Introduction
Bone undergoes constant remodeling via a balance
between bone formation and resorption, which maintains adult
skeletal health; osteoporosis is a bone-associated disease caused
by disruption of this balance. It is characterized by a reduction
in bone mass or bone mineral density, and the deterioration of
micro-architecture that frequently leads to a high risk of fracture
(1,2). Osteoblasts induce bone formation
whereas osteoclasts promote bone resorption.
Mechanical loading, including strain, compression
and fluid shear, are stimuli that regulate the function of
osteoblasts and osteoclasts. Therefore, bone mass (3) and tissue are maintained and have
sufficient strength to withstand such loads. By contrast, unloading
may disrupt this balance and lead to rapid bone loss or
osteoporosis. For example, astronauts may lose up to 2% in hip bone
density per month in space (4).
Bone cells, including osteocytes and osteoblasts, have been
extensively studied in response to mechanical loading, and reports
have suggested that strain or fluid shear may serve a significant
role in bone development (5–7). In
addition, the effect of bone cell compression has been investigated
in vivo and in vitro (8–11).
However, the majority of previous studies have examined the effect
of mechanical load on monolayer culture. Therefore, the aim of the
present study was to determine the effect of compressive load on
osteoblast differentiation in a 3D model in vitro.
Mechanical force may influence biochemical signaling
in osteoblasts and osteocytes by altering transcription factor
translocation, gene expression and osteoblast differentiation
(12). This response may be
associated with various intracellular signaling pathways, including
wingless-type (Wnt)/β-catenin, bone morphogenetic protein (BMP),
mitogen activated protein kinase and osteoprotegerin (OPG)-receptor
activation of nuclear factor (NF)-κB (RANK)-RANK ligand (RANKL)
(10). The Wnt/β-catenin signaling
pathway is important in bone biology, as it may promote osteoblast
lineage differentiation of mesenchymal precursors (13–15).
This may enhance osteoblast proliferation and terminal
differentiation (16–18), and inhibit apoptosis of osteoblasts
and osteocytes (19). Runt-related
transcription factor 2 (Runx2) and osterix (Osx) are key downstream
transcription factors of the Wnt/β-catenin and BMP signaling
pathways, and regulate osteoblast differentiation (20,21).
Accompanied with differentiation of osteoblasts, the expression of
bone matrix proteins, alkaline phosphatase (ALP) and osteocalcin
(OCN) increases, which may lead to mineralization and bone
formation.
Sawakami et al (22) revealed that mice lacking the Wnt
co-receptor, functional lipoprotein receptor-related protein
(Lrp)5, have an impaired cortical bone response to ulna loading. A
further study demonstrated that high and low magnitudes of strain
enhanced β-catenin in mesenchymal stem cells, promoted osteogenesis
and suppressed adipogenesis (23).
Mechanical loading additionally regulates osteoblast activity via
downregulation of sclerostin (SOST) and Dickkopf-related protein 1
(DKK-1) expression, which negatively regulate the Wnt signaling
pathway (9,11,24).
Conversely, other studies reported that a high magnitude of
mechanical load enhanced the expression levels of interleukin
(IL)-6, IL-8, RANKL and fibroblast growth factor-2, and reduced the
expression levels of OPG and ALP activity (25–28).
This reduced the differentiation of osteoblasts and enhanced the
activation of osteoclasts. Thus, the underlying mechanism by which
magnitude and duration of cyclic compressive load affect osteoblast
differentiation remains to be fully understood. The present study
aimed to investigate the effect of different magnitudes and
durations of cyclic compression on osteoblast differentiation and
determine whether this is associated with activation of the Wnt
signaling pathway.
Materials and methods
Cell culture
The mouse MC3T3-E1 osteoblast-like cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA; CRL-2594) and cultured in α-modified Eagle's medium (α-MEM;
HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), and 1% penicillin and streptomycin (P/S;
Beyotime Institute of Biotechnology, Haimen, China). Cells were
cultured in a humidified incubator at 37°C and 5% CO2.
Cells were passaged at 70–80% confluence using 0.25% trypsin-EDTA
(Beyotime Institute of Biotechnology).
3D scaffold preparation
Confluent cells were detached using 0.25%
trypsin-EDTA and counted. Cells were suspended at 4×107
cells/ml in 2X α-MEM containing 20% FBS and 2% P/S, prior to mixing
with an equal volume of 4% low melting point agarose (cat. no.
A9045; Sigma; Merck KGaA, Darmstadt, Germany) at 37°C. The mixture
was added to 6-well Biopress™ compression culture plates (Flexcell
International Corporation, Burlington, NC, USA) and allowed to set
at room temperature. The resulting 3×5 mm cylinder agarose gel
containing 2×107 cells/cm3 established the 3D
scaffold model. Cells within the 3D scaffolds were cultured in 1X
α-MEM. During the cyclic compression experiment, 50 mg/ml ascorbic
acid and 10 mM β-glycerophosphate were added to the medium.
