Introduction
Lung cancer is one of the frequent clinical
malignancies (1). Chemotherapy has
been adopted as the primary treatment method. However, the majority
of lung cancer patients have not been sufficiently sensitive to
traditional chemotherapy treatment (2). Matrine
(C15H24N2O) is an alkaloid
(3), which may inhibit the
proliferation of A549 human lung adenocarcinoma cells (4) and non-small-cell lung cancer cells
(5).
p53 is a well-known tumor suppressor gene (6), and in numerous types of cancer
patients often have a dysfunctional p53 (7). p21 is an important member of the Cip
family (8) and the
cyclin-dependent kinase inhibitor family (9). A mutation in the p21 gene is
associated with pancreatic cancer (10) and the differentiation of other
malignant tumor types, including to the depth of invasion,
proliferation and metastasis. Additionally, p21 has prognostic
value; p21 expression has been documented as a poor prognostic
marker in human lung cancer (11).
Proliferating cell nuclear antigen (PCNA) is a protein expressed in
proliferative stage cells or tumor cells (12). PCNA expression levels have been
observed to change periodically throughout the cell cycle (13). The detection of PCNA expression in
cells may be used as an indicator of the proliferation status of
pancreatic cancer cells (14).
Eukaryotic initiation factor 4E (eIF4E) is a cytokine associated
with pancreatic cancer (15) and
other malignant tumor types, which is important in the initiation
of protein synthesis (16) and is
closely associated with tumorigenesis, invasion and metastasis.
Therefore, the present study investigated if matrine
is capable of reducing the proliferation of A549 lung cancer cells
via the p53/p21/PCNA/eIF4E signaling pathway.
Materials and methods
Cell culture
Human lung adenocarcinoma A549 cells, were obtained
from the Chinese Academy of Medical Sciences Tumor Cell Bank
(Beijing, China). These cells were maintained in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and incubated at 37°C in a 5% CO2
atmosphere.
Cell toxicity and proliferation
assays
Adherent A549 cells were cultured by digestion. The
cells were seeded at a density of 1.0×105 cells/well
into 96-well plates. A control group and four groups of cells
treated with different concentrations (60, 120, 180 and 240 mg/l)
of matrine (National Institute for the Control of Pharmaceutical
and Biological Products, Beijing, China) were used. The control
group was exposed to identical quantities of medium. The drug was
diluted with distilled water and served as a vehicle control. Every
48 h, the medium was replaced with fresh medium containing the
specified concentration of the drug. The cell groups were cultured
for 6 days. A total of 20 µl (5 mg/ml)
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.,
Hangzhou, China) were added to each well, and the samples were
incubated for 4 h at 37°C. Following incubation, the absorbance (A)
values of the microplate wells were determined at 570 nm. Cell
proliferation inhibition rate = (l - Atreatment group /
Acontrol group) × 100.
Transwell assay
Cells were seeded at 4×105/ml in two
6-well plates and treated with 1 ml 10% FBS, 1%
penicillin/streptomycin and RPMI-1640. After 24 h, varying
concentrations (60, 120, 180 and 240 mg/l) of matrine were
administered to the different groups and the cells were maintained
at 37°C for 48 h. The top of the transwell chamber contained 150 µl
suspension (1×106 cell/ml), and the bottom contained 600
µl 10% FBS. The cells were gently removed from the top chamber with
a cotton swab prior to staining with crystal violet. A total of
three visual fields were randomly selected and images were captured
using a fluorescence microscope (BX53; Olympus Corporation, Tokyo,
Japan). Migrating cells were counted and statistical analysis was
performed on the data obtained. The inhibition of cell migration
inhibition = (1 - number of migrated cells in the intervention
group / number of migrating cells in the control group) × 100.
Flow cytometry
The A549 cell suspension (3×104/ml) was
placed at 2 ml per well into a 6-well plate and incubated for 12 h
at 37°C in a an atmosphere of 5% CO2. After 12 h, the
media was aspirated and the matrine was added at the aforementioned
concentrations. Media with no cell suspension served as a blank
control. Each treatment was performed in triplicate. The cells were
trypsinized and collected, washed with cold phosphate-buffered
saline (PBS) twice and 100 μl 1X annexin-binding buffer (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) was added. Annexin V (5
µl) was added, followed by 100 μg/ml propidium iodide (1 µl).
Following a 15-min incubation in the dark at room temperature, 400
µl 1X annexin-binding buffer was added. Flow cytometry was
performed using a BD FACSVerse™, BD Biosciences, Franklin Lakes,
NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following treatment, the A549 cells were collected
from the control group and the group treated with 240 mg/l matrine.
The total RNA was extracted using TRIzol reagent (Thermo Fisher
Scientific, Inc.) and was reverse transcribed to obtain cDNA.
β-actin was used as an internal reference. The qPCR primers
(β-actin, p53, p21, PCNA and eIF4E) were synthesized by the
Shanghai Biological Engineering Technology Services Limited
(Table I). The CFX Connect
Real-Time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and the CFX Manager (Bio-Rad Laboratories, Inc.) were used for
analysis according to the manufacturer's instructions. Each
reaction contained 500 ng cDNA, 10 µl of 2X master mix, 0.5 µl
primers and water for a final volume of 30 µl. A SuperReal Premix
Plus (SYBR Green) from Tiangen Biotech Co., Ltd. (Beijing, China)
was used. The threshold cycle value, that represents the cycle
number at which sample fluorescence rises to a statistically
significant level above the background, was calculated. CFX Manager
uses the comparative Cq method for relative quantitative analysis,
and the gene expression levels are presented as a fold change
(17). All amplifications were run
in triplicate and the mean value was applied for all
calculations.
 | Table I.Primer sequences for polymerase chain
reaction. |
Table I.
