Introduction
In China, the yearly mortality rate for end-stage
liver disease is >300,000 patients (1). Of the >30 million patients with
chronic liver disease in China, ~80% are infected with the
hepatitis B virus (HBV) (2). The
most effective treatment for HBV-associated end-stage liver disease
is liver transplantation. However, without effective prophylaxis,
the risk of HBV re-infection following transplantation may reach
>80% (3,4). The current treatment protocol of
nucleos(t)ide analogues combined with hepatitis B immunoglobulin
(HBIG) following liver transplantation, greatly reduces the
hepatitis B recurrence rate (2,5,6).
However, the high cost remains a heavy burden for patients
(7,8), and the long-term use of nucleos(t)ide
analogues may lead to HBV resistance (9,10).
Application of the HBV vaccine following liver transplantation may
potentially lead to the withdrawal of nucleoside analogues and HBIG
therapy, however the vaccine is less effective due to the use of
immunosuppressants following transplantation (11,12).
Therefore, it is important to identify novel methods to prevent
hepatitis B recurrence following liver transplantation.
Bone marrow-derived mesenchymal stem cells (BM-MSCs)
have demonstrated anti-inflammatory (13,14)
and angiogenesis-enhancing effects (15,16)
with low immunogenicity (17,18).
In addition, BM-MSCs exhibit immunomodulatory capabilities in
animal models of rejection following transplantation (19–21),
which may represent a promising method for inducing immune
tolerance. Transfusions of umbilical cord-derived MSCs for patients
with HBV-associated acute-on-chronic liver failure resulted in
improved liver function and alleviated liver damage (22). However, the biological effects of
BM-MSCs on HBV have not yet been reported. In the present study,
the effect of BM-MSCs on HBV replication and genome mutation in
vitro was investigated, as well as its associated mechanisms.
The results of the current study may provide innovative strategies
for the prevention of hepatitis B recurrence following liver
transplantation.
Materials and methods
Animals and cell lines
A total of 12 specific pathogen-free Brown Norway
(BN) male rats (age, 4–5 weeks; body weight, 200–220 g) were used
for the isolation and identification of BM-MSCs. Inbred male BN
rats were kept 2 rats per cage at 24°C, with 50% humidity and a 12
h light and dark cycle, with free access to water and food. An
additional 6 specific pathogen-free BN male rats (age, 4–5 weeks;
body weight, 200–220 g) were used for the extraction of splenic
lymphocytes (SLCs), and were kept under the same conditions as
described above. All animals were purchased from the Chinese
Academy of Military Medical Sciences (Beijing, China). The use of
animals and the animal experimental procedures employed for the
purposes of this study were approved by the Ethics Committee of
Tianjin First Central Hospital (Tianjin, China). The human
hepatocellular carcinoma cell line HepG2.2.15 was donated by
Professor Wei Lai (Hepatology Institute of Peking University
Affiliated Hospital, Beijing, China), and contained the complete
HBV genome, as well as expressed HBV-associated antigens and
secreted whole Dane particles (23,24).
Instruments and reagents
The following instruments and reagents were used:
Dulbecco's modified Eagle's medium (DMEM) and DMEM/F12 media (1:1;
Hyclone, Logan, UT, USA), G418 (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), fetal bovine serum (FBS; Biowest, Nuaille,
France), transwell plates (Corning, Inc., Corning, NY, USA), MTT
reagent (Beijing Dingguo Changsheng Biotechnology, Co., Ltd.,
Beijing, China), dimethyl sulfoxide (DMSO; Amresco, Solon, OH,
USA), lymphocyte separation medium (Beijing Dingguo Changsheng
Biotechnology, Co., Ltd.), TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.), antibodies directed against CD29 (cat. no.
102207), CD90 (cat. no. 202503), RT1A (cat. no. 205208), CD45 (cat.
no. 202207) and RT1B (cat. no. 205305) for the identification of
BM-MSCs (Biolegend, Inc., San Diego, CA, USA), CD34 (cat. no.
sc-7324; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), CD3-APC
mAb (cat. no. 11-0040-82), CD8a-PE-Cy7 (cat. no. 12-0084-82), and
CD4-FITC mAb (cat. no. 11-0040-82; eBiosciences, Inc., San Diego,
CA, USA), a cell genomic DNA extraction kit (Beijing Kangwei
Century Biotech Co. Ltd., Beijing, China) and enzyme-linked
immunosorbent assay (ELISA) kits for measuring IL-10 (cat. no.
R1000), IL-22 (cat. no. M2200), and IFN-γ (cat. no. RIF00; R&D
Systems, Inc., Minneapolis, MN, USA). Primer sequences used for
quantitative polymerase chain reaction (PCR) assay analysis for the
detection of HBV covalently closed circular DNA (cccDNA) were as
follows: cccDNA, forward, 5′-GTGTGCACTTCGCTTCAC-3′, and reverse,
5′-GGGTCAATGTCCATGCC-3′ (designed by Shanghai Jikang Biotechnology
Company, Co., Ltd., Shanghai, China). The TaqMan probe (5′-FAM-ATG
TCC TAC TGT TCA AGC CTC CAA-BHQ-3′) was designed by Takara Bio,
Inc. (Otsu, Japan). Instruments included the CO2
incubator (Sheldon Manufacturing, Inc., Cornelius, OR, USA), an
inverted fluorescence microscope (Olympus Corporation, Tokyo,
Japan), the FACSCalibur flow cytometer (BD Biosciences), the ABI
PRISM® 3700 DNA Analyzer and the fluorescence-based 7500
Fast Real-Time PCR system (Applied Biosystems™; Thermo Fisher
Scientific, Inc.), the automatic fluorescence quantitative flow
cytometer (PerkinElmer, Inc., Waltham, MA, USA), and the RT-6000
automatic microplate reader (Omega Bio-Tek, Inc., Norcross, GA,
USA). Serum levels of alanine transaminase (ALT) and aspartate
aminotransferase (AST) were determined using a 7180 clinical
chemistry analyzer (Hitachi High-Technologies Corporation, Tokyo,
Japan).
Isolation and identification of
BM-MSCs
BM-MSCs were aseptically isolated from the femur and
tibia of 12 male BN rats. Red blood cells were lysed using 0.1
mol/l NH4Cl, and the remaining cells were washed,
resuspended and cultured in DMEM/F12 (1:1) media containing 100
U/ml penicillin, 100 mg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.), and 15% FBS. BM-MSCs were cultured in an
incubator at 37°C and 5% CO2 with saturating humidity.
The medium was refreshed every 48 h. When cells at passage 3 had
reached 80% confluence, cells were trypsinized, washed, centrifuged
at 300 × g for 5 min at room temperature, and resuspended at
1×107 cells/ml in phosphate-buffered saline (PBS).
