Introduction
Chronic kidney disease (CKD) is increasingly
recognized as a worldwide public health issue (1). Chronic hypoxia often occurs in the
kidney tissues of several patients with CKD. Tubulointerstitial
fibrosis is a pathological characteristic of the development of
almost all CKDs (2).
Tubulointerstitial fibrosis occurs and leads to increased thickness
of the tubular basement membrane (TBM) and accumulation of
interstitial ECM (3–6). Matrix metalloproteinase-2 (MMP-2) is
involved in the breakdown of ECM, degrading type IV collagen
(Coll-IV), a major component of the basement membrane (7–11).
The activity of MMP-2 in kidney tissues of patients with CKD is
decreased, leading to progressive renal dysfunction and organ
failure (12), although the
mechanism remains to be fully elucidated.
As is already known, hypoxia alters the expression
levels of the components of the tissue inhibitors of
metalloproteinases (TIMP) in human renal tubular endothelia,
interstitial fibroblasts and microvascular endothelial cells
(13). In vitro studies
have found that the activity of MMP-2 is decreased in proximal
tubular cells under hypoxic conditions (14). Our previous study demonstrated
altered expression and activity of MMP-2 in hepatic stellate cells
under hypoxic conditions (15),
however, the mechanism remains to be fully elucidated.
Previous studies have shown that autophagy also has
a close complex link with hypoxia (16). Extensive data suggests that
autophagy has a dual effect in hypoxia-induced cell injury
(17). The adaptation to hypoxia
at the cellular level is regulated by a dual mechanism; hypoxia
leads to augmentation in the efficiency of energy-producing
pathways, and decreases energy consuming processes, including the
activity of Na, K-ATPase (18). As
a cellular adaptive response, hypoxia decreases the activity of Na,
K-ATPase by triggering the endocytosis of its α1 subunit in the
alveolar epithelia (19). These
studies indicated that hypoxia induces autophagy and endocytosis.
Another study showed that autophagy and endocytosis lead to
reshaping of the cell membrane (20). Membrane-type 1 MMP (MT1-MMP) is a
zinc-dependent proteinase found in cholesterol-rich lipid rafts on
the plasma membrane. MT1-MMP activates proMMP-2 (21), and the cleavage of proMMP-2 to an
active form in a ternary complex with TIMP (22,23).
Therefore, the present study hypothesized that autophagy and
endocytosis triggered by hypoxia can lead to alteration of the
plasma membrane, which can alter the expression of molecules
regulating the activation of MMP-2, and eventually resulting in a
decrease in the activity of MMP-2.
The present study investigated the association
between autophagy and endocytosis and the activity of MMP-2 in
human proximal tubular cells under hypoxia in vitro. As
proximal tubular cells are key in the development of renal fibrosis
(24), the results of the present
study may provide useful clues to understand the mechanism by which
hypoxia alters the activity of MMP-2 in HK-2 cells.
Materials and methods
Cell culture and hypoxic
treatment
The HK-2 human renal proximal tubular epithelial
cell line was purchased from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). The
HK-2 cells were cultured in high-glucose DMEM supplemented with 10%
fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml
streptomycin (Thermo Fisher Scientific, Inc., Waltham, MA, USA) in
humidified air containing 5% CO2 at 37°C. At the second
passage of the cell culture, the viability of the cells was optimal
for experiments, which was the phase identified as the logarithmic
growth phase. Cells in the logarithmic growth phase were used in
all experiments.
The cells were seeded on 6-well plates at a density
of 5×105 cells/well and cultured for 24 h. To mimic
hypoxic conditions, the cells in the culture medium were then
subjected to low-oxygen conditions. The oxygen concentrations were
maintained at 1–3% using a three gas incubator (Thermo Fisher
Scientific, Inc.), which was held under a positive pressure in an
atmosphere of 94–92% N2/5% CO2/1–3%
O2 for 24 h. For the hypoxic experiments, there were
four groups of triplicate wells, comprising a control group,
hypoxic group, hypoxic+3-methyladenine (3-MA) group (5 mM 3-MA;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and a
hypoxic+filipin group (filipin 2.5 µg/ml; Sigma-Aldrich; Merck
KGaA) group for 24 h at 37°C. The control cells were cultured under
normoxic conditions.
