Introduction
Cataracts account for the most common cause of
blindness worldwide, affecting over half (51.8%) of all individuals
with blindness, which renders it one of the most prevalent eye
diseases (1). It has been accepted
that age is the most important risk factor for cataracts, followed
by environmental and genetics factors. Currently, there are three
primary types of age-related cataracts, which differ in optical
age, size, density, shape, color and location (2). The cataracts most associated with age
are cortical cataracts, in which the initial opacity is confined to
the outer lens shells. Cortical cataracts are more common in
individuals aged >65 years (3).
In nuclear cataracts, the opacity first appears in the center of
the lens, which is the second most prevalent group. The age at
which nuclear cataracts are most common is also >65 years. The
third types of cataracts are posterior subcapsular cataracts (PSC),
a less common group, which lie in the outermost layers of the
cortex at the posterior pole.
In the last few decades, an increasing number of
studies have been performed to investigate the developmental
mechanism of cataracts to identify potential prevention and
treatment strategies (4–6). Sufficient evidence has demonstrated
that, in addition to aging, genetics and environmental factors,
including UV exposure and X-ray irradiation, are involved in
cataract development. For example, Ji et al (2015) found
that prolonged UV exposure can increase cataract risk (7). Other environmental factors, including
diabetes, glaucoma and myopia, are also important in cataract
development (8). For example,
diabetes is reported to be associated with age-related PSCs and
cortical cataracts, but not nuclear cataracts (9). However, cigarette smoking has
consistently been confirmed as a risk factor for nuclear cataracts,
and possibly PSCs, but not cortical cataracts. In another previous
report, diabetes was identified to be associated with the
development of PSCs, and cigarette smoking may increase the risk of
the formation of nuclear cataracts (10). The additional effects of these
factors in cataract development have also been investigated. For
example, in a report by Pan et al (2013), myopia,
particularly high myopia, was determined to predispose to PSCs
(11).
To date, proteome analyses have been used in various
ocular diseases, including diabetic retinopathy, glaucoma and
cataracts. It allows the simultaneous identification of a large
number of proteins at a given time in specific cells, tissues or
body fluids, which aims to identify proteomic peptide biomarkers of
disease for the diagnosis of eye diseases, and elucidating the
disease mechanism for providing novel therapies (12–16).
For example, Izzotti et al (6) investigated the association between
aqueous humor (AH) protein content and the pathogenesis of
open-angle glaucoma (POAG). It was determined that certain proteins
underwent marked variation, which was associated with pathogenetic
events characterizing POAG, including oxidative damage,
mitochondrial damage, neural degeneration and apoptosis. These
results not only indicated that proteomic analysis of the AH is a
novel tool for POAG diagnosis, but provided an improved
understanding of the mechanisms involved in the pathogenesis of
POAG.
There have been several proteomic investigations of
ocular fluids, including AH, using two-dimensional gel
electrophoresis (2-DE). However, this traditional technology is
known to be poor for analyzing low quantities of protein (17). However, one type of high throughput
technology, termed isobaric tagging for relative and absolute
protein quantification (iTRAQ) labeling combined with mass
spectrometry (MS) has been development for protein identification
and relative quantification (18).
Therefore, the present study aimed to take advantage of this
technology to perform proteomic profiling of AH from three types of
patient and their controls using iTRAQ combined with reversed-phase
liquid chromatography (RPLC)/RPLC-MS. The results revealed marked
proteomic differences between the controls and patients with high
myopia, glaucoma or diabetes. Of note, 44 cataract-associated
proteins were determined, five of which were randomly selected and
identified using enzyme-linked immunosorbent assays (ELISA). The
biological functions of these 44 cataract-associated proteins were
analyzed using Gene Ontology (GO)/pathway annotation.
Protein-protein interaction network analysis was also performed to
incorporate known evidence of physical/functional interactions. The
results aimed to provide potential AH biomarkers and offer insights
into the mechanisms underlying cataract development associated with
myopia, glaucoma and diabetes.
Materials and methods
Subjects
A total of 24 subjects in four groups were recruited
for the present study, including six patients with high myopia, six
patients with glaucoma, six patients with diabetes and six control
patients (Table I). All patients
were of Asian ethnicity and all patients with glaucoma were
determined to have POAG, whose intraocular pressure was controlled
between 10 and 21 mm Hg by topical intraocular pressure-lowering
medications. The cup-to-disc ratios of these patients were
significantly increased, and they had early and middle visual field
defects. The patients with diabetes were treated via oral
administration of drugs to control blood sugar levels (<6.1
mM/l), with no microangiopathy and no insulin therapy. The control
groups were age-related patients with cataracts but without
glaucoma, high myopia or diabetes.
 | Table I.Summary of human aqueous humor
samples. |
Table I.
Summary of human aqueous humor
samples.
| Group (Labeled
ID) | Patient ID | Gender | Age (Years
old) | Axial length | LOCSIII |
|---|
| Controls (117) | 1 | Male | 73 | 25.29 | 3 |
|
| 2 | Female | 78 | 24.78 | 4 |
|
| 3 | Female | 81 | 22.64 | 4 |
|
| 4 | Female | 63 | 23.88 | 3 |
|
| 5 | Male | 60 | 23.73 | 3 |
|
| 6 | Male | 72 | 23.56 | 3 |
| Patients with high
myopia (119) | 1 | Male | 68 | 26.22 | 3 |
|
| 2 | Male | 70 | 27.3 | 3 |
|
| 3 | Female | 65 | 29.87 | 3 |
|
| 4 | Male | 85 | 25.87 | 4 |
|
| 5 | Female | 59 | 26.34 | 3 |
|
| 6 | Female | 63 | 27.65 | 4 |
| Patients with
glaucoma (118) | 1 | Female | 75 | 22.61 | 3 |
|
| 2 | Female | 59 | 22.84 | 3 |
|
| 3 | Female | 81 | 23.81 | 4 |
|
| 4 | Male | 79 | 22.78 | 3 |
|
| 5 | Female | 76 | 23.21 | 3 |
|
| 6 | Male | 83 | 23.52 | 4 |
| Patients with
diabetes (121) | 1 | Female | 70 | 23.68 | 3 |
|
| 2 | Female | 78 | 24.35 | 4 |
|
| 3 | Male | 76 | 23.75 | 3 |
|
| 4 | Female | 62 | 24.65 | 3 |
|
| 5 | Female | 68 | 22.78 | 3 |
|
| 6 | Male | 88 | 23.65 | 4 |
None of the subjects had a history or slit-lamp
evidence of ocular trauma, nor use of systemic anti-metabolites,
immunosuppressants or corticosteroids. No ocular diseases, other
than cataracts, were present to meet inclusion criteria. The
average age of these patients was ~72 years (range 59–88 years) and
that of the control subjects was 71 years (range 60–81 years).
Other characters of these subjects, including axial length and
clinical application of the lens opacities classification system
III (19), are demonstrated in
Table I. The study protocol was
reviewed and approved by the Ethics Committee of Putuo Hospital,
Shanghai University of Traditional Chinese Medicine (Shanghai,
China) and informed written consent was obtained from the
patients.
