3.38±0.16 |
| Hb (g/dl) |
13.29±0.60a |
7.84±0.33b | 6.37±0.35 |
| Hct (%) |
39.0±1.61a | 24.77±0.98 | 22.51±0.95 |
| MCV (fl) |
85.29±1.48a |
60.45±2.26b | 67.22±2.06 |
| MCH (pg) |
29.20±0.49a | 19.11±0.72 | 20.02±0.68 |
| MCHC (g/dl) |
34.65±0.10a |
31.59±0.17b | 29.76±0.42 |
| Reticulocyte count
(1×109 cells/l) |
49.76±4.23a |
137.70±19b | 456.70±53.73 |
| Platelet count
(1×103 cells/ml) |
288.72±26.73a |
220.90±20.08b | 684.92±75.92 |
| Number of
splenectomized patients | 0 | 0 | 10 |
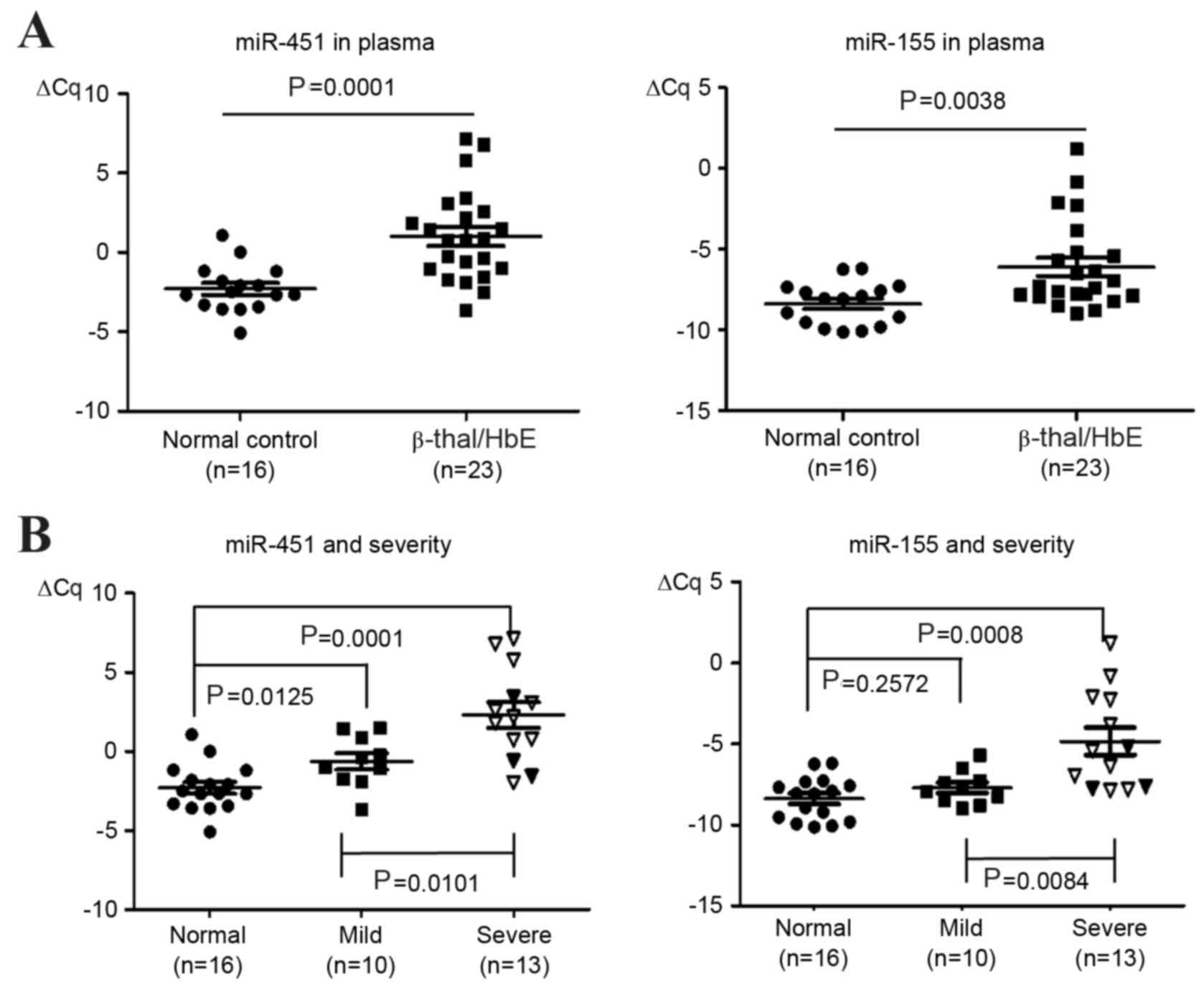
Increased levels of plasma miR-451 and
miR-155 in patients with β0-thalassemia/HbE
The present study used the synthetic miRNA,
cel-miR-39 as an internal control. Cel-miR-39 was recovered from
normal controls and β0-thalassemia/HbE plasma samples at
the same level as indicated by the average Cq values of
27.97±0.46 in control subjects and 28.37±0.47 in the patients with
β0-thalassemia/HbE (P=0.5037) (data not shown).