Cyclic compression experiments
Following 20 h incubation of the 3D scaffold, the
Flexcell-5000C™ Compression system (Flexcell International
Corporation) was utilized to apply the compressive load. Previously
published data revealed that 6% compressive force corresponds to 1
MPa (29). Therefore, the present
study used a sinusoidal wave with 0.33, 0.5 and 1 MPa (equivalent
to 2, 3 and 6% magnitude) and 1 Hz frequency (25,29).
Unloaded 3D scaffold samples served as controls. Samples were
analyzed at 4, 6 and 8 h. To determine whether the Wnt/β-catenin
signaling pathway was involved in the osteoblast response to
mechanical compression, an inhibitor of Wnt signaling, DKK-1 (50
ng/ml), was added to the 1X culture medium 1 h prior to compressive
loading (6 h and 0.5 MPa).
ALP assay
Following cyclic compression, cell-gel samples were
washed 3 times with phosphate-buffered saline (PBS) and homogenized
with 1 ml 0.3% Triton X-100. ALP activity was quantified in the gel
supernatants with the Alkaline Phosphatase assay kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol, using para-nitrophenol as a substrate, as previously
described (30).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Following compressive loading, total RNA was
isolated from cell-gel samples using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. RNA purity and concentration were
determined by measuring the absorbance at wavelengths of 260 and
280 nm. cDNA was obtained by RT using the PrimeScript®
kit (Takara Bio, Inc., Otsu, Japan). qPCR was subsequently
performed with the FastStart SYBR®-Green Master kit
(Roche Applied Science, Mannhein, Germany) on an ABI 7300 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
with 40 cycles of 94°C for 30 sec and 60°C for 90 sec. Forward and
reverse PCR primers were designed based on mouse sequence
information, and are listed in Table
I. The quantitation cycle (Cq) value was calculated for gene
expression quantification using the 2−ΔΔCq method
(31). GAPDH served as an internal
standard.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer sequence
(5′-3′) |
|---|
| ALP_F |
ACTGGCTGTGCTCTCCCTAC |
| ALP_R |
GACCTCTCCCTTGAGTGTGG |
| OCN_F |
CAGGGAGGCAGTGACTCTTC |
| OCN_R |
AGTGTGGAAAGTGTGGAGTT |
| Runx2_F |
GGTGAAACTCTTGCCTCGTC |
| Runx2_R |
AGTCCCAACTTCCTGTGCT |
| Osx_F |
TGGTACAAGGCAGGCATCCA |
| Osx_R |
GGAGCAAAGTCAGATGGGTAAGT |
| Lrp5_F |
GAGCACGTGATTGAGTTTG |
| Lrp5_R |
TCAGTCCAGTAGATGTAGC |
| SOST_F |
GAAGGGAGTGTGGAACGAAAG |
| SOST_R |
CCAGGTCAGGGTCAGAAACC |
| GAPDH_F |
GACAACTTTGGCATTGTGGA |
| GAPDH_R |
ATGCAGGGATGATGTTCTGG |
Western blot analysis
Following compressive loading, cell-gel samples were
washed in PBS. Total protein was extracted using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). The protein concentration was determined in each
sample using the Bicichoninic Acid Protein assay kit (Beyotime
Institute of Biotechnology). A total of 20 ml of each sample was
loaded onto 8 to 10% gels and subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, prior to transfer onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA) via electroblotting. Membranes were blocked for 1 h with 5%
fat-free milk in Tris-HCl-buffered saline containing 0.1% Tween-20
(TBST) at room temperature. Subsequently, membranes were probed
with the following primary antibodies: Rabbit anti-phosphorylated
(p)-β-catenin (dilution, 1:800; catalog no. 9561; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit anti-β-catenin
(dilution, 1:1,000; catalog no. ab6302; Abcam, Cambridge, MA, USA),
rabbit anti-Wnt1 (dilution, 1:1,000; catalog no. ab15251; Abcam),
rabbit anti-β-actin (dilution, 1:1,000; catalog no. 4970; Cell
Signaling Technology, Inc.) and rabbit anti-disheveled segment
polarity protein-2 (DVL2; dilution, 1:1,500; catalog no.
12037-1-AP; ProteinTech Group, Inc., Chicago, IL, USA) at 4°C
overnight. Following washing with TBST, membranes were incubated
with a horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary antibody (dilution, 1:5,000; catalog no. 7074; Cell
Signaling Technology, Inc.) for 2 h at room temperature. An
Enhanced Chemiluminescence Detection kit (EMD Millipore) was used
to detect the immunoreactive signals, which were exposed using the
Tanon 5200 Multi system (Tanon Science and Technology Co., Ltd.,
Shanghai, China). Protein bands were quantified using Image J
software version 1.49 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
of three independent experiments. Figures were prepared using
GraphPad Prism software version 5.01 (GraphPad Software, Inc., La
Jolla, CA, USA). Two-way analysis of variance followed by the
Bonferroni post hoc test was performed to determine the effect of
cyclic compression magnitude and duration on osteoblast
differentiation. Statistical calculations were performed using SPSS
software version 13.0 (SPSS, Inc., Chicago, IL, USA), and P<0.05
was considered to indicate a statistically significant
difference.
