Introduction
Cerebral hemorrhage is the most common human type of
cerebrovascular disease (accounting for 20–30% of cases) and the
least treatable subtype of hemorrhagic stroke, with a mortality
rate ~30–40% (1). The most common
clinical manifestations are cerebral arteriosclerosis, hypertension
and intracranial vascular malformations (2). Cerebral hemorrhage is often provoked
by exertion and emotion and the majority of patients present sudden
onset during activity. Cerebral hemorrhage usually causes severe
dysfunction of the cerebral nervous system and the loss of mobility
and independence, often resulting in burden to family and carers
(3,4). Subarachnoid hemorrhage is one of the
most serious types of cerebral hemorrhage and usually results in
mortality as blood bleeds into the subarachnoid space (5,6).
Although orally administered anticoagulants or surgical resection
are used as the main clinical treatments of cerebral hemorrhage,
there is no effective therapeutic schedule to improve functional
outcomes in patients with cerebral hemorrhage, especially
subarachnoid hemorrhage (7–9).
Therefore, there is an urgent requirement for potential therapeutic
agents targeting cerebral hemorrhage and an improved understanding
of the molecular mechanisms of human cerebrovascular disease.
The majority of cerebral hemorrhages are
non-traumatic and caused by rupture of vessels in brain parenchyma
(10). An increasing number of
detection methods are used for measuring the disease progression of
intracranial hemorrhage, including intracranial pressure, positron
emission tomography, computed tomography, and magnetic resonance
imaging (11). In addition,
numerous molecules have been proposed that may aid the diagnosis
of, and therapy for, cerebral hemorrhage by targeting
cell-associated hemostasis (12,13).
Previous studies (14,15) have identified epidermal growth
factor receptor (EGFR) and hepatocyte growth factor (HGF) as
important risk factors for bleeding in the development,
rehabilitation and recurrence of cerebral hemorrhage. In addition,
literature and clinical studies indicate that EGFR and HGF are
prospective candidate molecules for targeted molecular therapy in
cerebrovascular disease (16).
Vascular endothelial growth factor (VEGF) serves as
a crucial promoter for angiogenesis in physiological and
pathological conditions, and is identified as a survival factor and
specific mitogen in endothelial cells (17). Previous studies reported that VEGF
was a target for drug therapy including bevacizumab, sorafenib and
sunitinib, and as a predictive marker for hypertension (18–20).
Hypertension is the primary risk factor for cerebrovascular
disease, and may be a consequence of an inductor in the
microvascular network of the brain, resulting in abnormal
regulation of functions. The VEGF-mediated angiogenesis target of
rapamycin-mediated regulation of cell growth, cell proliferation,
cellular metabolism and angiogenesis have been identified as key
factors in the development of cerebrovascular disease (21). Therefore, targeting VEGF by
inhibiting the VEGF pathway by binding with VEGF receptor (VEGFR)
has demonstrated preclinical benefits in cerebrovascular disease
(22).
HGF is known for its important role in the
regulation of cell proliferation, morphogenesis wound healing,
motility and angiogenesis (23). A
previous study (16) demonstrated
that HGF and HGF receptor (HGFR) served a vital function in the
formation and progression of human cerebrovascular disease by
regulating cell proliferation. HGF expression was closely
associated with the state of patients with cerebral hemorrhage. Chu
et al (24) reported that
mRNA stabilization of HGF mediated by hypoxia and HGF may be a risk
factor in cerebral hemorrhage.
Materials and methods
Animals
Healthy specific-pathogen-free male Wistar rats
(n=50; 40 weeks old; weight, 350±20 g) were purchased from the
Animal Center of Hebei Medical University [Shijiazhuang, China;
Certification No. SCXK (Hei) 20100026] and used to establish a
cerebral hemorrhage model as described in a previous study
(25). Following the confirmation
of brain hemorrhage, the rats were divided into primary, moderate
and severe (n=10 in each group) according to the Tarlov scale. The
maximum tolerated doses of VEGFR and HGFR were determined by
anesthetization with 50 mg/kg pentobarbital followed by slow
injection of various doses of VEGFR and HGR into the right
ventricle wall of rats. The rats were treated intravenously with
VEGFR or/and HGFR once daily with PBS as a control. The rats were
sacrificed by cervical dislocation following 30 days of treatment
and the excised brains were studied by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
expression of HGF and VEGF mRNA was examined and analyzed. The
remaining rats were observed until 180 days following the
commencement of treatment, and every 10 days the state according to
the Tarlov scale and the survival rate was recorded. All surgery
procedures and euthanasia were performed to cause minimal
suffering. All experimental protocols were approved by the Ethics
Committee of Second Hospital of Hebei Medical University and were
performed in accordance with the guidelines by the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (26).