Western blot analysis
The cells were plated at a density of
5×105 cells/well on 6-well plates and cultured in DMEM
supplemented with 10% FBS for 24 h. Following treatment under
hypoxia for 24 h, the cells were washed three times with ice-cold
1X PBS and lysed with immunoprecipitation assay buffer containing
protease inhibitors. Following incubation on ice for 15 min to
ensure complete lysis, the cell lysates were centrifuged at 14,000
g for 10 min at 4°C. The protein concentrations of the lysates were
examined using a Bradford protein assay kit. The cell lysates were
boiled and 40 µg of proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
transferred onto a polyvinylidene difluoride membrane via semi-dry
transfer (Bio-Rad Laboratories Inc., Hercules, CA, USA). The
membranes were washed in Tris-buffered saline containing 0.1%
Tween-20 (TBST), blocked with 5% non-fat milk in TBST for 1 h at
room temperature, and incubated with primary rabbit monoclonal
antibody against LC3B (ab48394; Abcam, Cambridge, UK; 1:1,000),
anti-MT1-MMP (ab51074; Abcam; 1:1,000), anti-caveolin-1
(16447–1-AP; Protech Technology, Inc., China; 1:1,000) or
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 10494-1-AP;
Protech Technology, Inc.; 1:5,000) overnight at 4°C. The membranes
were washed three times in TBST, followed by incubation with the
appropriate horseradish peroxidase (HRP)-linked secondary
anti-rabbit antibodies (SA00001-1; Proteintech, Wuhan, China;
1:5,000) for 1 h at room temperature. The specific proteins on the
blots were developed using enhanced chemiluminescence (ECL; Vazyme
Biotech Co., Ltd., Nanjing, China) and visualized as bands on
CL-XPosure film (Thermo Fisher Scientific, Inc.). The optical
densities of the bands were measured on the GS710 Densitometer and
analyzed using Quantity One image analysis software version 4.6
(Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the HK-2 cells using
the RNeasy Mini kit (Vazyme Biotech Co., Ltd.), and DNase digestion
was executed using an RNase-free DNase set (Vazyme Biotech, Co.,
Ltd.). RT-qPCR was performed to determine the expression of human
MMP-2 using SYBR Green PCR Master mix (Vazyme Biotech Co., Ltd.)
using the StepOnePlus™ Real-Time PCR Detection system (Step One
Plus 2.1 software) with universal thermal cycling parameters,
Reverse transcription was performed in a reaction system containing
5 µg RNA, reverse transcriptase, RNase inhibitor and random
primers. The obtained cDNAs were used to run qPCR with pairs of
primers. The thermal cycling conditions were as follows: 10 sec at
95°C followed by the amplification reaction consisting of 40 cycles
of denaturation for 10 sec at 95°C and annealing for 30 sec at
60°C. The resulting data were analyzed with the comparative Ct
method for relative gene expression quantification against
housekeeping gene, GAPDH. The following primers were used: MMP-2
forward 5′-GAGAACCAAAGTCTGAAGAG-3′ and reverse
5′-GGAGTGAGAATGCTGATTAG-3′; GAPDH forward
5′-GGAAGGTGAAGGTCGGAGTCA-3′ and reverse
5′-GCAACAATATCCACTTTACCAG-3′. The gene expression level of GAPDH
served as the control of target genes for reaction efficiency. The
2−ΔΔCq method (25) was
used to determine the relative quantities of products.
Enzyme-linked immunosorbent assay
(ELISA) to quantify MMP-2 and Collagen-IV (Col-IV) in culture
media
ELISA kits were used to detect the protein contents
of MMP-2 and Col-IV in culture media, according to the
manufacturer's protocol (E-11117 and E-13368 respectively; Shanghai
Hengyuan Biological Technology, Co., Ltd., Shanghai, China).
Purified MMP-2 or Col-IV antibody was used to coat the microtiter
plate wells, and the culture supernatants were then mixed into the
wells and incubated at 37°C for 2 h. Following washing of the
plates with PBS, another MMP-2 antibody or Col-IV antibody labeled
with HRP was added to the plates at 37°C for 2 h. The plates were
then washed thoroughly and 3,3′,5,5′-tetramethylbenzidine substrate
solution was added. The reaction was terminated by the addition of
a sulfuric acid solution, and color was measured by
spectrophotometry at a wavelength 450 nm. The protein contents of
MMP-2 or Col-IV in the samples were then calculated according to
the standard curve using Origin version 8.0 (OriginLab,
Northampton, MA, USA).