Results
Mechanical compressive load stimulates
osteoblast differentiation
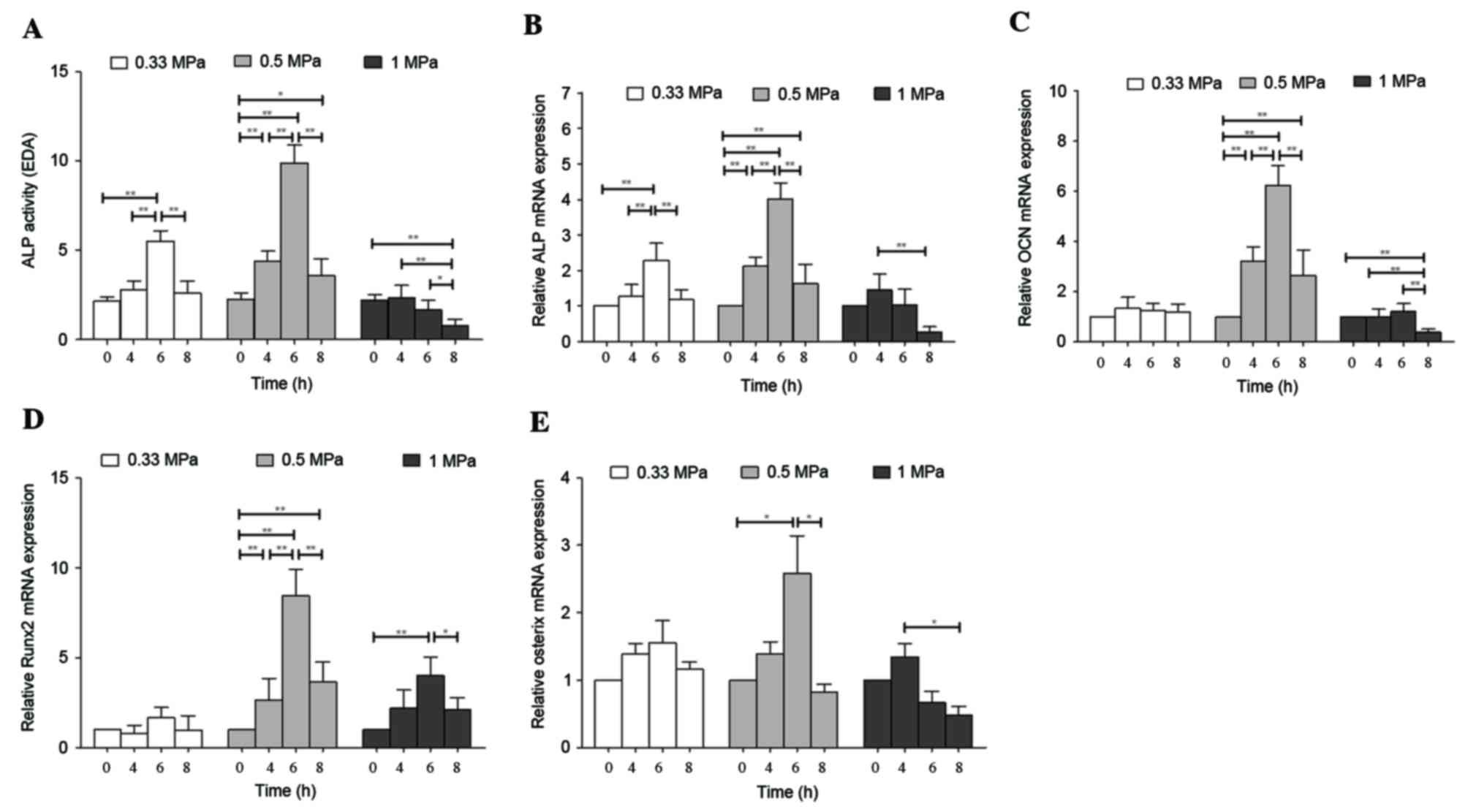
ALP and OCN are indicators of early and late
osteoblast differentiation, respectively. The present study
revealed that 0.5 MPa compression significantly increased
expression of ALP activity by 4.40-fold after 6 h compared with
cells at 0 h (P<0.01). In addition, compared with cells at 0 h,
a 2.57-fold increase in ALP activity was observed following 0.33
MPa compression for 6 h (P<0.01), whereas 1 MPa induced a
significant reduction in ALP activity after 8 h (P<0.01;
Fig. 1A).
Similarly, compression of cells induced a
significant increase in the mRNA expression levels of ALP
(Fig. 1B) and OCN (Fig. 1C), particularly following treatment
with 0.5 MPa for 6 h (P<0.01). In addition, a significant
increase in ALP mRNA expression levels was observed
following treatment with 0.33 MPa for 6 h (P<0.01); however, no
significant increase in OCN expression levels were observed
under this condition. Conversely, application of 1 MPa
significantly reduced the expression levels of ALP (Fig. 1B) and OCN (Fig. 1C) after 8 h (P<0.01). These
results indicated that the ability of compressive load to stimulate
osteoblast differentiation is dependent on the duration and
magnitude of the force applied.
