Introduction
Although many efforts have been made to improve the
efficacy of therapeutic strategies for the treatment of pancreatic
cancer, current chemotherapeutic options remain unsatisfactory
(1). In 2016, pancreatic cancer
was reported as the 4th leading cause of cancer-associated
mortality in the USA, the estimated death in males was 21,450 and
in females 20,330 (2), stressing
the need for the development of novel therapeutic agents for
pancreatic cancer. Tanshinone (Tan)-IIA
(C19H18O3) is one of the active
constituents of the plant-derived traditional Chinese medicine
Danshen (3,4). Tan-IIA has been reported to possess
anti-cancer potential; it has been demonstrated to induce apoptosis
in prostate cancer cells (5).
Tan-IIA has also been reported to inhibit the proliferation of A549
human non-small cell lung cancer cells via decreasing the
expression of vascular endothelial growth factor and its receptor
(6). Yang et al reported
that Tan-IIA inhibited the growth of human glioma stem cells via
inducing apoptosis in vitro and in vivo in a
dose-dependent manner (7).
Munagala et al suggested that Tan-IIA may have potential as
a therapeutic agent for the prevention and treatment of cervical
and other human papilloma virus-related types of cancer (8). It has previously been reported that
Tan-IIA may exert cytotoxic effects in human pancreatic cancer
MIAPaCa-2 (9) and BxPC-3 cells
(10), and it induced endoplasmic
reticulum (ER) stress to inhibit the growth of BxPC-3 cells in
vitro (11). Further in
vivo studies are required to elucidate the mechanisms
underlying the ER-related effects of Tan-IIA in BxPC-3 cells. The
present study investigated the in vivo effects of Tan-IIA on
the expression of ER stress-related proteins in BxPC3-derived
xenograft tumors.
Materials and methods
Chemicals and reagents
The BxPC-3 human pancreatic cancer cell line was
obtained from the Food Industry Research and Development Institute
(Hsinchu, Taiwan). Tan-IIA, sodium deoxycholate, leupeptin, Triton
X-100, Tris-HCl, sodium pyruvate, HEPES, RPMI-1640, trypsin-EDTA,
mouse anti-β-actin antibody (cat. no. A5441; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), penicillin-streptomycin, dimethyl
sulfoxide, potassium phosphates and were obtained from Merck KGaA.
Fetal bovine serum (FBS) and glutamine were obtained from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Tris-glycine-SDS buffer (10X), Tween-20 and glycine were obtained
from Ameresco, Inc. (Framingham, MA, USA). BioMax film was obtained
from Kodak (Rochester, NY, USA). Anti-protein kinase RNA-like
endoplasmic reticulum kinase (PERK) (cat. no. 9956),
anti-inositol-requiring enzyme 1α (IRE1α) (cat. no. 9956),
anti-phosphorylated (p)-c-Jun N-terminal (JNK) (cat. no. 9910),
anti-CCAAT-enhancer-binding protein homologous protein (CHOP) (cat.
no 9956) and anti-caspase-3 (cat. no 9661) antibodies were obtained
from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Anti-caspase 12 (cat. no. ab62484), anti-activating transcription
factor 6 (ATF6) (cat. no. ab37149) and anti-eukaryotic initiation
factor 2α (elF2α) (cat. no. ab5369) antibodies were obtained from
Abcam (Cambridge, UK). Anti-B-cell lymphoma 2 (Bcl-2) antibody
(cat. no. NB100-92142) was obtained from Novus Biologicals, LLC
(Littleton, CO, USA).
Cell culture
Human pancreatic adenocarcinoma BxPC-3 cells were
cultured as previously described (10,11).
Briefly, BxPC-3 cells were maintained in RPMI-1640 medium
supplemented with 10% FBS, 10,000 U/ml penicillin and 10 mg/ml
streptomycin, at 37°C in a humidified atmosphere containing 5%
CO2.
In vivo studies
Cultured BxPC3 cells (2×106/0.2 ml) were
implanted into 4-week old, male nude severe combined
immunodeficiency (SCID) mice (n=30) via subcutaneous injection over
the flank area. Mice were maintained in a pathogen-free environment
(Laboratory Animal Center of Tzu Chi University, Hualien, Taiwan).