Surgical sample preparation and iTRAQ
labeling
The collection and preparation of AH was performed
by the same operator in a manner similar to that described in a
previous report (10). All samples
collected were digested with trypsin and labeled using iTRAQ
according to the manufacturer's protocol (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The labeled
digests were injected onto a 2.1×150 mm XBridge BEH 300 column
(Waters Corporation, Milford, MA, USA) on a Shimadzu LC-20AD
(Shimadzu Corporation, Kyoto, Japan) with UV detection at 214/280
nm. High-performance liquid chromatography solvent A consisted of
20 mM ammonium formate in water (pH 10) and solvent B consisted of
20 mM ammonium formate in water (pH 10) with 100% ACN. Peptides
were separated at a flow rate of 200 µl/min and eluted from the
column with a 5 min gradient from 0 to 5% solvent B, followed by a
35 min gradient from 5 to 35% solvent B, a 5 min gradient from 35
and 80% solvent B, a 5 min gradient of 80% solvent B, and a final 1
min gradient of from 80 to 0% solvent B, followed by termination
for 9 min. A total of 20 gradient-based fractions were collected
and were reduced using rotation vacuum concentrators (Christ RVC
2-25; Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am
Harz, Germany), following which they were reconstituted to 50 µl
mixtures containing 5% ACN and 0.1% ammonium formate.
Protein identification and
quantification
MS was performed on a Triple TOF 5600 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) coupled with an
Eksigent 1D plus with a ZORBAX 300SB-C18 column (5 µm; 300Å;
0.1×150 mm; Agilent Technologies, Inc., Santa Clara, CA, USA).
Solvents for LC-MS separation of mixed peptides were as follows:
Solvent A consisted of 5% ACN and 0.1% ammonium formate; solvent B
consisted of 95% ACN and 0.1% ammonium formate. The peptides were
eluted from the analytical column with a 65 min gradient ranging
from 5 to 45% solvent B, followed by a 2 min gradient from 45 to
80% solvent B, a 5 min gradient for 80% solvent B, a 3 min gradient
from 80 to 5% solvent B, and a final 10 min gradient from 5 to 0%
solvent B. Triple TOF 5600 MS was performed in data-dependent mode
to automatically switch between MS and MS/MS acquisition. The MS
spectra were obtained across the mass range of 350–1,250 m/z in
high resolution mode using a 250 msec accumulation time per
spectrum. Tandem MS were scanned from 100 to 1,250 m/z in high
sensitivity mode with rolling collision energy. The 20 most intense
precursors were selected for fragmentation per cycle with a dynamic
exclusion time of 9 sec. Protein identification was performed
according to the following parameters: Cysteine alkylation,
iodoacetic acid; ID focus, biological modification; digestion,
trypsin; species: Homo; database: Swissprot human (177,396 entries;
http://www.expasy.ch/sprot/); search
effort, thorough ID. Protein Pilot software version 4.5 (ABSciex,
Foster City, CA, USA) was used for relative quantification of
proteins.
Elisa
The alterations in the expression levels of five
randomly selected proteins were confirmed using ELISA (JRDun
Biotech, Shanghai, China). These five proteins were isoform 5 of
chordin-like protein 1 (CRDL1), amyloid-like protein 2 (APLP2),
dickkopf-related protein 3 (F6SYF8), cytochrome P450 2S1 (CP2S1)
and fibulin-1 (F8W7M9). The ELISA was performed according to the
manufacturer's protocol of the kits (cat. nos. DRE13425, DRE10132,
DRE12755, DRE12757 and DRE12481). A total of three repeat
determinations were performed for all samples.
Bioinformatics analyses
The 44 differently expressed proteins were annotated
according to the GO database (http://www.geneontology.org/). The pathway annotations
of proteins were established by searching against the Kyoto
Encyclopedia of Genes and Genomes database (http://www.genome.jp/kegg/pathway.html). In addition,
the functional regulatory networks of the proteomics-identified
proteins were analyzed using the Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING; version 9.0; http://string.embl.de/) database. Statistical
analyses, including independent t-tests were performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA. P≤0.05 was considered
to indicate a statistically significant difference.
Results
Proteome analysis of human AHs
To perform comprehensive proteomic profiling of
human AH in the present study, AH samples were collected from
patients with high myopia, glaucoma, diabetes and controls. Using
an iTRAQ methodology, a total of 445 proteins were identified in
these patients, which revealed the broadest human AH proteome,
compared with those in previous studies (20,21).
Among these proteins, 334 proteins have previously been reported in
eyes in the AH, cornea, vitreous humor, tears, choroid and retina,
whereas the remaining 111 proteins were novel, according to the
human eye proteome project (22).
Of note, for 224 proteins, it was the first time they have been
detected in AH, showing the value of this proteomic profiling
technology in examining human AHs. Further functional
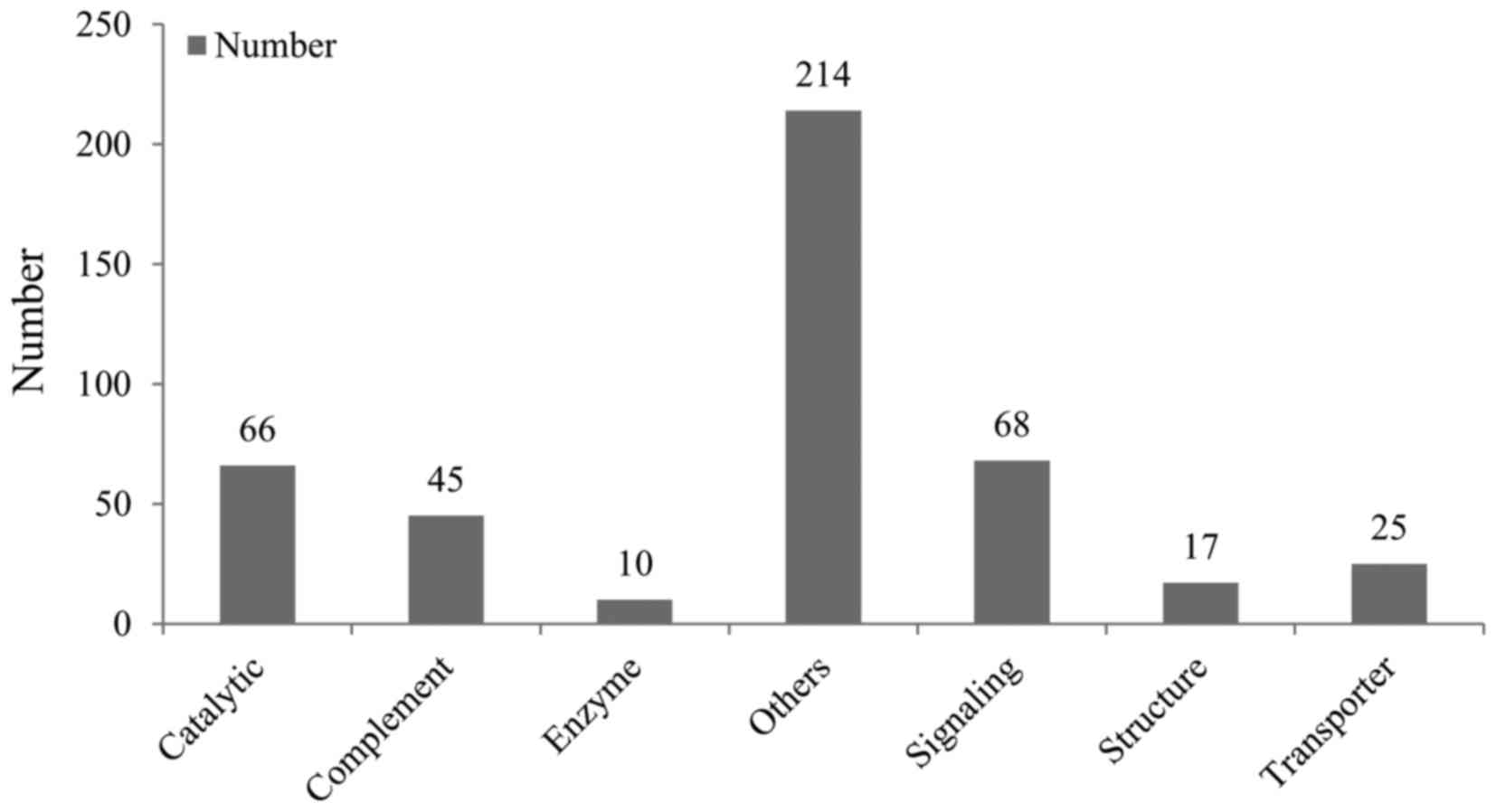
classification revealed that the 445 proteins identified reflected
the most common and abundant proteins in human AHs, the functions
of which included complement, signaling, catalytic, enzyme,
structural and transporter functions (Fig. 1).
