Introduction
The incidence of pelvic organ prolapse (POP) is
increasing in aging populations due to the prolonged life span
following the menopause (1,2), and
is becoming one of the most common forms of female pelvic floor
dysfunction. POP causes various complications and significantly
affects the quality of life of patients (3). The occurrence of POP is closely
associated with elasticity, toughness and functional alterations in
the connective tissue of the pelvic support tissue (4). Normal pelvic structure support and
function depend on the pelvic floor connective tissues. It has
previously been reported that alterations in extracellular matrix
(ECM) proteins of the supporting ligament are associated with POP
(5).
Bone marrow mesenchymal stem cells (BMSCs) originate
from the mesoderm, have multilineage capacity and generate a
variety of cell types (6). BMSCs
may induce and regulate the development of bone marrow
hematopoietic stem cells and stromal cells. In addition, they may
promote the assembly and ordered arrangement of ECM molecules
(7). Following injury, endogenous
or exogenous BMSCs migrate into lesions for repair. Increasing
evidence suggests that in the process of ligament injury repair,
different pluripotent stem cells are released from bone marrow.
These stem cells are similar to the surrounding ligament cells, and
move to and accumulate in the injury sites (8,9).
Young et al studied BMSCs in vivo (10). Following replacement of the
Achilles tendon of rabbits with collagen fibers and BMSCs, assembly
of the fibers was improved compared with replacement with collagen
fibers only. The freshly produced ligament fibers were thicker
compared with the original (9). In
a separate study, BMSCs or fibroblasts in a liquid fibrin matrix
were injected into the damaged knee tendon and patellar tendon of
immunodeficient rats. This study reveals that an injection of BMSCs
or fibroblasts in a liquid fibrin matrix altered tissue morphology,
ultrastructure and the mRNA expression levels of ECM proteins,
which facilitate damage repair (11). Therefore, BMSCs have the potential
to improve recovery from injury.
Tissue repair depends on intrinsic and extrinsic
processes, requiring blood supply, fibroblasts, ECM proteins and
growth factors for proliferation, repair and remodeling (12). In connective tissue, evidence
suggests that constant mechanical stress regulates the synthesis of
ECM proteins, including collagen, elastin, tenascin and integrin
ligands, and their arrangement (13). These ECM proteins regulate
mechanical signal transduction and promote the secretion of growth
factors (14). Simultaneously, via
integrins, mechanical stress signals are transmitted to the
cytoskeleton to assemble in a certain physiological range and
pattern of dynamics (15).
Transforming growth factor (TGF)-β is a known stimulator of ECM
protein production in fibroblasts and mediates the response of
fibroblasts to mechanical stress (16). Tenascin-C is an ECM component that
is upregulated in fibroblasts following mechanical stress
stimulation. In addition, tenascin-C is highly expressed during
wound healing and tissue remodeling (17–19).
However, little is known regarding the tenascin-C pathway during
BMSC differentiation.
Therefore, the present study aimed to determine the
effect of pelvic ligament fibroblasts following stretch stimulation
on tenascin-C expression and its pathway in BMSCs, using a
co-culture system. This may facilitate further understanding of
BMSC differentiation potential and characteristics, which may aid
in the development of novel therapeutic strategies for the
treatment of POP.
Materials and methods
Experimental animals and primary
reagents
Female, 7-week-old Sprague Dawley rats (n=20;
225–275 g) were purchased from the Experimental Animal Center of
The Fourth Military Medical University (Xi'an, China). They were
maintained under controlled conditions of 22–26°C, 12 h light/dark
cycle and a relative humidity of 50–70%. Animals had access to food
and water throughout the experiment. All studies were conducted in
accordance with the standards of humane animal care described in
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (20), using
protocols approved by Zhengzhou University Institutional Animal
Care and Research Advisory Committee (Zhengzhou, China). Low
glucose Dulbecco's modified Eagle's medium (LG-DMEM) and trypsin
were purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA), fetal bovine serum (FBS) was obtained from Hangzhou
Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou,
China). Penicillin and streptomycin sulfate were purchased from
North China Pharmaceutical Group Co., Ltd. (Shijiazhuang, China),
and Percoll separation medium was purchased from Pfizer, Inc. (New
York, NY, USA). For flow cytometry, the mouse anti-rat monoclonal
antibodies cluster of differentiation (CD)44-fluorescein
isothiocyanate (FITC; cat. no. 561859), CD90-phycoerythrin (PE;
cat. no. 551401), CD45-FITC (cat. no. 551401), and the isotype
control antibodies mouse anti-rat IgG1-FITC (cat. no. 553892) and
mouse IgG1-PE (cat. no. 554680) were obtained from BD Biosciences
(Franklin Lakes, NJ, USA); the mouse anti-rat CD34-FITC monoclonal
antibody (cat. no. sc-7324 FITC) was purchased from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). The Total Protein assay kit
was purchased from Beyotime Institute of Biotechnology (Shanghai,
China). The following antibodies were used for western blotting and
immunofluorescence: Alexa Fluor® 555 Phalloidin (cat.
no. 8953; Cell Signaling Technology, Inc., Danvers, MA, USA); mouse
anti-rat F-actin antibody (cat. no. ab205, Abcam, Cambridge, UK);
mouse anti-rat GAPDH antibody (cat. no. SC-47724; Santa Cruz
Biotechnology, Inc.); rabbit anti-rat tenascin-C antibody (cat. no.
SC-20932; Santa Cruz Biotechnology, Inc.); goat anti-rabbit
IgG-FITC (cat. no. SC-2012; Santa Cruz Biotechnology, Inc.);
horseradish peroxidase-conjugated goat anti-mouse IgG secondary
antibodies (cat. no. 1706516; Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and anti-rabbit IgG secondary antibodies (cat. no.
1706515; Bio-Rad Laboratories, Inc.). One-Step SYBR PrimeScript
RT-PCR kit II was obtained from Takara Biotechnology Co., Ltd.,
(Dalian, China) for reverse transcription-quantitative polymerase
chain reaction (RT-qPCR).
Isolation and analysis of rat
BMSCs
Isolation, culture and passage of rat BMSCs from
7-day-old Sprague Dawley rats were performed as previously
described (21) following
sacrifice of rats by cervical dislocation. Briefly, under sterile
conditions, a syringe was used to flush out the bone marrow. Bone
marrow cells were isolated with Percoll separation medium. The
interface layer of mononuclear cells was washed twice with cold PBS
and maintained in complete LG-DMEM (10% FBS; 100 U/ml penicillin
and 100 mg/ml streptomycin sulfate). These primary cells were
subcultured at 80–90% confluence. Fourth passage BMSCs were used
for experimental analysis.