BM-MSCs (100 µl) were incubated with the following
fluorescence-labeled antibodies at 4°C for 30 min in the dark:
CD29-PE (1:80), CD34-FITC (1:20), CD45-PE (1:80), CD90-FITC
(1:200), RT1A-PE (1:80) and RT1B-FITC (1:200). Cells were then
washed with PBS and analyzed by flow cytometry (FACSCalibur; BD
Biosciences) to determine the phenotype and purity of BM-MSCs.
Harvesting of rat SLCs
Spleens of 6 rats were extracted following sacrifice
by cervical dislocation under aseptic conditions, disassociated by
grinding, and then filtered through a 200-µm nylon mesh. Cell
suspensions were transferred to a centrifuge tube containing
Percoll lymphocyte separation medium (1.083 g/ml; Beijing Dingguo
Changsheng Biotechnology, Co., Ltd., Beijing, China). Following
centrifugation at 670 × g for 20 min at room temperature,
the white middle layer was extracted and centrifuged at 330 ×
g for 8 min at room temperature, before the supernatant was
discarded. After washing with PBS, the lymphocytes were counted and
cultured in RPIM 1640 media (Gibco; Thermo Fisher Scientific, Inc.)
containing 100 U/ml penicillin, 100 mg/ml streptomycin, 1 mmol/l
glutamine, and 10% FBS (5×105 cells/ml).
HepG2.2.15 cell culture
HepG2.2.15 cells were cultured in high glucose-DMEM
(Hyclone; GE Healthcare Life Sciences), which contained 10%
heat-inactivated FBS, 200 mg/l G418, 6 mmol/l glutamine, 100 U/ml
penicillin and 100 mg/l streptomycin, in an incubator at 37°C and
5% CO2 with saturating humidity. The medium was
refreshed every 48 h, and healthy cells were selected for
downstream experiments.
Co-culture of different cell
types
The following experimental groups were studied:
Group 1, SLCs; group 2, HepG2.2.15 cells; group 3, BM-MSCs +
HepG2.2.15 cells; group 4, SLCs + HepG2.2.15 cells; and group 5,
SLCs + BM-MSCs + HepG2.2.15 cells. HepG2.2.15 cells were plated in
the lower chamber of a 6-well transwell dish (pore size, 0.4 µm;
Corning, Incorporated) at 1×105 cells/well, and SLCs and
BM-MSCs were inoculated in the upper chamber of the transwell plate
at 5×105 cells/well. Plates were cultured at 37°C and 5%
CO2 with saturating humidity in an incubator for 24, 48
or 72 h. Each group was plated in triplicate wells for each time
point. At each time point, supernatants and cells were collected
for further analysis.
MTT cell viability assay
Cell suspensions (200 µl) from each experimental
group were added to each well of a 96-well plate (SLCs,
2×104 cells/well; BM-MSCs, 2×104 cells/well;
HepG2.2.15 cells, 4×103 cells/well), which was incubated
at 37°C with 5% CO2. Cells were cultured for 24, 48 or
72 h. MTT solution (15 µl at 5 g/l) was added to each well and
incubated for 3 h. The medium was subsequently aspirated and DMSO
(100 µl) was added to each well before the plates were placed on a
shaker for 10 min to fully dissolve the formazan crystals. The
absorbance (A) at 490 nm was measured using an automated microplate
reader, and the cell survival rate was calculated using the
following formula: Survival rate = (Atest
well-Ablank well) / (Acontrol
well-Ablank well) ×100%.
Detection of supernatant HBV DNA and
intracellular cccDNA of HepG2.2.15 cells and BM-MSCs
The supernatant HBV DNA levels were measured using a
real-time PCR kit according to the manufacturer's instructions
(Shanghai Kehua Bioengineering Co., Ltd.), using an ABI 7500
Real-Time PCR system. Genomic DNA was extracted from HepG2.2.15
cells (2×106 cells) or BM-MSCs (5×106 cells)
using a UniversalGen DNA kit (CWBio, Co., Ltd., Beijing, China),
and 2 µg HBV DNA or cccDNA was subjected to quantitative PCR
analysis using an optimized quantitative PCR method described
previously (25).
HBV genomic DNA extraction and
sequencing analysis
HBV genomic DNA was extracted from the supernatants
of co-cultured HepG2.2.15 cells using a Viral DNA Isolation kit
(DAAN Gene, Co., Ltd., of Sun Yat-sen University, Guangzhou, China)
according to the manufacturer's instructions. Briefly, cell
supernatants were added to virus lysis buffer, and lysates were
loaded onto a spin column. After viral DNA was bound to the
membrane, each column was washed and the viral DNA was eluted.
PCR was performed using HBV genomic DNA as a
template to amplify the P, S, X and C regions using the primer
sequences listed in Table I. The
PCR conditions were as follows: Initial denaturation at 94°C for 2
min, followed by 35 cycles of 94°C for 30 sec, 55°C for 30 sec,
72°C for 1 min and a final extension at 72°C for 10 min. PCR
products were resolved by 2% agarose gel electrophoresis, and the
bands were visualized under ultraviolet light following ethidium
bromide staining. The DNA was recovered from the agarose gel using
a MiniBEST Agarose Gel DNA Extraction kit (Takara Bio, Inc.)
according to the manufacturer's protocol, and the amplified DNA was
subjected to sequencing analysis by Sangon Biotech (Shanghai,
China).
 | Table I.Sequences of the primers used for
polymerase chain reaction in the present study. |
Table I.
Sequences of the primers used for
polymerase chain reaction in the present study.
| Primer name | Sequence
(5′-3′) | Length (bp) |
|---|
| HBV-F1 |
GGGTCACCATATTCTTGGGAAC | 22 |
| HBV-R1 |
ATTGAGAGAAGTCCACCACGAGT | 23 |
| HBV-F2 |
TAGGACCCCTGCTCGTGTTACAG | 18 |
| HBV-R2 |
GAACCACTGAACAAATGGCACTAG | 24 |
| HBV-F3 |
GAACCTCTATGTTTCCCTCT | 20 |
| HBV-R3 |
TGCGTCAGCAAACACTT | 17 |
| HBV-F4 |
CCTATTGATTGGAAAGTATG | 20 |
| HBV-R4 |
ATGAGAAGGCACAGACG | 17 |
| HBV-F5 |
CCGATCCATACTGCGGAACTCC | 22 |
| HBV-R5 |
GCTTGGAGGCTTGAACAGTAGGACA | 25 |
| HBV-F6 |
TACTAGGAGGCTGTAGGCATAA | 22 |
| HBV-R6 |
GTGTTGATAAGATAGGGGCATTT | 23 |
| HBV-F7 |
GGTGTCTTTTGGAGTGTGGA | 20 |
| HBV-R7 |
TTGTTCCCAAGAATATGGTGA | 21 |
| HBV-F8 |
AGAACTCCCTCGCCTCG | 17 |
| HBV-R8 |
TTGAAGTCCCAATCTGGATT | 20 |
Detection of lymphocyte surface
markers CD4 and CD8 in the CD3+ cell by flow
cytometry
SLCs were harvested and centrifuged at 300 ×
g for 5 min at 4°C following culture for 24, 48 or 72 h.
