Introduction
The anthracycline anticancer drug doxorubicin (DOX)
is one of the most effective and frequently used chemotherapeutic
agents in the treatment of a number of human malignancies (1,2).
However, its clinical use is limited by side effects, particularly
cardiotoxicity, which is a major adverse effect. It has been
previously reported that ~11% patients treated with DOX develop
acute cardiotoxicity within 2–3 days of administration, and ~1.7%
patients develop chronic DOX cardiotoxicity (3,4). The
prognosis of patients diagnosed with congestive heart failure is
poor (~50% mortality/year), and there are currently no reliable
pharmacological therapies available (3,4).
Dexrazoxane treatment may limit the cardiotoxic potential of
high-dose DOX treatment (>300 mg/m2), however, this
agent also demonstrates side effects, including second primary
malignancies (5). Therefore, an
improved understanding of the underlying mechanisms associated with
DOX-induced cardiotoxicity is required in order to develop a more
specific and effective treatment with fewer or no side effects.
Estrogen, primarily produced in the ovaries of
premenopausal women, is the primary female sex hormone, which is
responsible for the development and regulation of the female
reproductive system and secondary sex characteristics. The
association between estrogen and the pathogenesis of cardiovascular
diseases has been widely investigated (6–9). The
incidence of cardiovascular disease is lower in premenopausal women
than in men, and significantly increases in postmenopausal women,
which indicates that estrogen may have cardioprotective effects
(6). In animal studies,
ovariectomies inhibited the female-specific protection against
volume-induced cardiac remodeling (7), and attenuated the effect of age on
ventricular remodeling in rats (8). By contrast, estradiol administration
to ovariectomized female rodents attenuated hypertrophy associated
with cardiac pressure overload (9)
and aging (8).
Although the cardioprotective effects of estrogen
have been studied extensively in females, it has not been fully
investigated in males. As in postmenopausal women, men produce
estrogen in a number of extragonadal sites, including fat tissue,
the liver, brain and adrenal glands (10). Estrogen receptors have been
identified in female and male hearts (11–13).
Previous studies have indicated that estrogen may serve an
important role in the maintenance of cardiac structure and function
in men and male animals (14,15).
Clinical results have demonstrated that an imbalance of circulating
estradiol may be associated with increased mortality in men with
chronic systolic heart failure (14). In addition, a previous study
demonstrated that 17β-estradiol (E2) may promote survival in male
mice with cardiomyopathy (15).
The worldwide mortality and morbidity rates for
cancer are high, and DOX is often administered to a greater number
of males than females (16).
Therefore, it is important to investigate the effects of estrogen
on DOX-induced cardiotoxicity in males. The aim of the present
preclinical study was to evaluate the protective effects of E2 on
DOX-induced cardiac injury and the associated mechanisms in male
Sprague-Dawley rats.
Materials and methods
Animals
A total of 26 male Sprague-Dawley rats (age, 14
weeks; average body weight, 402±17 g) were obtained from Shandong
University School of Medicine Laboratory Animal Center (Jinan,
China). All animal study protocols were approved by the
Institutional Animal Research and Ethics Committee of Shandong
University. The animals were housed at 2 rats/cage in a
light-controlled environment at 18–22°C, with 12 h light/dark
cycles, 50±15% humidity, and with access to food and water ad
libitum throughout the experimental period.
Experimental protocol
Rats were randomly assigned into the following three
groups: The control group (n=9), the DOX-treated group (DOX–V
group; n=8), and the DOX plus E2-treated group (DOX-E2; n=9).
Doxorubicin hydrochloride (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was dissolved in saline, and 4 mg/2 ml/kg body weight was
administered by intraperitoneal injection twice per week for 2
weeks, with a cumulative dose of 16 mg/kg. Age-matched rats
injected with saline (2 ml/kg) were used as controls. A total of 2
mg/kg body weight/day E2 (water soluble; Sigma-Aldrich; Merck KGaA)
was administered subcutaneously, commencing at 3 days prior to DOX
treatment until sacrifice. Rats were then maintained in animal
housing until 3 weeks following the first DOX injection.
At the end of the experiment, and following
echocardiographic evaluation, rats were euthanized at 3 weeks
following the first DOX injection, via exsanguination by cardiac
puncture while under ketamine/xylazine anesthesia (ketamine HCL 60
mg/kg; xylazine HCL 5 mg/kg; Sigma-Aldrich; Merck KGaA). The serum
was used to examine the different parameters associated with
cardiac damage. Whole hearts were isolated and further dissected to
isolate the left ventricle. Tissue weights and tibial lengths were
measured using an analytical scale and a micrometer, respectively.
The left ventricle was divided into sections for RNA, western blot,
and histological analyses.
Evaluation of left ventricular
systolic function by M-mode echocardiography
Rats were anesthetized with 80 mg/kg ketamine and 12
mg/kg xylazine mixture at 1 week following the last injection of
DOX. The chest was shaved, and the animals were positioned on their
left side. Diastolic interventricular septum thickness (IVSTd),
systolic interventricular septum thickness (IVSTs), ejection
fraction (EF) and fractional shortening (FS) parameters were
measured as described previously (17), using a Philips 5500
echocardiography system and a 12 MHz phased array probe (Philips
Medical Systems, Inc., Bothell, WA, USA).
Cardiac injury-associated blood
biomarkers
Different parameters associated with cardiac injury,
including serum alanine aminotransferase (ALT), aspartate
aminotransferase (AST), lactate dehydrogenase (LDH) and creatine
kinase (CK) were assayed using commercial kits from Sigma-Aldrich
(Merck KGaA) as follows: ALT (cat. no. MAK052), AST (cat. no.
MAK055), LDH (cat. no. MAK066), and CK activity assay kits (cat no.
MAK116). Enzyme activities were measured according to the
manufacturer's instructions using a SpectraMax M2e microplate
reader (Molecular Devices, Sunnyvale, CA, USA), and expressed as
international units (U/l).