SCID mice implanted with BxPC-3 cells were randomly divided into 3
groups (n=10 per group) to receive 3 different weekly doses of
Tan-IIA (0, 30 and 90 mg/kg). Tan-IIA was dissolved in corn oil and
administered intraperitoneally on weeks 1, 3 and 5 following
xenotransplantation. Volumes of the xenograft tumors were measured
every other week. Tumor volume was estimated according to the
following formula: Tumor volume (mm3)=LxW2/2,
where L refers to tumor length and W refers to tumor width. On day
35 following xenotransplantation SCID mice were sacrificed by
CO2 inhalation, the xenograft tumors were dissected and
total protein was extracted from the tumors. Subsequently, protein
expression levels of PERK, ATF6, caspase-12/caspase-3, IRE1α,
eIF2α, p-JNK, CHOP and Bcl-2 in the xenograft tumors were assessed
using western blot analysis.
All experimental procedures were approved by the
Institutional Animal Care and Use Committee of Tzu Chi University
(approval no. CCH-AE-101-010).
Protein preparation
Total protein was extracted from xenograft tumors.
Following dissection, tumors were homogenized and lysed in ice-cold
whole cell lysis buffer containing protease inhibitors (BioVision,
Inc., Milpitas, CA, USA). The lysates were incubated for 30 min at
4°C with agitation and were centrifuged at 12,281 × g for 10 min.
Protein concentration was measured using the bicinchoninic acid
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.).
Western blot analysis
Western blot analysis was conducted as previously
described (10,11). Briefly, equal amounts of extracted
protein samples (10 µg) were separated by 12% SDS-PAGE (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and transferred onto
polyvinylidene difluoride membranes, which were blocked for 1 h at
4°C with blocking buffer [5% dried skimmed milk in solution
containing 50 mM Tris-HCl (pH 8.0), 2 mM CaCl2, 80 mM
sodium chloride, 0.05% Tween-20 and 0.02% sodium azide (Merck
KGaA)]. The membranes were incubated for 2 h at room temperature
with the following primary antibodies: PERK, ATF6, caspase-3,
caspase-12, IRE1α, eIF2α, p-JNK, CHOP, Bcl-2 (all diluted to
1:1,000) and β-actin (diluted to 1:5,000). Subsequently, they were
incubated at room temperature for 1 h with anti-rabbit (cat. no.
sc-2004) or anti-mouse (cat. no. sc-2005) immunoglobulin
G-horseradish peroxidase-conjugated secondary antibodies (1:5,000;
Santa Cruz Biotechnology Inc., Dallas, TX, USA). The membranes were
washed 3 times for 10 min with 1X PBS with 0.05% Tween-20. The
protein bands were visualized on X-ray film using an enhanced
chemiluminescence detection system (PerkinElmer, Inc., Waltham, MA,
USA) and quantified using ImageJ version 1.44 (National Institute
of Health, Bethesda, MD, USA).
Statistical analysis
The statistical significance of the difference
between groups was assessed by one-way analysis of variance
followed by Dunnett's test. Data are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference. The analysis was performed
using IBM SPSS software version 20.0 (IBM SPSS, Armonk, NY,
USA).