Primer sequences for polymerase chain
reaction.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) | Length (bp) |
|---|
| β-actin |
CCCATCTATGAGGGTTACGC |
TTTAATGTCACGCACGATTTC | 150 |
| p53 |
ACCTATGGAAACTACTTCCTGAAA |
CTGGCATTCTGGGAGCTTCA | 141 |
| p21 |
AGTCAGTTCCTTGTGGAGCC |
CATTAGCGCATCACAGTCGC | 184 |
| PCNA |
CAGAGCTCTTCCCTTACGCA |
GTCCTTGAGTGCCTCCAACA | 200 |
| eIF4E |
TTCCTTCTGACTGGGGGACT |
CCTCCTCTGTAGTCGGGGGA | 192 |
Western blotting
The cells were cultured for 24 h in 6-well plates at
a density of 1.0×106 cells/cm2. Following
incubation, the cells were collected and washed three times with
ice-cold PBS. The cells were subsequently centrifuged for 5 min at
300 × g, and the supernatant was discarded. The total protein was
extracted using RIPA (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Protein concentration was determined
using the bicinchoninic acid assay method (Enhanced BCA Protein
assay kit; Beyotime Institute of Biotechnology, Shanghai, China).
Aliquots of 150 µg total protein were separated by 8%
polyacrylamide gel electrophoresis. Tris-buffered saline/Tween-20
solution containing 1% bovine serum albumin (Sangon Biotech Co.,
Ltd., Shanghai, China) and non-fat milk was used to block the
membranes overnight at 4°C. Following blocking, the membranes were
incubated with the following primary antibodies at a dilution of
1:1,000: Polyclonal rabbit anti-p53 (cat. no. sc-6243), polyclonal
rabbit anti-PCNA (cat. no. sc-7907), monoclonal mouse anti-p21
(cat. no. sc-271532) and monoclonal mouse anti-eIF4E (cat. no.
sc-271480; Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA). The
β-actin antibody (cat. no. sc-47778; dilution, 1:2,000 from Santa
Cruz Biotechnology, Inc.) served as the loading control. The
proteins were visualized using the following secondary antibodies:
Goat anti-rabbit (1:4,000; cat. no. bs-0295G) and goat anti-mouse
(1:4,000; cat. no. bs-0368Gs) antibody, purchased from Beijing
Biosynthesis Biotechnology Co., Ltd. (Beijing, China). The bands
were developed using an ECL system according to the manufacturer's
protocols. ImageJ 1.48 software (National Institutes of Health,
Bethesda, MD, USA) was used to determine the density of the bands
in all blots.
Statistical analysis
When comparing two groups of data a paired samples
t-test was performed. When comparing multiple groups a one-way
analysis of variance followed by a post-hoc Tukey's test was used.
Statistical analyses were conducted using SPSS version 22.0 (IBM
SPSS, Armonk, NY, USA) and data are presented as means ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibitory action of matrine on A549
cell proliferation
A549 cells were treated with different
concentrations of matrine (0, 60, 120, 180 and 240 mg/l) for 6
days. The A570 values in each group were measured using an MTT
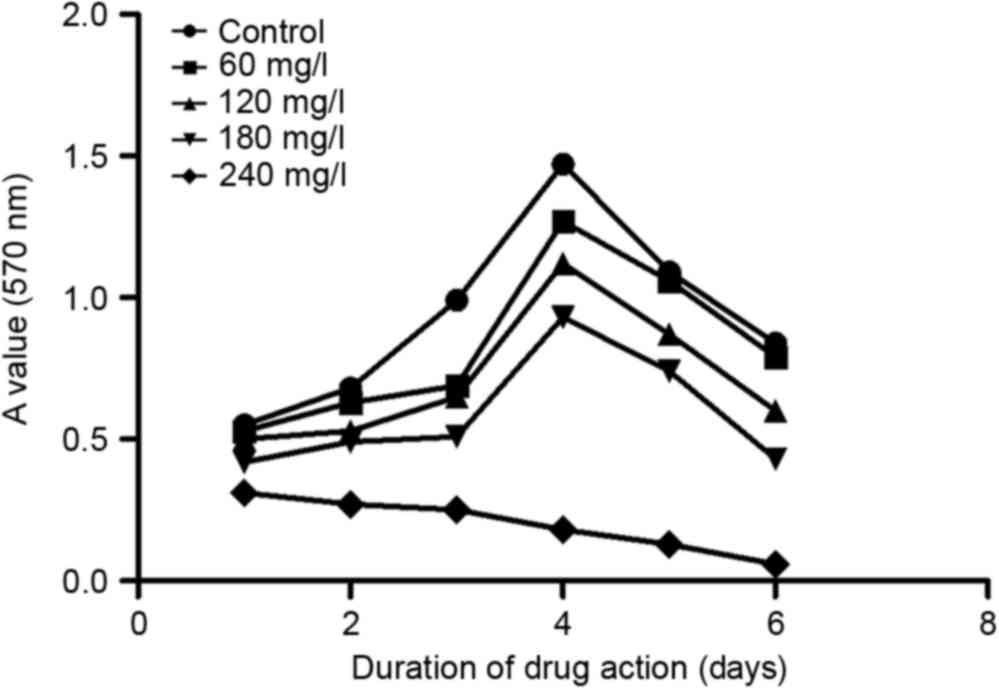
assay and the obtained data were utilized to produce a growth curve
(Fig. 1).