The expression levels of miR-451 and miR-155, which
are expressed in mature and early erythroid cells (19), respectively, were compared between
the plasma samples of the 16 control subjects and 23 patients with
β0-thalassemia/HbE. The average Cq values of
miR-451 and miR-155 were 30.5±0.37 and 36.57±0.33, in control
subjects, respectively, and 27.375±0.67 and 34.475±0.58 in patients
with β0-thalassemia/HbE, respectively (data not shown).
As indicated by the ∆Cq values, the levels of plasma
miR-451 and miR-155 following normalization to 1 fmol synthetic
cel-miR-39 were upregulated in the β0-thalassemia/HbE
plasma samples when compared with the control samples (miR-451,
P=0.0001; miR-155, P=0.0038; Fig.
1A).
Correlation between plasma miR-451,
miR-155 levels and disease severity
The correlation of miR-451 and miR-155 with disease
severity, as well as additional clinical data was then analyzed.
The severity grading was categorized using the criteria proposed by
Sripichai et al (29). The
level of miR-451 expression was significantly associated with
disease severity (Fig. 1B). The
level of miR-451 was significantly higher in severe cases than that
observed in mild cases (P=0.0101; Fig.
1B). In addition, the miR-451 levels in mild cases were
significantly higher when compared with the levels observed in
normal control subjects (P=0.0125; Fig. 1B). Similarly, the miR-155 levels in
severe cases were significantly higher than those observed in the
mild cases (P=0.0084; Fig. 1B).
However, no significant difference in miR-155 levels was observed
between mild cases and the normal controls (Fig. 1B). In addition, significantly
higher levels of plasma miR-451 were observed in patients that had
been splenectomized (n=10) when compared to those that were not
(n=13; P=0.0005), with the miR-451 levels in the 3 severe patients
that had not undergone a splenectomy falling within the range
exhibited by the mild cases (Fig.