Flow cytometry was performed to determine the
surface expression of CD44, CD90, CD45 and CD34 on rat BMSCs. On
reaching 80–90% confluence, fourth passage BMSCs (1×105
cells/ml) were stained with 1 µl CD44-FITC (1:1,000), CD90-PE
(1:1,000), CD45-FITC (1:1,000), CD34-FITC (1:1,000), IgGl-FITC
(1:1,000) or IgGl-PE (1:1,000) at room temperature in the dark for
30 min. The labeled cells were centrifuged at 208 × g for 5
min at 4°C and resuspended in PBS for analysis using a FACScan flow
cytometer (BD Biosciences). CellQuest™ Pro Version 4.0 (BD
Biosciences) was used for analysis. As described previously
(4), the control cells were
cultured in complete medium (LG-DMEM; 10% FBS; 100 U/ml penicillin
and 100 mg/ml streptomycin sulfate) and the treatment group was
cultured with osteogenic and adipogenic induction, respectively.
After 12 and 14 days, cells were fixed with paraformaldehyde and
stained with alizarin red or Oil-red-O solution for observation
under microscope (22,23).
Growth factors and inhibitors
SB 431542 (a TGF-β receptor type I inhibitor), PD
98059 [a mitogen-activated protein kinase (MAPK) kinase (MEK-1)
inhibitor] and SB 203580 (a p38 MAPK inhibitor) were purchased from
EMD Millipore (Billerica, MA, USA). Stock solutions of SB 431542,
PD 98059 and SB 203580 were prepared in dimethyl sulfoxide (DMSO).
Neutralizing anti-TGF-β1 antibodies were obtained from R&D
Systems, Inc. (Minneapolis, MN, USA). Human recombinant TGF-β1 was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and
prepared in bovine serum albumin (BSA; Sigma-Aldrich; Merck
KGaA)-containing stock solutions according to the manufacturer's
protocol. Growth factor and inhibitor concentrations used in assays
were optimized based on the literature (24–26),
our preliminary experiments and the manufacturer's data sheets, and
the following final concentrations were selected: Neutralizing
anti-TGF-β1 antibodies, 100 ng/ml; SB 431542, 10 µM; PD 98059, 10
µM; SB 203580, 3 µM; and TGF-β1, 5 ng/ml.
Rat pelvic ligament fibroblasts and
fibroblast traction injury model
Rat pelvic ligament fibroblasts were prepared as
previously described (21) and
maintained in LG-DMEM containing 10% FBS, and 100 U/ml penicillin
and 100 mg/ml streptomycin sulfate. Briefly, ligament was removed
and immediately placed into serum-free Hanks' balanced salt
solution (HBSS) and minced into 1 mm3, followed by
treatment with 0.25% trypsin and 0.01% EDTA at 37°C for 2 days with
gentle rotation. The supernatant, which contained the fibroblasts,
was washed with HBSS and DMEM with 10% heat-inactivated FBS. The
cell suspension was spun down twice to remove collagenase. The
pellets were resuspended in growth medium and plated in tissue
culture flasks. The medium was changed every other day. Prior to
stretching, a small number of fourth passage fibroblasts were
collected for immunofluorescence staining to confirm that they were
fibroblasts. Passage 3 fibroblasts (3×105/membrane) were
seeded onto gelatin-coated silicone membranes and cultured for 24
h, following culture, cells were considered to be passage 4.
Subsequently, 10% of the load transformation was exerted on cells
with 1 Hz horizontal stretch stimulation for various durations (0,
3, 12 or 24 h) under 5% CO2 and 37°C conditions. Cells
were fixed in paraformaldehyde and stained with 5 µg/ml DAPI. Cell
morphology was examined under a confocal microscope (Olympus
Corporation, Tokyo, Japan). Prior to co-culture, ligament
fibroblasts were subjected to mechanical stretch stimulation for 12
h and subsequently seeded into the upper chamber at
5×104/well. BMSCs (2×105/well) were seeded
into the lower chamber of a 6-well Transwell plate. Following
indirect co-culture for 3, 6 or 12 days, cells were collected for
RT-qPCR and western blot analysis.
Treatment with growth factor and
inhibitors
BMSCs (2×105 cells/well in 2 ml) in the
lower chamber and fibroblast cells (5×104 cells/well in
2 ml) in the upper chamber were cultured in 6-well Transwell
plates. Protein kinase inhibitors and growth factors were diluted
with medium at double the final concentration stated previously:
Neutralizing anti-TGF-β1 antibodies, 200 ng/ml; SB 431542, 20 µM;
PD 98059, 20 µM; SB 203580, 6 µM; and TGF-β1, 10 ng/ml. The control
group was treated with medium containing 0.1% DMSO. Control- or
inhibitor-containing medium (2 ml) was placed into each well and
incubated for 30 min. Subsequently, 2 ml medium with or without
TGF-β1 was added to the upper and lower compartments of each well.
Cells were collected at different time points following co-culture
(3, 6 or 12 days), which was followed by lysis and total RNA
extraction as described below.
TGF-β1 ELISA
TGF-β1 was detected using a commercially available
ELISA kit (cat. no. MB100B, R&D Systems). Representative
samples were analyzed for the presence of active TGF-β1; however,
the concentration was below the detection limit of the assay.
Therefore, all samples were activated by acidification to separate
TGF-β1 from its binding proteins, allowing for measurement of total
TGF-β1. For activation of samples containing FBS, 2.5 M acetic
acid+10 M urea was used, and 1 M HCl was used for microdialysis
samples (as recommended by the supplier). Activated samples were
neutralized using 2.7 M NaOH + 1 M
4-(2-hydroxyethyl)-1-piperazinee-thanesulfonic acid (HEPES) for
samples with FBS and 1.2 M NaOH+0.5 M HEPES for microdialysis (as
recommended by the supplier). Samples were loaded onto ELISA plates
immediately following neutralization. All samples were measured in
duplicate.
RT-qPCR
RNA was isolated using TRIzol® reagent
(Takara Biotechnology Co., Ltd.). The RNA yield was determined by
measuring the absorbance at a wavelength of 260 nm. RNA was
subsequently reverse-transcribed to cDNA, and the cDNA was
amplified by qPCR using the SYBR®-Green reporter that
was included in the kit used for qPCR and the following
thermocycling conditions: Pre-denaturation for 2 min at 94°C;
followed by 45 cycles of 30 sec at 60°C and 60 sec at 55°C. The
specificity of the produced amplification product was confirmed by
examination of dissociation reaction plots. Each sample was tested
in triplicate, and samples obtained from 3 independent experiments
were used for analysis of relative gene expression, normalization
to GAPDH expression was performed using the ∆∆Cq method (27–29).
The following primers were designed by Takara Biotechnology Co.,
Ltd.: GAPDH, 5′-GACATCAAGAAGGTGGTGAAGC-3′ (forward) and
5′-TGTCATTGAGAGCAATGCCAGC-3′ (reverse); and tenascin-C,
5′-ACCATGCTGAGATAGATGTTCCAAA-3′ (forward) and
5′-CTTGACAGCAGAAACACCAATCC-3′ (reverse).