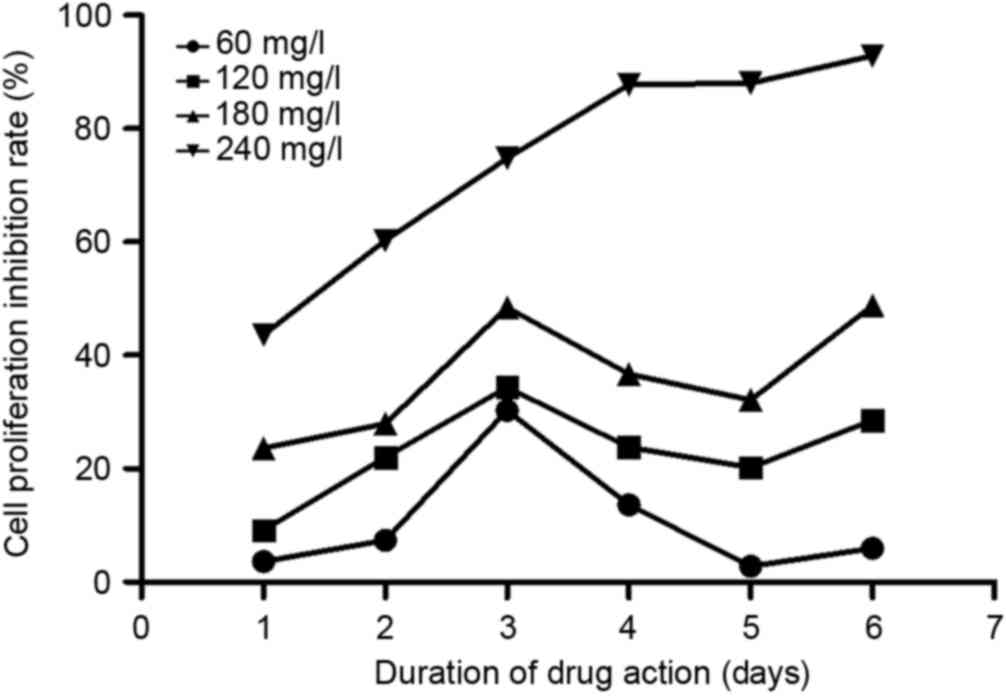
As shown in Fig. 2,
matrine could effectively inhibit A549 cell proliferation. At each
time point, matrine exerted inhibitory effects in a dose-dependent
manner, from 60–240 mg/l. In addition, on day 3 following
treatment, the inhibition reached its maximum at all concentrations
except for 240 mg/l. Based on this observation, one possible
explanation is that between days 3 and 4, the cells had already
occupied all the culturing space and could not proliferate any
further; therefore, the inhibitory rate dropped on day 4.
Matrine inhibits cell migration
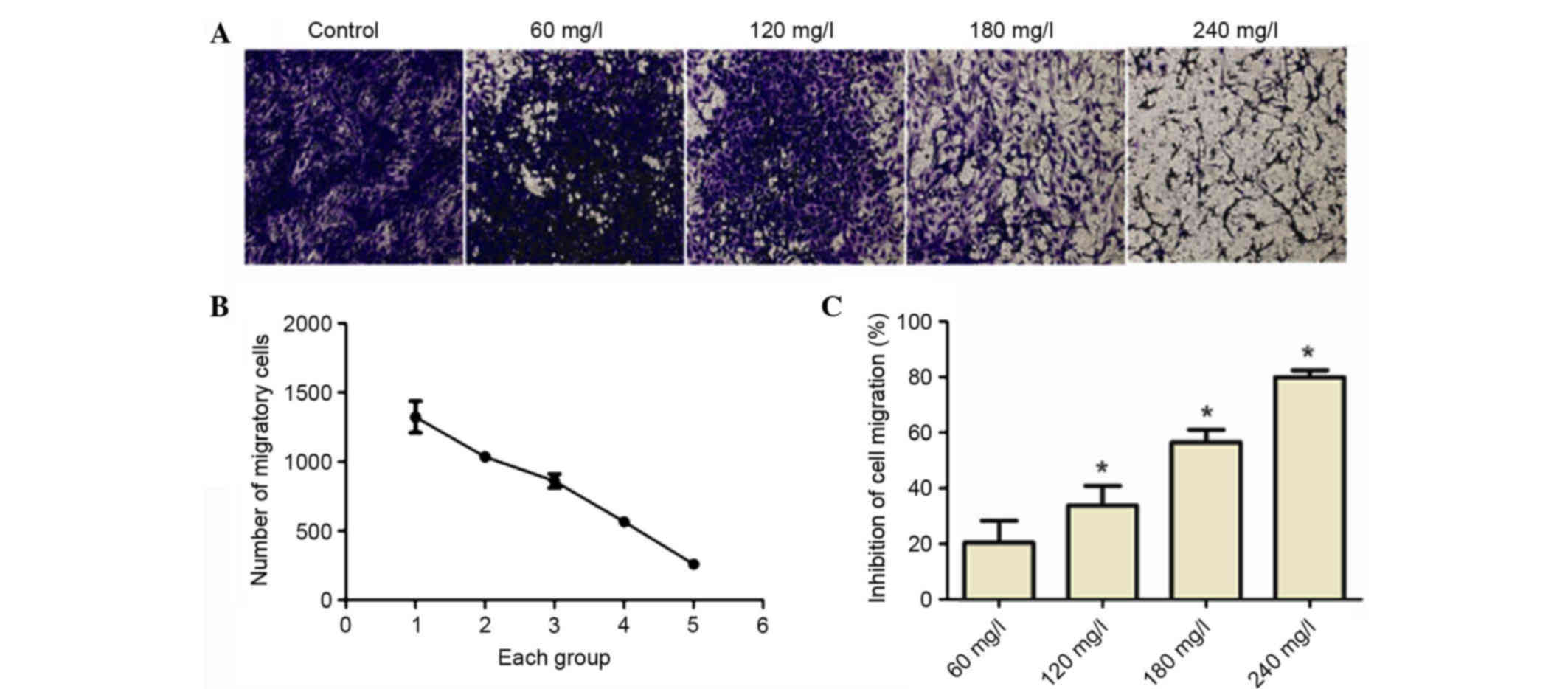
As depicted in Fig.
3, A549 cell migration was reduced as the concentration of
matrine was increased. Therefore, the inhibition rate of cell
migration increased in a dose-dependent manner.