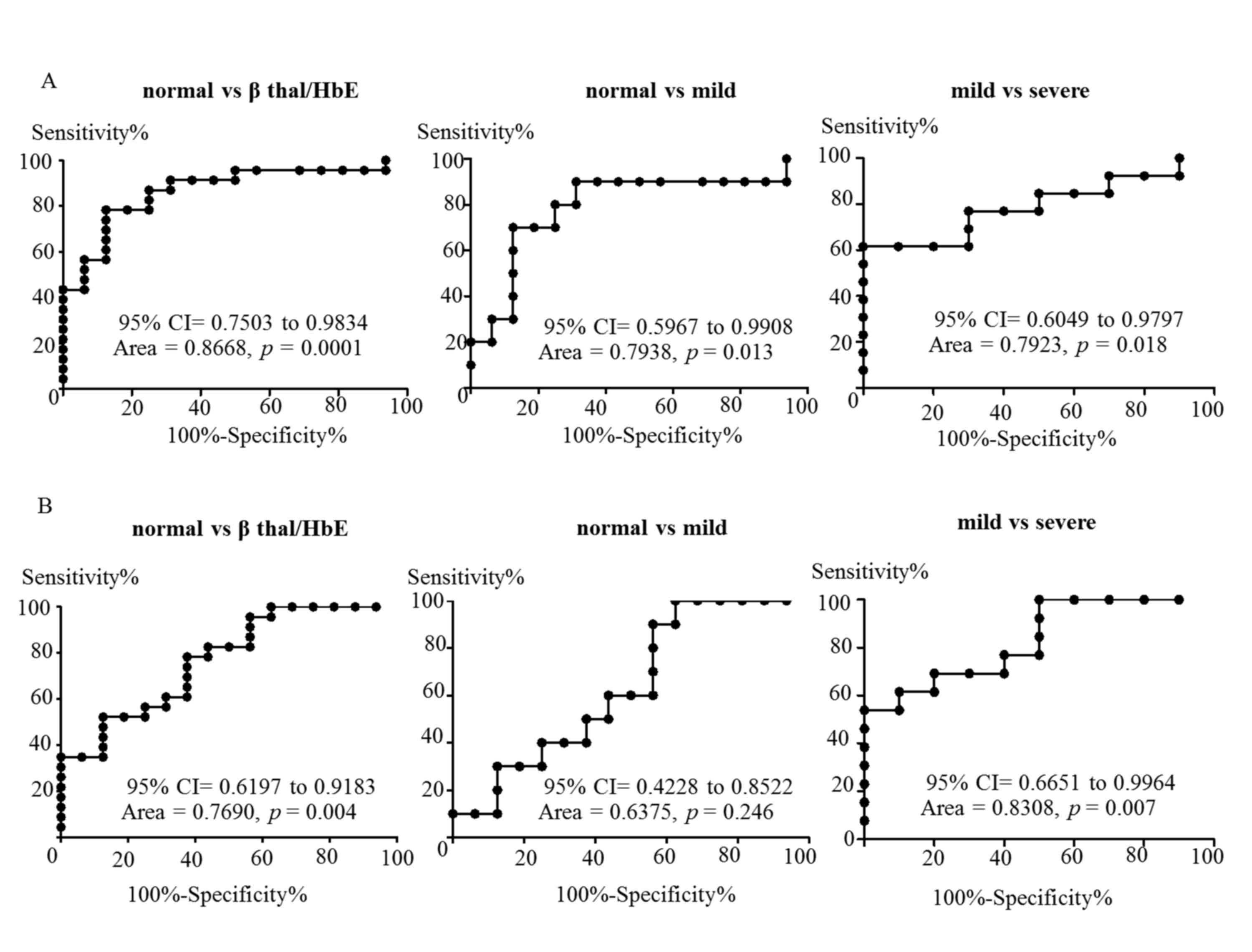
1). In order to evaluate the diagnostic value of plasma miR-451
and miR-155, the ∆Cq values of miR-451 and miR-155 from
the two subject groups were used to construct ROC curves. The
discriminating data between control and
β0-thalassemia/HbE groups was observed with an AUC of
0.8668 (P=0.0001) for miR-451 and 0.7690 (P=0.004) for miR-155
(Fig. 2). As shown in Fig. 2A, plasma miR-451 may discriminate
between normal controls and patients with mild disease (AUC=0.7938;
P=0.013), or between patients with mild and severe disease
(AUC=0.7923; P=0.018). However, the AUC for miR-155 demonstrated
that this sequence may only discriminate between normal controls
and patients with β0-thalassemia/HbE or between mild and
severe cases (AUC=0.8308; P=0.007), but not between normal and mild
cases (AUC=0.6375; P=0.246; Fig.
2B).