Western blot analysis
Cells were lysed using 200 µl
radioimmunoprecipitation assay lysis buffer (cat. no. R0278,
Sigma-Aldrich; Merck KGaA). Equal quantities of proteins (20 µg)
were loaded onto 10% Tris-Glycine gels following denaturation. The
proteins were subsequently transferred to polyvinylidene difluoride
membranes. Membranes were blocked with 5% milk in PBS containing
0.1% Tween-20 (PBST), followed by incubation with primary
antibodies against F-actin (1:1,000), and GAPDH (1:1,000) at 4°C
overnight. The membranes were washed with PBST and incubated for 1
h at room temperature with horseradish peroxidase-conjugated
secondary antibodies (1:2,000). Following washing, protein bands
were visualized using a Chemiluminescence Protein Detection kit
(Pierce; Thermo Fisher Scientific, Inc.). Each experiment was
repeated in triplicate. GAPDH served as the internal control.
Immunofluorescent staining
Cells (fibroblasts and BMSCs) were cultured on
coverslips in a 6-well plate. Following mechanical stress or
co-culture, cells were fixed with 3% paraformaldehyde at 4°C in PBS
containing 1 mM calcium chloride for 10 min and permeabilized by
the use of 0.2% Triton X-100 at room temperature for 5 min.
Subsequently, cells were incubated with blocking solution
(containing 5% goat serum, 0.3% Triton X-100, and 0.1% BSA in PBS)
at room temperature for 30 min. Following 3 rinses, slides were
incubated with Alexa Fluor 555 Phalloidin (1:20) for 1 h at 37°C.
Rinsed slides were incubated with anti-tenascin-C (1:500) primary
antibody at 4°C overnight. After washing and the addition of goat
anti-rabbit IgG-FITC (1:500) for 1 h at 37°C, nuclear staining was
performed with DAPI (1:20,000; Sigma-Aldrich; Merck KGaA) for 5
min. Slices were subsequently mounted and observed under a
fluorescent microscope.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical significance was analyzed using one-way analysis of
variance followed by Tukey's post hoc test, and was performed using
SPSS software version 11.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Isolation and characterization of
BMSCs
As described in our previous studies (21,30),
scattered small BMSCs colonies formed, fused and stacked following
several passages. Following 3–4 passages, cells exhibited more
homogeneous morphology. These homogeneous cells were collected for
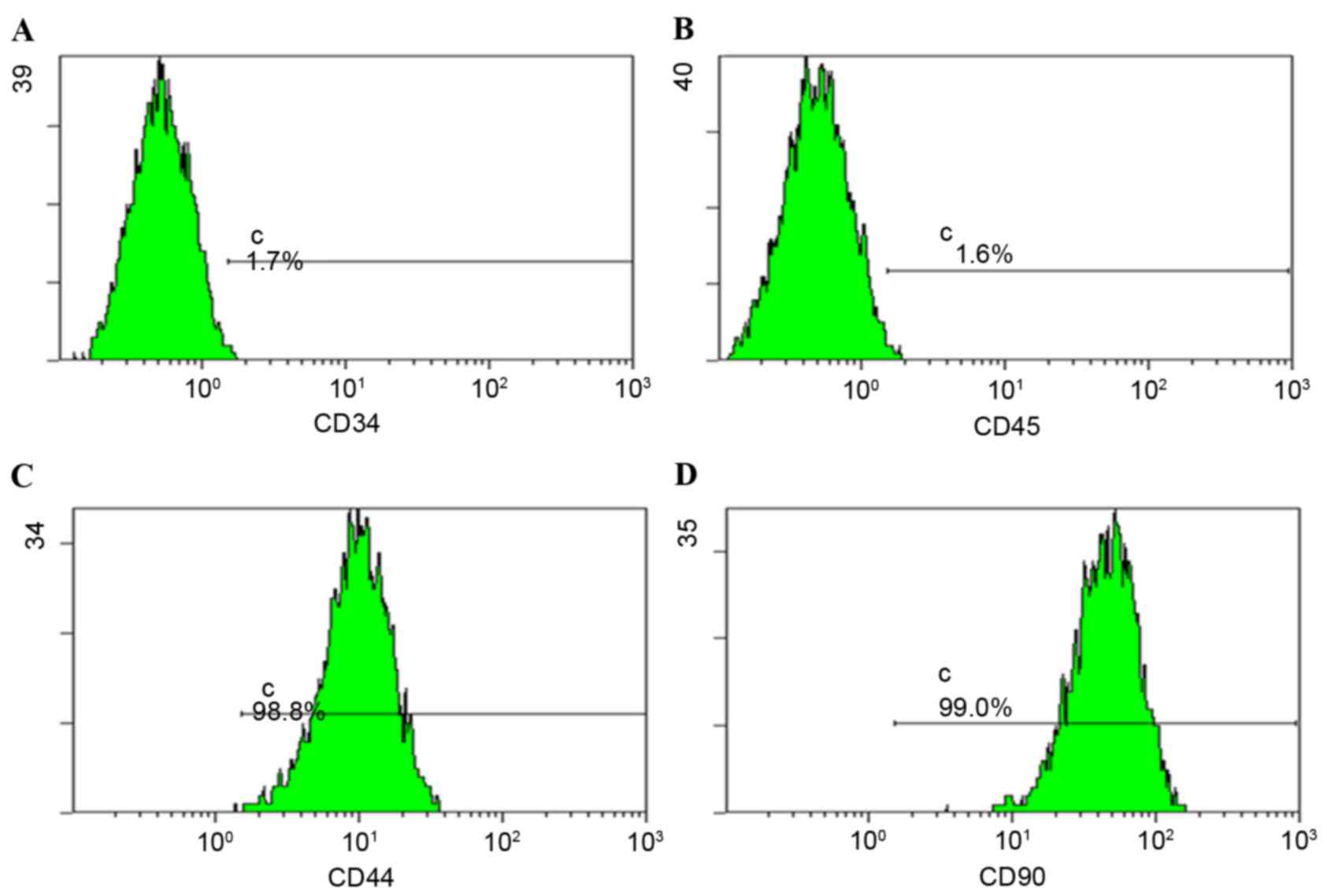
expression profile analysis by flow cytometry. Cells expressed CD44
and CD90 (Fig. 1) and may be
induced to differentiate into osteoblasts and adipogenic cells
(data not shown).