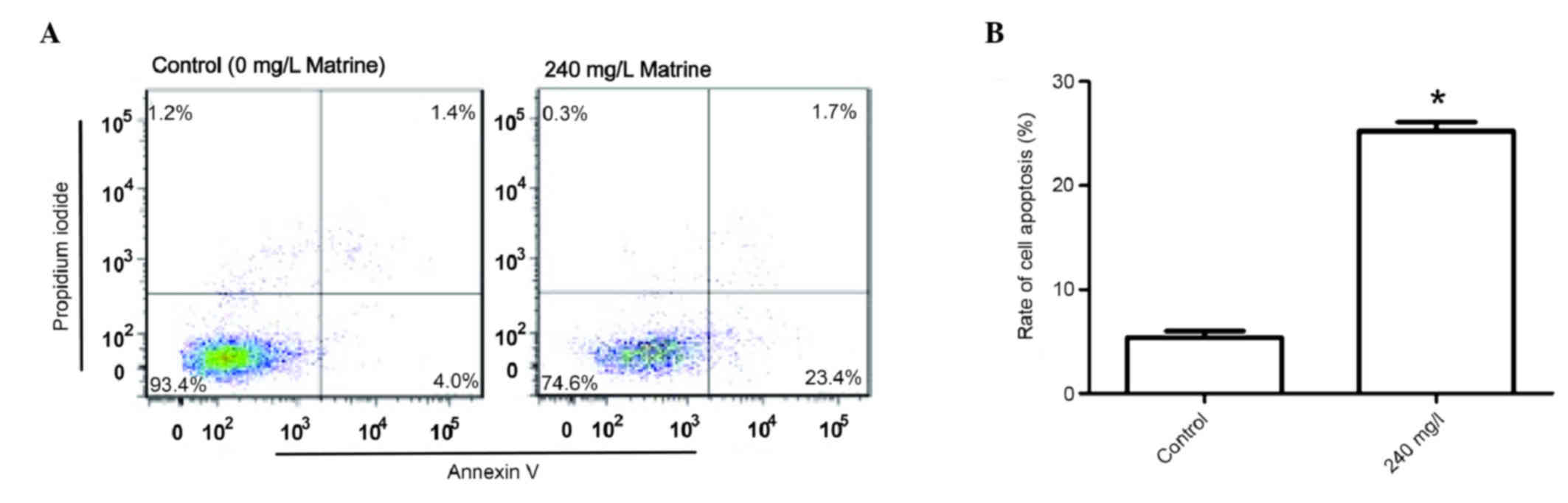
Apoptosis is increased in cells
treated with a high concentration of matrine
Based on the growth curve of A549 cells (Fig. 1), matrine at 240 mg/l exhibited the
strongest inhibitory effects 48 h after treatment; therefore, 240
mg/l of matrine was chosen as the treatment condition. Even higher
concentrations of matrine may lead to early cell death, thus
indicating that other types of cell death may have occurred.
Therefore, only 240 mg/l matrine was used to treat cells in the
apoptosis assay. A549 cells were treated with 240 mg/l matrine in
order to investigate its effect on the apoptotic rate compared with
that of the control group. Flow cytometry indicated (Fig. 4) that the apoptotic rate in the 240
mg/l matrine-treated group was significantly higher when compared
with the control group (P<0.05).
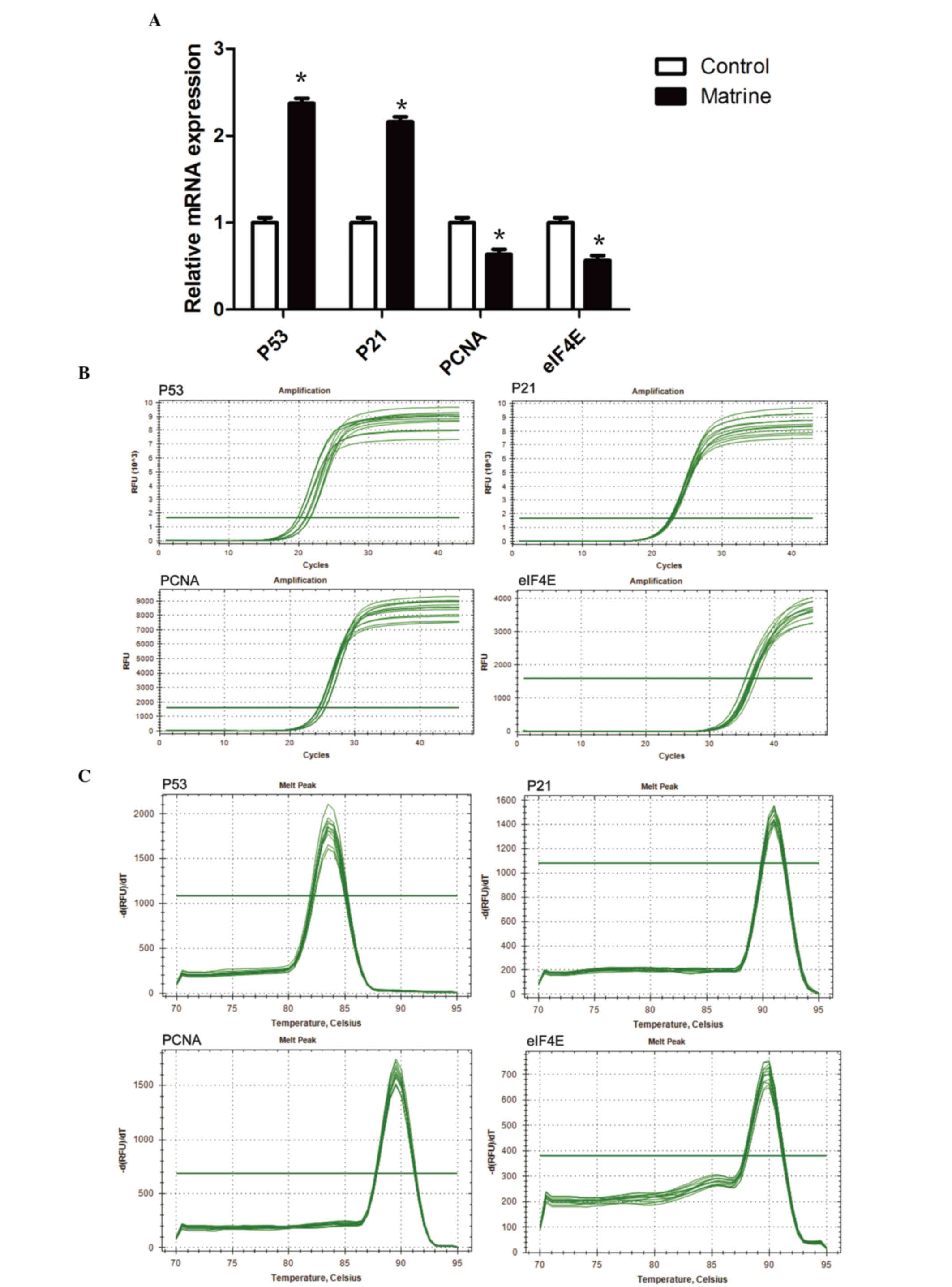
Matrine treatment increases the mRNA
expression levels of p53 and p21, whilst reducing that of PCNA and
eIF4E
The RT-qPCR findings indicated that the mRNA
expression levels of p53 and p21 were significantly higher in the