Mechanical compressive load increases
the expression of key transcription factors involved in osteoblast
differentiation
Runx2 and Osx are transcription factors involved in
osteoblast differentiation. Consistent with ALP activity and mRNA
expression levels, 6 h compression at magnitude of 0.5 MPa induced
the greatest increase in Runx2 (P<0.01; Fig. 1D) and Osx (P<0.05; Fig. 1E) mRNA expression levels by 8.44-
and 2.51-fold, respectively, compared with the 0 h control.
Application of 0.33 MPa did not significantly alter Runx2 and Osx
mRNA expression levels. Compression at 1 MPa induced a significant
increase in the mRNA expression levels of Runx2 after 6 h
(P<0.01), whereas mRNA expression levels of Osx were reduced
after 8 h (P<0.05). Thus, 0.5 MPa compressive load for 6 h
increased the mRNA expression levels of key transcription factors,
suggesting that osteoblast differentiation was enhanced in MC3T3-E1
cells.
Mechanical compressive load stimulates
osteoblast differentiation via the Wnt/β-catenin signaling
pathway
The present study examined whether Wnt/β-catenin
signaling was involved in compression-induced regulation of
osteoblast differentiation. Compared with cells at 0 h, a
compressive load of 0.5 MPa significantly enhanced the mRNA
expression levels of Lrp5 at 6 h (P<0.01; Fig. 2A), and reduced SOST mRNA expression
levels at 4 and 6 h (P<0.01; Fig.
2B). In addition, the protein expression levels of Wnt1,
β-catenin and DVL2 significantly increased (P<0.01) following
mechanical treatment, particularly after 6 h compression treatment
at 0.5 MPa (Figs. 2C and D).
Furthermore, 6 h compressive load reduced the protein expression
levels of p-β-catenin at 0.5 MPa, decreasing the ratio of
p-β-catenin/β-catenin (P<0.01), which suggested that compression
stabilized β-catenin by inhibiting its phosphorylation at residues