Proteomic alterations in the AH from
patients with high myopia
Previous reports have demonstrated that myopia is
closely associated with cataracts. Accordingly, the present study
performed a proteomic analysis of the AH composition between
patients with high myopic eyes and controls with non-myopic
cataracts to identify the possible mechanism underlying cataract
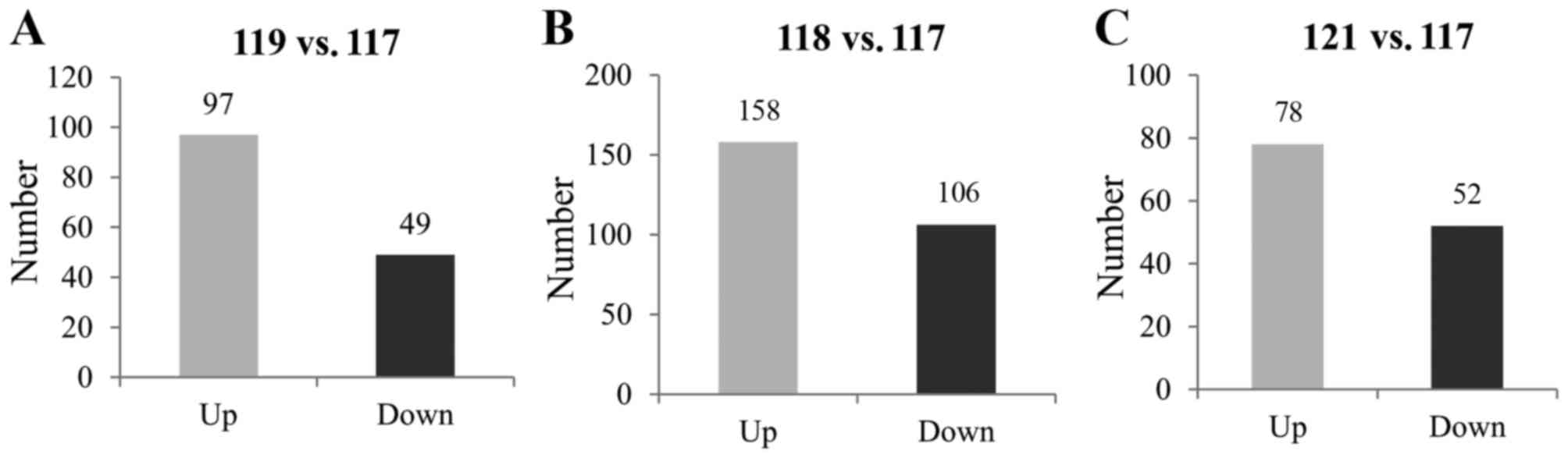
development. The result revealed that there were 146 differently
expressed proteins (Fig. 2A).
Among the proteins, 49 proteins were downregulated, including the
probable phospholipid-transporting ATPase IK, which was the most
markedly altered, and was involved in acrosome reactions and
binding of spermatozoa to zonapellucida. Two proteins belonging to
the keratin family, keratin, type II cytoskeletal 2 (K22E) and
keratin, type I cytoskeletal 10 (K1C10) were also included, which
are markers for various pathways of keratinocyte differentiation
(23). By contrast, 97 proteins
were upregulated in patients with high myopia. Notably, six
crystalline proteins, including α-crystallin B chain, β-crystallin
A4, α-crystallin A chain, β-crystallin B1, β-crystallin B2 and
β-crystallin A3, were upregulated, and these are known to be
critical for lens clarity and refraction, and involved in cataract
formation (24,25). The levels of four keratins,
keratin, type II cytoskeletal 6B (K2C6B), keratin, type II
cytoskeletal 6A (K2C6A), keratin, type I cytoskeletal 14 (K1C14)
and keratin, type I cytoskeletal 16 (K1C16) were also
increased.
Proteomic alterations of AH from
patients with glaucoma
Previous studies have also revealed that cataract
progression frequently follows glaucoma (26). To determine the possible mechanism
underlying cataract formation due to glaucoma, the present study
performed a comparative proteomic analysis of AH between patients
with glaucoma and controls. As demonstrated in Fig. 2B, a total of 264 proteins were
differently expressed, including 158 upregulated proteins and 106
downregulated proteins. Similarly, six keratin proteins (type I and
II) were found in patients with glaucoma, whereas four keratins
[K1C10, keratin, type I cytoskeletal 9 (K1C9), keratin, type II
cytoskeletal 1 (K2C1) and K22E, were downregulated, and keratin,
type II cytoskeletal 5 (K2C5) and K2C6A] were upregulated
>2.14-fold. In addition, all the crystalline proteins were
upregulated, with the exception of β-crystallin A3. Of note, two
lysozyme C proteins were upregulated the most, with fold-changes
>195, and these had a bacteriolytic function.
Proteomic alterations of AH from
patients with diabetes
There is considerable evidence that diabetes is
associated with increased oxidative stress, which may be prone to
cataract formation (27).
Therefore, the present study determined the differently expressed
proteins between patients with diabetes and controls to identify
the proteomic alterations. As demonstrated in Fig. 2C, 130 proteins were differently
expressed. Among these proteins, the levels of 78 proteins were
upregulated, although only one keratin protein (K2C6B) was
upregulated. Similarly, the expression levels of all three
crystallin proteins, including β-crystallin B1 (CRBB1),
α-crystallin A chain (CRYAA) and β-crystallin A4 (CRBA4) were
increased. By contrast, 52 proteins were downregulated in the
patients with diabetes, including the five keratins, K1C10, K2C1,
K1C9, K22E and K1C16.
Proteomic alterations of AH associated
with cataract development
According to the differently expressed proteins
between the above-mentioned three groups of patients and their
respective controls, the present study further determined the
proteomic alterations of AH associated with cataract development.