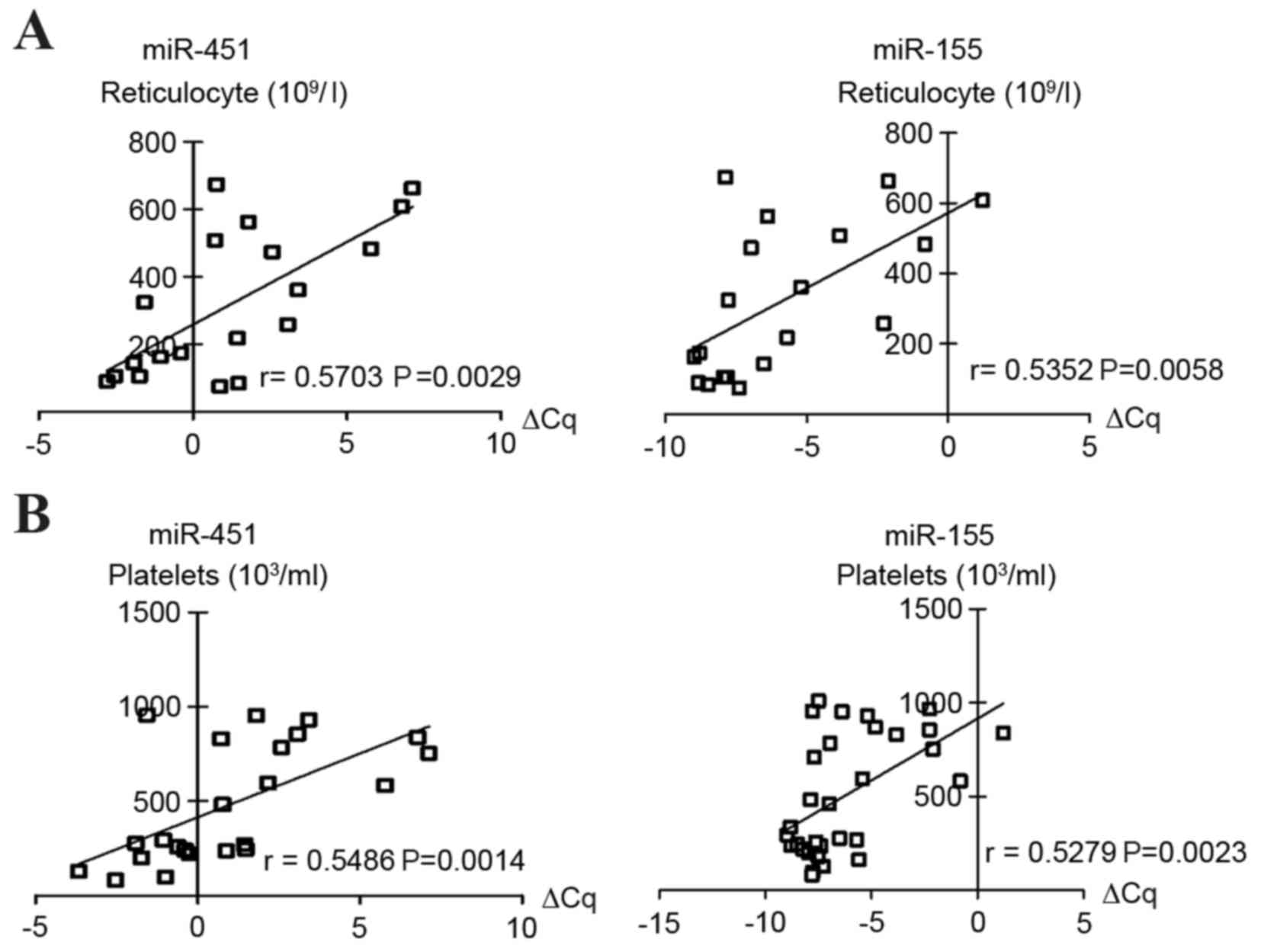
Correlation between plasma miR-451 and
miR-155 levels and clinical features
The association between plasma miR-451 or miR-155
and clinical features, including RBC count, Hb, Hct, MCV, MCH and
MCHC was investigated. Notably, elevated plasma levels of miR-451
and miR-155 were significantly correlated with reticulocyte and
platelet counts (Fig. 3). No
statistically significant association between the levels of miR-451
and miR-155 and any additional clinical parameters, including RBC
count, Hb, Hct, MCV, MCH or MCHC, was observed (data not
shown).