Then SLCs (1×106 cells) were resuspended in 100 µl PBS
for detection, and the fluorescence-labeled antibodies anti-CD3-APC
(1:80), anti-CD4-FITC (1:200), and anti-CD8a-PE-Cy7 (1:160) were
added for incubation at 4°C for 30 min in the dark, to detect the
expression intensity of each cell surface marker by flow
cytometry.
Detection of supernatant
cytokines
Concentrations of IFN-γ, IL-10, and IL-22 in the
cell supernatants were determined using an ELISA kit (R&D
Systems, Inc.) according to the manufacturer's protocol. The
absorbance at 450 nm was measured using an automated microplate
reader.
Statistical analysis. SPSS 17.0 (SPSS,
Inc., Chicago, IL, USA) was used for statistical analysis
Normally distributed data were presented as the mean
± standard deviation. Additional data sets were compared by
analysis of variance, and Dunnett's method was used when the
variance was not homogenous. Linear correlation analysis was used
to test the interdependence of the variables. P<0.05 was
considered to indicate a statistically significant difference.
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA) was used to plot data for presentation.
Results
Morphology and phenotypic analysis of
HepG2.2.15 cells and BM-MSCs
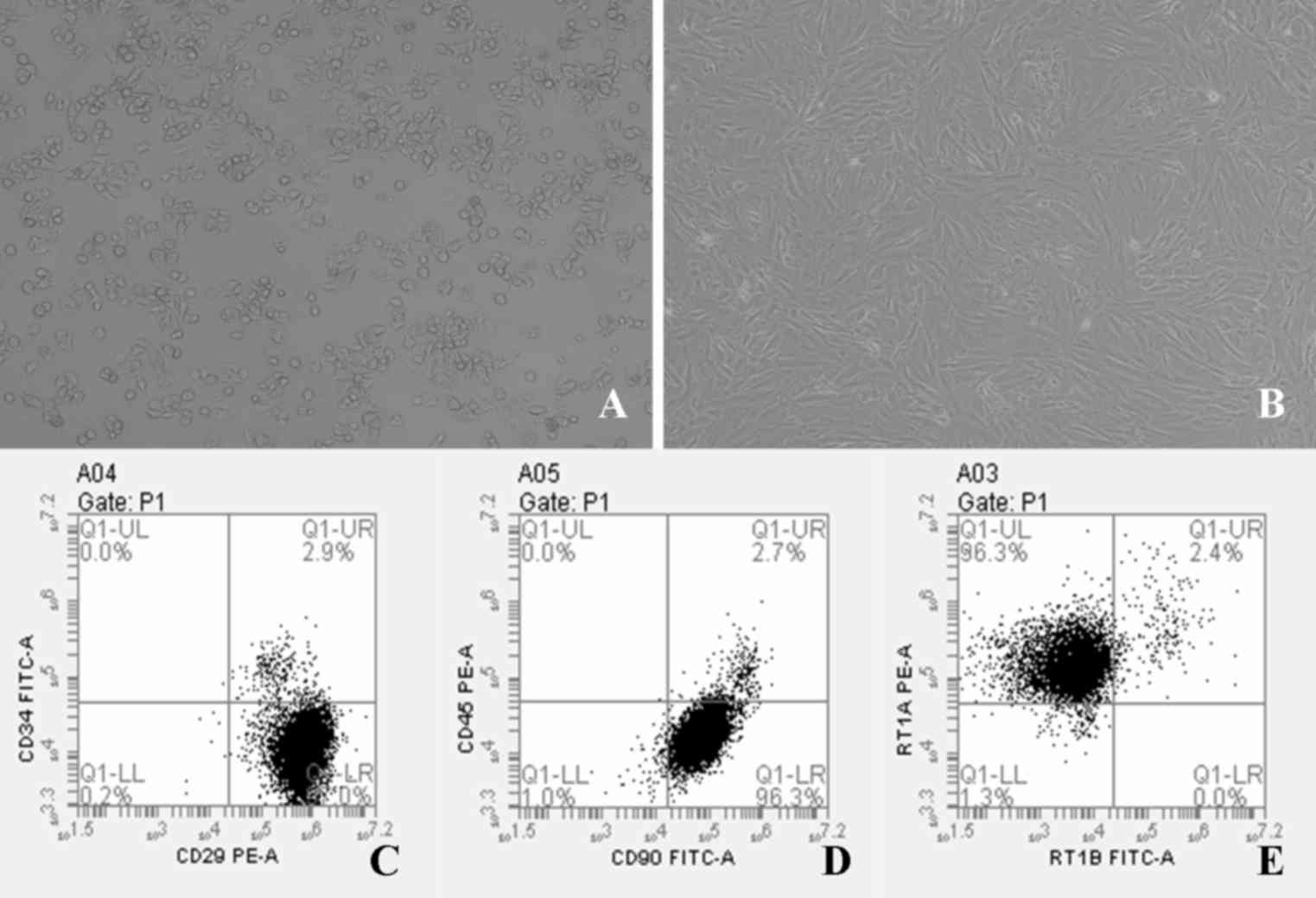
HepG2.2.15 cells were confirmed to be
plastic-adherent cells with a spindle-shaped morphology (Fig. 1A). Rat BM-MSCs were successfully
established in culture and proliferated in vitro.
Morphological and phenotypic examination revealed that BM-MSCs were
confirmed to be plastic-adherent cells with a spindle-shaped
morphology under standard culture conditions, as determined by
microscopy, and some of the cells exhibited a whirlpool or
chrysanthemum pattern (Fig. 1B).
BM-MSCs were incubated with antibodies against CD29, CD90, RT1A,
CD34, RT1B and CD45, and were analyzed by flow cytometry.
Phenotypic examination of BM-MSCs at passage 3 demonstrated that
97.0% of cells expressed CD29, 96.3% of cells expressed CD90, and
96.3% of cells expressed RT1A (Fig.
1C-E). By contrast, >95% of BM-MSCs were negative for CD34,
CD45 and RT1B (Fig. 1C-E), which
was in accordance with the results of a previous study (26).
Detection of liver enzymes in
supernatants
When co-cultured with xenogeneic SLCs or BM-MSCs, no
significant difference in liver enzyme levels in HepG2.2.15 cell
supernatants was observed (Table
II). This suggested that neither BM-MSCs nor SLCs induced
rejection of the human hepatocellular carcinoma cell line,
HepG2.2.15.
 | Table II.Supernatant ALT and AST levels in
different groups at different time points. |
Table II.