Results
Effects of Tan-IIA on BxPC3-derived
tumor xenografts
Mice implanted with BxPC-3-derived tumor xenografts
were treated with 3 doses of Tan-IIA (0, 30 and 90 mg/kg) for 4
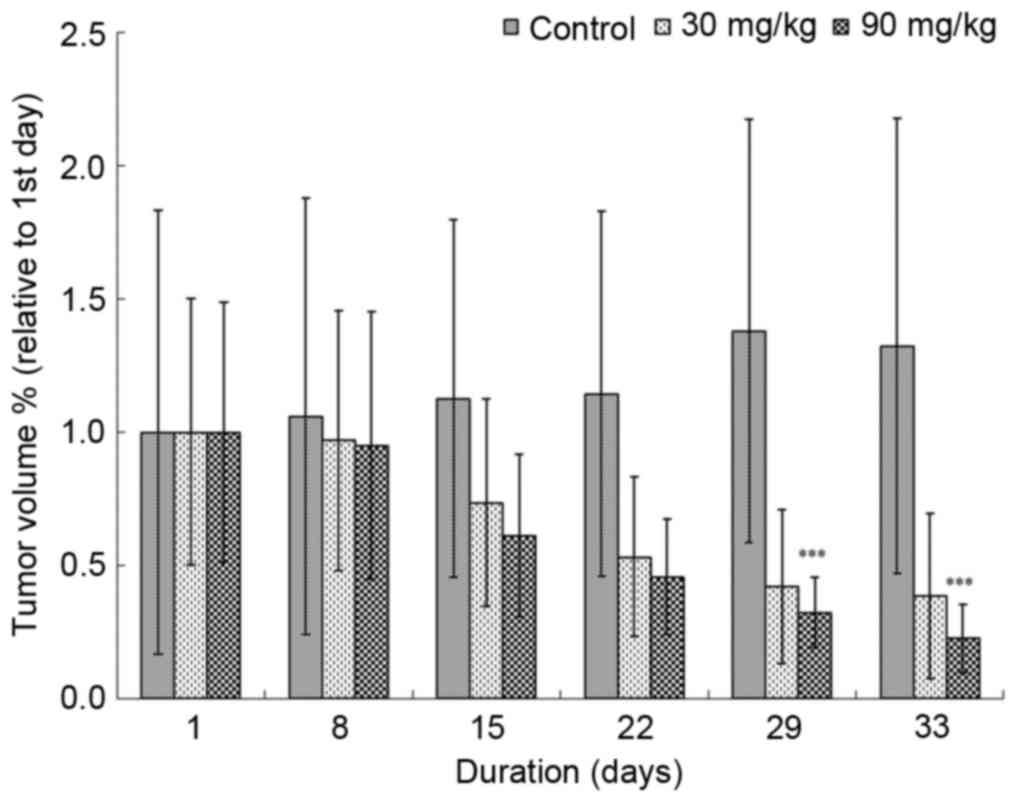
weeks. Tan-IIA was demonstrated to impair xenograft tumor growth in
a dose-dependent manner (Fig. 1).
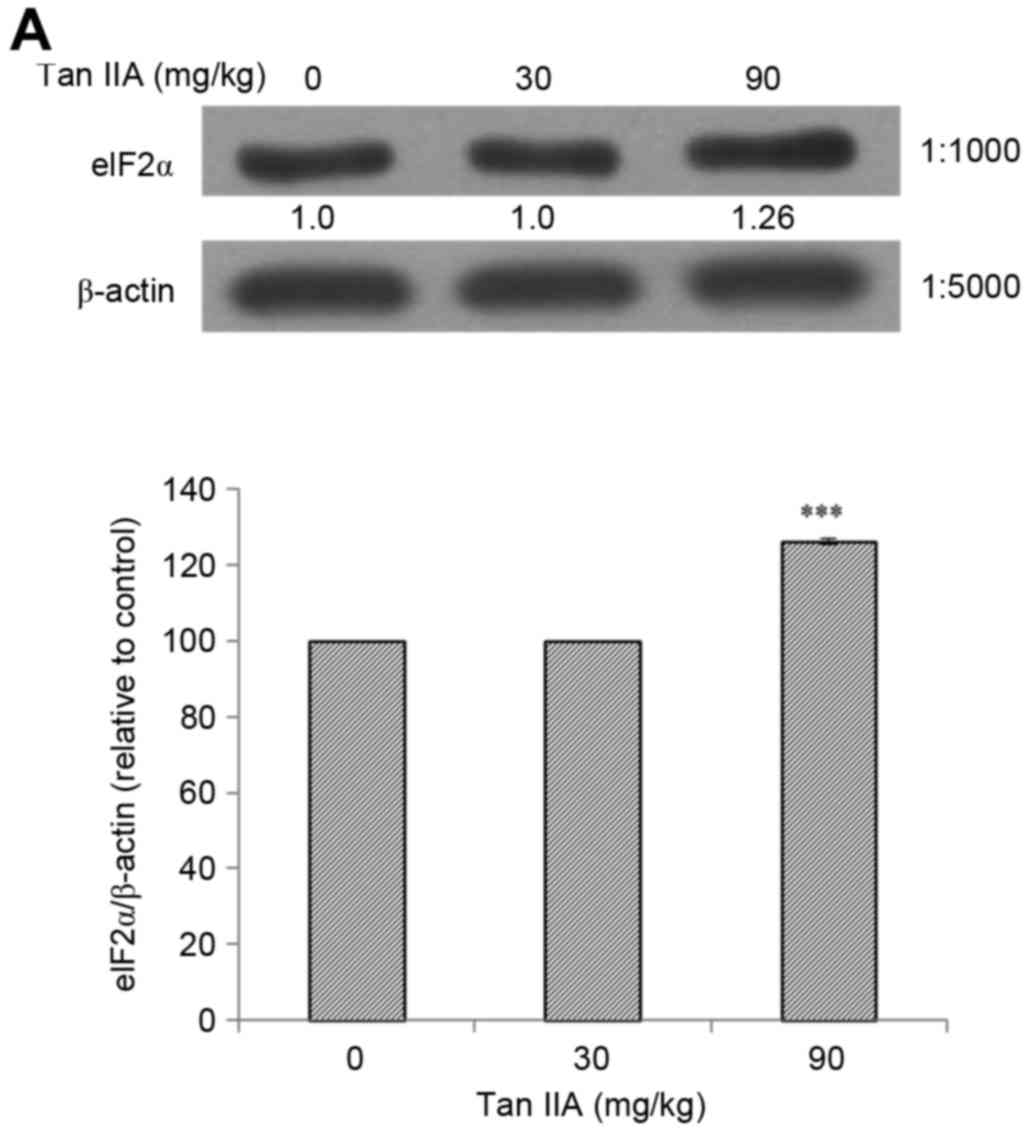
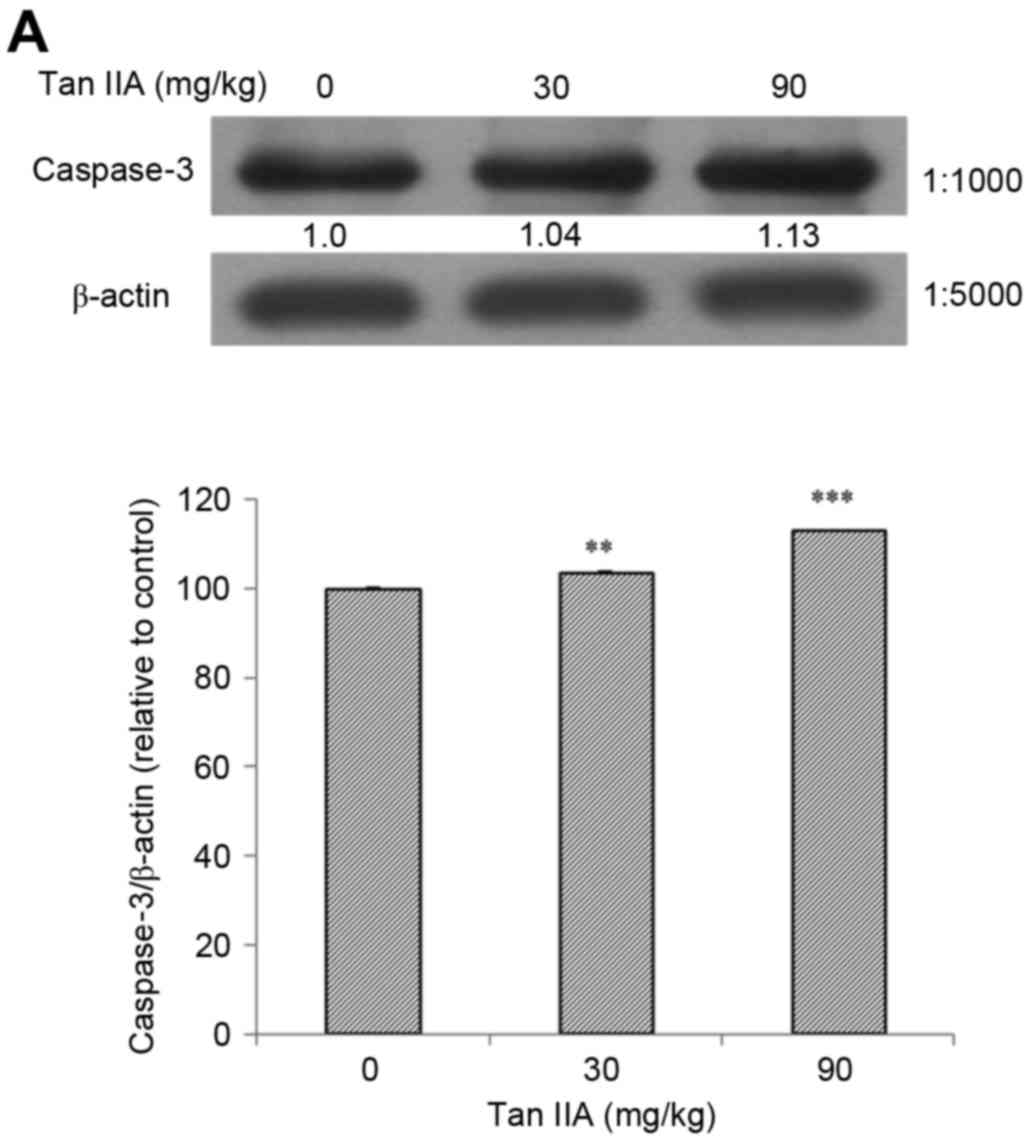
In addition, protein expression of PERK, ATF6, caspase-3,
caspase-12, IRE1α, eIF2α, p-JNK, CHOP and Bcl-2 was assessed using
western blot analysis, with β-actin as an internal control. The
present results revealed that Tan-IIA significantly increased the
protein expression levels of PERK (Fig. 2A), ATF6 (Fig. 2B), caspase-12 (Fig. 2C), IRE1α (Fig. 2D), elF2α (Fig. 3A), p-JNK (Fig. 3B), CHOP (Fig. 3C) and caspase-3 (Fig. 4A) in a dose-dependent manner.
Conversely, treatment with Tan-IIA resulted in a significant
dose-dependent decrease in Bcl-2 protein expression levels