Histological examination of
tissues
The left ventricle sections were fixed in 10%
formalin at room temperature for 24 h, dehydrated through a graded
alcohol series and embedded in paraffin wax. Paraffin sections were
cut into 4 µm thick sections, and were deparaffinized by immersion
in xylene and rehydrated. Slides were stained with hematoxylin and
eosin (H&E) at room temperature (0.5% haematoxylin for 5 min
and 0.1% eosin for 1 min), dehydrated using a graded alcohol
series, immersed in xylene, and mounted for histological
examination using a Zeiss Axiophot microscope (Zeiss AG,
Oberkochen, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to measure mRNA levels in the
tissues. Total RNA was extracted from frozen left ventricles using
TRIzol reagent (Thermo Fisher Scientific, Inc. Waltham, MA, USA),
and processed according to the manufacturer's recommendations.
Complementary first strand DNA was synthesized from 2 µg of
oligo(dT)-primed total RNA, using the Omniscript RT kit (Qiagen,
Inc., Valencia, CA, USA). Relative quantification of mRNA levels by
RT-qPCR was performed using a SYBR green PCR kit (Qiagen, Inc.).
Amplification and detection were performed using the ABI 7500
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). PCR amplification conditions were as follows:
Denaturation at 95°C for 15 min followed by 40 cycles of
denaturation at 94°C for 15 sec, annealing at 60°C for 1 min and
extension at 72°C for 30 sec. The PCR products were quantified
using quantification cycle (Cq) values, which were
defined as the fractional cycle number at which the fluorescence
signal exceeded a fixed threshold. ΔCq represented the
difference in expression between the target gene and the GAPDH
endogenous control. The normalized relative target mRNA level
(ΔΔCq) was calculated using the following equation:
ΔΔCq = ΔCq (treated animals)-ΔCq
(control animals). The normalized relative target mRNA level in
each sample was calculated as 2−∆∆Cq (18). RT-qPCR was performed in duplicate
and a non-template control was included in each run to test for
contamination. Sequence-specific oligonucleotide primers were
designed according to published GenBank sequences (https://www.ncbi.nlm.nih.gov/genbank;
Table I).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer | Primer
sequence | Product size
(bp) | Accession
number |
|---|
| NOX2 | Sense |
5′-GTGGAGTGGTGTGTGAATGCC-3′ | 164 | NM_023965 |
|
| Antisense |
5′-ATGCCAGCCAACCGAGTCACA-3′ |
|
|
| NOX4 | Sense |
5′-CTGCATCTGTCCTGAACCTCAA-3′ | 101 | XM_008759643 |
|
| Antisense |
5′-TCTCCTGCTAGGGACCTTCTGT-3′ |
|
|
| BAX | Sense |
5′-GAGCGGCTGCTTGTCTGGAT-3′ | 161 | NM_017059 |
|
| Antisense |
5′-CAAGGCAGCAGGAAGCCTCA-3′ |
|
|
| Caspase 3 | Sense |
5′-GACTGCGGTATTGAGACAGA-3′ | 209 | NM_012922 |
|
| Antisense |
5′-CGAGTGAGGATGTGCATGAA-3′ |
|
|
| GAPDH | Sense |
5′-GGCAAGTTCAATGGCACAGT-3′ | 151 | NM_017008 |
|
| Antisense |
5′-TGGTGAAGACGCCAGTAGACTC-3′ |
|
|
Western blot analysis
Left ventricle homogenates were prepared using
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Protein samples (20 µg) were
separated by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes for western blot analysis. Immunoblots were
probed using antibodies against nicotinamide adenine dinucleotide
phosphate oxidase (NOX) 2 (1:170 dilution; cat. no. ab129068;
Abcam, Cambridge, UK), NOX4 (1:200 dilution; cat. no. ab109225;
Abcam), B-cell lymphoma 2-associated X protein (BAX; 1:200
dilution; cat. no. sc-6236; Santa Cruz Biotechnology, Inc.), and
caspase 3 (1:200 dilution; cat. no. sc-70497; Santa Cruz
Biotechnology, Inc.). GAPDH (1:1,000 dilution; cat. no. ab8245;
Abcam) was used as a loading control. All the antibodies were
incubated with membranes overnight at 4°C. Horseradish
peroxidase-conjugated secondary antibodies (1:1,000 dilution; cat.
no. ab6721 for rabbit, cat. no. ab6728 for mouse; Abcam) were
incubated at room temperature for 1 h and immune complexes were
visualized by an enhanced chemiluminescence (ECL) Western Blotting
Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The bands were digitized using MCID image analysis software
(version 7.0; InterFocus Imaging Ltd., Cambridge, UK). The density
of each band was expressed in arbitrary units and normalized to
that of GAPDH.