Detection of the activity of MMP-2
using zymography
The activity of MMP-2 was determined via gelatin
zymography using an MMP Zymography Assay kit (Applygen
Technologies, Inc., Beijing, China) according to the manufacturer's
protocol. Following MMP-2 separation using SDS-PAGE, the SDS was
extracted from the gels using Triton X-100, and then incubated for
48 h at 37°C. The gels were stained with coomassie brilliant blue
G250 and decolorized. The clear band against a blue background
represented the activity of MMP-2 and was measured using a gel
image system (Image Master 1D analysis software; GE Healthcare Life
Sciences, Shanghai, China) and recorded as the total area (area of
clear band×mean area) (26).
Statistical analysis
Statistical analyses were performed using the SPSS
13.0 statistical software package (SPSS, Inc., Chicago, IL, USA).
The data are expressed as the mean ± standard deviation.
Differences between groups were analyzed using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
3-MA and filipin inhibit
hypoxia-induced autophagy and endocytosis in HK-2 cells,
respectively
To examine the inhibition of 3-MA and filipin in the
HK-2 cells subjected to hypoxia, the expression of LC3 and
Cavelin-1 were detected n in HK-2 cells under hypoxia using western
blot analysis. The results of the western blot analysis of LC3
confirmed that there was increased transformation from LC3-I to
LC3-II in the hypoxia-treated HK-2 cells, compared with the cells
under normoxic conditions, which demonstrated that hypoxia induced
autophagy. However, in the hypoxic cells treated with 3-MA, the
transformation from LC3-I to LC3-II was significantly reduced.
These data suggested that hypoxia induced autophagy in HK-2 cells,
which was inhibited by 3-MA (Fig. 1A
and B). Simultaneously, it was found that hypoxia upregulated
the protein expression of Caveolin-1. When the HK-2 cells were
treated with filipin under hypoxia, the expression of Caveolin-1
was inhibited (Fig. 1A and C).
These results showed that hypoxia promoted autophagy and Caveolae
endocytosis, which were inhibited by 3-MA and filipin in HK-2 cells
under hypoxia, respectively.
Inhibition of autophagy and
endocytosis alters the activity of MMP-2 in culture media of
hypoxia-treated HK-2 cells
The results of the gel zymography of MMP-2 showed
two major bands, one band at 62 kDa representing the active form of
MMP-2 and another at 72 kDa representing pro-MMP-2. The activity of
MMP-2 in the hypoxic group (0.36±0.07) was found to be lower,
compared with that in the normoxic group (1.00±0.00). When the HK-2
cells were treated with 3-MA, active MMP-2 (0.25±0.04) in the
hypoxic+3-MA group was further decreased, compared with that in the
hypoxic group. By contrast, the activity of MMP-2 was significantly
elevated in the group treated with filipin and hypoxia (0.60±0.08),
compared with that in the hypoxic group (P<0.01; Fig. 2A and B).
Inhibition of autophagy and
endocytosis enhances the mRNA expression of MMP-2 in
hypoxia-treated HK-2 cells
To examine the expression of MMP-2 and its
correlation with autophagy and endocytosis, the present study
investigated the expression of MMP-2 in HK-2 cells using RT-qPCR
analysis. Compared with the normoxic group, the mRNA level of MMP-2
was not affected in the cells exposed to hypoxia. However, 3-MA and
filipin significantly increased the mRNA levels of MMP-2 in the
hypoxic cells (P<0.01), and 3-MA exhibited more marked induction
of the mRNA expression of MMP-2, compared with filipin. The
relative expression of MMP-2 was 1.24±0.16 in the hypoxic group,
15.53±2.12 in the hypoxic+3MA group and 6.40±4.22 in the
hypoxic+filipin group (Fig.
2C).