240 mg/l matrine group compared with the control group (P<0.05;
Fig. 5A). The mRNA expression
levels of PCNA and eIF4E were significantly lower in the 240 mg/l
matrine group compared with the control (P<0.05).
The Cq values of the RT-qPCR amplification curves of
p53, p21, PCNA and eIF4E genes (Fig.
5B) were between 20 and 40. The results indicated that the p53,
p21, PCNA and eIF4E have been successfully amplified. There was a
steep peak in all RT-qPCR amplification curves, indicating that
only one DNA product was formed during the reaction.
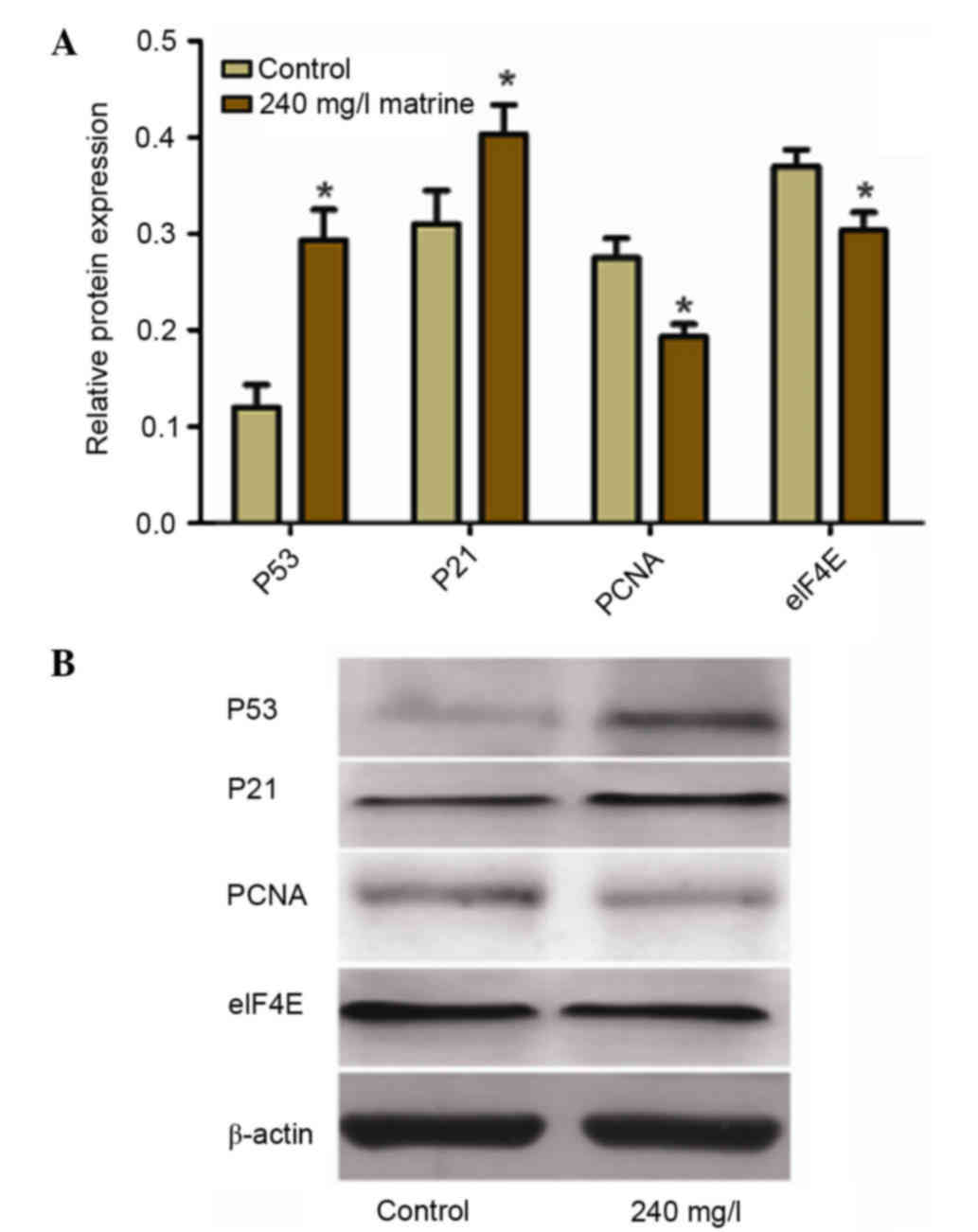
Matrine treatment increases the
protein expression levels of p53 and p21, whilst reducing that of
PCNA and eIF4E
Western blot analysis (Fig. 6) revealed that the protein
expression levels of p53 and p21 were significantly higher in the
240 mg/l matrine-treated group compared with the control group
(P<0.05). The protein expression levels of PCNA and eIF4E were
significantly lower in the 240 mg/l matrine-treated group compared
with the control group (P<0.05).