Statistical analysis
Data are presented as the mean ± standard error. For
all endpoints, one-way analysis of variance was used to determine
the significant differences among groups. The significance of
interactions among the groups was determined using Tukey's post-hoc
tests. Statistical analyses were performed using GraphPad Prism 6.0
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
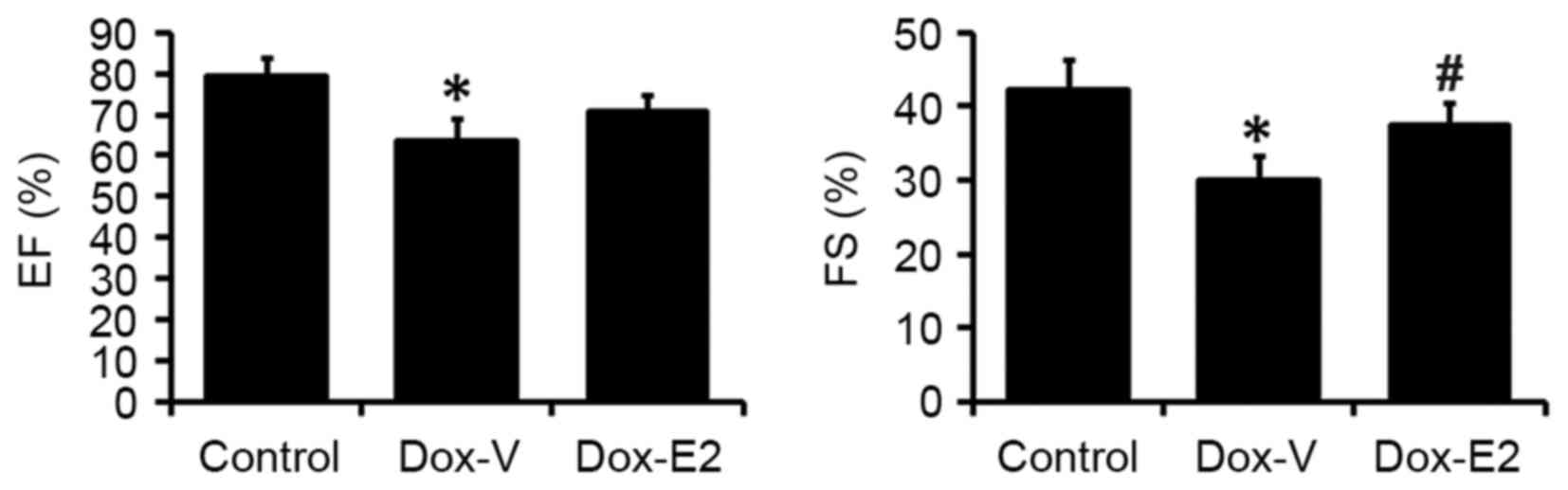
Estrogen attenuates DOX-induced
systolic dysfunction
As shown in Fig. 1,
the echocardiograph results demonstrated that chronic DOX
administration significantly decreased cardiac EF by ~20%
(P<0.05) when compared with the control. The DOX-induced
decrease in cardiac EF was attenuated by E2 treatment (Fig. 1). Similarly, cardiac FS
significantly decreased in the DOX-treated rats by 29% (P<0.05)
when compared to vehicle-treated rats. This effect was
significantly attenuated prevented by E2 treatment (P<0.05;
Fig. 1).
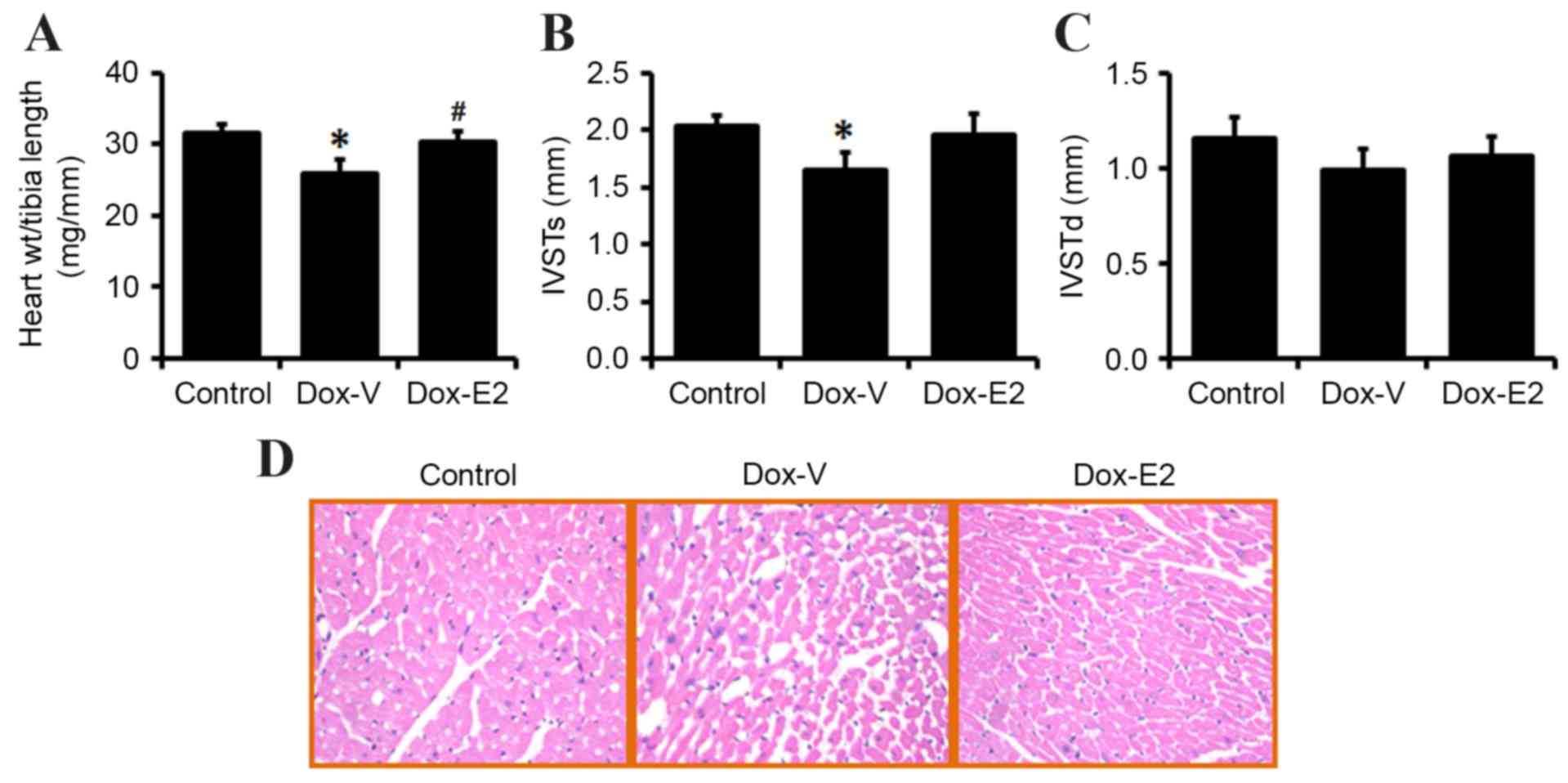
Heart weight, structure and
histological alterations
Heart weight, corrected by tibial length decreased
by 18% in DOX vs. vehicle control-treated rats (P<0.05), and E2
treatment significantly restored heart weight (P<0.05; Fig. 2A). Similarly, the echocardiograph
results demonstrated that IVSTs significantly decreased in the
DOX-treated group when compared with control rats (1.64±0.15 vs.