Supernatant ALT and AST levels in
different groups at different time points.
|
| 24 h | 48 h | 72 h |
|---|
|
|
|
|
|
|---|
| Group | ALT (IU/l) | AST (IU/l) | ALT (IU/l) | AST (IU/l) | ALT (IU/l) | AST (IU/l) |
|---|
| HepG2.2.15 | 1.17±0.41 | 9.53±1.63 | 1.50±0.38 | 13.25±2.65 | 1.47±0.27 | 19.82±1.64 |
|
BM-MSCs+HepG2.2.15 | 1.20±0.36 | 11.30±0.40 | 1.40±0.33 | 15.65±1.02 | 1.77±0.59 | 23.12±2.22 |
|
SLCs+HepG2.2.15 | 1.45±0.37 | 11.02±2.95 | 1.72±0.20 | 17.62±3.26 | 1.83±0.43 | 23.42±3.49 |
|
SLCs+BM-MSCs+HepG2.2.15 | 1.17±0.40 | 11.70±3.37 | 1.43±0.14 | 15.93±0.68 | 1.73±0.19 | 21.27±0.74 |
| P-value | 0.442 | 0.099 | 0.862 | 0.447 | 0.056 | 0.145 |
Effects of BM-MSCs on the activity of
SLCs and HepG2.2.15 cells
The viability of SLCs in group 5 was significantly
lower when compared with that of group 4 at each time point (24 h,
P<0.05; 48 h, P<0.01; 72 h, P<0.01; Fig. 2A), which suggested that BM-MSCs may
reduce the viability of SLCs.
 | Figure 2.Viability of SLCs and adherent cells
as determined using the MTT assay. The viability of (A) SLCs in
groups 1, 4 and 5, and (B) adherent cells in groups 2, 3, 4 and 5.
*P<0.05 and **P<0.01 as indicated. SLCs, splenic lymphocytes;
1, SLCs alone; 2, HepG2.2.15 cells alone; 3, bone marrow-derived
mesenchymal stem cells (BM-MSCs) + HepG2.2.15 cells; 4, SLCs +
HepG2.2.15 cells; 5, SLCs + BM-MSCs + HepG2.2.15 cells. |
The viability of adherent cells in group 3 was
significantly lower when compared to that of groups 2 at 48 and 72
h, respectively (P<0.01 at 48 and 72 h; Fig. 2B). These results suggested that
BM-MSCs may inhibit the viability of HepG2.2.15 cells. In contrast,
the viability of adherent cells in group 5 was significantly higher
when compared to that of groups 3 at 24, 48 and 72 h, respectively
(P<0.01 at 24, 48 and 72 h; Fig.
2B). These results suggested that BM-MSCs exhibited stimulatory
effects on HepG2.2.15 cell viability when co-cultured with
SLCs.
Effects of BM-MSCs on the supernatant
levels of HBV DNA in HepG2.2.15 cells
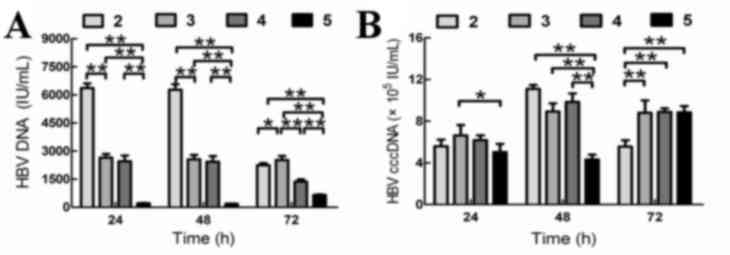
The quantity of supernatant HBV DNA in group 5 was
significantly lower when compared to that of groups 2, 3 and 4 at
24, 48 and 72 h, respectively (Fig.
3A).
When co-cultured with BM-MSCs and SLCs (group 5),
the intracellular quantity of HBV cccDNA in HepG2.2.15 cells was
lower than that of groups 2 and 4 at 24 h, however this did not
reach statistical significance. The intracellular quantity of HBV
cccDNA in group 5 was statistically higher than that of group 2 at
72 h (P<0.01; Fig. 3B). The
intracellular quantity HBV cccDNA in group 5 was significantly
lower than that of groups 2, 3 and 4 at 48 h (Fig. 3B). These findings suggested that
BM-MSCs and SLCs may inhibit HBV replication in HepG2.2.15 cells,
and that the inhibitory effect was more significant when HepG2.2.15
cells were co-cultured with BM-MSCs and SLCs.
Detection of intracellular HBV cccDNA
in BM-MSCs
No intracellular HBV cccDNA was detected in the
BM-MSCs in any of the groups (data not shown), which suggested that
BM-MSCs co-cultured with HepG2.2.15 cells were not be infected by
HBV.
HBV gene sequencing
No mutations in the C or X regions of the HBV genome
were detected in HepG2.2.15 cells co-cultured with BM-MSCs, SLCs,
or both types of cells (Table
III). However, a T45 N mutation in the S region, and an rtR192S
mutation in the P region was identified in the supernatants of
BM-MSCs + HepG2.2.15 and SLCs + HepC2.2.15 groups, respectively
(Table III).
 | Table III.Effect of BM-MSCs and SLCs on the HBV
gene sequence. |
Table III.
Effect of BM-MSCs and SLCs on the HBV
gene sequence.
|
| HBV gene
sequence |
|---|
|
|
|
|---|
| Group | Mutation in C
region | Mutation in X
region | Mutation in S
region | Mutation in P
region |
|---|
| HepG2.2.15 | No mutation | No mutation | No mutation | No mutation |
|
BM-MSCs+HepG2.2.15 | No mutation | No mutation | T45N | No mutation |
|
SLCs+HepG2.2.15 | No mutation | No mutation | No mutation | rtR192S |
|
SLCs+BM-MSCs+HepG2.2.15 | No mutation | No mutation | No mutation | No mutation |
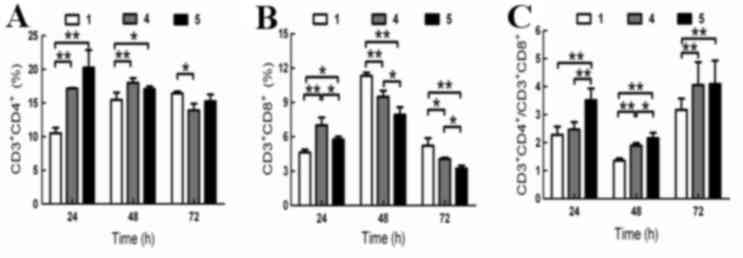
Effect of BM-MSCs on lymphocyte
subsets
Detection of lymphocyte surface markers by flow
cytometry revealed that the percentage of
CD3+CD4+ cells in group 5 was higher than
that of group 4 at 24 and 72 h, but was lower at 48 h. These
differences did not reach statistical significance (Fig. 4A).