Discussion
Matrine (C15H24N2O)
is usually derived from the legume, Sophora flavescens Ait
(18), however the bioactive
compound may also be extracted from other species belonging to the
same genus, including Sophora subprostrata (19) and Sophora alopecuroides
(20). Matrine exerts excellent
pharmacological activity and is beneficial for lowering body
temperature, promoting diuresis, detoxifying, treating jaundice and
preventing liver fibrosis. Previous studies indicated that matrine
may be used as an effective treatment for various types of cancer,
including liver (21) and lung
cancer (22), gastric (23) and colon cancer (24), cervical cancer (25), leukemia (26), osteosarcoma (27), glioma (28) and nasopharyngeal carcinoma
(29), and exhibits potent
activity against various other types of cancer. Lung cancer occurs
in the bronchial epithelium (30)
and is characterized by a high morbidity rate (31), a considerable degree of malignancy,
a great metastatic propensity in the early stages and the tendency
to recur following tumor excision (32). The present study aimed to
investigate the effects of matrine on the proliferation of A549
human lung adenocarcinoma cells and on the mRNA and protein
expression levels of p53, p21, PCNA and eIF4E. Additionally the
current study aimed to determine the possible mechanisms by which
matrine affects in order to inhibit A549 cell proliferation.
The main cell invasion and metastasis processes of
A549 human lung adenocarcinoma cells and other tumor cells involves
the adhesion of tumor cells to the basement membrane, which leads
to the degradation of the extracellular matrix, promoting migration
and invasion of cancerous cells, resulting in metastasis.
Therefore, in the event that any of these stages are inhibited, the
metastasis process would likely be inhibited, leading to of human
lung adenocarcinoma A549 cells.
Initially, the present study used different
concentrations (60, 120, 180 and 240 mg/l) of matrine as a
preliminary dosage. Via growth curves, it was established that all
matrine concentrations used had a slight effect on the
proliferation of A549 human lung adenocarcinoma cells. However, the
most marked effect was in cells treated with 240 mg/l matrine
Migration of A549 cells and other tumor cells is an
important stage between the invasion and metastasis progression.
Therefore, Transwell cell migration experiments were used to
determine the influence of 60, 120, 180 and 240 mg/l matrine on
A549 lung cancer cell migration. As matrine concentration was
increased, the rate of A549 cell migration was inhibited in a
dose-dependent manner.
Apoptosis is a fundamental biological phenomenon in
the cell life cycle, which has been important for the evolution of
organisms as it allows for the removal of unhealthy or
dysfunctional cells. The present study used flow cytometry to
investigate the effect of 240 mg/l matrine on the apoptosis of A549
human lung adenocarcinoma cells. The results suggested that the
apoptotic rate following the application of 240 mg/l matrine was
significantly higher when compared with the control group. It was
concluded that a concentration of 240 mg/l matrine induced
considerable levels of apoptosis in A549 cells.
The p53 gene is a tumor suppressor gene (33), which is frequently termed a
‘genetic guardian’. When DNA is damaged, the expression levels of
p53 increase rapidly (34). If the
p53 gene harbors a mutation, the activity of the p53 protein is
reduced, leading to uncontrolled cell division and ultimately, the
occurrence of cancer (35).
In recent years, p21 has been identified as an
important member of the cyclin-dependent kinase inhibitor family
(36), which is located downstream
of the p53 gene (37). p21 and p53
contribute to the regulation of the G1 checkpoint of the cell
cycle. As the damaged DNA cannot pass the G1 checkpoint without
repair, the replication and the accumulation of damaged DNA can be
reduced substantially. Therefore, the G1 checkpoint is important
for tumor suppression (38).
Previous studies (11,22) have determined that the p21 gene is
associated with tumor differentiation, depth of invasion,
proliferation and metastasis of tumors, and is also valuable as a
prognostic sign for tumor progression.
PCNA may be used as an indicator to evaluate the
state of cell proliferation (39).
A close association exists between PCNA and DNA synthesis (40). PCNA is also important for the
initiation of cell proliferation. Previous experimental findings
indicated that the expression of PCNA is associated with the stage
of lung cancer, and in the later phases of lung cancer progression,
the expression of PCNA is higher (41).
eIF4E is a cap-binding protein (42), which is important for the initial
process of translation in eukaryotes. It is also closely associated
with tumor occurrence, infiltration and metastasis; therefore, it
is highly expressed in human lung cancer and various other
malignancies (43).
In the present study, RT-qPCR results indicated that
the mRNA expression levels of p53 and p21 in the 240 mg/l matrine
group were higher compared with the control group. Additionally,
western blotting revealed that the protein expression levels of p53
and p21 in the 240 mg/l matrine group were also elevated compared
with the control group. Therefore, matrine may promote the
expression of p53 and p21 in A549 cells.
The RT-qPCR results also indicated that the mRNA
expression levels of PCNA and eIF4E in the treatment group were
lower compared with that in the control group. Furthermore, the
reduction of PCNA and eIF4E expression levels following 240 mg/l
matrine treatment, was less than that of the control group.
Therefore, it is possible that matrine inhibits the gene expression
of PCNA and eIF4E in A549 cells.
In conclusion, the findings of the present study
indicated that matrine may inhibit the proliferation of A549 cells.