2.03±0.1 mm; P<0.05; Fig. 2B).
However, the decrease in IVSTd exhibited by DOX-treated rats was
not significantly different when compared with the controls
(0.99±0.11 vs. 1.15±0.12 mm; P=0.13; Fig. 2C). The decrease in IVSTs and IVSTd
induced by DOX treatment were inhibited by E2 treatment, however,
this did not reach statistical significance (Fig. 2B and C).
Histopathological evaluation by H&E staining of
cardiac tissues revealed that DOX induced disorganization of
myofibrillar morphology, myofibrillar loss in ~80% cells and
cytoplasmic vacuolization (Fig.
2D). In rats treated with DOX and supplemented with E2,
histopathological examination with H&E staining revealed
similar myocardial fibers and architecture to that observed in the
control rats (Fig. 2D).
Serum biomarkers for cardiac
injury
Consistent with the echocardiography results and
histopathological evaluations, all serum biomarkers for cardiac
injury assayed in the present study, including ALT, AST, LDH and
CK, were significantly increased by 63 and 150% in the DOX-treated
group when compared with vehicle control-treated rats (P<0.05;
Fig. 3). This effect was
significantly attenuated by E2 treatment (P<0.05; Fig. 3).
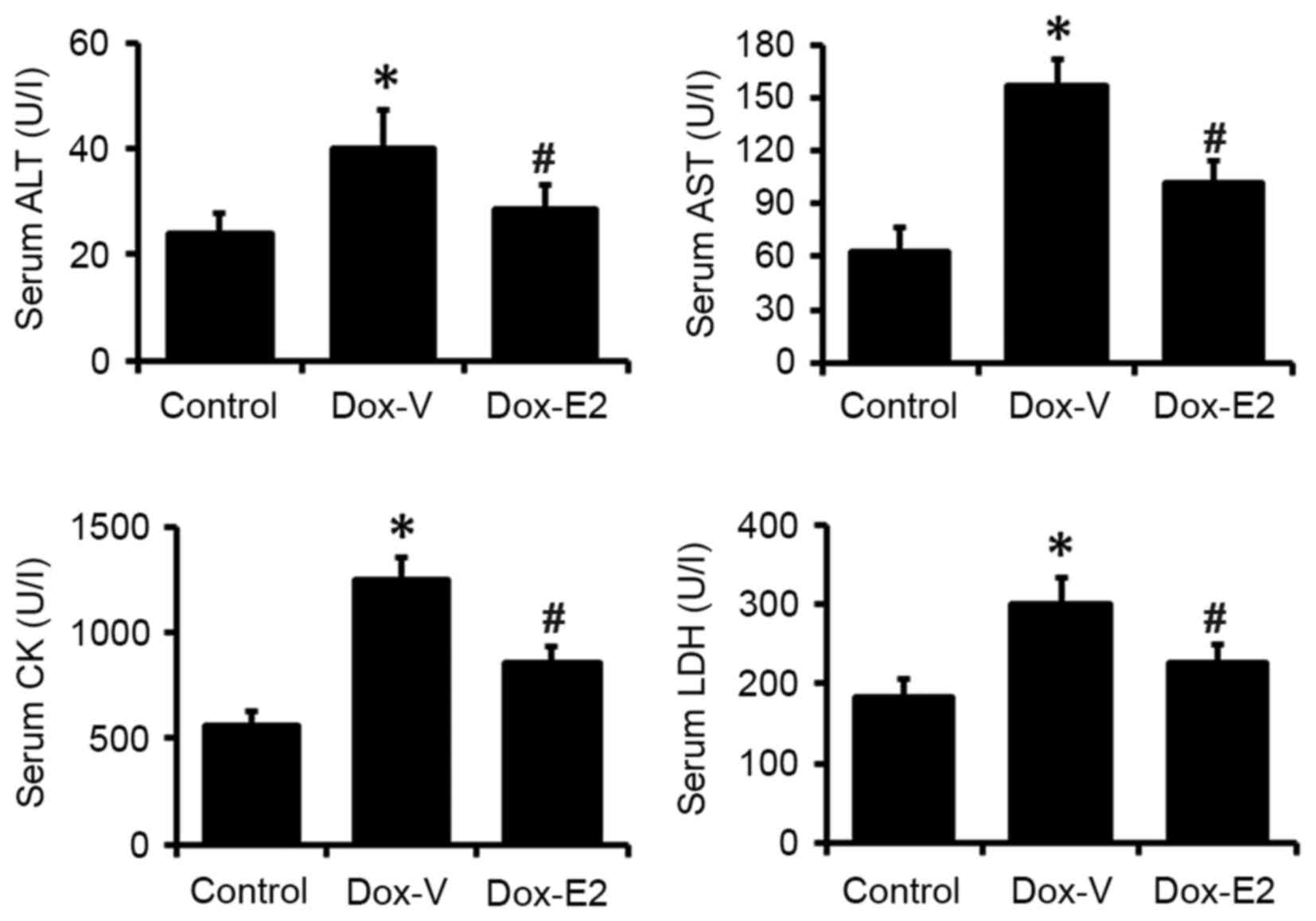 | Figure 3.Level of serum biomarkers, ALT, AST,
CK and LDH, for cardiac injury. Values are presented as the mean ±
standard error (n=8/9). *P<0.05 vs. control;
#P<0.05 vs. DOX-V-treated group. ALT, alanine
aminotransferase; AST, aspartate aminotransferase; LDH, lactate
dehydrogenase, CK, creatine kinase; DOX, doxorubicin; DOX-V, DOX +
vehicle treatment; E2, 17β-estradiol. |
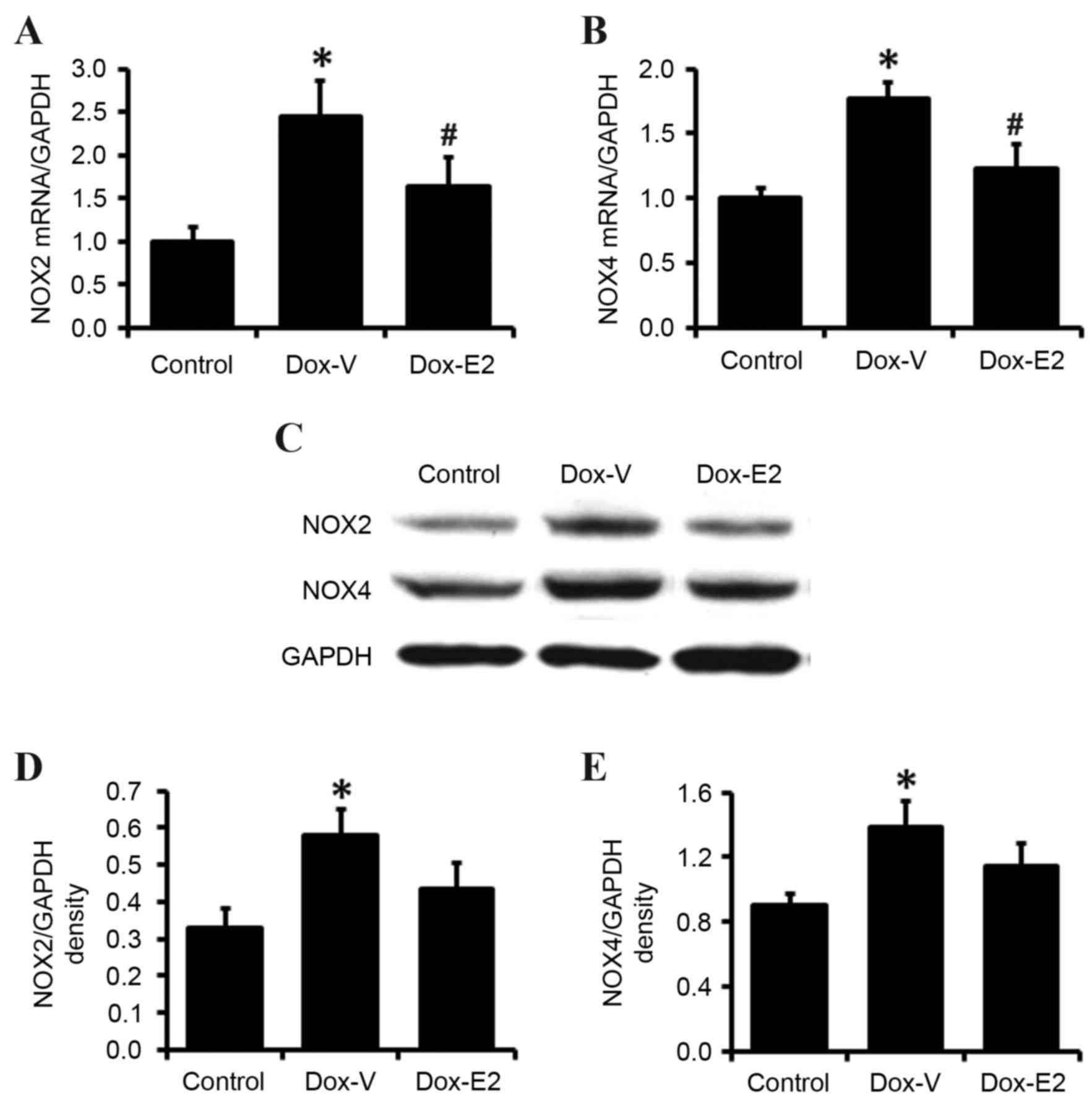
Cardiac NOX2 and NOX4 gene
expression
In order to investigate the mechanisms underlying
the cardioprotective effects of E2 on DOX-induced cardiotoxicity
further, NOX2 and NOX4 gene expression was measured at the mRNA and
protein levels by RT-qPCR and western blot analyses, respectively.
As shown in Fig. 4A and B, cardiac
NOX2 and NOX4 mRNA levels increased by 144 and 77% in DOX-treated
rats, respectively, when compared to control rats (P<0.05). E2
treatment significantly attenuated the DOX-associated increase in
cardiac NOX2 and NOX4 mRNA levels (P<0.05; Fig. 4A and B). These results were
confirmed by western blot analysis, which demonstrated that E2
inhibited DOX-induced increases in cardiac NOX2 and NOX4 protein
expression levels (Fig. 4C-E).