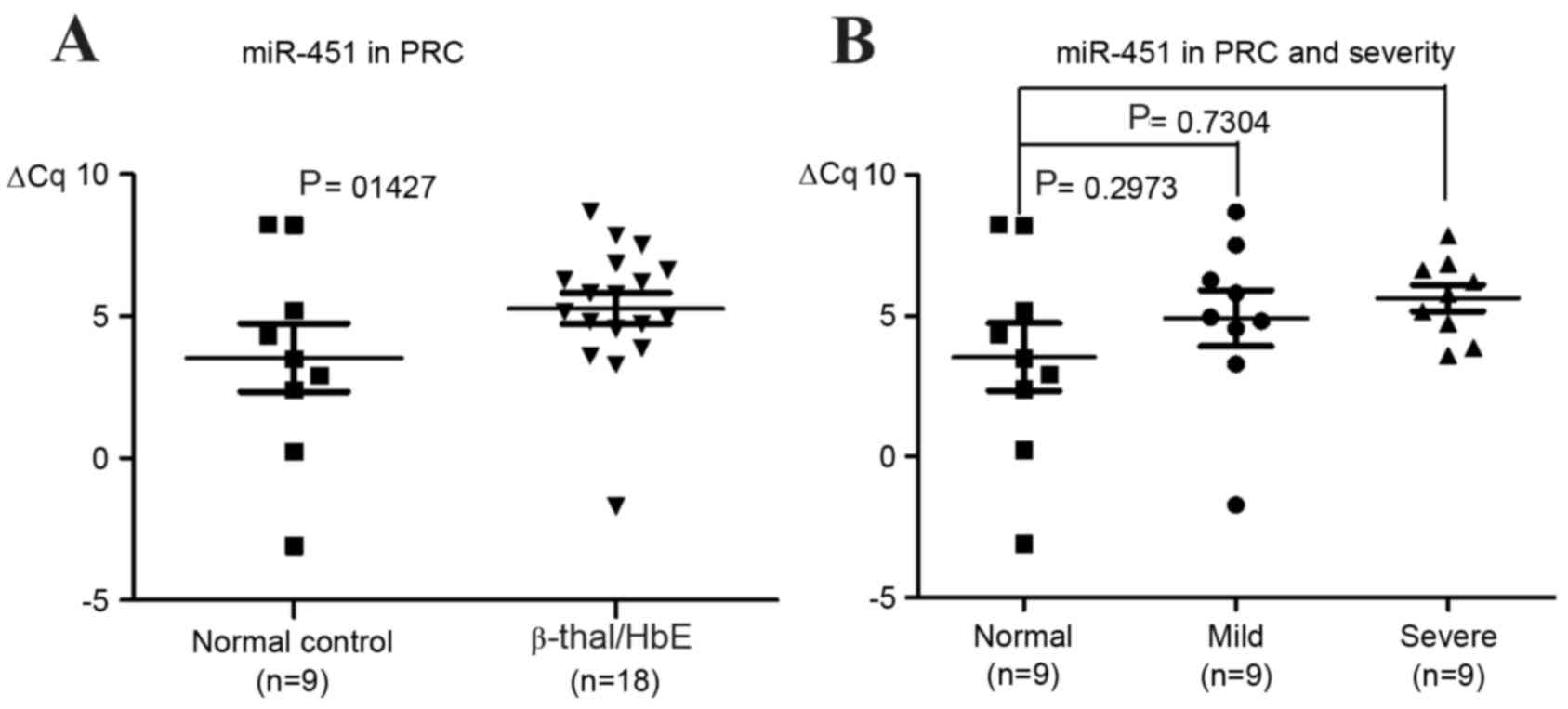
Level of miR-451 in packed red
cells
In order to investigate whether the level of
cellular miR-451 expression was deregulated in RBCs from patients
with β0-thalassemia/HbE, miR-451 levels in mature RBCs
collected from the same subject cohort were analyzed. The levels of
cellular miR-451 were not significantly different in control
subjects when compared with patients with β-thalassemia/HbE, or in
patients with mild compared with severe cases of the disease
(Fig. 4).
Discussion
Patients with β0-thalassemia/HbE exhibit
major RBC abnormalities as a result of precipitation of excess α
globin chains which are unable to form a stable tetramer.