Relative mRNA levels by RT-qPCR
Total cellular RNA was extracted from the
hippocampus, midbrain, cerebral cortex and white matter and was
subjected to synthesis of cDNA (2 µg) by RT-qPCR. RT was performed
using the SuperScript® First-Strand Synthesis System for
RT-PCR kit (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) according to the manufacturer's protocol. The cycling
conditions for RT were as follows: Initial cycling for 5 min at
95°C, followed by 40 cycles for 15 sec at 95°C, 30 sec at 60°C and
30 sec at 72°C.
Samples were subjected to qPCR using
SYBR® Premix Taq (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on ABI PRISM 7900 thermocycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.) to analyze expression
changes of VEGF and HGF. The following primers were used: VEGF,
5′-CTCATCGCAGATGCCTGGAA-3′ (forward) and
5′-TTCAGGTAATAGGCACCCTTGAAGA-3′ (reverse); HGF,
5′-CTCAGCCAGATGCAATCAAT-3′ (forward) and 5′-GCTTCTTTGGGACACTTGCT-3′
(reverse); and housekeeping gene GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′
(forward) and 5′-TGGTGAAGACGCCAGTGGA-3′ (reverse). The cycling
conditions for qPCR were as follows: An initial denaturation step
at 95°C for 4 min, followed by 35 cycles at 94°C for 20 sec, 55°C
for 30 sec, 72°C for 20 sec, 72°C for 2 min and a final elongation
step at 72°C for 10 min. The mean value in the control group was
identified as the calibrator and the results are expressed as the
n-fold difference relative to control (n=3; relative expression
levels).
Behavioral assessments
The behavior of the rats was assessed on days 7, 14,
21 and 30 following surgery. The assessment parameters, including
left limb movement and co-ordination of movement were evaluated
using modified Tarlov scores as follows: Severe level, possible
limb movement and partial limb paralysis (1–4 points); moderate
level, failure to jump and stand normally (4–7 points); primary
level, failure to stand and with joint movement (7–9 points).
Healthy rats were scored 9–10 points.
Efficacy safety assessments
Efficacy assessments included the maximum toxicity
dose in cerebral hemorrhage and the dose-limiting toxicity in the
presence and absence of VEGFR (0.18 mg) and/or HGFR (0.24 mg). A
significant decrease of median percent change in cerebral
hemorrhage was observed following 30-day treatment in the VEGFR
plus HGFR-treated group. Safety assessments included the incidence
rates (>10%) of the most frequent treatment-emergent adverse
events in the 30-day treatment period in the experimental and
control groups. The efficacy and safety data included all rats with
cerebral hemorrhage receiving the therapeutic drugs and
control.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA) and Microsoft
Excel (Microsoft, Redmond, WA, USA). All data were reported as the
means and standard error. Statistical significance of differences
between mean values was assessed by Student's t-test for unpaired
data. Comparisons of data between multiple groups were performed
with analysis of variance. *P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristic of rats with cerebral
hemorrhage
The rats were induced to develop cerebral hemorrhage
through autologous blood injection and were designated into three
categories of cerebral hemorrhage according to the severity of the
illness. The rats were divided randomly into four groups (n=40) in
each category subsequent to the confirmation of cerebral
hemorrhage. The characteristics of the rats with cerebral
hemorrhage are presented in Table
I. All rats received therapy with at least one agent and the
control group received normal saline.
 | Table I.Characteristics of the male rats (age,
40 weeks; weight, 350±20 g; treatment cycle, 30 days). |
Table I.
Characteristics of the male rats (age,
40 weeks; weight, 350±20 g; treatment cycle, 30 days).
| Characteristic | Primary | Moderate | Severe |
|---|
| Total number | 80 | 80 | 80 |
| Received drugs | VEGFR or/plus
HGFR | VEGFR or/plus
HGFR | VEGFR or/plus
HGFR |
| Score | 8.4±0.3 | 5.6±0.4 | 3.4±0.2 |
| Pressure (cm
H2O) | 3.23±0.52 | 7.24±0.63 | 13.97±0.64 |
Duration of treatment, maximum
tolerated dose, and dose-limiting toxicity
Median overall duration of treatment was 14 days in
all dosing cohorts. These were 0.06, 0.12, 0.18, 0.24 and 0.30 mg
VEGFR, and 0.08, 0.16, 0.24, 0.30 and 0.36 mg HGFR for the
respective cohorts. The maximum tolerated dose was 0.18 mg of VEGFR
and 0.24 mg of HGFR once daily, identified by slow injection into
the right ventricle wall in the preclinical study. The lowest-dose
cohort of VEGFR and HGFR had the fewest number of VEGFR and HGFR
dose reductions. Rats with primary, moderate or severe cerebral
hemorrhages received a minimum of dose of therapy with a post
baseline safety evaluation included in the safety population in the
present study. The most common treatment-associated adverse events
were hypertension and proteinuria during the treatment with VEGFR
and HGFR in rats with moderate or severe of cerebral hemorrhage
(Table II).
 | Table II.Treatment-associated hypertension and
proteinuria by common toxicity criteria grade. |
Table II.