(Fig. 4B).
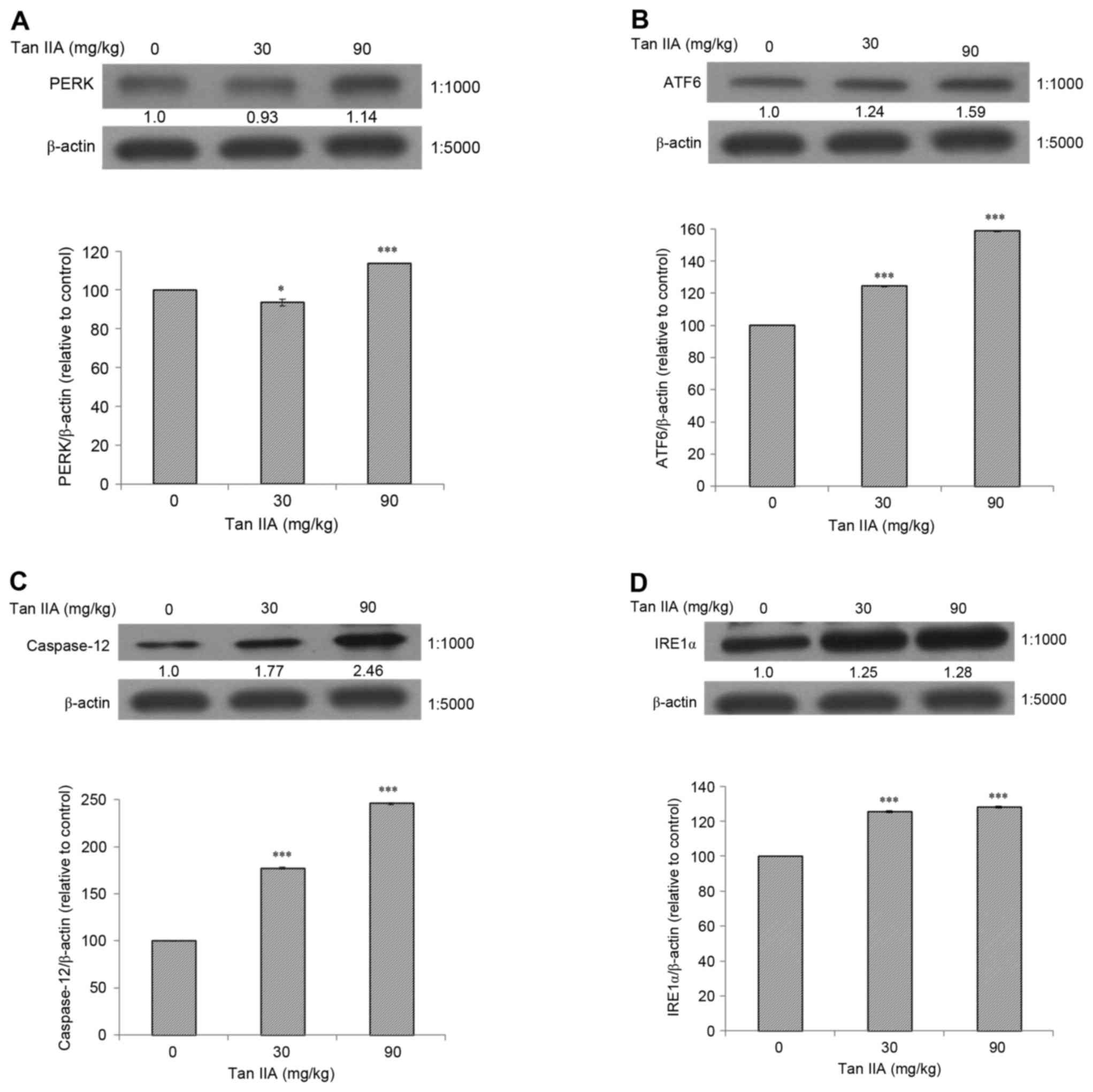 | Figure 2.Protein expression levels of PERK,
ATF6, caspase-12 and IRE1α in BxPC-3-derived xenograft tumors
following treatment with Tan-IIA. Mice bearing BxPC-3-derived
xenograft tumors were treated with various doses of Tan-IIA (0, 30
and 90 mg/kg). Tumors were dissected and western blot analysis was
performed to assess protein expression levels. Tan-IIA increased
the protein expression levels of (A) PERK, (B) ATF6, (C) caspase-12
and (D) IRE1α in a dose-dependent manner. Data are expressed as the
mean ± standard deviation. *P<0.05, ***P<0.001 compared with
the control group. PERK, protein kinase RNA-like endoplasmic
reticulum kinase; ATF, activating transcription factor; IRE,
inositol-requiring enzyme; Tan, Tanshinone. |
Discussion
The induction of ER stress is one of the underlying
mechanisms involved in therapeutic strategies for cancer (12). It has previously been reported that