Ser33/37/Thr41. These results indicated that compressive load
increased expression of key regulators of the Wnt/β-catenin
signaling pathway, thus promoting osteoblast differentiation.
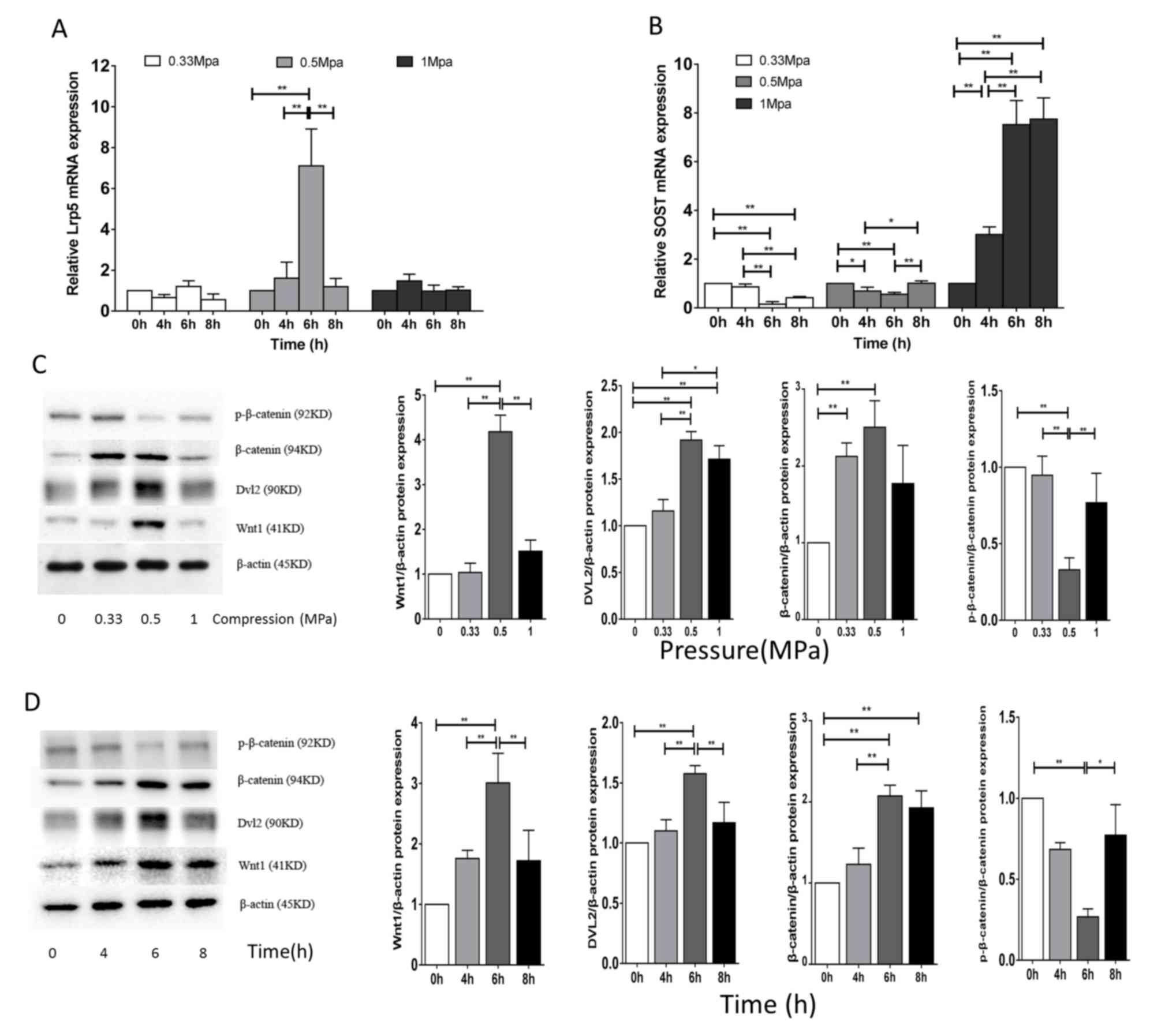 | Figure 2.Effect of cyclic compressive load on
Wnt/β-catenin signaling in MC3T3-E1 cells. Using a 3D scaffold
model, cells were exposed to compressive loads of 0.33, 0.5 or 1
MPa for 0, 4, 6 or 8 h. mRNA expression levels of (A) Lrp5 and (B)
SOST were determined by reverse transcription-quantitative
polymerase chain reaction. Protein expression levels of
p-β-catenin, β-catenin, DVL2 and Wnt1 were evaluated by western
blotting comparing (C) different compression pressures (MPa) for 6
h and (D) different time points with 0.5 MPa; β-actin served as an
internal control. Bands were quantified via densitometry to obtain
relative protein expression levels. Data are expressed as the mean
± standard deviation of three independent experiments. *P<0.05;
**P<0.01. Lrp5, low density lipoprotein receptor-related protein
5; SOST, sclerostin; p, phosphorylated; DVL2, disheveled segment
polarity protein-2; Wnt1, wingless-type. |
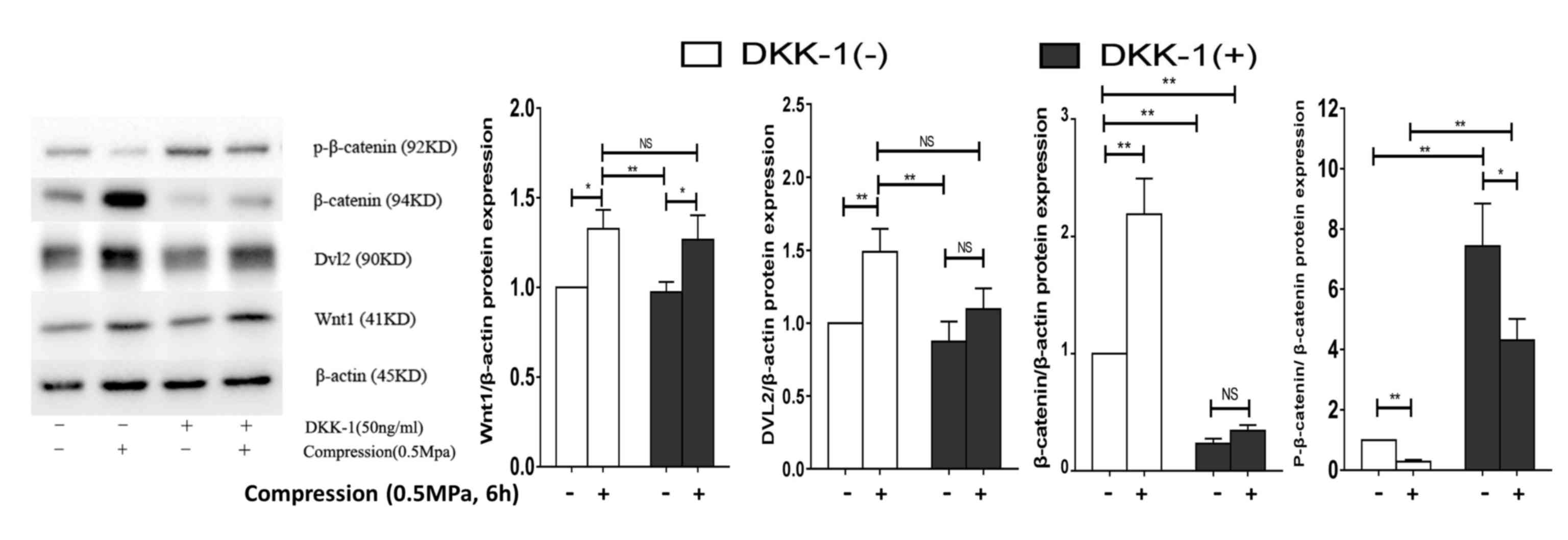
Subsequently, DKK-1 was utilized to inhibit the
binding of Wnt ligands to Lrp5, decreasing the protein expression
of β-catenin and increasing p-β-catenin/β-catenin, with no change
in Wnt1 or DVL2. Notably, compression loading (0.5 MPa, 6 h) and
DKK-1 treatment stimulated a significant increase in the protein
expression levels of Wnt1 (P<0.05), decrease in
p-β-catenin/β-catenin (P<0.05) and no change in β-catenin or
DVL2 when compared with DKK-1 treatment without compression loading
(Fig. 3). Therefore, this
suggested that DKK-1 decreased but did not prevent compressive
load-induced Wnt/β-catenin signaling.