Therefore, a globin chains precipitate as inclusion bodies leading
to the ineffective erythropoiesis and immature destruction of RBCs
(5). β0-thalassemia/HbE
is traditionally classified as a disease with extravascular
hemolysis in addition to ineffective erythropoiesis (5). However, previous studies have
provided evidence of intravascular hemolysis as indicated by
increased serum-free Hb levels and depleted levels of plasma
haptoglobin protein (30,31). Several conventional techniques have
been developed for the assessment of hemolysis, including the
detection of lactate dehydrogenase (LDH), serum haptoglobin and
serum-free Hb levels, as well as urine-free Hb, urine hemosiderin,
and erythrocyte survival and indirect bilirubin tests (32,33).
A number of conventional techniques may not be suitable for routine
clinical use, such as the detection of erythrocyte survival with a
radionuclide chromium 51 labeling method, which requires exclusive
equipment for radioactive materials and a prolonged examination
period with a series of blood samples (34). LDH is considered to be a useful
clinical marker of intravascular hemolysis; however, it is not
specific as LDH is released from neoplastic cells, the liver, or
from damaged organs (35). The
present study aimed to develop a novel and specific method for
assessing the degree of hemolysis in β0-thalassemia/HbE
patients. Previous studies have demonstrated that miRNAs are
present in the circulation with high stability, and the profile of
circulating miRNAs reflects the pathophysiological processes of a
number of human diseases (18).
A number of miRNAs serve important roles in the
regulation of erythropoiesis (16,19).
A previous study revealed that miR-451 expression may be associated
with the production of erythroid cells in a lineage- and
stage-specific manner (20).
Analysis of the expression patterns of miR-451 during normal
erythropoiesis revealed that the expression of miR-451 was
upregulated during late erythropoiesis (19). A previous study demonstrated that
circulating RBCs contain high levels of miR-451 expression, with
~10,000-fold greater levels than granulocytes (19). Expression of miR-451 is dependent
on the Argonaute 2 protein and is restricted to the erythroid
lineage. Mice deficient in the miR-451 cluster exhibited impaired
erythroid maturation, ineffective erythropoiesis and mild anemia
(36). The present study evaluated
the possibility that intracellular hemolysis may result in
increased levels of plasma miR-451. The levels of plasma miR-451 in
patients with β0-thalassemia/HbE were elevated and miRNA
levels correlated with disease severity. To the best of our
knowledge, the present study demonstrated the suitability of using
plasma miR-451 as a biomarker for erythroid cell destruction in
β0-thalassemia/HbE patients for the first time.
Consistent with the results of a previous study (24), no alteration in the levels of
cellular miR-451 in erythrocytes between control subjects and
β0-thalassemia/HbE patients was observed. This indicated
that the increased levels of plasma miR-451 may be as a result of
erythroid cell destruction and not from the increased miR-451
levels in RBCs. The level of miR-451 was strongly associated with
disease severity, and the authors hypothesize that variations in
plasma miR-451 levels may result from differences in the degree of
intravascular hemolysis in each patient. Notably, the patients that
had undergone a splenectomy exhibited higher levels of plasma
miR-451 than those that had not. Splenectomy is used to manage
severe cases of β0-thalassemia to reduce spleen-induced
extravascular hemolysis. In addition, intravascular hemolysis has
been reported in splenectomy patients (10). The results of the present study
suggest that elevated levels of circulating miRNA-451 may be a
result of red cell destruction via the intravascular hemolysis
pathway in splenectomy patients.
In the present study, the level of plasma miRNA-155,
which is expressed in early erythroid cells, was measured. miR-155
has been previously characterized as a multifunctional miRNA that
serves important roles in the pathological processes of a number of
diseases, particularly cancer, inflammation, immunity and
cardiovascular diseases (25). In
addition, the overexpression of miR-155 as an oncogenic miRNA in
hematological malignancies and solid tumors has been reported
(26,27). The levels of plasma miR-155 in
patients with β0-thalassemia/HbE were significantly
higher when compared with control subjects. However, no correlation
between miR-155 expression and disease severity was observed, as
the levels of miR-155 in control subjects and mild cases were not
significantly different. The level of plasma miR-155 is very low,
and is detected at >35 Cq. Therefore, plasma miR-155
expression may be undetected, which may lead to false-negative
results in some cases.