Treatment-associated hypertension and
proteinuria by common toxicity criteria grade.
|
|
| 0.18 mg VEGFR
(n=24) | 0.24 mg HGFR
(n=24) | 18 mg VEGFR + 0.24 mg
HGFR (n=4) |
|---|
|
|
|
|
|
|
|---|
| Adverse event | Total (n=72) | Primary | Moderate | Severe | Primary | Moderate | Severe | Primary | Moderate | Severe |
| Hypertension | 38 | 1 | 4 | 6 | 2 | 5 | 6 | 3 | 5 | 6 |
| Grade 1 | 11 | 0 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 |
| Grade 2 | 11 | 0 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 1 |
| Grade 3 | 16 | 1 | 2 | 3 | 1 | 2 | 3 | 0 | 1 | 3 |
| Proteinuria | 28 | 0 | 2 | 4 | 1 | 4 | 6 | 1 | 5 | 5 |
| Grade 1 | 4 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| Grade 2 | 10 | 0 | 1 | 1 | 0 | 1 | 2 | 1 | 2 | 2 |
| Grade 3 | 14 | 0 | 1 | 2 | 1 | 2 | 3 | 0 | 3 | 2 |
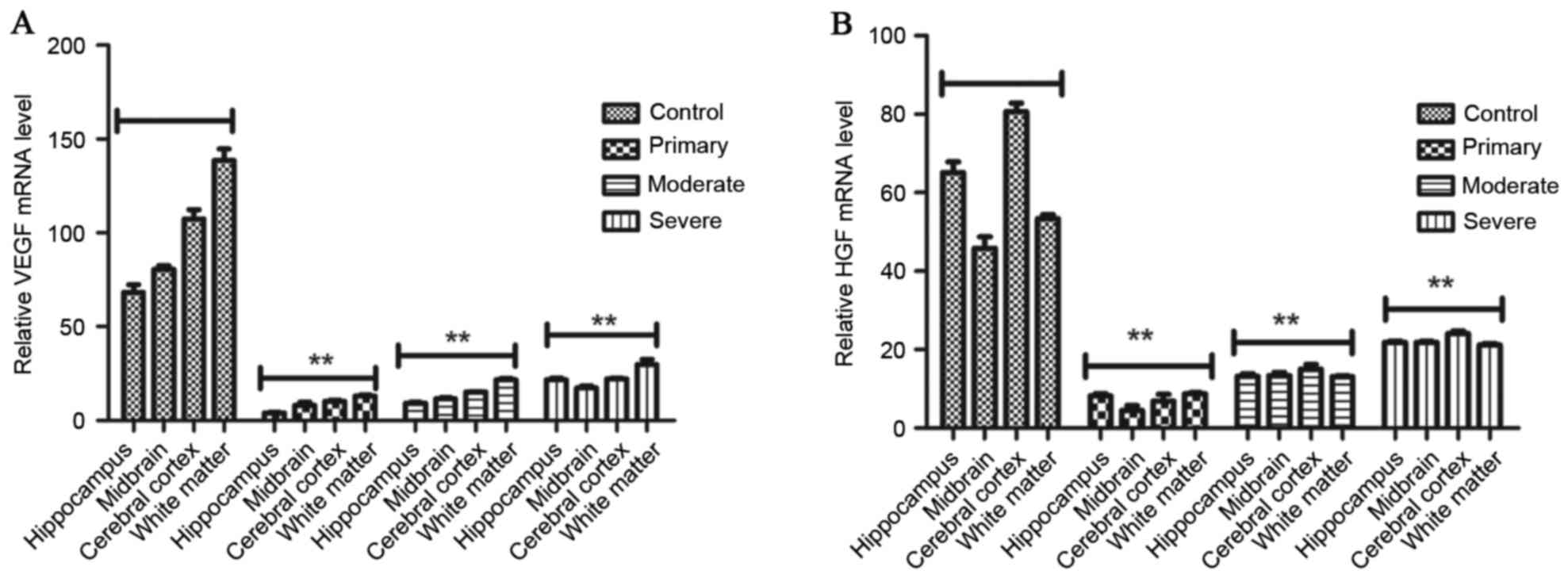
The mRNA expression of VEGF and HGF in
rats with cerebral hemorrhage
RT-qPCR was performed to detect the
expression levels of HGF and VEGF mRNA and protein in the cerebral
tissue of adult rats with cerebral hemorrhage after treatment with
VEGFR or/and HGFR. The results (Fig.