As demonstrated in Table II, 44
proteins were identified to be associated with cataract
development, which were markedly altered in all three groups of
patients. Among these proteins, eight proteins were synchronously
downregulated and 33 proteins were synchronously upregulated in the
patients with high myopia, glaucoma or diabetes. Of the eight
downregulated proteins, Ig µ chain C region was decreased the most,
which is known to be important in primary defense mechanisms
(28). The other two most
decreased proteins were K22E and K1C10, with average fold-changes
of ~0.18 and 0.30, respectively. By contrast, β-crystallin A4 and
α-crystallin A were upregulated. The remaining three proteins were
downregulated or upregulated in only one or two groups of patients.
For example, the levels of K1C16 were higher in patients with high
myopia and those with glaucoma, but were lower in patients with
diabetes.
 | Table II.Proteomic changes of aqueous humor
related to cataract development. |
Table II.
Proteomic changes of aqueous humor
related to cataract development.
| Accession no. | Average
(119/117) | Average
(118/117) | Average
(121/117) |
|---|
|
sp|P01871|IGHM_HUMAN | 0.10 | 0.42 | 0.22 |
|
sp|P35908|K22E_HUMAN | 0.20 | 0.15 | 0.58 |
|
sp|P13645|K1C10_HUMAN | 0.23 | 0.05 | 0.27 |
|
tr|F6SYF8|F6SYF8_HUMAN | 0.40 | 0.28 | 0.43 |
|
sp|Q08629|TICN1_HUMAN | 0.44 | 0.25 | 0.56 |
|
sp|P01833|PIGR_HUMAN | 0.46 | 64.78 | 3.63 |
|
sp|P26447|S10A4_HUMAN | 0.53 | 0.52 | 0.29 |
|
sp|Q9NQ79-3|CRAC1_HUMAN | 0.54 | 0.21 | 0.57 |
|
sp|Q9HCB6|SPON1_HUMAN | 0.57 | 0.37 | 0.30 |
|
sp|P81605|DCD_HUMAN | 1.53 | 7.48 | 2.90 |
|
sp|P07451|CAH3_HUMAN | 1.63 | 6.17 | 3.19 |
|
sp|Q16378|PROL4_HUMAN | 1.76 | 14.52 | 1.97 |
|
sp|P05543|THBG_HUMAN | 1.81 | 1.67 | 1.51 |
|
sp|P08185|CBG_HUMAN | 1.90 | 1.51 | 1.86 |
|
sp|P25311|ZA2G_HUMAN | 1.90 | 8.09 | 1.98 |
|
sp|P02760|AMBP_HUMAN | 1.96 | 44.48 | 1.91 |
|
sp|P02774-3|VTDB_HUMAN | 2.02 | 1.56 | 2.20 |
|
sp|P07357|CO8A_HUMAN | 2.08 | 1.78 | 1.96 |
|
tr|B0YIW2|B0YIW2_HUMAN | 2.27 | 8.87 | 1.71 |
|
sp|P01024|CO3_HUMAN | 2.46 | 2.18 | 2.36 |
|
tr|C9JEU5|C9JEU5_HUMAN | 2.46 | 15.86 | 4.08 |
|
sp|P01610|KV118_HUMAN | 2.49 | 4.92 | 2.62 |
|
sp|P02675|FIBB_HUMAN | 2.67 | 20.09 | 2.36 |
|
sp|P01877|IGHA2_HUMAN | 2.70 | 2.22 | 3.64 |
|
sp|P01861|IGHG4_HUMAN | 2.71 | 0.40 | 2.94 |
|
sp|P04217|A1BG_HUMAN | 2.77 | 2.62 | 2.05 |
|
tr|C9JV77|C9JV77_HUMAN | 2.92 | 5.30 | 1.72 |
|
sp|P02748|CO9_HUMAN | 3.15 | 4.70 | 1.77 |
|
sp|P19652|A1AG2_HUMAN | 3.24 | 3.29 | 2.73 |
|
sp|P01031|CO5_HUMAN | 3.79 | 7.59 | 3.35 |
|
sp|P02763|A1AG1_HUMAN | 4.00 | 8.90 | 5.61 |
|
sp|P01042-2|KNG1_HUMAN | 4.00 | 8.01 | 1.86 |
|
sp|P02649|APOE_HUMAN | 4.01 | 11.09 | 2.27 |
|
sp|P43652|AFAM_HUMAN | 4.01 | 2.74 | 1.62 |
|