In conclusion, the present study demonstrated that
reticulocyte counts in the circulation were increased in patients
with β0-thalassemia/HbE when compared to normal
controls. In addition, reticulocyte counts were associated with the
level of circulating miR-451. Reticulocytes are immature
erythrocytes, which are released from the bone marrow into the
blood circulation. Increased reticulocyte numbers reflect a
response to anemia induced by hemolysis or the loss of
erythrocytes, by accelerated erythropoiesis in the bone marrow. The
results of the present study indicate that the increased levels of
circulating miRNA-451 observed during β0-thalassemia/HbE
may result from the accelerated erythropoiesis-associated
destruction of erythroid cells. In conclusion, the observations
suggest that the measurement of plasma miR-451 may be a relevant
biomarker of intravascular hemolysis in patients with
β0-thalassemia/HbE.
Acknowledgements
The present study was supported, in part, by the
Thailand Research Fund (grant no. RMU5380001), the Research Chair
Grant, National Science and Technology Development Agency (NSTDA),
the Exchange Program for East Asian Young Hematologists, sponsored
by the Japan Society for the Promotion of Science and the
Chulalongkorn University Ratchadaphisek Somphot Endowment Fund. The
manuscript was corrected by Dr Valery Combes (Faculty of Sciences,
School of Life Sciences, University of Technology, Sydney, NSW,
Australia).
References
|
1
|
Olivieri NF: The beta-thalassemias. N Engl
J Med. 341:99–109. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fucharoen S and Winichagoon P: Clinical
and hematologic aspects of hemoglobin E beta-thalassemia. Curr Opin
Hematol. 7:106–112. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vichinsky E: Hemoglobin e syndromes.
Hematology Am Soc Hematol Educ Program. 79–83. 2007.PubMed/NCBI
|
|
4
|
Fucharoen S and Winichagoon P:
Hemoglobinopathies in southeast Asia. Hemoglobin. 11:65–88. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rund D and Rachmilewitz E:
Beta-thalassemia. N Engl J Med. 353:1135–1146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olivieri NF, Pakbaz Z and Vichinsky E: Hb
E/beta-thalassaemia: A common & clinically diverse disorder.
Indian J Med Res. 134:522–531. 2011.PubMed/NCBI
|
|
7
|
Thein SL: Pathophysiology of beta
thalassemia-a guide to molecular therapies. Hematology Am Soc
Hematol Educ Program. 31–37. 2005.PubMed/NCBI
|
|
8
|
Weatherall DJCJ: The thalassemia
syndromes. Malden MA Blackwell Science. 2001.
|
|
9
|
Conran N: Intravascular hemolysis: A
disease mechanism not to be ignored. Acta Haematol. 132:97–99.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Atichartakarn V, Chuncharunee S,
Archararit N, Udomsubpayakul U and Aryurachai K: Intravascular
hemolysis, vascular endothelial cell activation and thrombophilia
in splenectomized patients with hemoglobin E/β-thalassemia disease.
Acta Haematol. 132:100–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roth C, Rack B, Müller V, Janni W, Pantel
K and Schwarzenbach H: Circulating microRNAs as blood-based markers
for patients with primary and metastatic breast cancer. Breast
Cancer Res. 12:R902010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lawrie CH, Gal S, Dunlop HM, Pushkaran B,
Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J,
Wainscoat JS, et al: Detection of elevated levels of
tumour-associated microRNAs in serum of patients with diffuse large
B-cell lymphoma. Br J Haematol. 141:672–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Gelfond JA, McManus LM and
Shireman PK: Reproducibility of quantitative RT-PCR array in miRNA
expression profiling and comparison with microarray analysis. BMC
Genomics. 10:4072009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reid G, Kirschner MB and van Zandwijk N:
Circulating microRNAs: Association with disease and potential use
as biomarkers. Crit Rev Oncol Hematol. 80:193–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Masaki S, Ohtsuka R, Abe Y, Muta K and
Umemura T: Expression patterns of microRNAs 155 and 451 during
normal human erythropoiesis. Biochem Biophys Res Commun.