1) demonstrate that mRNA expression levels of HGF and
VEGF were significantly decreased in the hippocampus, midbrain,
cerebral cortex and white matter in the rat brains of the HGFR plus
VEGFR-treated groups. However, HGF and VEGF mRNA expression levels
were significantly increased after two cycles treatment in control
group. Furthermore, although rats with primary cerebral hemorrhage
also received two cycles of treatment, mRNA and protein expression
levels of HGF and VEGF were significantly decreased compared with
the moderate and severe cerebral hemorrhage groups.
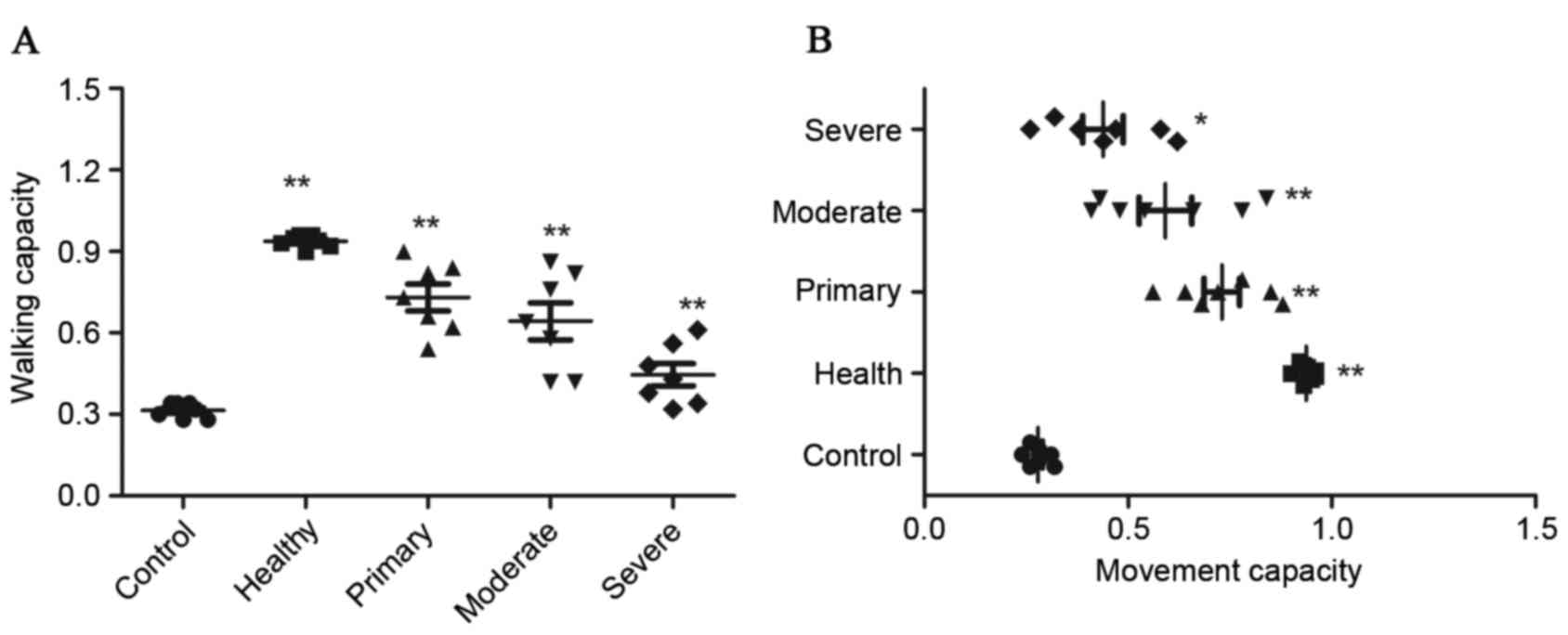
VEGFR and HGFR enhance healing and
prolong survival rates of rats
In order to explore whether the combination therapy
of VEGFR and HGFR is effective for rats with cerebral hemorrhage
in vivo, the recurrent activity of VEGFR and HGFR in the
cerebral hemorrhage rat model was studied. The results (Fig. 2) demonstrated that movement