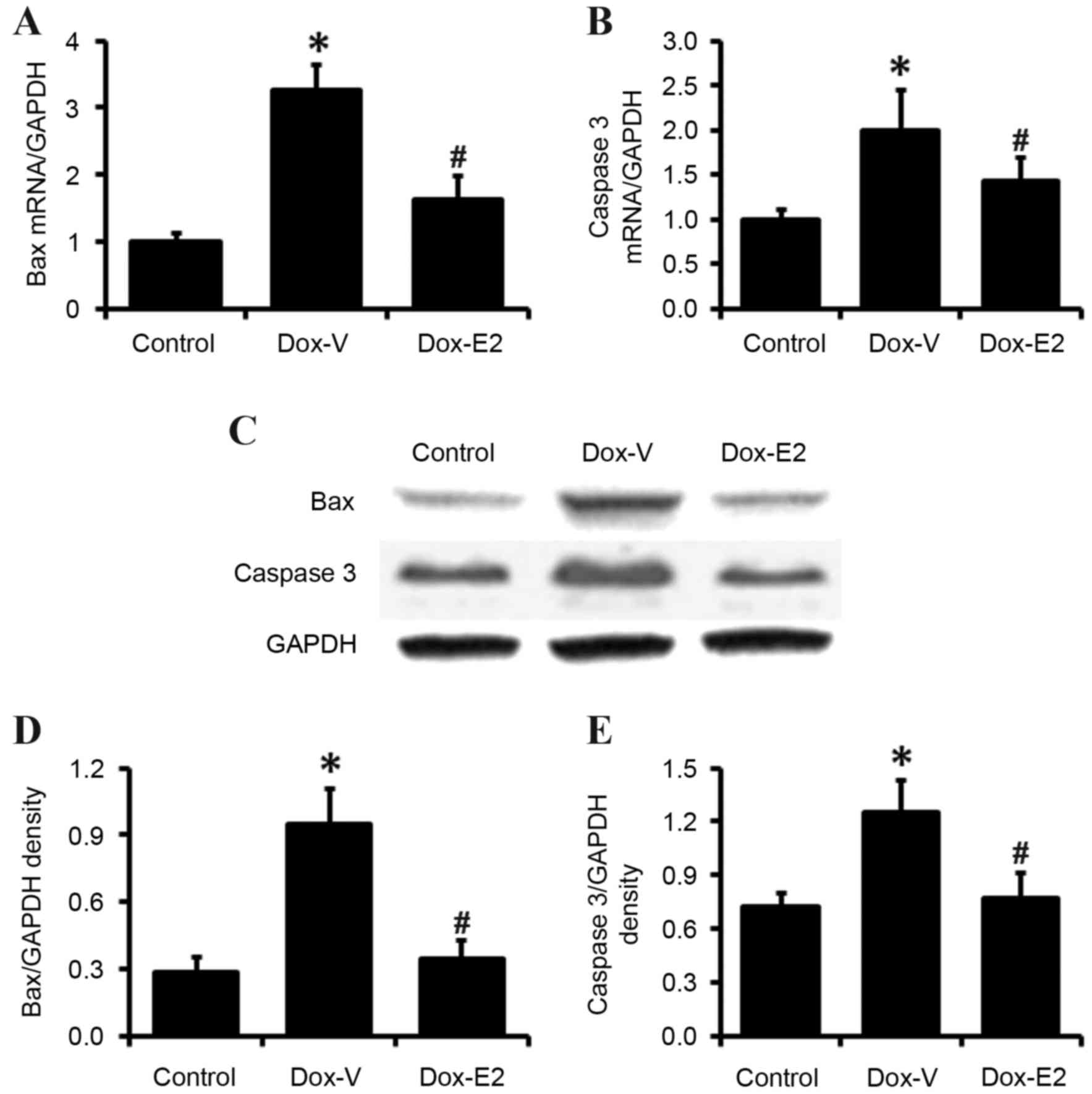
Cardiac BAX and caspase 3 gene
expression
The expression levels of the apoptosis-associated
genes BAX and caspase 3 were measured in the heart tissues of rats
from all experimental groups. As expected, RT-qPCR and western blot
results demonstrated that DOX significantly increased BAX and
caspase 3 mRNA and protein expression levels in heart tissues
(P<0.05; Fig. 5), which was
significantly inhibited by E2 treatment (P<0.05; Fig. 5).
Discussion
DOX, an effective anthracycline antitumor
antibiotic, is used extensively to treat a number of malignancies,
including rhabdomyosarcoma, acute leukemia and Hodgkin's disease
(1,2). However, due to its serious
cardiotoxic effects, DOX has limited clinical use (3,4).
Symptomatic congestive heart failure is the most severe
complication of DOX-based chemotherapy, with an incidence rate of 5
to 48%, depending on the cumulative dose received (3,4).
Therefore, understanding the underlying mechanisms of DOX-induced
cardiotoxicity is critical for the development of specific and
effective strategies to target this life-threatening side effect of
cancer treatment. In addition, investigating the effect of estrogen
on DOX-induced cardiac injury in males is of greater importance, as
the use of DOX, and cancer mortality and morbidity rates are
greater in males than in females (16). The results of the present study
have provided novel and significant evidence that E2 treatment may
protect the heart from DOX-induced cardiotoxicity in male rats.
These cardioprotective effects of E2 may be mediated via the
regulation of NOX2, NOX4 and apoptosis-associated genes. The
results may provide sufficient experimental evidence to support the
introduction of monitoring circulating estrogen levels in male
patients undergoing treatment with DOX.
The cardioprotective effects of estrogen in females
have been investigated in detail in clinical and experimental
studies (19,20). Similar to females, estrogen is
produced in males, and its receptors have been detected in the
hearts of male animals and humans (11–13).
However, few studies conducted thus far, have determined the role
of estrogen in the male heart. It is possible that the abnormal
circulating levels of estrogen in men may contribute to the
pathogenic processes and prognoses of a variety of cardiac
diseases. A clinical study involving 501 male patients with chronic
heart failure demonstrated that low and high concentrations of
circulating estradiol were significant predictors of a poor
prognosis, independent of gonadal and adrenal androgen deficiencies
and conventional clinical prognostic indicators (14). A prospective population-based study
with a 4.5-year follow-up period, involving healthy elderly men
aged 69–80 years, demonstrated that elderly men with low serum
estradiol levels exhibited a significantly increased risk of
mortality (21). These results
reveal the importance of estrogen levels in healthy men,
particularly elderly men, and in male patients with cardiac
diseases. In the present study, the dose of E2 used was based on
previous reports (22,23). However, more studies are needed to
determine the dose-response and the estrogen levels in male animals
under physiological and pathological conditions. Chronic E2
treatment at the dose of 2 mg/kg body weight/day attenuated the
DOX-induced cardiac systolic dysfunction associated with oxidative
stress and alterations to apoptosis-associated gene expression in
male rats. The present study indicates that the focus of future
research should involve investigating estrogen levels in male
patients undergoing DOX treatment. In addition, further studies may
be necessary to investigate the potential of estrogen-mediated
pathways as a novel target for treating DOX-induced
cardiotoxicity.
The major mechanism of DOX-induced cardiotoxicity is
associated with the excessive generation of myocardial reactive
oxygen species (ROS) and oxidative stress (24–26).
Experimental and clinical studies have demonstrated that the
adverse cardiac effects of DOX are inhibited by exogenous
antioxidant treatment or overexpression of an endogenous
antioxidant enzyme (27). In
addition, the major source of ROS in the heart is known to be NOX
(28,29) and among the 7 isoforms of this
enzyme, NOX2 and NOX4 are the primary isoforms expressed in the
heart (30). These isoforms have
been demonstrated to participate in DOX-induced cardiac ROS
generation and heart failure (31,32).
