Introduction
Cervical cancer (CC) is a common malignancy of the
female reproductive system. In China, >200,000 fatalities due to
CC occur each year, and the incidence in younger age groups is on
the increase (1). Current
treatment approaches to CC include surgery, chemotherapy and
radiotherapy in patients with primary tumors. However, due to
continuous use of various chemotherapy drugs, multidrug resistance
(MDR) may develop in tumor cells. Such resistance reduces the
sensitivity of tumor cells to chemotherapy, leading to failure of
treatment. MDR is defined as the resistance of cancer cells to a
diverse panel of structurally and functionally unassociated drugs
(2). This resistance can occur
naturally (inherent resistance) or acquired during the course of
chemotherapy (3). To date, current
research on MDR has focused on the transcriptional regulation of
the MDR gene. The multidrug-resistance 1 (MDR1) gene product
P-glycoprotein (P-gp) is an efflux pump that actively transports
substrates, such as glucocorticoids, out of the cell (4). LRP is a small subcellular structure
located at cytoplasmic vaults that may be in charge of subsequent
exocytosis of agents from the cell. A previous in vitro
study determined that LRP was associated with resistance to
melphalan, cisplatin and doxorubicin (5). GSTP1, which has an important role in
the detoxification of toxic substances, is a phase II metabolic
enzyme, protects cells from anticancer drug-induced injury
(6). To understand the role of the
MDR gene in primary drug resistance, the present study detected the
mRNA expression levels of MDR1, LRP and GSTP1
in 47 cases of CC and 20 healthy cervical tissue samples. In
addition, a preliminary study on the association between the
expression levels of these genes and cervical pathology was
conducted. The present study investigated mechanisms underlying
drug resistance in CC and may aid understanding of individual
responsiveness to chemotherapy and prognosis.
Materials and methods
Patients and specimens
A total of 47 fresh CC specimens were collected from
February 2008 to August 2010. Patients were aged between 43 and 65
years, with a mean age of 46.7 years. Histologic cell types
included 41 cases of squamous-cell carcinoma and 6 cases of
adenocarcinoma. Clinical staging was performed, and was as follows:
Stage I, 12 cases; stage II, 18 cases; and stage III, 17 cases. Of
these cases, 10 were well differentiated, 16 were moderately
differentiated and 21 were poorly differentiated carcinomas. All
diagnoses were confirmed by pathology. A total of 19 cases were ≥4
cm in size and 28 measured <4 cm. All patients had not received
chemotherapy and radiation therapy prior to sample collection. In
addition, 20 healthy cervical tissue samples were collected
(cancerous tissue from the same cervical margin tissue surgery
measures >2 cm from the edge, as the tumor edge was not clear,
an additional 27 cases were not collected). Samples were stored in
liquid nitrogen for future use. The integrity of clinical data was
maintained, and the patients were monitored for 6 to 60 months.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues using
TRIzol® reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. The
concentration and purity of total RNA was determined by measuring
the absorbance with a NanoDrop-2000 spectrophotometer at
wavelengths of 260 and 280 nm. cDNA was synthesized from total RNA
using the Reverse Transcription system (Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol. cDNA
was utilized immediately or stored at −80°C until use. Each qPCR
reaction contained 1.5 µl 2 mmol/l dNTP, 0.3 µl 5 U/µl Taq (Sangon
Biotech Co., Ltd., Shanghai, China), 2.5 µl 25 mmol/l
Mg2+, 3 µl 10X buffer, 2 µl forward primer, 2 µl reverse
primer, 5 µl cDNA (0.5 µg/µl), 1.0 µl 10X SYBR®-Green I
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The total
volume of each reaction was adjusted to 30 µl with sterile water.