capacities of limbs and coordination in walking were notably
improved in cases of moderate and severe hemorrhage lesions in the
VEGFR plus HGFR-treated group and mainly alleviated in cases of
primary hemorrhage lesion compared with rats in the single VEGFR or
HGFR-treated groups and the control group (**P<0.01). There were
no significant changes in arterial blood pressure, body weight or
body temperature, and injected arterial blood gas data were not
detected across all the experimental groups. The results of the
present study also demonstrated that there was no significant
difference between the VEGFR-treated and HGFR-treated groups as
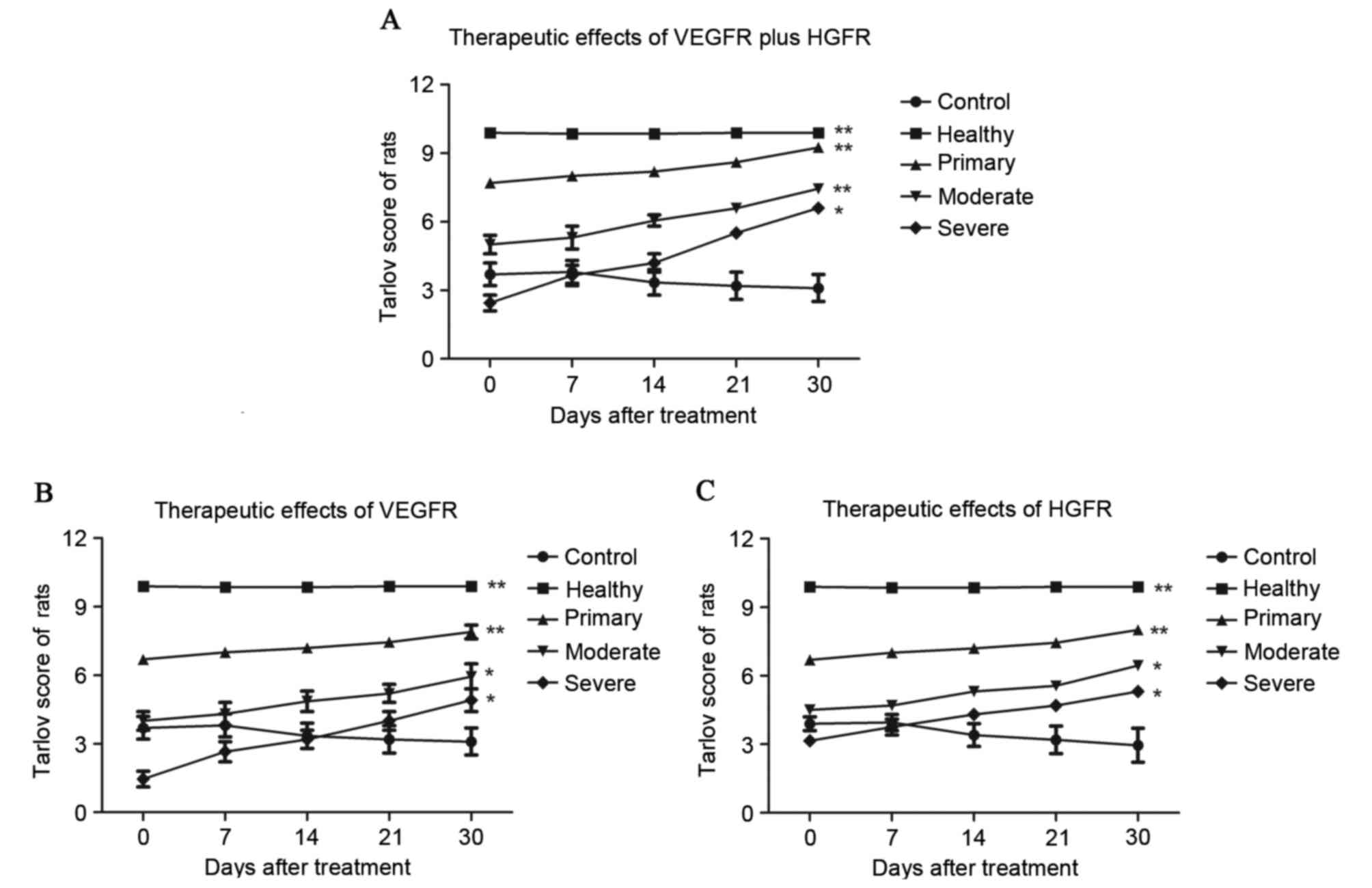
evaluated by Tarlov scores. Tarlov scores of the rats with cerebral
hemorrhage indicated that the therapeutic effects were significant
in the primary, moderate and severe hemorrhage categories in the
VEGFR plus HGFR group (*P<0.05, **P<0.01) compared with the
control group (Fig. 3).