sp|P01011|AACT_HUMAN | 4.33 | 6.95 | 1.51 |
|
tr|E7EMM4|E7EMM4_HUMAN | 5.02 | 8.10 | 3.80 |
|
sp|P02743|SAMP_HUMAN | 5.07 | 8.65 | 2.01 |
|
tr|B7ZKJ8|B7ZKJ8_HUMAN | 5.34 | 6.97 | 1.66 |
|
tr|V9GYM3|V9GYM3_HUMAN | 7.21 | 3.73 | 1.84 |
|
sp|P01009|A1AT_HUMAN | 7.47 | 13.49 | 2.21 |
|
sp|P01008|ANT3_HUMAN | 10.80 | 21.10 | 4.46 |
|
sp|P02489|CRYAA_HUMAN | 18.01 | 2.56 | 1.75 |
|
sp|P08779|K1C16_HUMAN | 20.77 | 2.56 | 0.59 |
|
sp|P53673|CRBA4_HUMAN | 21.37 | 3.46 | 2.11 |
Validation of alterations in protein
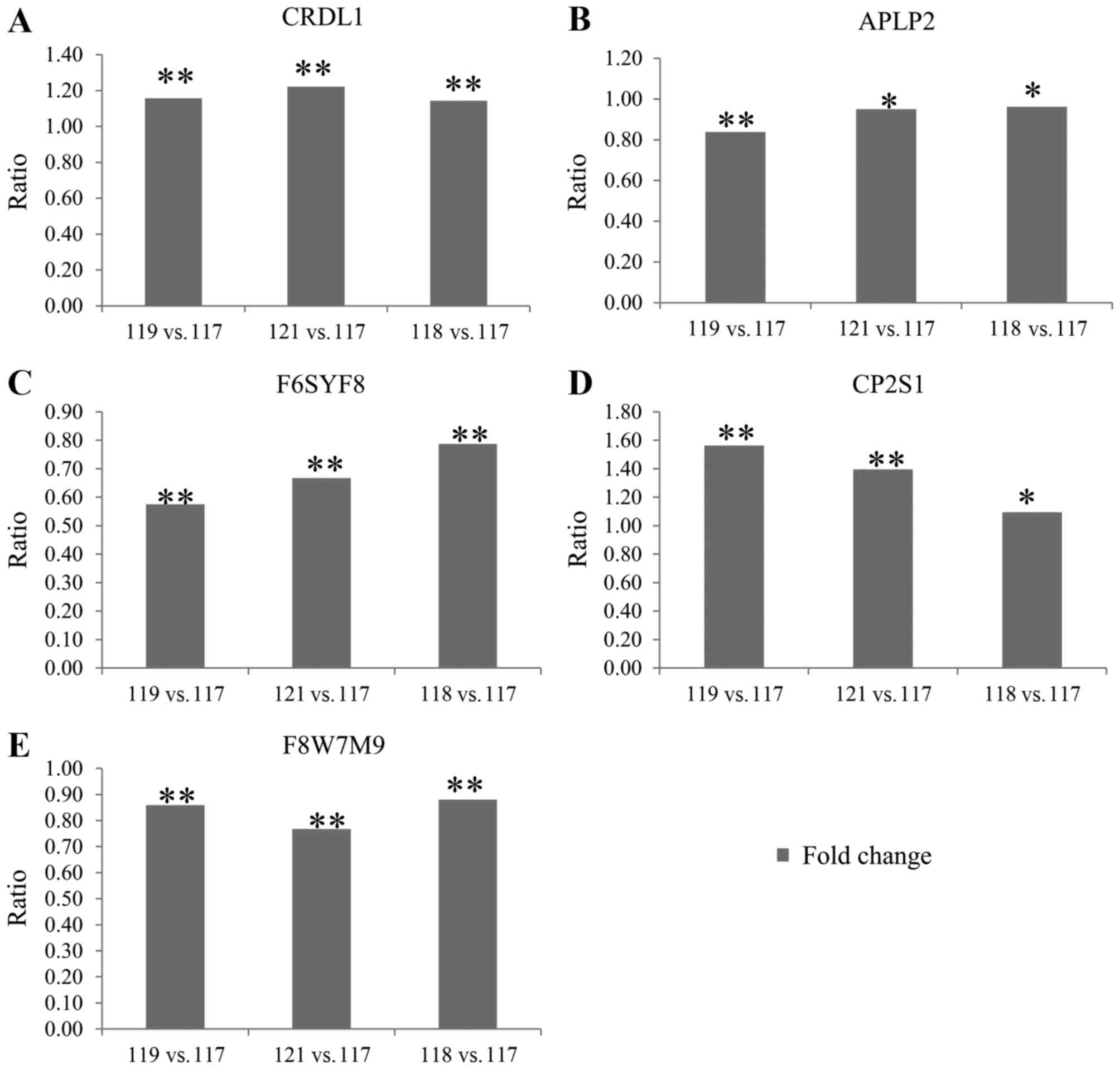
expression using ELISA analysis
To confirm the alterations in protein expression
demonstrated by the iTRAQ analysis, ELISA analysis was used to
examine the levels of five AH proteins (CRDL1, APLP2, F6SYF8, CP2S1
and F8W7M9), which were randomly selected from Table II. The results of the ELISA showed
that the alterations in the five differently expressed proteins
were all consistent with the proteomics data obtained using iTRAQ
analysis (Fig. 3A-E).
Functional analysis of
cataract-associated proteins
To obtain further insight into the functions of
cataract-associated proteins, GO annotation analysis of the 44
differentially expressed proteins was performed, which were grouped
into three functional groups: Cellular component, molecular
function and biological process. For the cellular component
ontology, there were nine GO terms, including cell part,
extracellular region part, macromolecular complex, organelle,
organelle part, extracellular region, membrane-enclosed lumen,
synapse and synapse part. For the molecular function ontology,
seven GO terms were identified, including binding, enzyme regulator
activity, structural molecule activity and catalytic activity. For
biological processes, the 44 GO terms were determined and included
the regulation of biological process, metabolic process, response
to stress, developmental process and establishment of localization
functions. In particular, 17 proteins were involved in terms of
binding for function ontology, whereas for biological processes,
19, 13 and 13 proteins were involved in the terms regulation of
biological process, metabolic process and response to stress,
respectively.
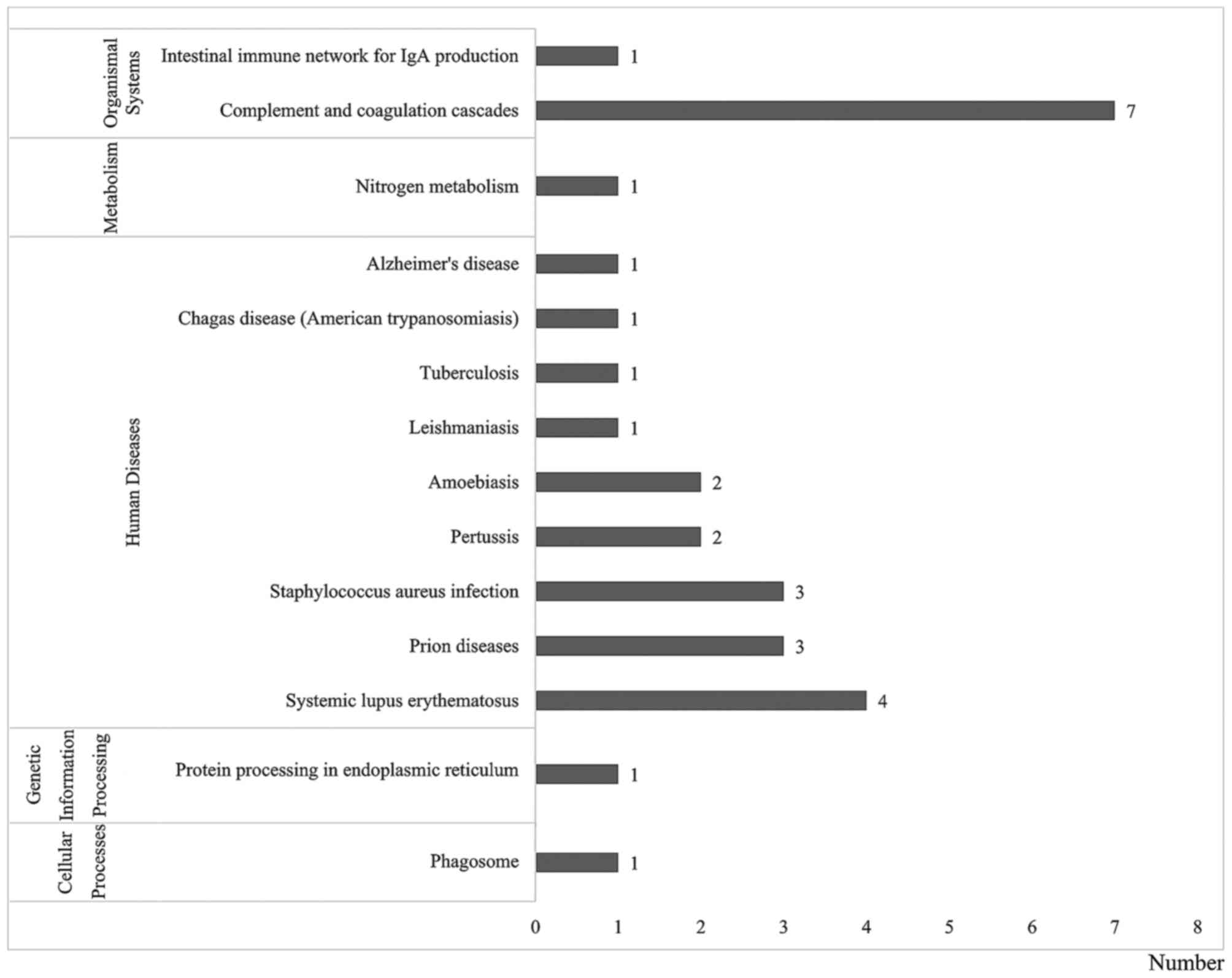
Pathway annotation analysis was also performed to
determine significant metabolic and/or signal transduction pathways
(Fig. 4). The results showed that
there were 14 significantly biological pathways, which belonged to
five groups: Cellular processes, genetic information processing,
human diseases, metabolism and organismal systems. Of these 14
pathways, 23 proteins were involved in infectious diseases,
neurodegenerative diseases, signal transduction, immune system, and
transport and catabolism.