Verification of the rat fibroblast
stretch injury model
Fibroblasts were subjected to 10% load and 1 Hz
stretch optimized according to our previous studies (21,30)
and preliminary experiments. Compared with the control group, this
mechanical stretch elongated the cell body and increased shape
index values at different time points (data not shown). Under a
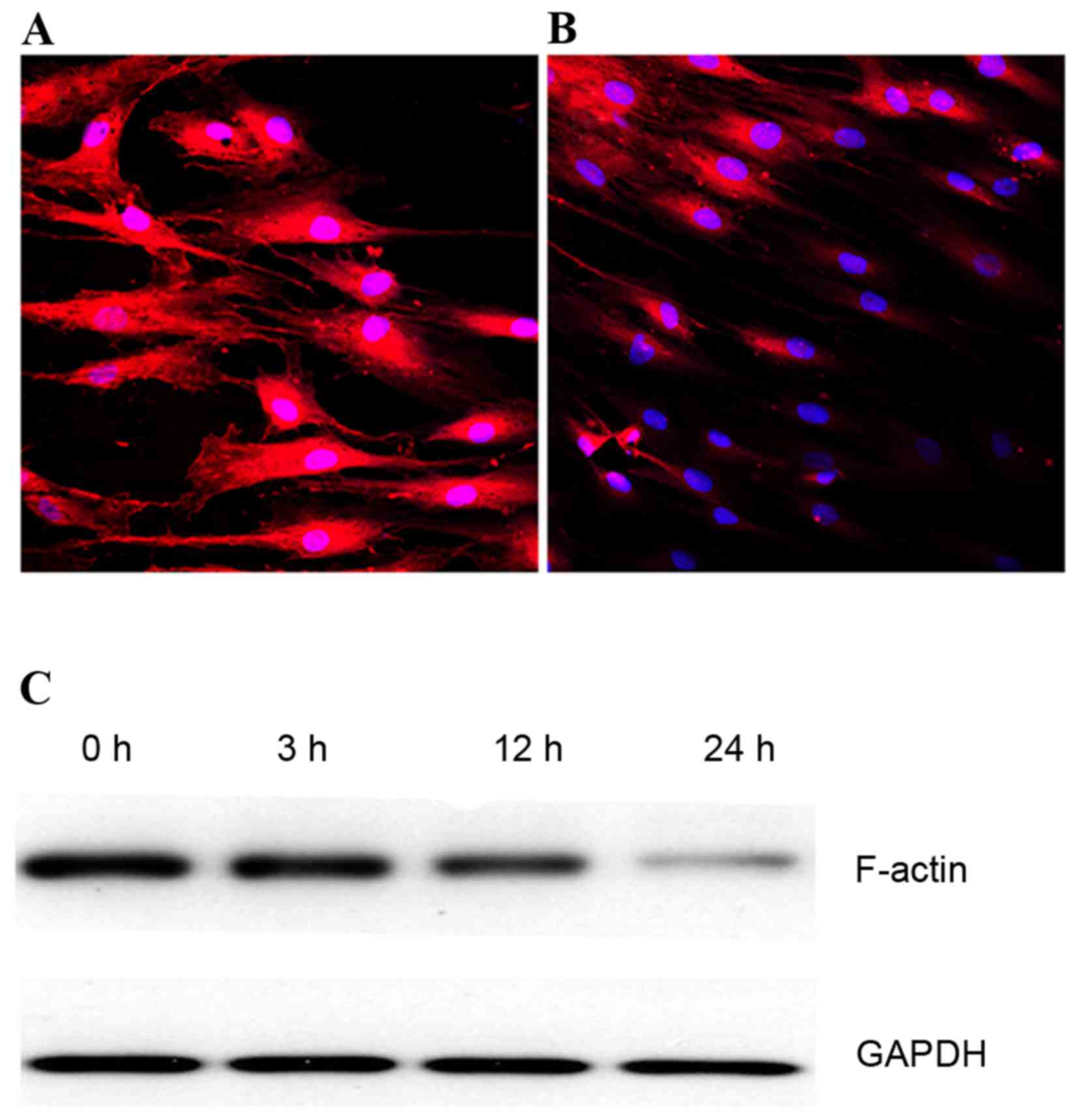
laser confocal scanning microscope, non-stretching rat ligament
fibroblasts exhibited the typical morphology of polygonal cells
with a thick F-actin fiber bundle (Fig. 2A). However, 24 h of mechanical
stress decreased the F-actin staining intensity (Fig. 2B); the effect on F-actin protein
expression levels was time-dependent, as demonstrated by western
blotting (Fig. 2C).
Time-dependent induction of tenascin-C
in BMSCs by mechanically stretched fibroblasts
Preliminary experiments (data not shown) revealed
that mechanical stress significantly and directly increased
tenascin-C expression in fibroblasts in a time-dependent manner.
However, whether indirect co-culture with mechanically stretched
ligament fibroblasts may regulate tenascin-C expression in BMSCs
remains controversial. Therefore, the present study used an
indirect co-culture system to investigate tenascin-C expression
levels and pathway in BMSCs cultured with stretched fibroblasts.
Following co-culture with stretched fibroblasts, tenascin-C mRNA
expression levels in BMSCs increased ~1.5-fold (P<0.05) after 3
days, ~2.4-fold (P<0.05) after 6 days and 4- to 5-fold
(P<0.05) after 12 days, compared with the control group
(Fig. 3A). The data indicated that
co-culture with mechanically stretched fibroblasts increased the
tenascin-C mRNA expression levels in BMSCs in a time-dependent
manner. In accordance with this, immunofluorescence staining
revealed that tenascin-C expression was increased in BMSCs
following co-culture with mechanically stimulated fibroblasts for
12 days, compared with control BMSCs (Fig. 3B).
Synergistic effects of TGF-β and
indirect co-culture with mechanically stretched fibroblasts on
tenascin-C expression levels
It was subsequently investigated whether TGF-β
treatment and co-culture with strained fibroblasts had synergistic
effects. TGF-β is a paracrine growth factor mediating the cellular
response to mechanical strain. This factor induces tenascin-C mRNA
expression in fibroblasts (31,32).