Cycling conditions were as follows: An initial predenaturation step
at 94°C for 5 min, followed by 35 cycles of denaturation at 95°C
for 30 sec, annealing at 55°C for 30 sec and extension at 72°C for
1 min. Subsequently, dissociation curve analysis was performed, and
the reaction products were separated by electrophoresis on a 2%
agarose gel and stained with ethidium bromide for confirmation of
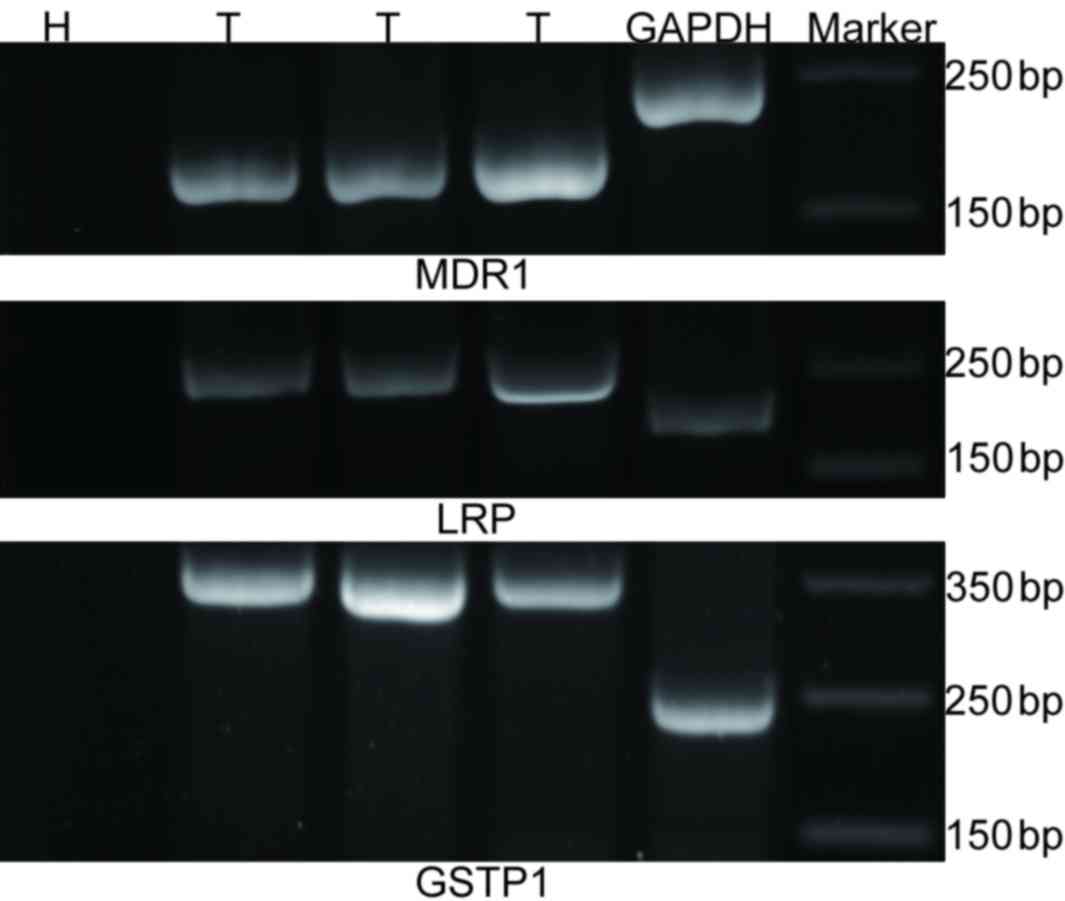
the PCR products. The mRNA expression levels of MDR1,
LRP and GSTP1 were expressed as a ratio relative to
GAPDH in each sample, the level of genes expression were
normalized to that of the internal (GAPDH) and were determined by
the 2−∆∆Cq method (7).
Primer pairs were as follows: Forward, 5′-CCCATCATTGCAATAGCAGG-3′
and reverse, 5′-TGTTCAAACTTCTGCTCCTGA-3′ for human MDR1;
forward, 5′-CCAGAACCAGGGAGGCAAGA-3′ and reverse,
5′-GAGGCGCCCCACATATGCT-3′ for human GSTP1; forward,
5′-GTCTTCGGGCCTGAGCTGGTGTCG-3′ and reverse,
5′-CTTGGCCGTCTCTTGGGGGTCCTT-3′ for human LRP; and forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′- GAA GAT GGT GAT GGG ATT
TC-3′ for human GAPDH. MDR1, LRP, GSTP1
and GAPDH primers yielded products of 158, 325, 240 and 226
bp, respectively.
Statistical analysis
Statistical calculations were performed using SPSS
software version 13.0 (SPSS, Inc., Chicago, IL, USA), and P<0.05
was considered to indicate a statistically significant difference.
The measured data were expressed as the mean ± standard deviation.