Inhibition of autophagy and
endocytosis alters the protein levels of MMP-2 and Col-IV in the
culture media of hypoxia-treated HK-2 cells
The protein expression levels of MMP-2 in cell
culture media was determined using ELISA to determine the
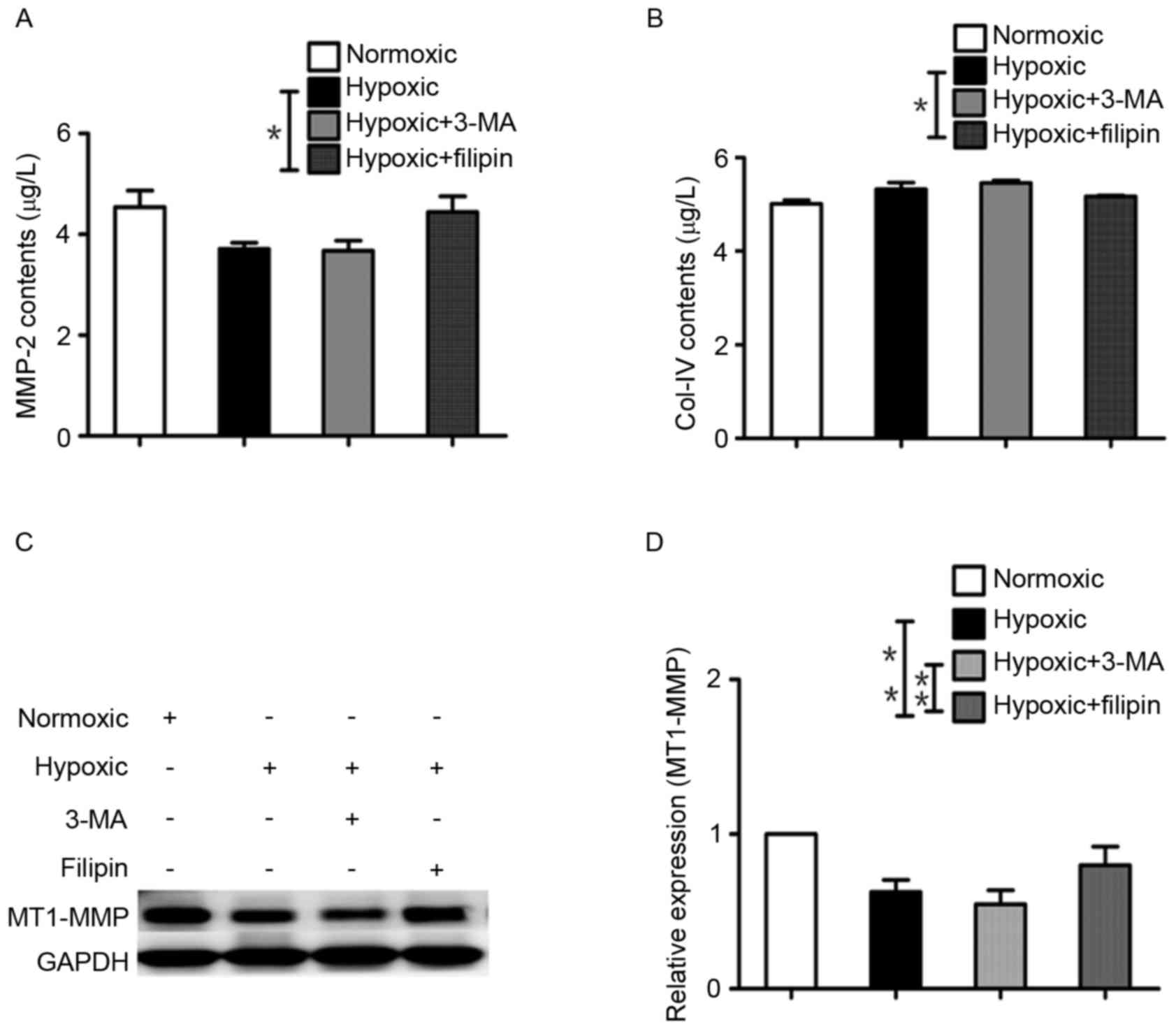
efficiency of protein secretion by the cells. As shown in Fig. 2D, the protein expression of MMP-2
(3.71±0.12 µg/l) was significantly reduced in the hypoxia-treated
cells, compared with that in the normoxic cells (4.54±0.33 µg/l;
P<0.05). When the cells were incubated with the inhibitor, 3-MA,
under hypoxia for 24 h, the protein expression of MMP-2 (3.67±0.20
µg/l) was further reduced, compared with the hypoxic group.