The percentage of CD3+CD8+
cells in group 5 was significantly lower than that of group 4 at
all time points (24 h, P<0.01; 48 h, P<0.05; 72 h, P<0.05;
Fig. 4B). When compared with group
4, the
CD3+CD4+/CD3+CD8+ ratio
in group 5 significantly increased at 24 and 48 h (P<0.01 and
P<0.05, respectively; Fig. 4C),
but no significant difference was observed at 72 h. The percentage
of CD3+CD8+ cells was positively correlated
with HBV DNA levels when co-cultured with BM-MSCs (24 h,
r=0.865; 48 h, r=0.766; 72 h, r=0.912;
P<0.05).
Effect of BM-MSCs on cytokine levels
in co-cultured SLCs and HepG2.2.15 cell supernatants
The supernatant concentrations of IFN-γ in group 5
were higher than those of groups 3 and 4 at 24, 48 and 72 h
(Table IV). By contrast, IL-10
and IL-22 levels in group 5 were lower than those of group 3 and
group 4 at 24, 48 and 72 h (Table
IV). IFN-γ secretion levels were negatively correlated with HBV
DNA levels (24 h, r=−0.900, 48 h, r=−0.982; 72 h,
r=−0.968; P<0.05), whereas IL-10 and IL-22 secretion
levels were positively correlated with HBV DNA levels (IL-10, 24 h,
r=0.860; 48 h, r=0.972; P<0.05; IL-22, 48 h,
r=0.858; 72 h, r=0.742; P<0.05). In group 5, the
supernatant IFN-γ levels at 48 h were significantly higher than
those at 72 h, and the supernatant levels of IL-10 at 48 h were
significantly lower than those detected at 24 and 72 h (Table IV). These findings suggested that
alterations in IFN-γ and IL-10 levels were most evident at 48 h
within the same group.
 | Table IV.Cytokine levels in cell culture
supernatants. |
Table IV.
Cytokine levels in cell culture
supernatants.
| Cytokine | Group | 24 h (pg/µl) | 48 h (pg/µl) | 72 h (pg/µl) |
|---|
| IFN-γ | SLCs |
848.557±11.409aa |
468.347±20.523aa |
528.111±15.640aa |
|
|
BM-MSCs+HepG2.2.15 |
636.650±47.047aa |
460.953±38.345aa |
603.735±26.848a |
|
|
SLCs+HepG2.2.15 |
675.637±19.046aa |
621.237±24.709aa |
517.170±31.331aa |
|
|
SLCs+BM-MSCs+HepG2.2.15 | 735.030±18.646 | 780.463±19.879 |
676.317±34.414bb |
| IL-10 | SLCs |
803.930±55.897aa |
297.040±32.246aa |
183.367±46.742aa |
|
|
BM-MSCs+HepG2.2.15 |
240.747±28.605aa |
206.609±13.669a |
259.580±30.070aa |
|
|
SLCs+HepG2.2.15 |
511.553±37.490a |
413.360±14.133aa |
553.133±54.416a |
|
|
SLCs+BM-MSCs+HepG2.2.15 |
420.227±23.235bb | 153.087±26.016 |
447.230±31.192bb |
| IL-22 | SLCs |
344.423±36.904aa |
180.337±4.672aa | 164.537±35.654 |
|
|
BM-MSCs+HepG2.2.15 |
183.135±18.123a |
166.264±23.206aa | 164.722±12.389 |
|
|
SLCs+HepG2.2.15 | 166.337±18.651 |
258.923±23.426aa |
305.053±14.766aa |
|
|
SLCs+BM-MSCs+HepG2.2.15 |
146.007±20.407bb | 208.537±6.499 | 210.857±22.527 |
Discussion
Liver-derived MSCs have been demonstrated to be
crucial for the repair of damaged hepatocytes and liver
regeneration (27–29). Oh et al (30) confirmed that BM-MSCs are potential
sources of hepatic oval cells. When the liver is severely damaged,
BM-MSCs differentiate into hepatic progenitor-like cells and
mediate repair of the liver (31–33).
The present study aimed to explore the effects of BM-MSCs on
hepatocytes infected with HBV. Previous studies have demonstrated
that human MSCs survive and exhibit protective effects on
neurological and lung injuries following transplantation into rats
(34–36). However, they may also stimulate an
allogeneic immune response to increase lymphocyte proliferation in
the host (37,38). Therefore, with the lack of stable
rat cell lines transfected with HBV, and the strict ethical limits
to acquire human stem cells, a xenotransplantation model was
employed in the present study.
The preliminary findings demonstrated that when
co-cultured with BM-MSCs, the proliferation of HepG2.2.15 cells was
inhibited and HBV DNA levels were decreased. When BM-MSCs were
co-cultured with SLCs, HBV DNA levels were markedly reduced.
Meanwhile, BM-MSCs induced very few HBV genome sequence mutations
and did not cause rejection between xenogeneic cells. To the best
of our knowledge the T45N mutation in the S region, and the rtR192S
mutation in the P region, are not known to be significant in the
clinical treatment of hepatitis B. In addition, the preliminary
results of the present study suggested that BM-MSCs may inhibit the
replication of HBV cccDNA in vitro. It is possible that
BM-MSCs may suppress the proliferation of co-cultured T cells in
vitro, thereby inhibiting immune responses to induce immune
tolerance (39–41). Alternatively, BM-MSCs may secrete
cytokines, including fibroblast growth factor (42,43),
epidermal growth factor (EGF) (44), and hepatocyte growth factor (HGF)
(43,45,46)
to inhibit HBV replication (47).
In addition, intracellular HBV cccDNA in BM-MSCs co-cultured with
HepG2.2.15 cells was not detected, which supports the conclusion
that HBV is unable to replicate in BM-MSCs (48,49).
BM-MSCs are a cell type that exert immunomodulatory
activities (19–21). They inhibit the proliferation and
activation of T cells and exhibit immunomodulatory functions
mediated by soluble factors (39,41).
Prostaglandin E2 (PGE2) and indoleamine
dioxygenase were observed to be potentially involved in the
immunomodulatory function of BM-MSCs (50). The majority of T lymphocytes can be
divided into CD4+ T cells and CD8+ T cells,
and the majority of CD8+ T cells are cytotoxic T
lymphocytes (CTL). T cell function is exhausted during chronic HBV
infection, and CTLs cannot effectively eliminate the virus. As a
result, the virus persists and the proportion of T cell subsets in
the peripheral blood is subsequently altered (51–53).
The findings of the present study suggested that the percentage of
CD8+ cells was positively correlated with HBV DNA
levels, which is consistent with a previous study demonstrating
that an imbalance of T cell subsets was closely associated with HBV
DNA levels (54,55). The CD4+/CD8+
ratio increased at 24 and 48 h, and then decreased at 72 h.
Furthermore, the reduction in the levels of intracellular HBV
cccDNA was the most significant at 48 h, which suggested that the
increased CD4+/CD8+ ratio was correlated with
inhibitory effects on HBV cccDNA replication. To further confirm
these results, the levels of cytokines were measured.