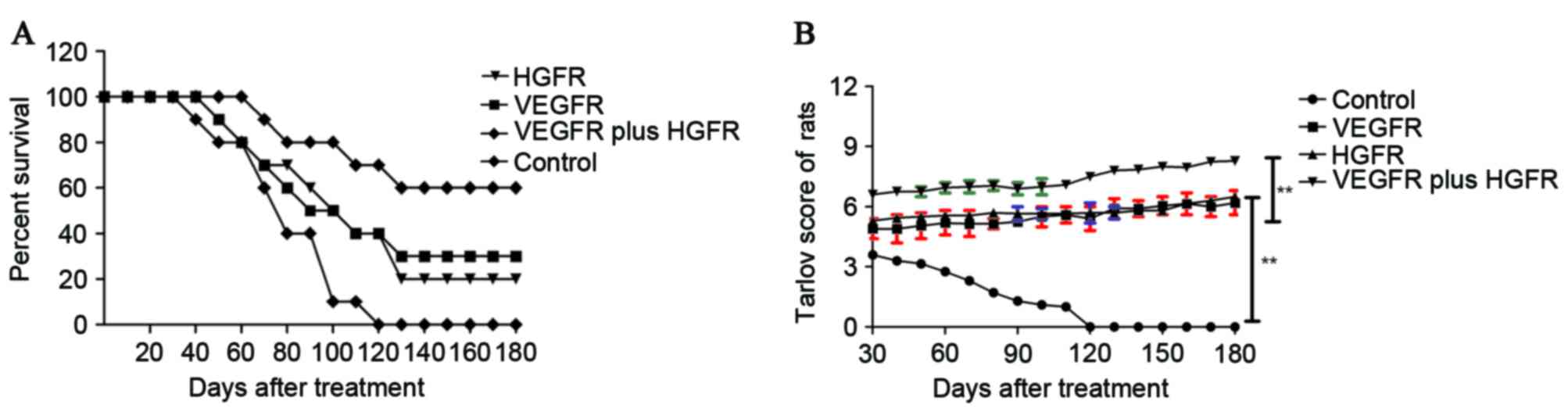
Furthermore, there was a long-term survival rate observation in the
severe cases of cerebral hemorrhage in the 180-day period after
treatment with VEGFR plus HGFR. The results (Fig. 4) demonstrated that that the
survival rates of the rats were extended, and Tarlov scores
improved, after treatment with VEGFR plus HGFR compared with the
control group (n=10 in each group). The results indicated that the
therapeutic agents used for rats with cerebral hemorrhage in the
VEGFR plus HGFR group are effective enough to relieve and heal the
animals, which translated into long-term survival.
Discussion
Spontaneous, non-dramatic cerebral hemorrhage leads
to high morbidity and mortality worldwide. Numerous studies have
demonstrated that cerebral hemorrhage causes neuronal damage and
further aggravates brain damage to the extent of developing
contralateral limb dysfunction (27–29).
In cerebral hemorrhage the blood often overflows directly into the
brain parenchyma. A previous study (11) indicated that the possible mechanism
may be associated with the leakage from small intracerebral
arteries caused by a function loss of endothelial growth cells.
Furthermore, this transformation may occur within one week of ictus
(12). Therefore, dysfunction of
endothelial growth cells may be an important pathophysiological
factor in cerebral hemorrhage and human cerebrovascular disease.
Chu et al (16) reported
that mRNA and protein expression levels of VEGF were increased in
the cerebral tissue of adult rats with chronic hydrocephalus after
subarachnoid hemorrhage. These observations suggested that the
inhibition of VEGF expression is beneficial in the reduction of the
degree of brain injury and the promotion of a functional recovery
(30). In the present study, the
function of VEGF was analyzed and the therapeutic effects of its
receptor were studied in rats with cerebral hemorrhage. The results
were consistent with previous studies and VEGFR exhibited a
beneficial treatment in the preclinical trial. However, in terms of
the final survival rate of the experimental rats, improvements in
therapy or combination therapy are required for an improved
therapeutic effect.
HGF is a multifunctional cytokine with numerous
roles in humans (31). Although
the underlying mechanism of HGF in the formation and development of
cerebral hemorrhage remains to be fully understood, HGF serves a
vital function in human cerebrovascular disease and it is
well-known that HGF is frequently unregulated in patients with
cerebral hemorrhage (31,32). Previous studies have demonstrated
that HGF increased damage by effectively promoting the growth of
other cells and the restoration of the nervous system on the
injured side (31,33). The present study hypothesized that
increasing HGF expression promoted the growth of endothelial growth
cells, exacerbating the increase of blood cells that aggravated the
state of illness in patients with cerebral hemorrhage. In the
present study, the therapeutic effects of the HGFR target for HGF
in rats with cerebral hemorrhage were tested. The observations
suggest that beneficial effects occurred and that HGFR may be an
effective candidate as a treatment for cerebral hemorrhage.
To the best of the authors' knowledge, the current
study is the first to investigate the preclinical therapeutic
effects of VEGFR and HGFR treatment in rats with cerebral
hemorrhage. The purpose of the present study was to evaluate the
efficacy and safety of VEGFR and HGFR for treating rats with
different levels of cerebral hemorrhage. During the treatment, the
maximum tolerated dose, and dose-limiting toxicities were analyzed
to ascertain the therapeutic dose. In the present study, VEGFR and
HGFR were demonstrated to be efficient and safe for controlling
bleeding in rats with cerebral hemorrhage and are recommended as
preferred agents in procedures. Notably, treatment of VEGFR plus
HGFR resulted in a statistically significant improvement in walking
and limb co-ordination in comparison with the control. In the
treatment-associated adverse events, it was also identified that
the main side effects of injection with VEGFR and HGFR are
lethargy, hypertriglyceridemia, fatigue and proteinuria. However,
further trials exploring the VEGFR plus HGFR treatment for cerebral
hemorrhage of are required.