It has been reported that oxidative stress modulates a number of
key processes underlying DOX-induced cardiotoxicity, including
extracellular matrix remodeling, cardiomyocyte apoptosis and
altered cardiac contractile properties (33–35).
In the present study, E2 treatment inhibited the DOX-induced
increase in NOX2 and NOX4 expression in the heart, which may
underlie its cardioprotective effects in this male rat model of
cardiac injury. This result is consistent with previous findings
observed in females, which suggests that estrogen, as an important
antioxidant molecule, affects NOX gene expression and activity in
animal models (36–38).
NOX-dependent ROS production induces cardiomyocyte
apoptosis, which may contribute to DOX-induced cardiotoxicity
(31,39,40).
As matured myocardial cells are terminally differentiated cells and
are unlikely to regenerate when suffering from lethal injury
(41), excessive apoptosis induces
a decrease in myocardial cells and leads to cardiac dysfunction,
which eventually progresses to heart failure. In the present study,
DOX decreased heart weight and echocardiography-derived IVSTs, of
which the mechanism might be due to the DOX-induced cardiomyocyte
apoptosis. While apoptosis was not directly determined in this
study, expression levels of apoptosis-related proteins BAX and
caspase 3 were increased following DOX treatment. Treatment with E2
attenuated these DOX-induced alterations in male rats, suggesting
that E2 may protect the heart from DOX-induced cardiotoxicity
potentially via the inhibition of the activated cardiac
NOX/ROS/apoptosis pathway induced by DOX treatment.
Estrogen is produced and binds to associated
receptors in cardiac tissues in males as well as females. However,
the role of estrogen in healthy men, and in the pathogenesis and
prognosis of cardiac diseases may be severely underestimated. The
present study demonstrated that E2 treatment inhibited DOX-induced
cardiotoxicity in male rats, and was associated with decreased
cardiac NOX2, NOX4 and apoptosis gene expression. The results may
provide a greater understanding of the underlying mechanisms of
DOX-induced cardiotoxicity in males and provide experimental
evidence for novel therapeutic approaches involving
estrogen-mediated pathways in the male heart.
Acknowledgements
The present study was funded by The National Natural
Science Foundation of China (grant no. 81270175) and The Doctoral
Research Grant of Shandong Province (grant no. BS2010YY005).
References
|
1
|
Batty N, Hagemeister FB, Feng L, Romaguera
JE, Rodriguez MA, McLaughlin P, Samaniego F, Copeland A, Dabaja BS
and Younes A: Doxorubicin, bleomycin, vinblastine and dacarbazine
chemotherapy with interferon for advanced stage classic Hodgkin
lymphoma: A 10-year follow-up study. Leuk Lymphoma. 53:801–806.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogilvie CM, Crawford EA, Slotcavage RL,
King JJ, Lackman RD, Hartner L and Staddon AP: Treatment of adult
rhabdomyosarcoma. Am J Clin Oncol. 33:128–131. 2010.PubMed/NCBI
|
|
3
|
Carvalho FS, Burgeiro A, Garcia R, Moreno
AJ, Carvalho RA and Oliveira PJ: Doxorubicin-induced
cardiotoxicity: From bioenergetic failure and cell death to
cardiomyopathy. Med Res Rev. 34:106–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Y, Moon M, Dawood S, McManus B and Liu
PP: Mechanisms and management of doxorubicin cardiotoxicity. Herz.
36:296–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Langer SW: Dexrazoxane for the treatment
of chemotherapy-related side effects. Cancer Manag Res. 6:357–363.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Molix L: Sex differences in cardiovascular
health: Does sexism influence women's health? Am J Med Sci.
348:153–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brower GL, Gardner JD and Janicki JS:
Gender mediated cardiac protection from adverse ventricular
remodeling is abolished by ovariectomy. Mol Cell Biochem.
251:89–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Arenas IA, Armstrong SJ and Davidge
ST: Estrogen modulation of left ventricular remodeling in the aged
heart. Cardiovasc Res. 57:388–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Eickels M, Grohé C, Cleutjens JP,
Janssen BJ, Wellens HJ and Doevendans PA: 17beta-estradiol
attenuates the development of pressure-overload hypertrophy.
Circulation. 104:1419–1423. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nelson LR and Bulun SE: Estrogen
production and action. J Am Acad Dermatol. 45:(3 Suppl). S116–S124.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ropero AB, Eghbali M, Minosyan TY, Tang G,
Toro L and Stefani E: Heart estrogen receptor alpha: Distinct
membrane and nuclear distribution patterns and regulation by
estrogen. J Mol Cell Cardiol. 41:496–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murphy E: Estrogen signaling and
cardiovascular disease. Circ Res. 109:687–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kunnas TA, Laippala P, Penttilä A,
Lehtimäki T and Karhunen PJ: Association of polymorphism of human
alpha oestrogen receptor gene with coronary artery disease in men:
A necropsy study. BMJ. 321:273–274. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jankowska EA, Rozentryt P, Ponikowska B,
Hartmann O, Kustrzycka-Kratochwil D, Reczuch K, Nowak J,
Borodulin-Nadzieja L, Polonski L, Banasiak W, et al: Circulating
estradiol and mortality in men with systolic chronic heart failure.
JAMA. 301:1892–1901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kadokami T, McTiernan CF, Higuichi Y, Frye
CS, Kubota T and Feldman AM: 17 Beta-estradiol improves survival in
male mice with cardiomyopathy induced by cardiac-specific tumor
necrosis factor-alpha overexpression. J Interferon Cytokine Res.