364:509–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pase L, Layton JE, Kloosterman WP,
Carradice D, Waterhouse PM and Lieschke GJ: miR-451 regulates
zebrafish erythroid maturation in vivo via its target gata2. Blood.
113:1794–1804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim M, Tan YS, Cheng WC, Kingsbury TJ,
Heimfeld S and Civin CI: MIR144 and MIR451 regulate human
erythropoiesis via RAB14. Br J Haematol. 168:583–597. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bruchova-Votavova H, Yoon D and Prchal JT:
miR-451 enhances erythroid differentiation in K562 cells. Leuk
Lymphoma. 51:686–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhan M, Miller CP, Papayannopoulou T,
Stamatoyannopoulos G and Song CZ: MicroRNA expression dynamics
during murine and human erythroid differentiation. Exp Hematol.
35:1015–1025. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Svasti S, Masaki S, Penglong T, Abe Y,
Winichagoon P, Fucharoen S and Umemura T: Expression of
microRNA-451 in normal and thalassemic erythropoiesis. Ann Hematol.
89:953–958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Faraoni I, Antonetti FR, Cardone J and
Bonmassar E: miR-155 gene: A typical multifunctional microRNA.
Biochim Biophys Acta. 1792:497–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu W, Qin W, Atasoy U and Sauter ER:
Circulating microRNAs in breast cancer and healthy subjects. BMC
Res Notes. 2:892009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eis PS, Tam W, Sun L, Chadburn A, Li Z,
Gomez MF, Lund E and Dahlberg JE: Accumulation of miR-155 and BIC
RNA in human B cell lymphomas. Proc Natl Acad Sci USA.
102:3627–3632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Winichagoon P, Saechan V, Sripanich R,
Nopparatana C, Kanokpongsakdi S, Maggio A and Fucharoen S: Prenatal
diagnosis of beta-thalassaemia by reverse dot-blot hybridization.
Prenat Diagn. 19:428–435. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sripichai O, Makarasara W, Munkongdee T,
Kumkhaek C, Nuchprayoon I, Chuansumrit A, Chuncharunee S,
Chantrakoon N, Boonmongkol P, Winichagoon P and Fucharoen S: A
scoring system for the classification of beta-thalassemia/HbE
disease severity. Am J Hematol. 83:482–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arce-Bejarano R, Lomonte B and Gutiérrez
JM: Intravascular hemolysis induced by the venom of the Eastern
coral snake, Micrurus fulvius, in a mouse model: Identification of
directly hemolytic phospholipases A2. Toxicon. 90:26–35. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ragab SM, Safan MA and Badr EA: Study of
serum haptoglobin level and its relation to erythropoietic activity
in Beta thalassemia children. Mediterr J Hematol Infect Dis.
7:e20150192015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okumiya T, Ishikawa-Nishi M, Doi T,
Kamioka M, Takeuchi H, Doi Y and Sugiura T: Evaluation of
intravascular hemolysis with erythrocyte creatine in patients with
cardiac valve prostheses. Chest. 125:2115–2120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rother RP, Bell L, Hillmen P and Gladwin
MT: The clinical sequelae of intravascular hemolysis and
extracellular plasma hemoglobin: A novel mechanism of human
disease. JAMA. 293:1653–1662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mock DM, Matthews NI, Zhu S, Strauss RG,
Schmidt RL, Nalbant D, Cress GA and Widness JA: Red blood cell
(RBC) survival determined in humans using RBCs labeled at multiple
biotin densities. Transfusion. 51:1047–1057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Painter PC, Van Meter S, Dabbs RL and
Clement GE: Analytical evaluation and comparison of Dupont aca
lactate dehydrogenase-1 (LD1) isoenzyme assay diagnostic efficiency
for acute myocardial infarction detection with other LD1 methods
and aca CK-MB. A two-site study. Angiology. 45:585–595. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rasmussen KD, Simmini S, Abreu-Goodger C,
Bartonicek N, Di Giacomo M, Bilbao-Cortes D, Horos R, Von Lindern
M, Enright AJ and O'Carroll D: The miR-144/451 locus is required
for erythroid homeostasis. J Exp Med. 207:1351–1358. 2010.
View Article : Google Scholar : PubMed/NCBI
|