Discussion
Published data have demonstrated that bone cells
respond to mechanical load, including tension, compression,
hydrostatic pressure and fluid shear stress, which regulate
osteoblast differentiation in vitro (6,8,10,32).
However, the majority of these studies investigated the effect of
loading on monolayer cell cultures. In the present study, a 3D
in vitro model was applied to determine the effect of cyclic
compressive load on osteoblast differentiation. Compressive load
increased ALP activity and mRNA expression levels of ALP,
OCN, Runx2 and Osx in osteoblasts, indicating
that mechanical load stimulates osteoblast differentiation, leading
to osteoblast maturation and bone formation.
Mechanical loads of 2,000–4,000 µ strain on long
bones from physical activity may promote bone remodeling, and 1,500
µ strain is considered to be the threshold for promoting this in
vivo (33,34). However, it is difficult to compare
the magnitude of strain in vitro with that in vivo,
and the effect of magnitude of mechanical stress on osteoblast
differentiation remains to be fully elucidated (8,28,32).
Thus, the present study investigated the effect of three different
magnitudes of compressive load on osteoblast differentiation. Data
revealed that 0.5 MPa compression effectively increased ALP
activity and the mRNA expression levels of osteoblast
differentiation markers. Compression at 0.33 MPa promoted ALP
expression and activity; however, to a lesser extent than 0.5 MPa,
whereas 1 MPa resulted in a reduction in ALP mRNA expression levels
and activity. This is consistent with a previous study that
demonstrated that Runx2 was up-regulated following 0.8 and
5% elongation for 6 h, and downregulated following 5, 10 and 15%
elongation for 24 and 48 h (26).
Sanchez et al (28)
demonstrated that 1-1.7 MPa compression lead to an increase in the
mRNA expression levels of IL-6 and cyclooxygenase-2, and a decrease
in the mRNA expression level of OPG in osteoblasts, which may
downregulate bone formation and promote osteoclast differentiation.
The results of the present study indicated that compressive load
promotes osteoblast differentiation in bone cells when an optimal
magnitude of 0.5 MPa (~3% elongation strain) is applied; however,
greater magnitudes prevent bone formation.
In the present study, the effect of different
durations of compressive load on osteoblast differentiation was
additionally investigated. Results demonstrated that 6 h
compressive load effectively promoted factors involved in
osteoblast differentiation, and was more efficacious than durations
of 4 or 8 h. A compression time of 8 h reduced the expression
levels of osteoblast differentiation-associated genes in MC3T3-E1
cells following treatment with a high magnitude of 1 MPa. Koike
et al (26) reported that
high strain magnitudes downregulated Runx2 mRNA expression
levels after 24 and 48 h in the stromal cell line, ST2, which is
consistent with data obtained in the present study.
Sittichockechaiwut et al (8) demonstrated that 2 h of 5% elongation
compression on MLO-A5 cells increased the mRNA expression levels of
type l collagen, osteopontin and OCN, and had a substantial effect
on osteoblast differentiation. These results indicated that the
response of bone cells to compressive load is dependent on a
combination of magnitude and duration. However, different cell
types have different responses to mechanical stress, which may lead
to variable results.
The Wnt/β-catenin signaling pathway is activated
when Wnt ligands, including Wnt1, Wnt3a and Wnt8 bind to the
frizzled (Fzd) receptors and coreceptors, Lrp5 and Lrp6. The
Wnt-Fzd-Lrp complex subsequently activates β-catenin signaling
(35). β-catenin is stabilized
when localized in the cytoplasm; however, its translocation to the
nucleus leads to activation of Wnt signaling (36,37),
which regulates osteoblastogenesis (13–18),
promotion of bone formation and repression of bone resorption
(38). Studies have demonstrated
that mechanical strain enhances the mRNA expression levels of Wnt3a
and Wnt10b (39); however, to the
best of our knowledge, no study to date has investigated whether
compressive strain increases expression levels of Wnt1. In the
present study, 0.5 MPa compressive load significantly increased the
protein expression level of Wnt1, and 6 h mechanical load was most
effective. This condition additionally increased the expression
levels of osteoblast differentiation markers, which suggested that
initial activation of Wnt/β-catenin signaling may be involved in
compression-induced osteoblast differentiation. A previous study
has revealed that compressive load activated DVL2, which promoted
the stabilization of β-catenin by disruption of the β-catenin
destruction complex (38), and
activated the Wnt/β-catenin signaling pathway. In the present
study, compressive load led to an increase in the protein
expression levels of β-catenin and decreased the inactivation of
β-catenin, as ascertained by measuring the protein expression
levels of p-β-catenin. In addition, compression enhanced mRNA
expression levels of Lrp5 and decreased those of
SOST. These results suggested that Wnt/β-catenin signaling
serves a role in the response to compressive load in MC3T3-E1 cells
and osteoblast differentiation.