Therefore, it was hypothesized that mechanical stretching of
fibroblasts may indirectly promote tenascin-C expression in BMSCs
via the release of TGF-β1. To investigate this possibility, BMSCs
were treated with exogenous TGF-β1 to determine whether this
synergistically increased the effects of mechanical stretch on
tenascin-C mRNA expression levels. With or without addition of
TGF-β1, BMSCs were cultured with mechanically stretched fibroblasts
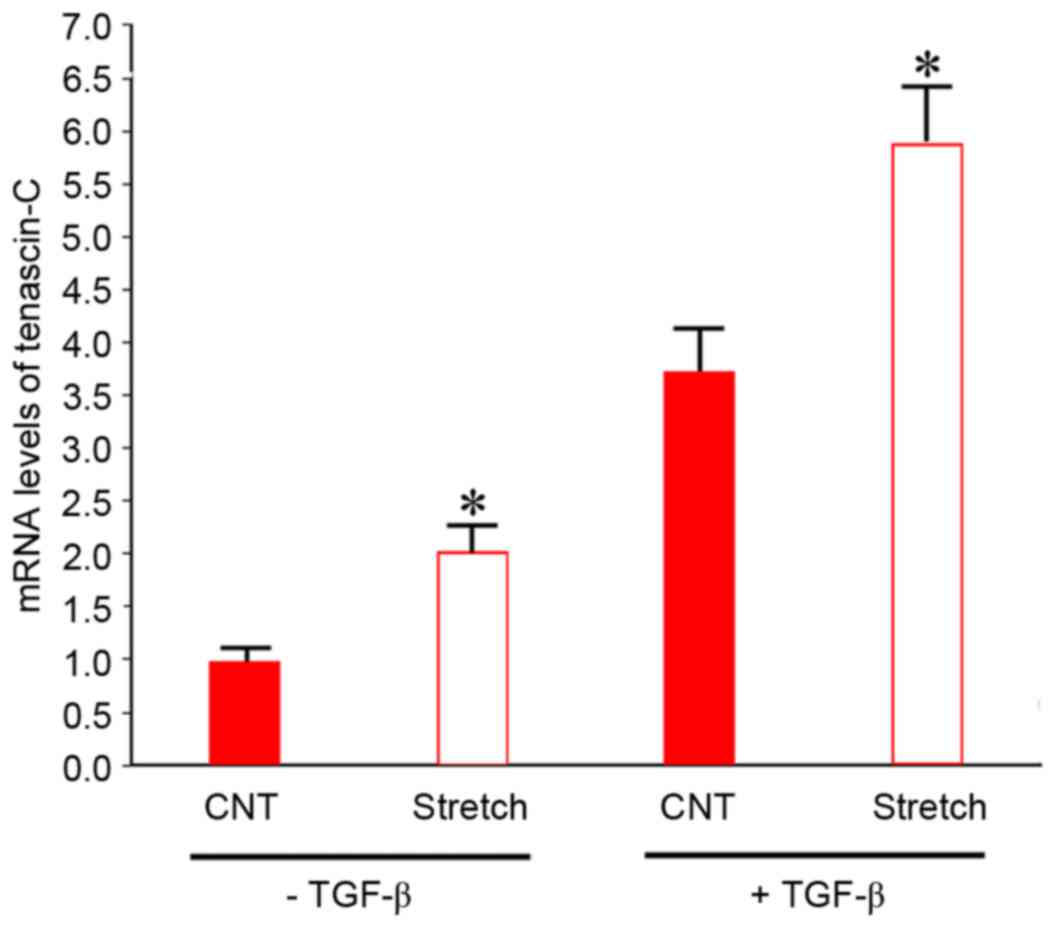
for 6 days. As presented in Fig.
4, in the absence of TGF-β1, mRNA expression levels of
tenascin-C in BMSCs co-cultured with mechanically stimulated
fibroblasts were 2-fold greater (P<0.05) compared with control
BMSCs co-cultured with fibroblasts without mechanical stimulation.
The addition of 5 ng/ml TGF-β1 significantly increased tenascin-C
mRNA expression levels ~3.8-fold (P<0.05) compared with BMSCs
cultured in the absence of TGF-β1. Tenascin-C mRNA expression
levels increased further following co-culture with mechanically
stretched fibroblasts. These data suggested that the effects of
TGF-β1 and indirect co-culture with strained fibroblasts on
tenascin-C expression in BMSCs were synergistic.
Induction of tenascin-C mRNA
expression via TGF-β1 and the MAPK signaling pathway
TGF-β1 has been reported to be a potent growth
factor with the capacity to induce tenascin-C expression in
fibroblasts (33). If indirect
co-culture with the stretched fibroblasts induces tenascin-C mRNA
expression level in BMSCs via the paracrine release of TGF-β1, the
effects of mechanical stimulation and TGF-β1 treatment may be
mediated via an intracellular signaling pathway. To test this
hypothesis, the effects of specific inhibitors on the increase of
tenascin-C mRNA expression levels by TGF-β1 and co-culture with
mechanically stretched fibroblasts were investigated. Treatment
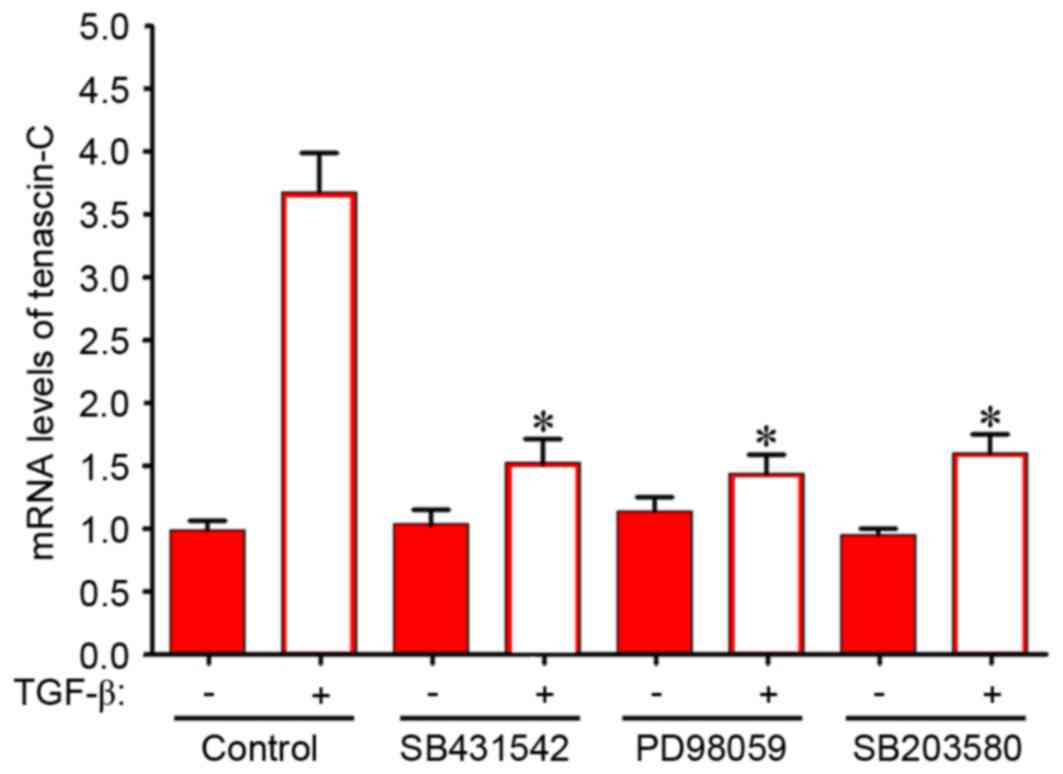
with 5 ng/ml TGF-β1 for 6 days led to a 2.4-fold increase in
tenascin-C mRNA expression levels in BMSCs (Fig. 5). SB 431542 is a specific inhibitor
of activin receptor-like kinase, the TGF-β receptor type I. As
presented in Fig. 5, 10 µM SB
431542 inhibited the TGF-β1-dependent increase of tenascin-C mRNA
expression levels by ~59%. A specific inhibitor of MEK-1, 10 µM PD
98059 significantly decreased tenascin-C mRNA expression levels in
BMSCs by ~61%. A similar inhibition was observed following
treatment with 3 µM SB 203580, a p38 MAPK inhibitor. These data
indicate that activation of the MEK/p38 MAPK signaling pathway may
contribute to the TGF-β1-dependent increase in tenascin-C
expression.