MSCs clearly inhibit the proliferation of allogeneic
lymphocytes, and immunosuppression is mediated by CD8+
regulatory cells (56).
CD8+ cells are divided into the Tc1 and Tc2 subtypes,
and control of the Tc1/Tc2 cell ratio is necessary to maintain
normal immune function (57,58).
Therefore, IFN-γ and IL-10 cytokine levels were ascertained in the
present study, as they are secreted by Tc1 and Tc2 cells,
respectively. The results demonstrated that BM-MSCs may influence
the expression of IFN-γ and IL-10 by inhibiting CD8+ T
cells, as well as inhibit the replication and reduce the levels of
HBV DNA.
BM-MSCs secrete various cytokines that affect the
function of hematopoietic cells, and release a number of
neurotrophic factors, including nerve growth factor, EGF, ciliary
neurotrophic factor and IFN-γ (59). The IFN-γ cytokine induces BM-MSCs
to constitutively express increased levels of immunosuppressive
cytokines, such as PGE2, HGF, and transforming growth
factor (TGF)-β1 (60). Thus, the
cytokine expression results obtained in the current study indicate
that BM-MSCs may secrete cytokines that affect HBV. However,
testing this hypothesis will require further study.
IL-22 was first discovered in the year 2000
(61). As it demonstrates 22%
amino acid sequence similarity with IL-10, it was classified as an
IL-10 family member (61).
However, whether IL-22 exhibits anti- or pro-inflammatory effects
on HBV infection remains controversial. Previous studies have
demonstrated that intra-hepatic expression of IL-22 was increased
in patients with acute and chronic hepatitis B (62). When infected with the virus, T
cells mediate antiviral immunity, and cause inflammatory injury to
the liver. Meanwhile, inflammation and injury leads to compensatory
increases in levels of cytokines (e.g. IL-22) that may protect
hepatocytes from inflammation and repair liver damage (63). The results of the present study
indicated that IL-22 and IL-10 secretion were reduced significantly
when SLCs were co-cultured with BM-MSCs, which suggested that IL-22
exerted anti-inflammatory effects in HBV infection.
HBV-associated end-stage liver disease poses a
serious threat to human health, and liver transplantation is
currently the only effective treatment. BM-MSC transplantation has
been proposed as a novel strategy for the treatment of HBV, and may
represent a new method for prophylaxis and the treatment of HBV
re-infection following liver transplantation. In addition, studies
of the effects of BM-MSCs on HBV cccDNA levels may provide novel
strategies to screen for preventative treatments against HBV
re-infection. Although HBV does not affect the phenotype or
differentiation ability of BM-MSCs, it has been demonstrated to
inhibit the proliferation of BM-MSCs in vitro (64). Therefore, a number of issues
require further investigation before BM-MSCs may be used as a
clinical treatment option, and will be a focus of future
research.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81270528, 81170444,
81441022 and 81670574) and the Natural Science Foundation of
Tianjin (grant nos. 08JCYBJC08400, 11JCZDJC27800 and
12JCZDJC25200), and the Technology Foundation of Health Bureau in
Tianjin (grant nos. 2011KY11 and 10KG101).
Glossary
Abbreviations
Abbreviations:
|
BM-MSCs
|
bone marrow-derived mesenchymal stem
cells
|
|
SLCs
|
splenic lymphocytes
|
|
HBV
|
Hepatitis B virus
|
|
BN
|
Brown Norway
|
|
UC-MSCs
|
umbilical cord-derived mesenchymal
stem cells
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
MTT
|
methylthiazolyl tetrazolium
|
|
EGF
|
epidermal growth factor
|
|
HGF
|
hepatocyte growth factor
|
References
|
1
|
Zhuang H: Current status and goals of
hepatitis B prevention and treatment. Zhonghua Nei Ke Za Zhi.
47:793–795. 2008.(In Chinese). PubMed/NCBI
|
|
2
|
Shen Zhong-Yang, Zhu Zhi-Jun, Deng
Yong-Lin, Sun Liying, Qu Wei, Rao Wei, Sun Xiao-Ye, Zheng Hong, Pan
Cheng and Liu Yi-He: Combination of low-dose HBIg and Nucleoside
analogues to prevent recurrent hepatitis B virus after liver
transplantation: A retrospective analysis of 1506 cases. Chinese J
Hepatobiliary Surg. 17:364–366. 2011.
|
|
3
|
Avolio AW, Nure E, Pompili M, Barbarino R,
Basso M, Caccamo L, Magalini S, Agnes S and Castagneto M: Liver
transplantation for hepatitis B virus patients: Long-term results
of three therapeutic approaches. Transplant Proc. 40:1961–1964.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samuel D, Feray C and Bismuth H: Hepatitis
viruses and liver transplantation. J Gastroenterol Hepatol.
12:(Suppl). S335–S341. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campsen J, Zimmerman M, Trotter J, Hong J,
Freise C, Brown R, Cameron A, Ghobrial M, Kam I, Busuttil R, et al:
Liver transplantation for hepatitis B liver disease and concomitant
hepatocellular carcinoma in the United States with hepatitis B
immunoglobulin and nucleoside/nucleotide analogues. Liver Transpl.
19:1020–1029. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cholongitas E, Goulis J, Akriviadis E and
Papatheodoridis GV: Hepatitis B immunoglobulin and/or nucleos(t)ide
analogues for prophylaxis against hepatitis b virus recurrence
after liver transplantation: A systematic review. Liver Transpl.
17:1176–1190. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishigami M, Onishi Y, Ito T, Katano Y, Ito
A, Hirooka Y, Kiuchi T and Goto H: Anti-hepatitis B surface
immunoglobulin reduction in early postoperative period after liver
transplantation in hepatitis B virus-positive patients. Hepatol
Res. 41:1189–1198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saab S, Ham MY, Stone MA, Holt C and Tong
M: Decision analysis model for hepatitis B prophylaxis one year
after liver transplantation. Liver Transpl. 15:413–420. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen ZY, Zheng WP, Deng YL and Song HL:
Variations in the S and P regions of the hepatitis B virus genome
under immunosuppression in vitro and in vivo. Viral Immunol.
25:368–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Limquiaco JL, Wong J, Wong VW, Wong GL,
Tse CH, Chan HY, Kwan KY, Lai PB and Chan HL: Lamivudine
monoprophylaxis and adefovir salvage for liver transplantation in
chronic hepatitis B: A seven-year follow-up study. J Med Virol.