DKK-1 inhibits Wnt/β-catenin signaling by binding to
Lrp5/6. When treated with DKK-1, compression loading (0.5 MPa, 6 h)
stimulated a significant increase in the protein expression levels
of Wnt1, and a significant decrease in the ratio of
p-β-catenin/β-catenin when compared to DKK-1 treatment without
compression loading; however, the expression level of DVL2 was not
altered. These results suggested that signaling pathways other than
Wnt may be contributing to load-induced osteoblast differentiation
(40). Previous studies have
demonstrated that mechanical stimuli activated protein kinase B,
inhibited glycogen synthase kinase 3β via phosphorylation, which
directly activated β-catenin (23,41).
Therefore, the osteoblast response to compressive loading may be
regulated by cross-talk between different signaling pathways. A
report by Ponik et al (6)
demonstrated that Lrp5-positive transgenic mice were more sensitive
to mechanical load, and this was associated with upregulated Wnt
target gene expression. Therefore, Wnt/β-catenin signaling may
regulate osteoblast differentiation in response to compressive
load.
In conclusion, the present study demonstrated that
compressive loading stimulates osteoblast differentiation in a
magnitude- and duration-dependent manner. The Wnt/β-catenin
signaling pathway may serve a role in load-induced osteoblast
differentiation; however, other signaling pathways are likely to be
involved. The findings of the present study provide further
understanding of the effect of compressive load on osteoblast
differentiation and bone formation, which may ultimately help
prevent the development of osteoporosis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81170323), the
Shanghai Key Laboratory of Human Sport Competence Development and
Maintenance (Shanghai University of Sport; grant no. 11DZ2261100)
and the Innovation Project of Shanghai University of Sport Graduate
Education (grant no. yjscx2015007).
References
|
1
|
NIH Consensus Development Panel on
Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis
prevention, diagnosis, and therapy. JAMA. 285:785–795. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bliuc D, Alarkawi D, Nguyen TV, Eisman JA
and Center JR: Risk of subsequent fractures and mortality in
elderly women and men with fragility fractures with and without
osteoporotic bone density: The dubbo osteoporosis epidemiology
study. J Bone Miner Res. 30:637–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zaidi M: Skeletal remodeling in health and
disease. Nature Med. 13:791–801. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lang T, LeBlanc A, Evans H, Lu Y, Genant H
and Yu A: Cortical and trabecular bone mineral loss from the spine
and hip in long-duration spaceflight. J Bone Miner Res.
19:1006–1012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim CH, Kim KH and Jacobs CR: Effects of
high frequency loading on RANKL and OPG mRNA expression in ST-2
murine stromal cells. BMC Musculoskelet Disord. 10:1092009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponik SM, Triplett JW and Pavalko FM:
Osteoblasts and osteocytes respond differently to oscillatory and
unidirectional fluid flow profiles. J Cell Biochem. 100:794–807.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carinci F, Pezzetti F, Spina AM, Palmieri
A, Carls F, Laino G, De Rosa A, Farina E, Illiano F, Stabellini G,
et al: An in vitro model for dissecting distraction osteogenesis. J
Craniofac Surg. 16:71–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sittichockechaiwut A, Scutt AM, Ryan AJ,
Bonewald LF and Reilly GC: Use of rapidly mineralising osteoblasts
and short periods of mechanical loading to accelerate matrix
maturation in 3D scaffolds. Bone. 44:822–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong Z, Zeng XL, Ni JH and Huang XF:
Comparison of the biological response of osteoblasts after tension
and compression. Eur J Orthod. 35:59–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson WR, Rubin CT and Rubin J:
Mechanical regulation of signaling pathways in bone. Gene.
503:179–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robling AG, Niziolek PJ, Baldridge LA,
Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido
TM, Harris SE and Turner CH: Mechanical stimulation of bone in vivo
reduces osteocyte expression of Sost/sclerostin. J Biol Chem.
283:5866–5875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wozniak M, Fausto A, Carron CP, Meyer DM
and Hruska KA: Mechanically strained cells of the osteoblast
lineage organize their extracellular matrix through unique sites of
alphavbeta3-integrin expression. J Bone Miner Res. 15:1731–1745.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Day TF, Guo X, Garrett-Beal L and Yang Y:
Wnt/beta-catenin signaling in mesenchymal progenitors controls
osteoblast and chondrocyte differentiation during vertebrate
skeletogenesis. Dev Cell. 8:739–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bennett CN, Longo KA, Wright WS, Suva LJ,
Lane TF, Hankenson KD and MacDougald OA: Regulation of
osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA.
102:3324–3329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Canalis E: Wnt signalling in osteoporosis:
Mechanisms and novel therapeutic approaches. Nat Rev Endocrinol.
9:575–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato M, Patel MS, Levasseur R, Lobov I,
Chang BH, Glass DA II, Hartmann C, Li L, Hwang TH, Brayton CF, et
al: Cbfa1-independent decrease in osteoblast proliferation,
osteopenia and persistent embryonic eye vascularization in mice
deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 157:303–314.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krishnan V, Bryant HU and Macdougald OA:
Regulation of bone mass by Wnt signaling. J Clin Invest.