Mechanically stretched fibroblasts
indirectly induce tenascin-C expression in BMSCs via the MEK/p38
MAPK signaling pathway
TGF-β1-dependent induction of tenascin-C expression
may require activation of MEK/p38 MAPK in BMSCs. In addition,
mechanical stretching significantly induced TGF-β1 expression in
stretched fibroblasts (Fig. 6A)
and BMSCs co-cultured with stretched fibroblasts (Fig. 6B). Therefore, the involvement of
the MEK/MAPK signaling pathway was investigated in the induction of
tenascin-C expression in BMSCs by co-culture with mechanically
stretched fibroblasts. As presented in Fig. 6C, the increase in tenascin-C mRNA
expression levels induced by indirect co-culture with mechanically
stretched fibroblasts was significantly inhibited by neutralizing
anti-TGF-β1 antibodies and SB 431542. Similarly, PD 98059 and SB
203580 significantly attenuated the increase in tenascin-C
expression levels in BMSCs. These results suggested that in the
indirect co-culture system, the intracellular MEK/p38 MAPK
signaling pathway is responsible for the upregulation of tenascin-C
expression levels induced by mechanical stretch stimulation.
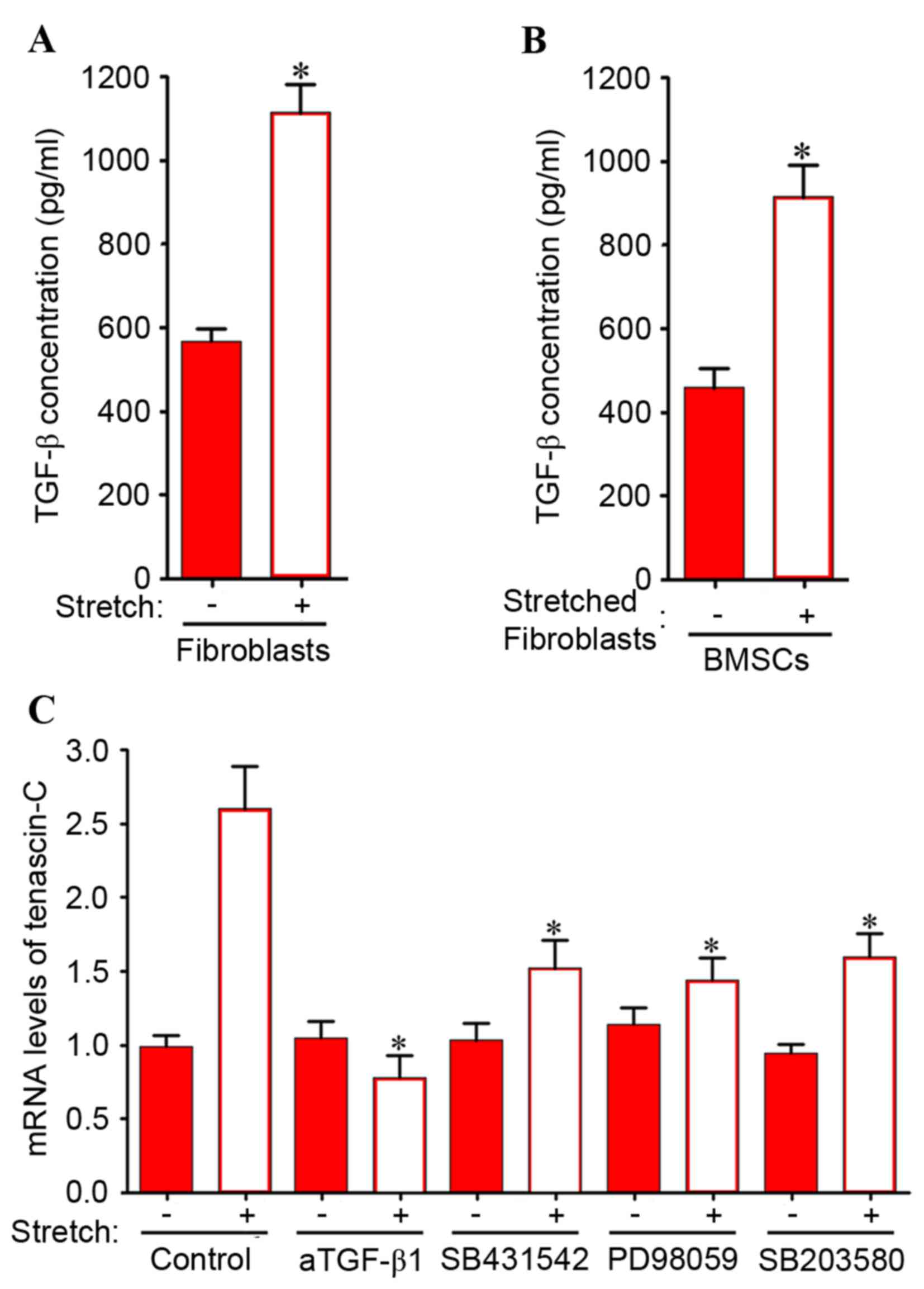 | Figure 6.(A) Fibroblasts only were subjected
to direct mechanical stretch with 10% load and 1 Hz. (B) BMSCs were
co-cultured with stretched ligament fibroblasts. An ELISA was used
to examine the TGF-β concentrations of stretched ligament
fibroblasts, and BMSCs co-cultured with stretched ligament
fibroblasts. Data are expressed as the mean ± standard deviation.
*P<0.05 vs. unstretched control fibroblasts or BMSCs cultured
with unstretched fibroblasts, respectively. (C) Increase in
tenascin-C mRNA expression levels in BMSCs co-cultured with
ligament fibroblasts was inhibited by neutralizing anti-TGF-β1
antibodies and inhibitors of TGF-β receptor type I, MEK-1 and p38
MAPK. BMSCs co-cultured with ligament fibroblasts were incubated in
medium containing DMSO (control), 10 µM SB 431542 (a TGF-β receptor
type I inhibitor), 10 µM PD 98059 (a MEK-1 inhibitor) or 3 µM SB
203580 (a p38 MAPK inhibitor). Fibroblasts were subjected to
mechanical stretching with 10% load and 1 Hz. Following 6 days of
co-culture, BMSCs were collected for reverse
transcription-quantitative polymerase chain reaction. Data are
expressed as the mean ± standard deviation (n=5). *P<0.05 vs.
stretched control in the absence of inhibitors. BMSCs, bone marrow
mesenchymal stem cells; DMSO, dimethyl sulfoxide; TGF, transforming
growth factor; MEK, mitogen-activated protein kinase kinase; MAPK,
mitogen-activated protein kinase. |
Discussion
POP is a distressing morbidity that affects the
quality of life of patients in developed and developing countries
(34). Pelvic organ support is
maintained by complex interactions between levator ani muscles and
connective tissues of the urethra, vaginal wall and rectum.
Abnormalities of normal levator ani function are a key feature of
POP (35). Stem cells have the
potential to develop into numerous different specialized cells in
the body. There is increasing evidence to suggest that BMSCs may be
used as a novel cell-based therapy to repair ligament, tendon and
cartilage damage (36).