81:224–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosenau J, Hooman N, Hadem J, Rifai K,
Bahr MJ, Philipp G, Tillmann HL, Klempnauer J, Strassburg CP and
Manns MP: Failure of hepatitis B vaccination with conventional
HbsAg vaccine in patients with continuous HBIG prophylaxis after
liver transplantation. Liver Transpl. 13:367–373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wursthorn K, Wedemeyer H and Manns MP:
Managing HBV in patients with impaired immunity. Gut. 59:1430–1445.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han D, Wu C, Xiong Q, Zhou L and Tian Y:
Anti-inflammatory mechanism of bone marrow mesenchymal stem cell
transplantation in rat model of spinal cord injury. Cell Biochem
Biophys. 71:1341–1347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen ZY, Zhang J, Song HL and Zheng WP:
Bone-marrow mesenchymal stem cells reduce rat intestinal
ischemia-reperfusion injury, ZO-1 downregulation and tight junction
disruption via a TNF-α-regulated mechanism. World J Gastroenterol.
19:3583–3595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitkari B, Nitzsche F, Kerkelä E, Kuptsova
K, Huttunen J, Nystedt J, Korhonen M and Jolkkonen J: Human bone
marrow mesenchymal stem/stromal cells produce efficient
localization in the brain and enhanced angiogenesis after
intra-arterial delivery in rats with cerebral ischemia, but this is
not translated to behavioral recovery. Behav Brain Res. 259:50–59.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santhakumar R, Vidyasekar P and Verma RS:
Cardiogel: A nano-matrix scaffold with potential application in
cardiac regeneration using mesenchymal stem cells. PLoS One.
9:e1146972014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ball LM, Bernardo ME, Roelofs H, Lankester
A, Cometa A, Egeler RM, Locatelli F and Fibbe WE: Cotransplantation
of ex vivo expanded mesenchymal stem cells accelerates lymphocyte
recovery and may reduce the risk of graft failure in haploidentical
hematopoietic stem-cell transplantation. Blood. 110:2764–2767.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair-current views. Stem
Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Shen ZY, Song HL, Yang Y, Wu BJ,
Fu NN and Liu T: Protective effect of bone marrow mesenchymal stem
cells in intestinal barrier permeability after heterotopic
intestinal transplantation. World J Gastroenterol. 20:7442–7451.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perez-Basterrechea M, Obaya AJ, Meana A,
Otero J and Esteban MM: Cooperation by fibroblasts and bone
marrow-mesenchymal stem cells to improve pancreatic rat-to-mouse
islet xenotransplantation. PLoS One. 8:e735262013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roemeling-van Rhijn M, Khairoun M,
Korevaar SS, Lievers E, Leuning DG, Ijzermans JN, Betjes MG,
Genever PG, van Kooten C, de Fijter HJ, et al: Human bone marrow-
and adipose tissue-derived mesenchymal stromal cells are
immunosuppressive in vitro and in a humanized allograft rejection
model. J Stem Cell Res Ther. (Suppl 6). S207802013.
|
|
22
|
Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z,
Zhang A, Shi J, Chen L, Lv S, et al: Human mesenchymal stem cell
transfusion is safe and improves liver function in acute-on-chronic
liver failure patients. Stem Cells Transl Med. 1:725–731. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sells MA, Chen ML and Acs G: Production of
hepatitis B virus particles in Hep G2 cells transfected with cloned
hepatitis B virus DNA. Proc Natl Acad Sci USA. 84:1005–1009. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen ZY, Zheng WP, Liu T, Yang Y and Song
HL: Effects of dendritic cells from hepatitis B virus transgenic
mice-stimulated autologous lymphocytes on hepatitis B virus
replication: A study on the impact of specific sensitized effector
cells on in vitro virus replication. Viral Immunol. 28:85–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao YT, Han T, Li Y, Yang B, Wang YJ, Wang
FM, Jing X and Du Z: Enhanced specificity of real-time PCR for
measurement of hepatitis B virus cccDNA using restriction
endonuclease and plasmid-safe ATP-dependent DNase and selective
primers. J Virol Methods. 169:181–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harting M, Jimenez F, Pati S, Baumgartner
J and Cox C Jr: Immunophenotype characterization of rat mesenchymal
stromal cells. Cytotherapy. 10:243–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joshi MB, Patil P, He Z, Holgersson J,
Olausson M and Sumitran-Holgersson S: Fetal liver-derived
mesenchymal stromal cells augment engraftment of transplanted
hepatocytes. Cytotherapy. 14:657–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fouraschen SM, Pan Q, de Ruiter PE, Farid
WR, Kazemier G, Kwekkeboom J, Ijzermans JN, Metselaar HJ, Tilanus
HW, de Jonge J and van der Laan LJ: Secreted factors of human
liver-derived mesenchymal stem cells promote liver regeneration
early after partial hepatectomy. Stem Cells Dev. 21:2410–2419.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fouraschen SM, Hall SR, de Jonge J and van
der Laan LJ: Support of hepatic regeneration by trophic factors
from liver-derived mesenchymal stromal/stem cells. Methods Mol
Biol. 1213:89–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh SH, Hatch HM and Petersen BE: Hepatic
oval ‘stem’ cell in liver regeneration. Semin Cell Dev Biol.
13:405–409. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Facciorusso A, Antonino M, Del Prete V,
Neve V, Scavo MP and Barone M: Are hematopoietic stem cells
involved in hepatocarcinogenesis? Hepatobiliary Surg Nutr.
3:199–206. 2014.PubMed/NCBI
|
|
32
|
Lehwald N, Duhme C, Wildner M, Kuhn S,
Fürst G, Forbes SJ, Jonas S, Robson SC, Knoefel WT, Schmelzle M and
Schulte Am Esch J: HGF and SDF-1-mediated mobilization of CD133+
BMSC for hepatic regeneration following extensive liver resection.
Liver Int. 34:89–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zocco MA, Piscaglia AC, Giuliante F, Arena
V, Novi M, Rinninella E, Tortora A, Rumi C, Nuzzo G, Vecchio FM, et
al: CD133+ stem cell mobilization after partial hepatectomy depends
on resection extent and underlying disease. Dig Liver Dis.
43:147–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hosseini M, Moghadas M, Edalatmanesh MA
and Hashemzadeh MR: Xenotransplantation of human adipose derived
mesenchymal stem cells in a rodent model of Huntington's disease:
Motor and non-motor outcomes. Neurol Res. 37:309–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao F, Li Q, Hou L, Li Z, Min F and Liu Z:
Mesenchymal stem cell-based angiotensin-converting enzyme 2 in
treatment of acute lung injury rat induced by bleomycin. Exp Lung
Res. 40:392–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khabbal J, Kerkelä E, Mitkari B, Raki M,
Nystedt J, Mikkonen V, Bergström K, Laitinen S, Korhonen M and
Jolkkonen J: Differential clearance of rat and human bone
marrow-derived mesenchymal stem cells from the brain after
intra-arterial infusion in rats. Cell Transplant. 24:819–828. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chuang CK, Lin KJ, Lin CY, Chang YH, Yen
TC, Hwang SM, Sung LY, Chen HC and Hu YC: Xenotransplantation of
human mesenchymal stem cells into immunocompetent rats for
calvarial bone repair. Tissue Eng Part A. 16:479–488. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Chen X, Armstrong MA and Li G:
Survival of bone marrow-derived mesenchymal stem cells in a
xenotransplantation model. J Orthop Res. 25:926–932. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jorgensen C, Djouad F, Apparailly F and
Noël D: Engineering mesenchymal stem cells for immunotherapy. Gene
Ther. 10:928–931. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kong QF, Sun B, Wang GY, Zhai DX, Mu LL,
Wang DD, Wang JH, Li R and Li HL: BM stromal cells ameliorate
experimental autoimmune myasthenia gravis by altering the balance
of Th cells through the secretion of IDO. Eur J Immunol.