Bioinformatics analysis using the STRING (version
9.0) database was used to search known evidence of
physical/functional interactions between the identified
cataract-associated proteins. As demonstrated in Fig. 5, three groups comprising 11
proteins were determined in the biological networks. Among each
group, the confidence between any two proteins was >0.4. The
first notable group contained seven proteins, including afamin
(AFM), α-2-HS-glycoprotein (AHSG), α-1-antitrypsin (SERPINA1),
α-1-antichymotrypsin (SERPINA3), apolipoprotein E (APOE),
apolipoprotein A-II (APOA2) and apolipoprotein C-III (APOC3). A
number of their interactions were high-level, including the
interaction between APOE and APOC3C3 and C5 (score 0.997), and the
interaction between APOE and APOA2 (score 0.991). In addition, two
proteins (C3 and C5) were grouped, which belong to proteins of the
immune system with a central role in the complement system and
contribution to innate immunity (29). In addition, the other two proteins
(fibrinogen β and fibrinogen γ) interacted with each other (scoring
0.797), which were involved in blood clot formation and may
contribute to the pathology of thrombosis.
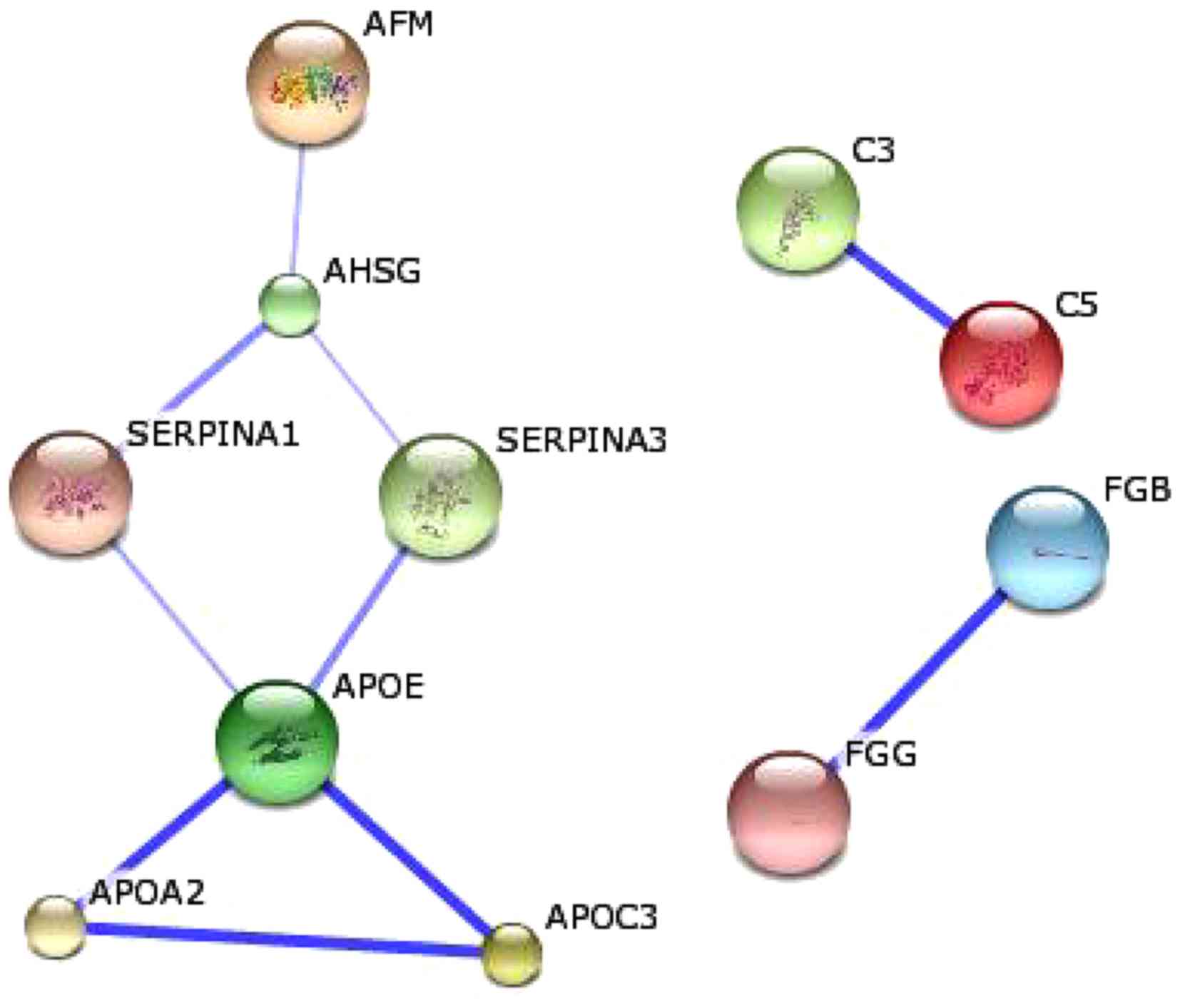 | Figure 5.Biological networks regulated by the
identified cataract-associated proteins. The blue line represents
the positive confidence between two proteins. The deeper the blue
color, the higher the confidence. AFM, afamin; AHSG,
α-2-HS-glycoprotein; SERPINA1, α-1-antitrypsin, SERPINA3,
α-1-antichymotrypsin; APOE, apolipoprotein E; APOA2, apolipoprotein
A-II; APOC3, apolipoprotein C-III; FGB, fibrinogen β; FGG,
fibrinogen γ. |
Discussion
As cataracts are the primary cause of blindness and
visual impairment, an increasing number of studies have focused on
proteomic analyses of ocular fluids to elucidate the mechanism.
Among these ocular fluids, the AH is important for the eye in
maintaining certain functions, including refraction, shape and
intraocular pressure (17).
Although proteomic analyses of cataract development have been
performed in AH, questions regarding the mechanism of cataract
formation remain due to the lack of integrative studies (30–33).
In addition, previous studies on high myopia or glaucoma-associated
cataracts were performed using traditional 2-DE analysis, which
usually results in lower levels of accuracy due to the lower
concentration of proteins in the AH. By subjecting samples to a
high throughput protein identification technique, labeled by iTRAQ,
the present study compared the AH proteome of three groups of
patients and their respective controls, which revealed significant
variation, not only in proteins levels, but in protein functions.
The proteomic differences in the AH among patients and controls
were then determined to be associated with cataract development.
Additional protein-protein interactions were also revealed, and the
differences in five randomly selected proteins were confirmed using
ELISA assays. Thus, the present study not only broadened the
proteomic profile of AH, compared with previous studies, but
provided novel insights into protein alterations and their
functions in cataract development (30–33).
The results of the present study assist in further extending the
current understanding of cataract formation resulting from high
myopia, glaucoma or diabetes, and may be useful for providing
potential prevention and treatment strategies.
Myopia is the most common visual impairment in the
world, and the worldwide prevalence of high myopia has been
estimated to be between 0.3 and 9.6%. The percentage has recently
been reported to be as high as 16% in some Asian countries
(30). High myopia is usually
defined as myopia >-6.00 diopters or with an axis length ≥26 mm.