A Student's t-test, two-tailed chi-squared test and multivariate
logistic regression analysis were used to compare groups. Survival
curves were compared using the two-sided data log-rank method.
Results
mRNA expression levels of MDR1, LRP
and GSTP1 in tissue samples
Of CC tissues, 63.8% were positive for MDR1,
76.6% were positive for LRP and 59.6% were positive for
GSTP1. Of healthy tissues, 10% were positive for
MDR1, 15.0% were positive for LRP and 5% were
positive for GSTP1, compared with the healthy tissues, if
the levels were ≥ 0.4, the expression was deemed to be positive;
otherwise, the expression was negative. Therefore, a greater
percentage of CC tissues expressed these genes compared with
healthy tissues (P<0.05). The relative mRNA expression levels of
the resistance genes MDR1, LRP and GSTP1 in
cancer tissues were 0.57±0.32, 0.58±0.29 and 0.44±0.24,
respectively. The relative mRNA expression levels of MDR1,
LRP and GSTP1 in normal cervical tissues were
0.19±0.10, 0.17±0.14 and 0.18±0.10, respectively. Therefore, the
mRNA expression levels of all three genes were greater in cancer
compared with healthy cervical tissues, and this was statistically
significant (P<0.001). In all cancer samples, the expression of
all three genes was detected in 14 cases, expression of two genes
was detected in 29 cases and expression of one gene was detected in
37 cases. MDR1, LRP and GSTP1 mRNA expression
levels are presented in Fig.
1.
mRNA expression levels of MDR1, LRP
and GSTP1 and clinicopathological data
No significant differences were identified between
the mRNA expression levels of LRP or GSTP1, and tumor
size, histological type or clinical stage (P>0.05; Table I). mRNA expression levels of
MDR1 were significantly greater in poorly-differentiated
compared with well and moderately differentiated carcinomas
(P<0.05; Table I); however, no
significant differences were identified between MDR1 mRNA
expression and the other clinicopathological factors assessed.
 | Table I.Association between MDR1, LRP
and GSTP1 mRNA expression levels and clinicopathological
parameters in cervical cancer. |
Table I.
Association between MDR1, LRP
and GSTP1 mRNA expression levels and clinicopathological
parameters in cervical cancer.
| Study groups | n | MDR1 | t | P-value | LRP | t | P-value | GSTP1 | t | P-value |
|---|
| Size (cm) |
|
|
|
|
|
|
|
|
|
| ≥4 | 19 | 0.48±0.27 | −1.29 | 0.21 | 0.51±0.33 | −1.34 | 0.19 | 0.42±0.25 | −0.49 | 0.63 |
|
<4 | 28 | 0.62±0.34 |
|
| 0.63±0.26 |
|
| 0.46±0.24 |
|
| Histological
type |
|
|
|
|
|
|
|
|