In conclusion, the present study focused on the
therapeutic effects of VEGFR and HGFR during cerebral hemorrhagic
injury, and reached no consistent conclusions on their efficiency.
In the current study, a rat model of cerebral hemorrhage was used
to investigate the effects the injecting into brain tissue of VEGFR
and HGFR. Although the conclusions of the present study were
inconsistent, certain results suggest that VEGFR and HGFR mitigated
brain injury after cerebral hemorrhage by decreasing VEGF and HGF
expression. Further investigation is required in order to establish
a preclinical foundation for the effects of VEGFR and HGFR
treatment in cerebral hemorrhage.
Acknowledgements
The present study was supported by the National
Science Foundation of China (grant no. 81401514 to Professor Z.
Dong).
References
|
1
|
Sussman ES and Connolly ES Jr: Hemorrhagic
transformation: A review of the rate of hemorrhage in the major
clinical trials of acute ischemic stroke. Front Neurol. 4:692013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi T, Tadokoro H, Odai T, Hibino T
and Waki K: A delayed cerebral vasospasm with infarction is
secondary to listeria monocytogenes meningitis: MRI and MRA are
diagnostically useful. Intern Med. 54:2935–2938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee WJ, Yeon JY, Jo KI, Kim JS and Hong
SC: Reversible cerebral vasoconstriction syndrome and posterior
reversible encephalopathy syndrome presenting with deep
intracerebral hemorrhage in young women. J Cerebrovasc Endovasc
Neurosurg. 17:239–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanamaru K, Suzuki H and Taki W: Cerebral
infarction after aneurysmal subarachnoid hemorrhage. Acta Neurochir
Suppl. 121:167–172. 2016.PubMed/NCBI
|
|
5
|
Brown RJ, Epling BP, Staff I, Fortunato G,
Grady JJ and McCullough LD: Polyuria and cerebral vasospasm after
aneurysmal subarachnoid hemorrhage. BMC Neurol. 15:2012015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jenson AV, Rodriguez GJ, Alvarado LA,
Cruz-Flores S and Maud A: Higher rate of intracerebral hemorrhage
in hispanic patients with cerebral cavernous malformation. J Vasc
Interv Neurol. 8:1–4. 2015.PubMed/NCBI
|
|
7
|
Dabus G and Nogueira RG: Current options
for the management of aneurysmal subarachnoid hemorrhage-induced
cerebral vasospasm: A comprehensive review of the literature.
Interv Neurol. 2:30–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sehba FA, Pluta RM and MacDonald RL: Brain
injury after transient global cerebral ischemia and subarachnoid
hemorrhage. Stroke Res Treat. 2013:8271542013.PubMed/NCBI
|
|
9
|
Matsumoto H and Yoshida Y: Rapid
progression of cerebral infarction after intraventricular
hemorrhage in adult moyamoya disease. J Korean Neurosurg Soc.
54:411–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu L and Chen G: Signaling pathway in
cerebral vasospasm after subarachnoid hemorrhage: News update. Acta
Neurochir Suppl. 121:161–165. 2016.PubMed/NCBI
|
|
11
|
Kidwell CS, Chalela JA, Saver JL, Starkman
S, Hill MD, Demchuk AM, Butman JA, Patronas N, Alger JR, Latour LL,
et al: Comparison of MRI and CT for detection of acute
intracerebral hemorrhage. JAMA. 292:1823–1830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roever L and Levine SR: Cerebral
hemorrhage following thrombolytic therapy for stroke: Are
neutrophils really neutral? Neurology. 85:1360–1361. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang DN, Hou XW, Yang BW, Lin Y, Shi JP
and Wang N: Quantity of cerebral microbleeds, antiplatelet therapy,
and intracerebral hemorrhage outcomes: A systematic review and
meta-analysis. J Stroke Cerebrovasc Dis. 24:2728–2737. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Wang R, Wang X, Xue X, Ran D and
Wang S: Relevance of IL-6 and MMP-9 to cerebral arteriovenous
malformation and hemorrhage. Mol Med Rep. 7:1261–1266.