The present study demonstrated that >95% of BMSCs
expressed CD90 and CD44, and these BMSCs may differentiate into
osteogenic and adipogenic cells. Therefore, the isolated primary
BMSCs in the present study possessed the characteristics of stem
cells. Our previous study revealed that indirect mechanical
stretching alters cell morphology and arrangement, and stimulates
the expression and secretion of ECM components including type I and
III collagen, elastin, lysyl oxidase and fibulin-5 in co-cultured
BMSCs (26). This indicated that
co-culture with mechanically stretched ligament fibroblasts
promotes BMSC differentiation into fibroblasts, consistent with a
separate report (9,37). Previous studies have reported that
tenascin-C expression is significantly increased in prolapsed
ligaments (34,38). As an ECM protein, tenascin-C is
transiently present in the ECM and is markedly upregulated in
tissue repair. Regulation of tenascin-C gene expression is complex
and may be involved in the process of BMSC differentiation.
However, the molecular mechanisms underlying the regulation of
tenascin-C expression in various conditions remain to be fully
elucidated. Therefore, the present study investigated the
mechanisms via which indirect mechanical stretching regulates
tenascin-C expression in BMSCs.
Mechanical stretching has been reported to
indirectly induce expression of ECM components (37). A mechanical signal has been
demonstrated to trigger the release of growth factors in an auto-
or paracrine manner in numerous cell types (39). These factors modify the
transcription rate of specific downstream genes including
tenascin-C and other matrix ingredients. A previous study has
revealed that tenascin-C expression levels are affected by various
growth factors including TGF-β, platelet-derived growth factor and
certain cytokines (40). TGF-β may
stimulate the production of ECM proteins by fibroblasts. Although
mechanical stretching is an important regulator of tenascin-C
expression, it may act indirectly to induce tenascin-C expression
via auto- or paracrine release of growth factors. For example,
release of TGF-β was induced by cyclic stretching in cardiac
fibroblasts, and was required to promote pro-collagen α1 (41). Therefore, TGF-β may indirectly
mediate the response to mechanical stimulation.
The results of the present study indicated that
mechanical stretching indirectly increased tenascin-C mRNA
expression levels in BMSCs, potentially via TGF-β, the production
of which was induced by stretching. The increase in tenascin-C
expression levels induced by mechanical stress was synergistic with
that induced by TGF-β treatment. Investigation of gene induction by
mechanical stretching has primarily been performed using serum free
medium (26). The present study
revealed that mechanical stretching-induced tenascin-C mRNA
expression may be further increased by TGF-β treatment. TGF-β
signaling is primarily mediated by the mothers against
decapentaplegic (SMAD)-dependent pathway. However, TGF-β has been
reported to additionally activate the MAPK signaling pathway, which
is SMAD-independent, and has been demonstrated to induce the
expression of ECM components with the involvement of the TGF-β/MAPK
pathway (42). Thus, TGF-β1 and
mechanical stretching-induced TGF-β1 may be inhibited by SB 431542
for the direct and indirect induction of tenascin-C expression. In
addition, specific inhibitors of MEK and MAPK significantly
inhibited the increase in tenascin-C mRNA expression levels induced
by TGF-β1 and indirect mechanical stretching. These results
suggested that regulation of tenascin-C expression levels in BMSCs
co-cultured with mechanically stretched pelvic ligament fibroblasts
is mediated via TGF-β1 and MEK/MAPK pathways.
The present study demonstrated that indirect
co-culture with mechanically stretched fibroblasts increased
tenascin-C expression levels in BMSCs. Consistent with this, it has
been reported that following co-culture with ligament fibroblasts
for 5 days, BMSCs may differentiate into fibroblasts (37). Conversely, a previous study has
demonstrated that indirect co-culture has no effect on the
differentiation of BMSCs (43).
The results of the present study suggested that indirect co-culture
is important for the differentiation of BMSCs as mechanically
stretched fibroblasts may produce soluble factors that activate and
regulate intracellular signaling pathways in the indirect
co-cultured BMSCs. The present study suggested that BMSCs may be
used as a potential novel cell-based therapy for the treatment of
POP and other diseases. Future studies aim to investigate the
detailed underlying mechanisms of intracellular pathways in the
differentiation of BMSCs co-cultured with injured ligament
fibroblasts.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81300469) and the
Key Technology Research and Development Program Foundation of Henan
Provincial Health Bureau (grant no. 201303093).
References
|
1
|
Choi KH and Hong JY: Management of pelvic
organ prolapse. Korean J Urol. 55:693–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giarenis I and Robinson D: Prevention and
management of pelvic organ prolapse. F1000Prime Rep. 6:772014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiegersma M, Panman CM, Kollen BJ, Berger
MY, Lisman-Van Leeuwen Y and Dekker JH: Effect of pelvic floor
muscle training compared with watchful waiting in older women with
symptomatic mild pelvic organ prolapse: Randomised controlled trial
in primary care. BMJ. 349:g73782014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Y, Ling O and Bo L: Expression and
significance of lysyl oxidase-like 1 and fibulin-5 in the cardinal
ligament tissue of patients with pelvic floor dysfunction. J Biomed
Res. 27:23–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aznal SS, Meng FG, Nalliah S, Tay A,
Chinniah K and Jamli MF: Biochemical evaluation of the supporting
structure of pelvic organs in selected numbers of premenopausal and
postmenopausal Malaysian women. Indian J Pathol Microbiol.
55:450–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wynn RF, Hart CA, Corradi-Perini C,
O'Neill L, Evans CA, Wraith JE, Fairbairn LJ and Bellantuono I: A
small proportion of mesenchymal stem cells strongly expresses
functionally active CXCR4 receptor capable of promoting migration
to bone marrow. Blood. 104:2643–2645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu YX, Chen L, Hou WK, Lin P, Sun L, Sun
Y, Dong QY, Liu JB and Fu YL: Mesenchymal stem cells treated with
rat pancreatic extract secrete cytokines that improve the
glycometabolism of diabetic rats. Transplant Proc. 41:1878–1884.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller MD, Nichols T and Butler CA:
Patella fracture and proximal patellar tendon rupture following
arthroscopic anterior cruciate ligament reconstruction.
Arthroscopy. 15:640–643. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Omoto M, Miyashita H, Shimmura S, Higa K,
Kawakita T, Yoshida S, McGrogan M, Shimazaki J and Tsubota K: The
use of human mesenchymal stem cell-derived feeder cells for the
cultivation of transplantable epithelial sheets. Invest Ophthalmol
Vis Sci. 50:2109–2115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Young RG, Butler DL, Weber W, Caplan AI,
Gordon SL and Fink DJ: Use of mesenchymal stem cells in a collagen
matrix for Achilles tendon repair. J Orthop Res. 16:406–413. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hankemeier S, Hurschler C, Zeichen J, van
Griensven M, Miller B, Meller R, Ezechieli M, Krettek C and
Jagodzinski M: Bone marrow stromal cells in a liquid fibrin matrix
improve the healing process of patellar tendon window defects.