|
| Squamous
carcinoma | 41 | 0.62±0.31 | 1.72 | 0.1 | 0.60±0.29 | 0.85 | 0.40 | 0.45±0.23 | 0.14 | 0.89 |
|
Adenocarcinoma | 6 | 0.47±0.39 |
|
| 0.49±0.30 |
|
| 0.43±0.33 |
|
| FIGO stages |
|
|
|
|
|
|
|
|
|
| I+II | 30 | 0.62±0.31 | 1.53 | 0.13 | 0.58±0.31 | −0.069 | 0.94 | 0.47±0.24 |
1.27 | 0.21 |
|
III | 17 | 0.46±0.32 | |
| 0.58±0.28 |
|
| 0.38±0.25 |
|
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
Well+moderately | 26 | 0.68±0.27 |
3.16 | 0.003 | 0.61±0.29 |
0.71 | 0.49 | 0.47±0.25 |
0.70 | 0.50 |
|
Poorly | 21 | 0.38±0.33 |
|
| 0.54±0.29 |
|
| 0.42±0.23 |
|
mRNA expression levels of
MDR-associated genes and survival rate of patients
Multivariate logistic regression analysis revealed
that the risk factors of CC were associated with high mRNA
expression levels of MDR1 and the state of differentiation;
however, there was no association between the mRNA expression
levels of LRP or GSTP1 (data not shown), and tumor
size or stage (Table II). The
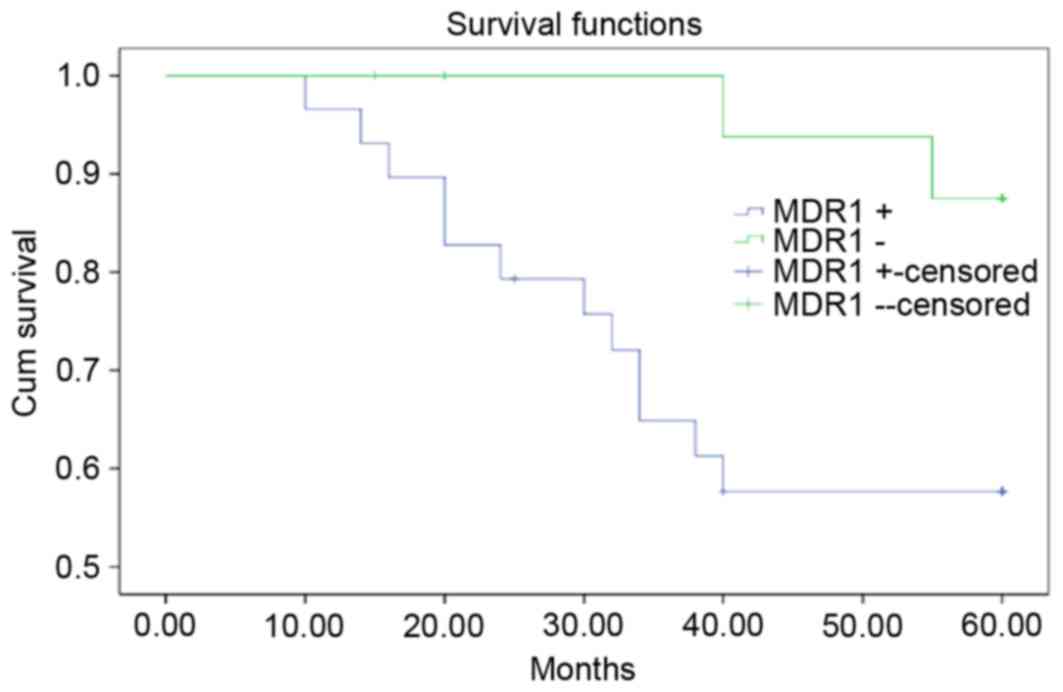
cancer samples were categorized into MDR1-positive (n=32)
and -negative (n=15) groups. The survival rate was significantly
greater in the MDR1-negative group compared with the
MDR1-positive group (Table
II; Fig. 2; P<0.05).
 | Table II.Multivariate logistics analysis on
factors that influence cervical cancer prognosis. |
Table II.
Multivariate logistics analysis on
factors that influence cervical cancer prognosis.
| Covariates | Deceased
patients/total | B | Exp (B) | 95% CI | P-value |
|---|
|
Differentiation |
|
|
|
|
|
|
Well+moderately | 6/26 | 2.83 | 16.89 | 1.71–166.48 | 0.015 |
|
Poorly | 8/21 |
|
|
|
|
| MDR1 |
|
|
|
|
|
|
Positive | 12/32 | 3.65 | 38.53 | 3.03–490.35 | 0.005 |
|
Negative | 2/15 |
|
|
|
|
Discussion
MDR may be divided into primary drug resistance that
exists prior to chemotherapy treatment, and acquired drug
resistance that may develop during chemotherapy. MDR was identified
as an important factor that contributes to the failure of cancer
treatment.
MDR is frequently associated with overexpression of
the MDR1 gene, which encodes the drug transporter,
P-glycoprotein (P-gp). P-gp is one of the ABC transporter proteins
that have similar trans-membrane domains that may pump
chemotherapeutic drugs out of cancer cells against a concentration
gradient in an ATP energy-dependent manner; therefore, reducing
intracellular accumulation of chemotherapeutic agents and
protecting cancer cells from toxicity (8). MDR1 genes and/or increased expression
of P-gp have been associated with drug resistance (9–11).