However, the addition of filipin increased the protein level of
MMP-2 in the culture media (4.44±0.31 µg/l), compared with that in
the hypoxic group (P<0.01; Fig.
3A).
As the protein level of Col-IV in the cell culture
media was opposite to the activity of MMP-2, the protein expression
of Col-IV was examined using ELISA to assess the enzymatic activity
of MMP-2. The results showed that the protein levels of Col-IV in
the hypoxic group (5.33±0.14 µg/l) and hypoxic+3-MA group
(5.46±0.06 µg/l) were increased, compared with that in the normoxic
group (5.02±0.07 µg/l), suggesting decreased activity of MMP-2. The
protein level of Col-IV was decreased in the hypoxic+filipin group
(5.18±0.02 µg/l), compared with that in the hypoxic group
(P<0.05; Fig. 3B), indicating
increased activity of MMP-2 in the presence of filipin.
Inhibition of endocytosis increases
the expression of MT1-MMP in hypoxia-treated HK-2 cells
Western blot analysis was used to detect the protein
levels of MT1-MMP in the cells. The protein expression of MT1-MMP
was normalized with the internal control, GAPDH (Fig. 3C). The results showed that the
protein expression of MT1-MMP was significantly higher in the
normoxic group, compared with that in the hypoxic group. Compared
with the hypoxic group, treatment with 3-MA under hypoxic
conditions for 24 h resulted in a further decrease in the protein
level of MT1-MMP, whereas filipin increased the protein level of
MT1-MMP in the cells. The fold increases in MT1-MMP protein were
0.54±0.09 and 0.80±0.12, compared with the normoxic group,
respectively (Fig. 3D).
Discussion
Renal hypoxia is an important factor in the
pathophysiology of the acute kidney injury (AKI) to CKD transition
(27). The reason for the reduced
MMP-2 activity in patients with CKD remains to be fully elucidated.
Studies have shown that renal proximal tubular cells may be the
primary target of a hypoxic insult (1,28).
Hypoxia induces autophagy and endocytosis (17–19).
However, whether hypoxic-induced autophagy and Caveolae-mediated
endocytosis can affect the activity of MMP-2 in human renal
proximal tubular epithelial cells remains to be elucidated. In the
present study, HK-2 cells were treated with hypoxia and with
specific inhibitors of autophagy and endocytosis (3-MA and filipin,
respectively), and decreased autophagy and endocytosis were
observed in the cells following exposure to hypoxia for 24 h. These
data suggested that 3-MA and filipin were effective inhibitors for
autophagy and Caveolae-mediated endocytosis in hypoxia,
respectively.
In the present study, the specific inhibitors for
autophagy and Caveolae-mediated endocytosis altered the expression
and activity of MMP-2 in hypoxia-treated HK-2 cells. 3-MA and
filipin significantly increased the mRNA levels of MMP-2 in
hypoxia-treated cells. In particular, the inhibition of
Caveolae-mediated endocytosis by filipin increased the activity of
MMP-2 in culture media under hypoxia. Although the inhibition of
autophagy considerably upregulated the expression of MMP-2 at the
mRNA level, 3-MA decreased the activity of MMP-2 in culture media
under hypoxia. In a report by Orphanides et al (14), data suggested that regulation of
the activity of MMP-2 was via a post-transcriptional mechanism, and
the results of the present study appeared to confirm this, with the
ELISA results for the protein expression levels of Col-IV and MMP-2
confirming these observations.
A previous study showed that Caveolin-1 inhibits the
activity of MT1-MMP, promoting the internalization of MT1-MMP from
the cell surface and reducing the activation of proMMP-2 (21). Another study demonstrated that
MT1-MMP is crucial for the activation of proMMP-2 (22). In the present study, the protein
level of MT1-MMP was increased, and the activity of MMP-2 was
augmented. These findings are consistent with these previous
reports. The expression of MT1-MMP induced the activation of
proMMP-2, however, the association requires further elucidation.