It is also termed pathological myopia as it may present with
retinal pathological alterations in the posterior pole with
progressive choroidal degeneration, in addition to other
complications. Increasing clinical evidence has revealed that high
myopia is a risk factor for cataracts and their association is well
established. For example, a previous study by Lim et al
(1999) suggested that early-onset myopia may be a reliable and
independent risk factor for PSC cataracts, and high myopia has been
associated with nuclear and PSCs (34). In another previous study, the
results showed that high myopia is a risk factor for dark nuclear
cataracts (35). The results
revealed an increased oxygen tension by earlier vitreous
liquefaction and the downregulation of αA-crystallin, a major
component of antioxidative mechanisms within the lens, which
functions in the pathogenesis of high-myopic dark nuclear
cataracts. In addition, Duan et al (36) used 2-DE to identify
disease-specific proteins in the AH between proteomes in patients
with cataracts (control) and those with high myopia. The results
indicated higher total protein concentrations, and the levels of
six proteins, including albumin, transthyretin and a vitamin
D-binding protein, were significantly increased in AH with high
myopia, compared with the non-myopia group. In the present study,
146 proteins were differently expressed between patients with high
myopia and controls. These results may provide potential biomarkers
for cataract development due to high myopia and extend current
understanding on the underlying mechanisms at the proteomic
level.
Excluding high myopia, previous studies have
revealed that cataract progression is frequently associated with
glaucoma, and there have been studies focusing on proteomic
differences between patients with various types of glaucoma and
controls (5,36–38).
These studies revealed several potential biomarkers, which may be
useful in the development of novel diagnostic markers and
therapeutic targets. For example, González-Iglesias et al
(2014) used several techniques in a comprehensive proteomic
workflow to determine the alterations in sera proteins between
patients with POAG, pseudoexfoliation glaucoma (PEXG) and healthy
controls (5). Their study offered
novel perspectives in the identification of glaucoma biomarkers in
the serum of patients with POAG and PEXG, which were considered to
be useful for the clinical prediction, prognosis, diagnosis and
monitoring of POAG and PEXG on a large scale. However, there has
been minimal reporting on proteomic alterations with glaucoma. In
the present study, the proteomic differences between patients with
glaucoma and their controls were compared, and 264 proteins were
differently expressed, which may be involved in cataract
development. These results may provide potential biomarkers for the
monitoring of cataract formation associated with glaucoma.
Diabetes has been reported to be associated with a
5-fold higher prevalence of cataracts and is a major cause of
blindness worldwide (39).
Proteomic studies on characterizing lenticular proteins and
elucidating the complex factors involved in the development of
diabetic cataracts have been performed (14,40).
For example, by using 2-D differential in-gel electrophoresis
coupled with MS, Su et al (2014) identified differential
alterations in proteins and metabolites underlying ‘fast’ type 1
and ‘slow’ type 2 diabetic cataract formation in rats (14). The results revealed alterations in
the abundance of crystallins and alterations in HMW noncrystallin
proteins, which assisted to in elucidating the shared and
differential pathological mechanisms associated with the two types
of diabetic cataracts. Similarly, Zhu et al (2013) used
2-DE, MS, and ELISA to investigate the differential lens proteomics
between diabetic cataracts, age-related cataracts and normal
subjects (40). The results
reflected the differential proteins in diabetic and age-related
cataract lenses, compared with normal subjects. In the present
study, the proteomic differences between patients with diabetes and
their controls were also compared using a high throughput
technology, rather than traditional 2-DE technology, which revealed
130 proteins were differently expressed. These results may provide
potential biomarkers for the monitoring of cataract formation
associated with diabetes, and may be assist with further
investigations on the principles and mechanisms of cataract
development.
To the best of our knowledge, cataracts usually
result from dysfunction of the lens, where the proteins are
photo-oxidatively damaged, aggregated and accumulated in lens
opacities (41). The results of
the present study demonstrated that there were proteomic
alterations among the three above-mentioned groups of patients, and
44 proteins were altered, which were considered to be
cataract-associated proteins. Further functional analyses of the 44
identified cataract-associated proteins were performed, and 14
pathways were annotated, including infectious diseases,
neurodegenerative diseases, signal transduction, signaling
molecules and interaction, and transport and catabolism. In
addition, physical/functional interactions between the identified
cataract-associated proteins indicated that 11 proteins in the
three groups were involved in the established biological network.
In particular, four crystallins and heat shock protein β-1, were
reported to be connected with nervous system disease and eye
disease, including cataracts.
In conclusion, the proteomic profiling data obtained
in the present study revealed marked proteomic differences between
the three groups of patients (high myopia, glaucoma and diabetes)
and their corresponding controls. Based on these differences, 44
cataract-associated proteins were determined, and five of these 44
cataract-associated proteins were randomly selected and confirmed
using ELISA assays. The biological functions and interaction
networks of these 44 proteins were also identified. The results
provide novel insights into the mechanism of cataract development,
and potential biomarkers for the diagnosis and monitoring of
cataract formation.
Acknowledgements
The present study was supported by the Research
Project of Health and Family Planning Commission in Shanghai (grant
no. 20124108).
References
|
1
|
Pascolini D and Mariotti SP: Global
estimates of visual impairment: 2010. Br J Ophthalmol. 96:614–618.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korsakova NV, Sergeeva VE and Petrov SB:
Immunohisto chemical analysis of lens cells on formation of
different types of age-related cataract in humans. Neurosci Behav
Physiol. 38:887–890. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salm M, Belsky D and Sloan FA: Trends in
cost of major eye diseases to medicare, 1991 to 2000. Am J
Ophthalmol. 142:976–982. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sachdeva R, Sears JE and Rychwalski PJ: A
novel case of bilateral high myopia, cataract and total retinal
detachment associated with interstitial 11q deletion. Ophthalmic
Genet. 31:84–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
González-Iglesias H, Álvarez L, García M,
Escribano J, Rodríguez-Calvo PP, Fernández-Vega L and Coca-Prados
M: Comparative proteomic study in serum of patients with primary
open-angle glaucoma and pseudoexfoliation glaucoma. J Proteomics.
98:65–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Izzotti A, Longobardi M, Cartiglia C and
Saccà SC: Proteome alterations in primary open angle glaucoma
aqueous humor. J Proteome Res. 9:4831–4838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji Y, Cai L, Zheng T, Ye H, Rong X, Rao J
and Lu Y: The mechanism of UVB irradiation induced-apoptosis in
cataract. Mol Cell Biochem. 401:87–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan L, Khan RA, Ahmed W, Rauf A, Khan MW,
Khan W, Durrani SA and Qayum S: Frequency, causes and cutting-edge
treatment of cataract: A review. AJBLS. 3:25–28. 2015.
|
|
9
|
Foster PJ, Wong TY, Machin D, Johnson GJ
and Seah SK: Risk factors for nuclear, cortical and posterior
subcapsular cataracts in the Chinese population of Singapore: The
tanjong pagar survey. Br J Ophthalmol. 87:1112–1120. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng CY, Liu JH, Chen SJ and Lee FL:
Population-based study on prevalence and risk factors of
age-related cataracts in Peitou, Taiwan. Zhonghua Yi Xue Za Zhi
(Taipei). 63:641–648. 2000.PubMed/NCBI
|
|
11
|
Pan CW, Boey PY, Cheng CY, Saw SM, Tay WT,
Wang JJ, Tan AG, Mitchell P and Wong TY: Myopia, axial length and
age-related cataract: The Singapore Malay eye study. Invest
Ophthalmol Vis Sci. 54:4498–4502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiang SY, Tsai ML, Wang CY, Chen A, Chou
YC, Hsia CW, Wu YF, Chen HM, Huang TH, Chen PH, et al: Proteomic
analysis and identification of aqueous humor proteins with a
pathophysiological role in diabetic retinopathy. J Proteomics.