The glutathione (GSH) system is responsible for the detoxification
of reactive oxygen and nitrogen species in cells. Tumor cells that
express glutathione s-transferase (GST) may catalyze the
conjugation of GSH to anticancer drugs, which are subsequently
removed from the cell by transporters. This protects cells from
anticancer drug-induced injury. GST expression is primarily induced
by alkylating agents, including platinum compounds and mitomycin-c
(12). LRP serves a role in drug
resistance in breast cancer (13),
ovarian cancer (14), lung cancer
(15) and various other tumor
types. Enhanced expression levels of LRP may cause tumor cells to
become resistant to traditional chemotherapy drugs, including
doxorubicin, cisplatin, vincristine and mitomycin (16). Therefore, findings regarding the
expression of LRP revealed that cervical cancer has an intrinsic
resistance of cisplatin (17). In
the present study, the mRNA expression levels of the resistance
genes MDR1, LRP and GSTP1 were measured in
cancer tissue specimens and compared with clinicopathological data,
to investigate their role in primary drug resistance in cancer.
Tolerance of tumor cells to drugs is associated with the expression
levels of drug resistance genes in cells. Therefore, the expression
levels of drug resistance genes indirectly reflect the resistance
of tumor cells to drugs. The present study demonstrated that the
mRNA expression levels of MDR1, LRP and GSTP1
in CC tissue specimens were greater compared with healthy cervical
tissues. Patients had not received chemotherapy or radiation
therapy. This suggested that primary drug resistance was associated
with CC.
In the present study, not all tumors expressed the
MDR-associated genes; 29.8% of tumors expressed MDR1,
LRP and GSTP1, and 61.7% expressed two of these
genes. This suggested that the expression levels of these genes in
CC are regulated by signaling pathways that contribute to MDR,
which are likely exhibit a high degree of complexity and
interconnectivity.
The mRNA expression levels of MDR1 were
greater in well and moderately differentiated carcinomas compared
with poorly differentiated carcinomas, which suggested that the
greater the degree of cancer differentiation, the greater the
resistance to chemotherapeutic drugs. However, the mRNA expression
levels of LRP and GSTP1 in the tumor tissues did not
exhibit a significant association with the clinicopathological
features of the patients. Greater numbers of specimens would be
necessary to confirm this finding. High mRNA expression levels of
LRP and GSTP1 may mediate drug resistance of tumor
cells to cisplatin, suggesting that primary MDR of CC to cisplatin
may be associated with the expression levels of LRP and
GSTP1 genes (18).
Sato et al (19) demonstrated that high expression
levels of P-gp were detected in lymph node metastasis of the colon.
In a follow-up study of 112 patients with CC, Nagai et al
(20) revealed that the five-year
survival rate of P-gp-positive patients was significantly reduced
compared with patients with P-gp-negative tumors. In the present
study, high MDR1 mRNA expression levels were associated with
poor CC survival rates, suggesting that MDR1 may be a
high-risk prognostic factor.
Reducing the expression levels of genes associated
with drug resistance is likely to enhance the efficacy of therapy.
The expression levels of the MDR1 gene and its corresponding
protein, P-gp, were significantly reduced in cancer cells
transfected with small interfering RNA targeting MDR1
(21). In vitro experiments
and RNA interference technology may effectively reverse drug
resistance in ovarian cancer cells (22). Studies that aim to reverse drug
resistance in solid tumors warrant further investigation.
In conclusion, MDR1, LRP and
GSTP1 genes may be involved in mechanisms underlying primary
MDR in CC. Detection of MDR1 expression levels may be utilized to
predict patient response to chemotherapy. Reversal of CC drug
resistance may strengthen the efficacy of chemotherapy and prolong
the survival of patients with CC.
References
|
1
|
Aggarwal P: Cervical cancer: Can it be
prevented? World J Clin Oncol. 5:775–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fojo A, Hamilton TC, Young RC and Ozols
RF: Multidrug resistance in ovarian cancer. Cancer. 60:(8 Suppl).
S2075–S2080. 1987. View Article : Google Scholar
|
|
3
|
Gong J, Jaiswal R, Mathys JM, Combes V,
Grau GE and Bebawy M: Microparticles and their emerging role in
cancer multidrug resistance. Cancer Treat Rev. 38:226–234. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lage H: Gene therapeutic approaches to
overcome ABCB1-Mediated drug resistance. Recent Results Cancer Res.
209:87–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Izquierdo MA, Shoemaker RH, Flens MJ,
Scheffer GL, Wu L, Prather TR and Scheper RJ: Overlapping
phenotypes of multidrug resistance among panels of human
cancer-cell lines. Int J Cancer. 65:230–237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu L, Shen Y, Peng X, Zhang S, Wang M, Xu
G, Zheng X, Wang J and Lu C: Aberrant promoter methylation of
cancer-related genes in human breast cancer. Oncol Lett.