39:800–809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Q, Sun B, Wang D, Ji Y, Kong Q, Wang
G, Wang J, Zhao W, Jin L and Li H: Murine bone marrow mesenchymal
stem cells cause mature dendritic cells to promote T-cell
tolerance. Scand J Immunol. 68:607–615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Ming L, Luo H, Liu W, Zhang Y, Liu
H and Jin Y: Integration of a calcined bovine bone and BMSC-sheet
3D scaffold and the promotion of bone regeneration in large
defects. Biomaterials. 34:9998–10006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hiwatashi N, Hirano S, Mizuta M, Tateya I,
Kanemaru S, Nakamura T and Ito J: Adipose-derived stem cells versus
bone marrow-derived stem cells for vocal fold regeneration.
Laryngoscope. 124:E461–E469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kwon DS, Gao X, Liu YB, Dulchavsky DS,
Danyluk AL, Bansal M, Chopp M, McIntosh K, Arbab AS, Dulchavsky SA
and Gautam SC: Treatment with bone marrow-derived stromal cells
accelerates wound healing in diabetic rats. Int Wound J. 5:453–463.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun L, Fan X, Zhang L, Shi G, Aili M, Lu
X, Jiang T and Zhang Y: Bone mesenchymal stem cell transplantation
via four routes for the treatment of acute liver failure in rats.
Int J Mol Med. 34:987–996. 2014.PubMed/NCBI
|
|
46
|
Li T, Zhu J, Ma K, Liu N, Feng K, Li X,
Wang S and Bie P: Autologous bone marrow-derived mesenchymal stem
cell transplantation promotes liver regeneration after portal vein
embolization in cirrhotic rats. J Surg Res. 184:1161–1173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schorr O, Borel C, Trepo C, Zoulim F and
Hantz O: Effects of liver growth factors on hepadnavirus
replication in chronically infected duck hepatocytes. J Hepatol.
44:842–847. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie C, Zheng YB, Zhu HP, Peng L and Gao
ZL: Human bone marrow mesenchymal stem cells are resistant to HBV
infection during differentiation into hepatocytes in vivo and in
vitro. Cell Biol Int. 33:493–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rong Q, Zhang L, Su E, Li J, Li J, Liu Z,
Huang Z, Ma W, Cao K and Huang J: Bone marrow-derived mesenchymal
stem cells are capable of mediating hepatitis B virus infection in
injured tissues. J Viral Hepat. 15:607–614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Matysiak M, Orlowski W, Fortak-Michalska
M, Jurewicz A and Selmaj K: Immunoregulatory function of bone
marrow mesenchymal stem cells in EAE depends on their
differentiation state and secretion of PGE2. J Neuroimmunol.
233:106–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Conroy MJ, Mac Nicholas R, Grealy R,
Taylor M, Otegbayo JA, O'Dea S, Mulcahy F, Ryan T, Norris S and
Doherty DG: Circulating CD56dim natural killer cells and CD56+ T
cells that produce interferon-γ or interleukin-10 are expanded in
asymptomatic, E antigen-negative patients with persistent hepatitis
B virus infection. J Viral Hepat. 22:335–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Murray JM and Goyal A: In silico single
cell dynamics of hepatitis B virus infection and clearance. J Theor
Biol. 366:91–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ito H, Ando T, Ando K, Ishikawa T, Saito
K, Moriwaki H and Seishima M: Induction of hepatitis B virus
surface antigen-specific cytotoxic T lymphocytes can be
up-regulated by the inhibition of indoleamine 2,3-dioxygenase
activity. Immunology. 142:614–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xue-Song L, Cheng-Zhong L, Ying Z and
Mo-Bin W: Changes of Treg and Th17 cells balance in the development
of acute and chronic hepatitis B virus infection. BMC
Gastroenterol. 12:432012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang JY, Song CH, Shi F, Zhang Z, Fu JL
and Wang FS: Decreased ratio of Treg cells to Th17 cells correlates
with HBV DNA suppression in chronic hepatitis B patients undergoing
entecavir treatment. PLoS One. 5:e138692010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Djouad F, Plence P, Bony C, Tropel P,
Apparailly F, Sany J, Noël D and Jorgensen C: Immunosuppressive
effect of mesenchymal stem cells favors tumor growth in allogeneic
animals. Blood. 102:3837–3844. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Uzawa A, Mori M, Hayakawa S, Masuda S,
Nomura F and Kuwabara S: Expression of chemokine receptors on
peripheral blood lymphocytes in multiple sclerosis and
neuromyelitis optica. BMC Neurol. 10:1132010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang T, Zhao H, Ren H, Guo J, Xu M, Yang R
and Han ZC: Type 1 and type 2 T-cell profiles in idiopathic
thrombocytopenic purpura. Haematologica. 90:914–923.
2005.PubMed/NCBI
|
|
59
|
Ma XQ, Ma QY, Wang YY, Gu P and Wang MW:
Protein secreted by bone marrow mesenchymal stem cells and its
function. J Clin Rehab Tiss Engin Res. 13:2763–2766. 2009.
|
|
60
|
Liang C, Chen SL, Wang M, Zhai WJ, Zhou Z,
Pang AM, Feng SZ and Han MZ: Synergistic immunomodulatory effects
of interferon-gamma and bone marrow mesenchymal stem cells.
Zhonghua Xue Ye Xue Za Zhi. 34:213–216. 2013.(In Chinese).
PubMed/NCBI
|
|
61
|
Dumoutier L, Van Roost E, Ameye G, Michaux
L and Renauld JC: IL-TIF/IL-22: Genomic organization and mapping of
the human and mouse genes. Genes Immun. 1:488–494. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang Y, Cobleigh MA, Lian JQ, Huang CX,
Booth CJ, Bai XF and Robek MD: A proinflammatory role for
interleukin-22 in the immune response to hepatitis B virus.
Gastroenterology. 141:1897–1906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Park O, Wang H, Weng H, Feigenbaum L, Li
H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS, et al: In vivo
consequences of liver-specific interleukin-22 expression in mice:
Implications for human liver disease progression. Hepatology.
54:252–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hu WH and Ren J: Impact of hepatitis B
virus infected serum on the hepatic differentiation of human bone
marrow mesenchymal stem cells. Beijing Da Xue Xue Bao. 40:459–464.
2008.(In Chinese). PubMed/NCBI
|