2013.PubMed/NCBI
|
|
15
|
Yang T, Gu J, Kong B, Kuang Y, Cheng L,
Cheng J, Xia X, Ma Y and Zhang J: Gene expression profiles of
patients with cerebral hematoma following spontaneous intracerebral
hemorrhage. Mol Med Rep. 10:1671–1678. 2014.PubMed/NCBI
|
|
16
|
Chu SH, Feng DF, Ma YB, Zhang H, Zhu ZA,
Li ZQ and Zhang ZH: Expression of HGF and VEGF in the cerebral
tissue of adult rats with chronic hydrocephalus after subarachnoid
hemorrhage. Mol Med Rep. 4:785–791. 2011.PubMed/NCBI
|
|
17
|
Rozman A, Silar M and Kosnik M: Angiogenin
and vascular endothelial growth factor expression in lungs of lung
cancer patients. Radiol Oncol. 46:354–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu CZ, Zhang L, Chang XH, Cheng YX, Cheng
HY, Ye X, Fu TY, Chen J and Cui H: Overexpression and
immunosuppressive functions of transforming growth factor 1,
vascular endothelial growth factor and interleukin-10 in epithelial
ovarian cancer. Chin J Cancer Res. 24:130–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hodorowicz-Zaniewska D, Kibil W, Malek A,
Szpor J, Kulig J and Sztefko K: Evaluation of serum concentrations
of vascular endothelial growth factor (VEGF) in breast cancer
patients. Pol J Pathol. 63:255–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dahlberg SE, Sandler AB, Brahmer JR,
Schiller JH and Johnson DH: Clinical course of advanced
non-small-cell lung cancer patients experiencing hypertension
during treatment with bevacizumab in combination with carboplatin
and paclitaxel on ECOG 4599. J Clin Oncol. 28:949–954. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim CH, Qi G, Dahlberg K and Li W:
Strontium-doped perovskites rival platinum catalysts for treating
NOx in simulated diesel exhaust. Science. 327:1624–1627. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palma J, Macedonia C, Deuster P, Olsen C,
Mozayeni BR and Crutchfield KE: Cerebrovascular dynamics and
vascular endothelial growth factor in acute mountain sickness.
Wilderness Environ Med. 17:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding Y, Adachi H, Katsuno M, Huang Z,
Jiang YM, Kondo N, Iida M, Tohnai G, Nakatsuji H, Funakoshi H, et
al: Overexpression of hepatocyte growth factor in SBMA model mice
has an additive effect on combination therapy with castration.
Biochem Biophys Res Commun. 468:677–683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chu SH, Feng DF, Ma YB, Zhu ZA, Zhang H
and Qiu JH: Stabilization of hepatocyte growth factor mRNA by
hypoxia-inducible factor 1. Mol Biol Rep. 36:1967–1975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He QS, Yang LF, Wang WB, Yuan B, Zhang LY
and Guo XJ: Vascular endothelial growth factor gene is associated
with hypertensive cerebellar hemorrhage and rehabilitative
treatment. Genet Mol Res. 14:9849–9857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals: Guide for the Care and Use
of Laboratory Animals. 8th. National Academies Press; Washington,
DC: 2011
|
|
27
|
Chamnanvanakij S, Margraf LR, Burns D and
Perlman JM: Apoptosis and white matter injury in preterm infants.
Pediatr Dev Pathol. 5:184–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riggs AJ and Riggs JE: Epilepsy's role in
the historical differentiation of religion, magic and science.
Epilepsia. 46:452–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao F, Guo Y, Zhang H, Wang S, Wang J, Wu
JM, Chen Z and Ding MP: Anterior thalamic nucleus stimulation
modulates regional cerebral metabolism: An FDG-MicroPET study in
rats. Neurobiol Dis. 34:477–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aliparasti MR, Almasi S, Sanaat Z,
Movasaghpoor A, Khalili-Dizaji R and Sadeghi-Bazargani H: Gene
expression of VEGF-A and VEGF-C in peripheral blood mononuclear
cells of iranian patients with acute myeloid leukemia. Turk J
Haematol. 30:137–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramezani A, Nägga K, Hansson O, Lönn J,
Sjöwall J, Katoozian F, Mansouri S and Nayeri F: Hepatocyte growth
factor in cerebrospinal fluid differentiates community-acquired or
nosocomial septic meningitis from other causes of pleocytosis.
Fluids Barriers CNS. 12:222015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou X and He QH: Hepatocyte growth factor
and male reproduction. Zhonghua Nan Ke Xue. 21:747–752. 2015.(In
Chinese). PubMed/NCBI
|
|
33
|
Ishibashi H, Tonomura H, Ikeda T, Nagae M,
Sakata M, Fujiwara H, Tanida T, Mastuda K, Kawata M and Kubo T:
Hepatocyte growth factor/c-met promotes proliferation, suppresses
apoptosis, and improves matrix metabolism in rabbit nucleus
pulposus cells in vitro. J Orthop Res. 34:709–716. 2016. View Article : Google Scholar : PubMed/NCBI
|