The underlying mechanism may be associated with the induction of
the expression levels of p53 and p21, and the inhibitory effect of
matrine on the mRNA expression levels of PCNA and eIF4E. PCNA
activity in DNA repair increases resistance to chemotherapy, and
activation of p53 in response to DNA damage is correlated with a
rapid increase in p53 expression level and enhanced p53 binding to
DNA. This leads to the activation of various genes, including p21,
and affects the phosphorylation of the eIF4E binding protein. The
present study provides evidence that matrine-induced apoptosis and
growth inhibition of lung cancer cells may be mediated by p53
activation, suggesting that matrine may serve as an adjuvant
chemotherapeutic agent for lung cancer.
Acknowledgements
The present study was supported by the Science and
Technology Department of Hubei Province (no. 20001P1804), the
Natural Science Foundation of Jiangxi Province (no. 20122BAB205077)
and the Natural Science Youth Foundation of Jiangxi Province (no.
20122BAB215042).
References
|
1
|
Kajatt Amorin E: Lung cancer: A review of
current knowledge, diagnostic methods and therapeutic perspectives.
Rev Peru Med Exp Salud Publica. 30:85–92. 2013.(In Spanish).
PubMed/NCBI
|
|
2
|
Spaans JN and Goss GD: Drug resistance to
molecular targeted therapy and its consequences for treatment
decisions in non-small-cell lung cancer. Front Oncol. 4:1902014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu ZG, Li MH, Wang JS, Wei DD, Liu QW and
Kong LY: Developmental toxicity and neurotoxicity of two
matrine-type alkaloids, matrine and sophocarpine, in zebrafish
(Danio rerio) embryos/larvae. Reprod Toxicol. 47:33–41. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu YQ, Li Y, Qin J, Wang Q, She YL, Luo
YL, He JX, Li JY and Xie XD: Matrine reduces proliferation of human
lung cancer cells by inducing apoptosis and changing miRNA
expression profiles. Asian Pac J Cancer Prev. 15:2169–2177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang CL, Liu SS, Ma YG, Liu YY, Xue YX and
Huang B: The influence of intraoperative pleural perfusion with
matrine-cisplatin or cisplatin on stromal cell-derived factor-1 in
non-small cell lung cancer patients with subclinical pleural
metastasis. Med Oncol. 29:574–581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rogler CE and Chisari FV: Cellular and
molecular mechanisms of hepatocarcinogenesis. Semin Liver Dis.
12:265–278. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong M, Nio Y, Yamasawa K, Toga T, Yue L
and Harada T: p53 alteration is not an independent prognostic
indicator, but affects the efficacy of adjuvant chemotherapy in
human pancreatic cancer. J Surg Oncol. 82:111–120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsui TA, Sowa Y, Murata H, Takagi K,
Nakanishi R, Aoki S, Yoshikawa M, Kobayashi M, Sakabe T, Kubo T and
Sakai T: The plant alkaloid cryptolepine induces p21WAF1/CIP1 and
cell cycle arrest in a human osteosarcoma cell line. Int J Oncol.
31:915–922. 2007.PubMed/NCBI
|
|
9
|
Fecteau JF, Corral LG, Ghia EM, Gaidarova
S, Futalan D, Bharati IS, Cathers B, Schwaederlé M, Cui B,
Lopez-Girona A, et al: Lenalidomide inhibits the proliferation of
CLL cells via a cereblon/p21 (WAF1/Cip1)-dependent mechanism
independent of functional p53. Blood. 124:1637–1644. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeo D, He H, Baldwin GS and Nikfarjam M:
The role of p21-activated kinases in pancreatic cancer. Pancreas.
44:363–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie D, Lan L, Huang K, Chen L, Xu C, Wang
R, Shi Y, Wu X, Wang L, Liu Y and Lu B: Association of p53/p21
expression and cigarette smoking with tumor progression and poor
prognosis in non-small cell lung cancer patients. Oncol Rep.
32:2517–2526. 2014.PubMed/NCBI
|
|
12
|
Freund G, Desplancq D, Stoessel A,
Weinsanto R, Sibler AP, Robin G, Martineau P, Didier P, Wagner J
and Weiss E: Generation of an intrabody-based reagent suitable for
imaging endogenous proliferating cell nuclear antigen in living
cancer cells. J Mol Recognit. 27:549–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halvorsen OJ: Molecular and prognostic
markers in prostate cancer. A study of cell-cycle regulators,
angiogenesis and candidate markers. APMIS. Suppl. 5–62. 2008.
|
|
14
|
Liu Y, Bi T, Wang G, Dai W, Wu G, Qian L,
Gao Q and Shen G: Lupeol inhibits proliferation and induces
apoptosis of human pancreatic cancer PCNA-1 cells through AKT/ERK
pathways. Naunyn Schmiedebergs Arch Pharmacol. 388:295–304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chakravarthy R, Clemens MJ, Pirianov G,
Perdios N, Mudan S, Cartwright JE and Elia A: Role of the eIF4E
binding protein 4E-BP1 in regulation of the sensitivity of human
pancreatic cancer cells to TRAIL and celastrol-induced apoptosis.