75:2950–2959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hadjistilianou T, Giglioni S, Micheli L,
Vannoni D, Brogi E, Cevenini G, Cortelazzo A, De Francesco S,
Menicacci F and Leoncini R: Analysis of aqueous humour proteins in
patients with retinoblastoma. Clin Exp Ophthalmol. 40:e8–e15. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su S, Leng F, Guan L, Zhang L, Ge J, Wang
C, Chen S and Liu P: Differential proteomic analyses of cataracts
from rat models of type 1 and 2 diabetes. Invest Ophthalmol Vis
Sci. 55:7848–7861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou HY, Yan H, Wang LL, Yan WJ, Shui YB
and Beebe DC: Quantitative proteomics analysis by iTRAQ in human
nuclear cataracts of different ages and normal lens nuclei.
Proteomics Clin Appl. 9:776–786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monteiro JP, Santos FM, Rocha AS,
Castro-de-Sousa JP, Queiroz JA, Passarinha LA and Tomaz CT:
Vitreous humor in the pathologic scope: Insights from proteomic
approaches. Proteomics Clin Appl. 9:187–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grus FH, Joachim SC and Pfeiffer N:
Proteomics in ocular fluids. Proteomics Clin Appl. 1:876–888. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu WW, Wang G, Baek SJ and Shen RF:
Comparative study of three proteomic quantitative methods, DIGE,
cICAT and iTRAQ, using 2D gel-or LC-MALDI TOF/TOF. J Proteome Res.
5:651–658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karbassi M, Khu PM, Singer DM and Chylack
LT Jr: Evaluation of lens opacities classification system III
applied at the slitlamp. Optom Vis Sci. 70:923–928. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bennett KL, Funk M, Tschernutter M,
Breitwieser FP, Planyavsky M, Mohien Ubaida C, Müller A, Trajanoski
Z, Colinge J, Superti-Furga G and Schmidt-Erfurth U: Proteomic
analysis of human cataract aqueous humour: Comparison of
one-dimensional gel LCMS with two-dimensional LCMS of unlabelled
and iTRAQ®-labelled specimens. J proteomics. 74:151–166.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qianqian Y, Yong Y, Zhaodong C, Yonghui T,
Jun S and Yuzheng H: Differential protein expression between type 1
diabetic cataract and age-related cataract patients. Folia Biol
(Praha). 61:74–80. 2015.PubMed/NCBI
|
|
22
|
Omenn GS: The human eye proteome project.
Proteomics. 13:2375–2376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang CK, Magnaldo T, Ohtsuki M, Freedberg
IM, Bernerd F and Blumenberg M: Epidermal growth factor and
transforming growth factor alpha specifically induce the
activation- and hyperproliferation-associated keratins 6 and 16.
Proc Natl Acad Sci USA. 90:6786–6790. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Héon E, Priston M, Schorderet DF,
Billingsley GD, Girard PO, Lubsen N and Munier FL: The
gamma-crystallins and human cataracts: A puzzle made clearer. Am J
Hum Genet. 65:1261–1267. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hejtmancik JF, Wingfield PT and Sergeev
YV: Beta-crystallin association. Exp Eye Res. 79:377–383. 2004.
View Article : Google Scholar
|
|
26
|
Brown RH, Zhong L and Lynch MG: Lens-based
glaucoma surgery: Using cataract surgery to reduce intraocular
pressure. J Cataract Refract Surg. 40:1255–1262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee AY and Chung SS: Contributions of
polyol pathway to oxidative stress in diabetic cataract. FASEB J.
13:23–30. 1999.PubMed/NCBI
|
|
28
|
Tisch R, Roifman CM and Hozumi N:
Functional differences between immunoglobulins M and D expressed on
the surface of an immature B-cell line. Proc Natl Acad Sci USA.
85:6914–6918. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Bruijn MH and Fey GH: Human complement
component C3: CDNA coding sequence and derived primary structure.
Proc Natl Acad Sci USA. 82:708–712. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan X, Lu Q, Xue P, Zhang H, Dong Z, Yang
F and Wang N: Proteomic analysis of aqueous humor from patients
with myopia. Mol Vis. 14:370–377. 2008.PubMed/NCBI
|
|
31
|
Chowdhury UR, Madden BJ, Charlesworth MC
and Fautsch MP: Proteome analysis of human aqueous humor. Invest
Ophthalmol Vis Sci. 51:4921–4931. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Richardson MR, Segu ZM, Price MO, Lai X,
Witzmann FA, Mechref Y, Yoder MC and Price FW: Alterations in the
aqueous humor proteome in patients with fuchs endothelial corneal
dystrophy. Mol Vis. 16:2376–2383. 2010.PubMed/NCBI
|
|
33
|
Yao J, Liu X, Yang Q, Zhuang M, Wang F,
Chen X, Hang H, Zhang W and Liu Q: Proteomic analysis of the
aqueous humor in patients with wet age-related macular
degeneration. Proteomics Clin Appl. 7:550–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lim R, Mitchell P and Cumming RG:
Refractive associations with cataract: The blue mountains eye
study. Invest Ophthalmol Vis Sci. 40:3021–3026. 1999.PubMed/NCBI
|
|
35
|
Zhu XJ, Zhou P, Zhang KK, Yang J, Luo Y
and Lu Y: Epigenetic regulation of αA-crystallin in high
myopia-induced dark nuclear cataract. PLoS One. 8:e819002013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duan X, Xue P, Wang N, Dong Z, Lu Q and
Yang F: Proteomic analysis of aqueous humor from patients with
primary open angle glaucoma. Mol Vis. 16:2839–2846. 2010.PubMed/NCBI
|
|
37
|
Bouhenni RA, Al Shahwan S, Morales J,
Wakim BT, Chomyk AM, Alkuraya FS and Edward DP: Identification of
differentially expressed proteins in the aqueous humor of primary
congenital glaucoma. Exp Eye Res. 92:67–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saccà SC, Centofanti M and Izzotti A: New
proteins as vascular biomarkers in primary open angle glaucomatous
aqueous humor. Invest Ophthalmol Vis Sci. 53:4242–4253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Obrosova IG, Chung SS and Kador PF:
Diabetic cataracts: Mechanisms and management. Diabetes Metab Res
Rev. 26:172–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu J, Shao J, Yao Y, Chu ZD, Yu QQ, Zhao
W, Lin Q and Zhang ZY: Differential proteomics analysis of proteins
from human diabetic and age-related cataractous lenses. J Res Med
Sci. 18:984–989. 2013.PubMed/NCBI
|
|
41
|
Kyselova Z: Mass spectrometry-based
proteomics approaches applied in cataract research. Mass Spectrom
Rev. 30:1173–1184. 2011. View Article : Google Scholar : PubMed/NCBI
|