Previous reports have shown that Caveolin-1 inhibits the activity
of MMP-2 in heart tissue (29) and
filipin decreases Caveolae-mediated endocytosis (30). In the present study, when filipin
inhibited the expression of Caveolin-1 under hypoxia, the levels of
Caveolin-1 significantly increased the activity of MMP-2, and this
result is similar to results of previous studies (29,30).
The activity of MMP-2 is key in preventing the
thickness of the TBM and the accumulation of interstitial ECM in
patients with CKD (3–6). The present study demonstrated the
association of hypoxia-induced autophagy and endocytosis with the
activity of MMP-2, respectively, providing novel insight into the
mechanisms underlying the interaction between the activity of MMP-2
and hypoxia. Furthermore, these results provide clues regarding the
mechanism underlying progressive fibrosis in patients with CKD. It
was hypothesized that the activity of MMP-2 increases in culture
media following the inhibition of autophagy and endocytosis as
molecules regulating the activation of MMP-2 may not be affected,
however, only the inhibition of endocytosis increased the activity
of MMP-2. It is likely that 3-MA may affect other signaling
molecules responsible for regulating the mRNA level and activity of
MMP-2 under hypoxic conditions. Further investigation in the in
vivo model is warranted.
In conclusion, the present study provided novel
findings suggesting that the inhibition of Caveolae-mediated
endocytosis may increase the activity of MMP-2 in the kidney
tissues of patients with CKD, and may be potentially used to
inhibit renal fibrogenesis, prevent TBM thickness and prevent the
accumulation of ECM in renal diseases. Simultaneously, the results
of the present study may provide novel insight into the mechanism
underlying progressive fibrosis.
Acknowledgements
The authors would like to thank Professor Wandong
Zhang at the National Research Council of Canada (Ottawa, ON,
Canada) for critically reading and editing the manuscript. The
present study was financially supported by the National Nature
Science Foundation of the People's Republic of China (grant no.
81370868) and the Fundamental Research Funds for the Central
Universities and Jiangsu Province Scientific Research Innovation
Project for Graduate Students (grant no. KYLX_0197).
Glossary
Abbreviations
Abbreviations:
|
ECM
|
extracellular matrix
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
MT1-MMP
|
membrane-type 1 matrix
metalloproteinase
|
|
CKD
|
chronic kidney disease
|
|
Col-IV
|
collagen-IV
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Levey AS, Atkins R, Coresh J, Cohen EP,
Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, et
al: Chronic kidney disease as a global public health problem:
Approaches and initiatives-a position statement from kidney disease
improving global outcomes. Kidney Int. 72:247–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schieppati A and Remuzzi G: Chronic renal
diseases as a public health problem: Epidemiology, social and
economic implications. Kidney Int Suppl. 98:S7–S10. 2005.
View Article : Google Scholar
|
|
3
|
Jacobson HR: Chronic renal failure:
Pathophysiology. Lancet. 338:419–423. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo X, Deng L, Lamsal LP, Xu W, Xiang C
and Cheng L: AMP-activated protein kinase alleviates extracellular
matrix accumulation in high glucose-induced renal fibroblasts
through mTOR signaling pathway. Cell Physiol Biochem. 35:191–200.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou X, Zhang J, Xu C and Wang W: Curcumin
ameliorates renal fibrosis by inhibiting local fibroblast
proliferation and extracellular matrix deposition. J Pharmacol Sci.
126:344–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eddy AA: Molecular insights into renal
interstitial fibrosis. J Am Soc Nephrol. 7:2495–2508.
1996.PubMed/NCBI
|
|
7
|
Ronco P and Chatziantoniou C: Matrix
metalloproteinases and matrix receptors in progression and reversal
of kidney disease: Therapeutic perspectives. Kidney Int.
74:873–878. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basile DP, Fredrich K, Weihrauch D, Hattan
N and Chilian WM: Angiostatin and matrix metalloprotease expression
following ischemic acute renal failure. Am J Physiol Renal Physiol.