Biol Cell. 105:414–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martineau Y, Azar R, Müller D, Lasfargues
C, El Khawand S, Anesia R, Pelletier J, Bousquet C and Pyronnet S:
Pancreatic tumours escape from translational control through 4E-BP1
loss. Oncogene. 33:1367–1374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bi W, Tian M and Row KH: Solid-phase
extraction of matrine and oxymatrine from Sophora flavescens
Ait using amino-imidazolium polymer. J Sep Sci. 33:1739–1745. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho CH, Chuang CY and Chen CF: Study of
the antipyretic activity of matrine. A lupin alkaloid isolated from
Sophora subprostrata. Planta Med. 343–345. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Guo S, Qian D, Qian Y and Duan JA:
Comparative analysis of quinolizidine alkaloids from different
parts of Sophora alopecuroides seeds by UPLC-MS/MS. J Pharm
Biomed Anal. 67–68:16–21. 2012. View Article : Google Scholar
|
|
21
|
Ou X, Chen Y, Cheng X, Zhang X and He Q:
Potentiation of resveratrol-induced apoptosis by matrine in human
hepatoma HepG2 cells. Oncol Rep. 32:2803–2809. 2014.PubMed/NCBI
|
|
22
|
Niu H, Zhang Y, Wu B, Zhang Y, Jiang H and
He P: Matrine induces the apoptosis of lung cancer cells through
downregulation of inhibitor of apoptosis proteins and the Akt
signaling pathway. Oncol Rep. 32:1087–1093. 2014.PubMed/NCBI
|
|
23
|
Li H, Xie S, Liu X, Wu H, Lin X, Gu J,
Wang H and Duan Y: Matrine alters microRNA expression profiles in
SGC-7901 human gastric cancer cells. Oncol Rep. 32:2118–2126.
2014.PubMed/NCBI
|
|
24
|
Zhang S, Cheng B, Li H, Xu W, Zhai B, Pan
S, Wang L, Liu M and Sun X: Matrine inhibits proliferation and
induces apoptosis of human colon cancer LoVo cells by inactivating
Akt pathway. Mol Biol Rep. 41:2101–2108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Wang T, Wen X, Wei Y, Peng X, Li
H and Wei L: Effect of matrine on HeLa cell adhesion and migration.
Eur J Pharmacol. 563:69–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Zhang ZN, Zhao HM, Tong ZC, Yang J,
Wang H and Liang XJ: Matrine inhibits the invasive properties of
human osteosarcoma cells by downregulating the ERK-NF-kB pathway.
Anticancer Drugs. 25:1035–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang S, Qi J, Sun L, Cheng B, Pan S, Zhou
M and Sun X: Matrine induces programmed cell death and regulates
expression of relevant genes based on PCR array analysis in C6
glioma cells. Mol Biol Rep. 36:791–799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie M, He G, Wang R, Shi S, Chen J, Ye Y,
Xie L, Yi X and Tang A: Matrine-induced apoptosis of human
nasopharyngeal carcinoma cells via in vitro vascular endothelial
growth factor-A/extracellular signal-regulated kinase1/2 pathway
inactivation. Horm Metab Res. 46:556–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Keith RL: Chemoprevention of lung cancer.
Proc Am Thorac Soc. 6:187–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saika K and Sobue T: Lung cancer: Progress
in diagnosis and treatments. Topics I. Epidemiology and
pathogenesis; 1. Epidemiology, prevention and screening. Nihon
Naika Gakkai Zasshi. 103:1255–1260. 2014.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nykainen A, Räsänen J, Salo J and Sihvo E:
Thoracoscopic surgery of lung cancer. Duodecim. 130:145–151.
2014.(In Finnish). PubMed/NCBI
|
|
33
|
Pflaum J, Schlosser S and Müller M: p53
family and cellular stress responses in cancer. Front Oncol.
4:2852014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Farnebo M, Bykov VJ and Wiman KG: The p53
tumor suppressor: A master regulator of diverse cellular processes
and therapeutic target in cancer. Biochem Biophys Res Commun.
396:85–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cadwell C and Zambetti GP: The effects of
wild-type p53 tumor suppressor activity and mutant p53
gain-of-function on cell growth. Gene. 277:15–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marone M, Bonanno G, Rutella S, Leone G,
Scambia G and Pierelli L: Survival and cell cycle control in early
hematopoiesis: Role of bcl-2 and the cyclin dependent kinase
inhibitors P27 and P21. Leuk Lymphoma. 43:51–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saegusa M, Hashimura M, Suzuki E, Yoshida
T and Kuwata T: Transcriptional up-regulation of Sox9 by NF-kB in
endometrial carcinoma cells, modulating cell proliferation through
alteration in the p14 (ARF)/p53/p21 (WAF1) pathway. Am J Pathol.
181:684–692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dimitrova N, Zamudio JR, Jong RM, Soukup
D, Resnick R, Sarma K, Ward AJ, Raj A, Lee JT, Sharp PA and Jacks
T: LincRNA-p21 activates p21 in cis to promote Polycomb target gene
expression and to enforce the G1/S checkpoint. Mol Cell.
54:777–790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen W, Yoshida S, Ohara N, Matsuo H,
Morizane M and Maruo T: Gonadotropin-releasing hormone antagonist
cetrorelix down-regulates proliferating cell nuclear antigen and
epidermal growth factor expression and up-regulates apoptosis in
association with enhanced poly (adenosine 5′-diphosphate-ribose)
polymerase expression in cultured human leiomyoma cells. J Clin
Endocrinol Metab. 90:884–892. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao X, Dan S, Xie Y, Qin H, Tang D, Liu X,
He QY and Liu L: 14-3-3ζ reduces DNA damage by interacting with and
stabilizing proliferating cell nuclear antigen. J Cell Biochem.
116:158–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oyama T, Mitsudomi T, Mizoue T, Ohgami A,
Osaki T, Nakanishi R and Yasumoto K: Proliferating cell nuclear
antigen may be superior to argyrophilic nucleolar organizer regions
in predicting shortened survival of patients with non-small cell
lung cancer. Surg Oncol. 4:83–89. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sonenberg N: eIF4E, the mRNA cap-binding
protein: From basic discovery to translational research. Biochem
Cell Biol. 86:178–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Y, Fan S, Koo J, Yue P, Chen ZG,
Owonikoko TK, Ramalingam SS, Khuri FR and Sun SY: Elevated
expression of eukaryotic translation initiation factor 4E is
associated with proliferation, invasion and acquired resistance to
erlotinib in lung cancer. Cancer Biol Ther. 13:272–280. 2012.
View Article : Google Scholar : PubMed/NCBI
|