25:254–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cook MB, McGlynn KA, Devesa SS, Freedman
ND and Anderson WF: Sex disparities in cancer mortality and
survival. Cancer Epidemiol Biomarkers Prev. 20:1629–1637. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Da Silva J, Alencar A, Zapata-Sudo
G, Lin MR, Sun X, Ahmad S, Ferrario CM and Groban L: Mast cell
inhibition attenuates cardiac remodeling and diastolic dysfunction
in middle-aged, ovariectomized Fischer344 × Brown Norway rats. J
Cardiovasc Pharmacol. 68:49–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blenck CL, Harvey PA, Reckelhoff JF and
Leinwand LA: The importance of biological sex and estrogen in
rodent models of cardiovascular health and disease. Circ Res.
118:1294–1312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knowlton AA and Korzick DH: Estrogen and
the female heart. Mol Cell Endocrinol. 389:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tivesten A, Vandenput L, Labrie F,
Karlsson MK, Ljunggren O, Mellström D and Ohlsson C: Low serum
testosterone and estradiol predict mortality in elderly men. J Clin
Endocrinol Metab. 94:2482–2488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balakrishnan B, Chiplunkar SV and Indap
MM: Methanol extract of euchelus asper prevents bone resorption in
ovariectomised mice model. J Osteoporos. 2014:3481892014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi M, Ma L, Zhou L and Fu P: Renal
protective effects of 17β-estradiol on mice with acute aristolochic
acid nephropathy. Molecules. 21:pii: E1391. 2016. View Article : Google Scholar
|
|
24
|
Ghosh J, Das J, Manna P and Sil PC: The
protective role of arjunolic acid against doxorubicin induced
intracellular ROS dependent JNK-p38 and p53-mediated cardiac
apoptosis. Biomaterials. 32:4857–4866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han XZ, Gao S, Cheng YN, Sun YZ, Liu W,
Tang LL and Ren DM: Protective effect of naringenin-7-O-glucoside
against oxidative stress induced by doxorubicin in H9c2
cardiomyocytes. Biosci Trends. 6:19–25. 2012.PubMed/NCBI
|
|
26
|
Ichihara S, Yamada Y, Kawai Y, Osawa T,
Furuhashi K, Duan Z and Ichihara G: Roles of oxidative stress and
Akt signaling in doxorubicin cardiotoxicity. Biochem Biophys Res
Commun. 359:27–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mut-Salud N, Álvarez PJ, Garrido JM,
Carrasco E, Aránega A and Rodríguez-Serrano F: Antioxidant intake
and antitumor therapy: Toward nutritional recommendations for
optimal results. Oxid Med Cell Longev. 2016:67195342016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bao MH, Dai W, Li YJ and Hu CP:
Rutaecarpine prevents hypoxia-reoxygenation-induced myocardial cell
apoptosis via inhibition of NADPH oxidases. Can J Physiol
Pharmacol. 89:177–186. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YS, He L, Liu B, Li NS, Luo XJ, Hu
CP, Ma QL, Zhang GG, Li YJ and Peng J: A novel pathway of NADPH
oxidase/vascular peroxidase 1 in mediating oxidative injury
following ischemia-reperfusion. Basic Res Cardiol. 107:2662012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nabeebaccus A, Zhang M and Shah AM: NADPH
oxidases and cardiac remodelling. Heart Fail Rev. 16:5–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilleron M, Marechal X, Montaigne D,
Franczak J, Neviere R and Lancel S: NADPH oxidases participate to
doxorubicin-induced cardiac myocyte apoptosis. Biochem Biophys Res
Commun. 388:727–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao Y, McLaughlin D, Robinson E, Harvey
AP, Hookham MB, Shah AM, McDermott BJ and Grieve DJ: Nox2 NADPH
oxidase promotes pathologic cardiac remodeling associated with
Doxorubicin chemotherapy. Cancer Res. 70:9287–9297. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spallarossa P, Altieri P, Garibaldi S,
Ghigliotti G, Barisione C, Manca V, Fabbi P, Ballestrero A,
Brunelli C and Barsotti A: Matrix metalloproteinase-2 and −9 are
induced differently by doxorubicin in H9c2 cells: The role of MAP
kinases and NAD(P)H oxidase. Cardiovasc Res. 69:736–745. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siwik DA, Pagano PJ and Colucci WS:
Oxidative stress regulates collagen synthesis and matrix
metalloproteinase activity in cardiac fibroblasts. Am J Physiol
Cell Physiol. 280:C53–C60. 2001.PubMed/NCBI
|
|
35
|
Grieve DJ, Byrne JA, Siva A, Layland J,
Johar S, Cave AC and Shah AM: Involvement of the nicotinamide
adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac
contractile dysfunction occurring in response to pressure overload.
J Am Coll Cardiol. 47:817–826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sumi D, Hayashi T, Matsui-Hirai H, Jacobs
AT, Ignarro LJ and Iguchi A: 17beta-estradiol inhibits NADPH
oxidase activity through the regulation of p47phox mRNA and protein
expression in THP-1 cells. Biochim Biophys Acta. 1640:113–118.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller AA, Drummond GR, Mast AE, Schmidt
HH and Sobey CG: Effect of gender on NADPH-oxidase activity,
expression, and function in the cerebral circulation: Role of
estrogen. Stroke. 38:2142–2149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang QG, Raz L, Wang R, Han D, De Sevilla
L, Yang F, Vadlamudi RK and Brann DW: Estrogen attenuates ischemic
oxidative damage via an estrogen receptor alpha-mediated inhibition
of NADPH oxidase activation. J Neurosci. 29:13823–13836. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hosseinzadeh L, Behravan J, Mosaffa F,
Bahrami G, Bahrami A and Karimi G: Curcumin potentiates
doxorubicin-induced apoptosis in H9c2 cardiac muscle cells through
generation of reactive oxygen species. Food Chem Toxicol.
49:1102–1109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kazama K, Okada M and Yamawaki H:
Adipocytokine, omentin inhibits doxorubicin-induced H9c2
cardiomyoblasts apoptosis through the inhibition of mitochondrial
reactive oxygen species. Biochem Biophys Res Commun. 457:602–607.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Claycomb WC and Palazzo MC: Culture of the
terminally differentiated adult cardiac muscle cell: A light and
scanning electron microscope study. Dev Biol. 80:466–482. 1980.
View Article : Google Scholar : PubMed/NCBI
|