116:1202–1209. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gong Y, Slee RB, Fukai N, Rawadi G,
Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et
al: LDL receptor-related protein 5 (LRP5) affects bone accrual and
eye development. Cell. 107:513–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui Y, Niziolek PJ, MacDonald BT, Zylstra
CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon
RA, et al: Lrp5 functions in bone to regulate bone mass. Nat Med.
17:684–691. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lian JB, Stein GS, Javed A, van Wijnen AJ,
Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ and Young DW:
Networks and hubs for the transcriptional control of
osteoblastogenesis. Rev Endocr Metab Disord. 7:1–16. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Javed A, Bae JS, Afzal F, Gutierrez S,
Pratap J, Zaidi SK, Lou Y, van Wijnen AJ, Stein JL, Stein GS and
Lian JB: Structural coupling of Smad and Runx2 for execution of the
BMP2 osteogenic signal. J Biol Chem. 283:8412–8422. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sawakami K, Robling AG, Ai M, Pitner ND,
Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, et al: The Wnt
co-receptor LRP5 is essential for skeletal mechanotransduction but
not for the anabolic bone response to parathyroid hormone
treatment. J Biol Chem. 281:23698–23711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sen B, Guilluy C, Xie Z, Case N, Styner M,
Thomas J, Oguz I, Rubin C, Burridge K and Rubin J: Mechanically
induced focal adhesion assembly amplifies anti-adipogenic pathways
in mesenchymal stem cells. Stem cells. 29:1829–1836. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR,
Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI and Bellido
T: Sost downregulation and local Wnt signaling are required for the
osteogenic response to mechanical loading. Bone. 50:209–217. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sanchez C, Pesesse L, Gabay O, Delcour JP,
Msika P, Baudouin C and Henrotin YE: Regulation of subchondral bone
osteoblast metabolism by cyclic compression. Arthritis Rheum.
64:1193–1203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koike M, Shimokawa H, Kanno Z, Ohya K and
Soma K: Effects of mechanical strain on proliferation and
differentiation of bone marrow stromal cell line ST2. J Bone Miner
Metab. 23:219–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jacobs C, Grimm S, Ziebart T, Walter C and
Wehrbein H: Osteogenic differentiation of periodontal fibroblasts
is dependent on the strength of mechanical strain. Arch Oral Biol.
58:896–904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sanchez C, Gabay O, Salvat C, Henrotin YE
and Berenbaum F: Mechanical loading highly increases IL-6
production and decreases OPG expression by osteoblasts.
Osteoarthritis Cartilage. 17:473–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fermor B, Weinberg JB, Pisetsky DS,
Misukonis MA, Banes AJ and Guilak F: The effects of static and
intermittent compression on nitric oxide production in articular
cartilage explants. J Orthop Res. 19:729–737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xue Y, Yan Y, Gong H, Fang B, Zhou Y, Ding
Z, Yin P, Zhang G, Ye Y, Yang C, et al: Insulin-like growth factor
binding protein 4 enhances cardiomyocytes induction in
murine-induced pluripotent stem cells. J Cell Biochem.
115:1495–1504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lviak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dumas V, Perrier A, Malaval L, Laroche N,
Guignandon A, Vico L and Rattner A: The effect of dual frequency
cyclic compression on matrix deposition by osteoblast-like cells
grown in 3D scaffolds and on modulation of VEGF variant expression.
Biomaterials. 30:3279–3288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
David V, Guignandon A, Martin A, Malaval
L, Lafage-Proust MH, Rattner A, Mann V, Noble B, Jones DB and Vico
L: Ex Vivo bone formation in bovine trabecular bone cultured in a
dynamic 3D bioreactor is enhanced by compressive mechanical strain.
Tissue Eng Part A. 14:117–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peptan AI, Lopez A, Kopher RA and Mao JJ:
Responses of intramembranous bone and sutures upon in vivo cyclic
tensile and compressive loading. Bone. 42:432–438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goel S, Chin EN, Fakhraldeen SA, Berry SM,
Beebe DJ and Alexander CM: Both LRP5 and LRP6 receptors are
required to respond to physiological Wnt ligands in mammary
epithelial cells and fibroblasts. J Biol Chem. 287:16454–16466.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Colaianni G, Cuscito C, Mongelli T,
Pignataro P, Tamma R, Oranger A, Colucci S and Grano M: Cellular
Mechanisms of Bone Regeneration: Role of Wnt-1 in Bone-Muscle
Interaction during Physical Activity39. J Biol Regul Homeost
Agents. 29:(4 Suppl). S39–S45. 2015.
|
|
37
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Robinson JA, Chatterjee-Kishore M,
Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P,
Brown EL, et al: Wnt/beta-catenin signaling is a normal
physiological response to mechanical loading in bone. J Biol Chem.
281:31720–31728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He XC, Zhang J, Tong WG, Tawfik O, Ross J,
Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al: BMP
signaling inhibits intestinal stem cell self-renewal through
suppression of Wnt-beta-catenin signaling. Nat Genet. 36:1117–1121.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Case N, Ma M, Sen B, Xie Z, Gross TS and
Rubin J: Beta-catenin levels influence rapid mechanical responses
in osteoblasts. J Biol Chem. 283:29196–29205. 2008. View Article : Google Scholar : PubMed/NCBI
|