Tissue Eng Part A. 15:1019–1030. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kushida T and Iida H: Bone marrow cell
transplantation efficiently repairs tendon and ligament injuries.
Front Cell Dev Biol. 2:272014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Humphrey JD, Dufresne ER and Schwartz MA:
Mechanotransduction and extracellular matrix homeostasis. Nat Rev
Mol Cell Biol. 15:802–812. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Provenzano PP and Keely PJ: Mechanical
signaling through the cytoskeleton regulates cell proliferation by
coordinated focal adhesion and Rho GTPase signaling. J Cell Sci.
124:1195–1205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang Y and Barakat AI: Dynamics of
mechanical signal transmission through prestressed stress fibers.
PLoS One. 7:e353432012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horiguchi M, Ota M and Rifkin DB: Matrix
control of transforming growth factor-β function. J Biochem.
152:321–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Midwood KS and Orend G: The role of
tenascin-C in tissue injury and tumorigenesis. J Cell Commun
Signal. 3:287–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mackie EJ, Halfter W and Liverani D:
Induction of tenascin in healing wounds. J Cell Biol.
107:2757–2767. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Imanaka-Yoshida K and Aoki H: Tenascin-C
and mechanotransduction in the development and diseases of
cardiovascular system. Front Physiol. 5:2832014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang SJ, Zhu B, Ren XX, Ben H, Li YQ and
Li YH: Effect of acupuncture of different acupoints on electrical
activities of hypothalamic sexual arousal stimulation-related
neurons at different stages of oestrous cycle in rats. Zhen Ci Yan
Jiu. 32:313–318. 2007.(In Chinese). PubMed/NCBI
|
|
21
|
Bing Z, Linlin L, Jianguo Y, Shenshen R,
Ruifang R and Xi Z: Effect of mechanical stretch on the expressions
of elastin, LOX and Fibulin-5 in rat BMSCs with ligament
fibroblasts co-culture. Mol Biol Rep. 39:6077–6085. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaminy A, Kashani Ragerdi I, Barbarestani
M, Hedayatpour A, Mahmoudi R and Nejad Farzaneh A: Osteogenic
differentiation of rat mesenchymal stem cells from adipose tissue
in comparison with bone marrow mesenchymal stem cells: Melatonin as
a differentiation factor. Iran Biomed J. 12:133–141.
2008.PubMed/NCBI
|
|
23
|
Jeong JY, Suresh S, Park MN, Jang M, Park
S, Gobianand K, You S, Yeon SH and Lee HJ: Effects of capsaicin on
adipogenic differentiation in bovine bone marrow mesenchymal stem
cell. Asian-Australas J Anim Sci. 27:1783–1793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilson E, Mai Q, Sudhir K, Weiss RH and
Ives HE: Mechanical strain induces growth of vascular smooth muscle
cells via autocrine action of PDGF. J Cell Biol. 123:741–747. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Millward-Sadler SJ, Wright MO, Lee H,
Nishida K, Caldwell H, Nuki G and Salter DM: Integrin-regulated
secretion of interleukin 4: A novel pathway of mechanotransduction
in human articular chondrocytes. J Cell Biol. 145:183–189. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto K, Dang QN, Kennedy SP,
Osathanondh R, Kelly RA and Lee RT: Induction of tenascin-C in
cardiac myocytes by mechanical deformation. Role of reactive oxygen
species. J Biol Chem. 274:21840–21846. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heid CA, Stevens J, Livak KJ and Williams
PM: Real time quantitative PCR. Genome Res. 6:986–994. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gibson UE, Heid CA and Williams PM: A
novel method for real time quantitative RT-PCR. Genome Res.
6:995–1001. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren CC, Ren RF, Zhao B, Zhang X and Jiang
YJ: Study on oriented differentiation of bone marrow mesenchymal
stem cells by fibroblast in rat uterine ligament with mechanical
stretch. Zhonghua Fu Chan Ke Za Zhi. 46:527–532. 2011.(In Chinese).
PubMed/NCBI
|
|
31
|
Wang JH, Thampatty BP, Lin JS and Im HJ:
Mechanoregulation of gene expression in fibroblasts. Gene.
391:1–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiquet M, Gelman L, Lutz R and Maier S:
From mechanotransduction to extracellular matrix gene expression in
fibroblasts. Biochim Biophys Acta. 1793:911–920. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jinnin M, Ihn H, Asano Y, Yamane K,
Trojanowska M and Tamaki K: Tenascin-C upregulation by transforming
growth factor-beta in human dermal fibroblasts involves Smad3, Sp1,
and Ets1. Oncogene. 23:1656–1667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ewies AA, Al-Azzawi F and Thompson J:
Changes in extracellular matrix proteins in the cardinal ligaments
of post-menopausal women with or without prolapse: A computerized
immunohistomorphometric analysis. Hum Reprod. 18:2189–2195. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boreham MK, Wai CY, Miller RT, Schaffer JI
and Word RA: Morphometric properties of the posterior vaginal wall
in women with pelvic organ prolapse. Am J Obstet Gynecol.
187:1501–1509. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kon E, Filardo G, Roffi A, Andriolo L and
Marcacci M: New trends for knee cartilage regeneration: From
cell-free scaffolds to mesenchymal stem cells. Curr Rev
Musculoskelet Med. 5:236–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee IC, Wang JH, Lee YT and Young TH: The
differentiation of mesenchymal stem cells by mechanical stress
or/and co-culture system. Biochem Biophys Res Commun. 352:147–152.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goepel C: Differential elastin and
tenascin immunolabeling in the uterosacral ligaments in
postmenopausal women with and without pelvic organ prolapse. Acta
Histochem. 110:204–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chiquet M, Sarasa-Renedo A and
Tunc-Civelek V: Induction of tenascin-C by cyclic tensile strain
versus growth factors: Distinct contributions by Rho/ROCK and MAPK
signaling pathways. Biochim Biophys Acta. 1693:193–204. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chiquet-Ehrismann R and Chiquet M:
Tenascins: Regulation and putative functions during pathological
stress. J Pathol. 200:488–499. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lindahl GE, Chambers RC, Papakrivopoulou
J, Dawson SJ, Jacobsen MC, Bishop JE and Laurent GJ: Activation of
fibroblast procollagen alpha 1(I) transcription by mechanical
strain is transforming growth factor-beta-dependent and involves
increased binding of CCAAT-binding factor (CBF/NF-Y) at the
proximal promoter. J Biol Chem. 277:6153–6161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang YE: Non-Smad pathways in TGF-beta
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li H, Yu B, Zhang Y, Pan Z, Xu W and Li H:
Jagged1 protein enhances the differentiation of mesenchymal stem
cells into cardiomyocytes. Biochem Biophys Res Commun. 341:320–325.
2006. View Article : Google Scholar : PubMed/NCBI
|