286:F893–F902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Racca MA, Novoa PA, Rodríguez I, Della
Vedova AB, Pellizas CG, Demarchi M and Donadio AC: Renal
dysfunction and intragraft proMMP9 activity in renal transplant
recipients with interstitial fibrosis and tubular atrophy. Transpl
Int. 28:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou TB, Qin YH, Lei FY, Huang WF and
Drummen GP: Prohibitin attenuates oxidative stress and
extracellular matrix accumulation in renal interstitial fibrosis
disease. PLoS One. 8:e771872013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeCoux A, Lindsey ML, Villarreal F, Garcia
RA and Schulz R: Myocardial matrix metalloproteinase-2: Inside out
and upside down. J Mol Cell Cardiol. 77:64–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fine LG and Norman JT: Chronic hypoxia as
a mechanism of progression of chronic kidney diseases: From
hypothesis to novel therapeutics. Kidney Int. 74:867–872. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Norman JT, Orphanides C, Garcia P and Fine
LG: Hypoxia-induced changes in extracellular matrix metabolism in
renal cells. Exp Nephrol. 7:463–469. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orphanides C, Fine LG and Norman JT:
Hypoxia stimulates proximal tubular cell matrix production via a
TGF-beta1-independent mechanism. Kidney Int. 52:637–647. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Fan R, Zhao S, Liu L, Guo S, Wu N,
Zhang W and Chen P: Reactive oxygen species released from hypoxic
hepatocytes regulates MMP-2 expression in hepatic stellate cells.
Int J Mol Sci. 12:2434–2447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bellot G, Garcia-Medina R, Gounon P,
Chiche J, Roux D, Pouysségur J and Mazure NM: Hypoxia-induced
autophagy is mediated through hypoxia-inducible factor induction of
BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol.
29:2570–2581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mazure NM and Pouysségur J:
Hypoxia-induced autophagy: Cell death or cell survival? Curr Opin
Cell Biol. 22:177–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Michiels C: Physiological and pathological
responses to hypoxia. Am J Pathol. 164:1875–1882. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dada LA, Welch LC, Zhou G, Ben-Saadon R,
Ciechanover A and Sznajder JI: Phosphorylation and ubiquitination
are necessary for Na, K-ATPase endocytosis during hypoxia. Cell
Signal. 19:1893–1898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kukulski W, Schorb M, Kaksonen M and
Briggs JA: Plasma membrane reshaping during endocytosis is revealed
by time-resolved electron tomography. Cell. 150:508–520. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HN and Chung HS: Caveolin-1 inhibits
membrane-type 1 matrix metalloproteinase activity. BMB Rep.
41:858–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sato H, Takino T, Okada Y, Cao J,
Shinagawa A, Yamamoto E and Seiki M: Matrix metalloproteinase
expressed on the surface of invasive tumour cells. Nature.
370:61–65. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Butler GS, Butler MJ, Atkinson SJ, Will H,
Tamura T, van Schade Westrum S, Crabbe T, Clements J, d'Ortho MP
and Murphy G: The TIMP2 membrane type 1 metalloproteinase
‘receptor’ regulates the concentration and efficient activation of
progelatinase A.A kinetic study. J Biol Chem. 273:871–880. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Amico G: Tubulo-interstitial damage in
glomerular diseases: Its role in the progression of the renal
damage. Nephrol Dial Transplant. 13:(Suppl 1). S80–S85. 1998.
View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takahara T, Furui K, Funaki J, Nakayama Y,
Itoh H, Miyabayashi C, Sato H, Seiki M, Ooshima A and Watanabe A:
Increased expression of matrix metalloproteinase-II in experimental
liver fibrosis in rats. Hepatology. 21:787–795. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka S, Tanaka T and Nangaku M: Hypoxia
as a key player in the AKI-to-CKD transition. Am J Physiol Renal
Physiol. 307:F1187–F1195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Norman J and Orphanides C: Hypoxia alters
extracellular matrix (ECM) synthesis and turnover in renal
fibroblasts. J Am Soc Nephrol. 7:A25771996.
|
|
29
|
Chow AK, Cena J, El-Yazbi AF, Crawford BD,
Holt A, Cho WJ, Daniel EE and Schulz R: Caveolin-1 inhibits matrix
metalloproteinase-2 activity in the heart. J Mol Cell Cardiol.
42:896–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schnitzer JE, Oh P, Pinney E and Allard J:
Filipin-sensitive caveolae-mediated transport in endothelium:
Reduced transcytosis, scavenger endocytosis, and capillary
permeability of select macromolecules. J Cell Biol. 127:1217–1232.
1994. View Article : Google Scholar : PubMed/NCBI
|