12:5145–5155. 2016.PubMed/NCBI
|
|
7
|
Karabulut S, Kaya Z, Amuran GG, Peker I,
Özmen T, Gūllūoḡlu BM, Kaya H, Erzik C, Ōzer A and Akkiprik M:
Correlation between the DNA methylation and gene expression of
IGFBP5 in breast cancer. Breast Dis. 36:123–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong X and Mumper RJ: Nanomedicinal
strategies to treat multidrug-resistant tumors: Current progress.
Nanomedicine (Lond). 5:597–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li WT, Aizimu A and Wang YH: Relationship
between the expression of P-glycoprotein and the therapeutic effect
of neoadjuvant chemotherapy on Uygur and Han patients with cervical
cancer. J Chin Pract Diagn Ther. 25:225–227. 2011.
|
|
10
|
Tydén E, Skarin M and Höglund J: Gene
expression of ABC transporters in Cooperia oncophora after field
and laboratory selection with macrocyclic lactones. Mol Biochem
Parasitol. 198:66–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Q, Wang J, He H, Liu H, Yan X and
Zou K: Trametenolic acid B reverses multidrug resistance in breast
cancer cells through regulating the expression level of
P-glycoprotein. Phytother Res. 28:1037–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stewart DJ: Tumor and host factors that
may limit efficacy of chemotherapy in non-small cell and small cell
lung cancer. Crit Rev Oncol Hematol. 75:173–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wood N and Streckfus CF: The expression of
lung resistance protein in saliva: A novel prognostic indicator
protein for carcinoma of the breast. Cancer Invest. 33:510–515.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu D, Shi HC, Wang ZX, Gu XW and Zeng YJ:
Multidrug resistance-associated biomarkers PGP, GST-π, Topo-II and
LRP as prognostic factors in primary ovarian carcinoma. Br J Biomed
Sci. 68:69–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YJ, Yu CH, Zhang W and Liu Y and Liu Y:
The relationship between expression of COX-2′LRP and metastasis and
prognosis in patients with non-small cell lung carcinoma. J Clin
Pulm Med. 15:1707–1709. 2010.
|
|
16
|
Jiao JW and Wen F: Tanshinone IIA acts via
p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and
lung-resistance protein in cisplatin-resistant ovarian cancer
cells. Oncol Rep. 25:781–788. 2011.PubMed/NCBI
|
|
17
|
Zhong H, Zuo Y, Wu X, Peng Y, He H, Yang
J, Guan C and Xu Z: Synergistic antitumor effect of amorphigenin
combined with cisplatin in human lung adenocarcinoma A549/DDP
cells. Zhongguo Fei Ai Za Zhi. 19:805–812. 2016.(In Chinese).
PubMed/NCBI
|
|
18
|
Daukantienė L, Kazbarienė B, Valuckas KP,
Didžiapetrienė J, Krikštaponienė A and Aleknavičius E: The
significance of reduced glutathione and glutathione S-transferase
during chemoradiotherapy of locally advanced cervical cancer.
Medicina (Kaunas). 50:222–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato A, Shimada K, Nakamachi M, Ushio J,
Yamamoto W, Kurihara M and Matsukawa M: Effectiveness of
doxifluridine (5′-DFUR)/docetaxel against advanced/recurrent
gastric cancer showing resistance to various anticancer drug
regimens. Gastric Cancer. 5:233–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagai N1, Shiroyama Y, Oshita T, Mukai K,
Shigemasa K, Fujii T, Katsube Y, Matsubayashi S, Murakami T and
Ohama K: Tumor dihydropyrimidine dehydrogenase activity in advanced
cervical carcinoma. Oncol Rep. 9:1033–1040. 2002.PubMed/NCBI
|
|
21
|
Lou JY, Peng ZL, Zheng Y, Wang H, He B and
Wang HJ: Reversal of multi-drug resistance in ovarian cancer cell
by RNA interference. Zhonghua Fu Chan Ke Za Zhi. 41:413–416.
2006.PubMed/NCBI
|
|
22
|
Gao GL, Tu KJ and Liu FJ: Expression and
reversal of multi-drug resistance gene in ovarian cancer. Chin Clin
Oncol. 15:870–873. 2010.
|