the activation of upstream elements, such as IRE1α and PERK,
consequently results in an increase in their downstream targets
eIF2α, p-JNK and CHOP (13). CHOP
has been demonstrated to inhibit the protein expression of Bcl-2.
When the unfolded protein response exceeds a threshold, damaged
cells become apoptotic, through a mechanism that may involve the
caspase-12- and ATF6-mediated induction of the CHOP signaling
pathway (14,15). Pan et al reported that
Tan-IIA may enhance the apoptosis of CaSki advanced cervical
carcinoma cells, through the activation of intrinsic mitochondrial
and ER stress-associated pathways (16). In addition, Chiu et al
demonstrated that Tan-IIA inhibited the growth of human prostate
cancer cells through the induction of ER stress in vitro and
in vivo (17). The present
study revealed that Tan-IIA suppressed the growth of BxPC-3-derived
xenograft tumors, as tumor volume was demonstrated to be decreased
in mice following 28 days of Tan-IIA treatment compared with in
untreated mice (Fig. 1). In
addition, Tan-IIA increased the protein expression levels of PERK,
ATF6, caspase-12, IRE1α, elF2α, p-JNK, CHOP and caspase-3 in
BxPC-3-derived xenograft tumors in a dose-dependent manner.
Conversely, treatment with Tan-IIA resulted in a dose-dependent
decrease of Bcl-2 protein expression levels in BxPC-3-derived
xenograft tumors. The present results indicated that Tan-IIA may
promote apoptosis through the induction of ER stress in xenograft
tumors derived from BxPC-3 cells. These results are in accordance
with an in vitro study that demonstrated that Tan-IIA
induced ER stress via increasing the expression of PERK, IRE1α,
caspase-12 and ATF6. These proteins stimulated the overexpression
of their downstream elements elF2α and p-JNK, and the target
protein CHOP, which resulted in decreased Bcl-2 expression,
mitochondrial dysfunction and increased caspase-3-mediated
apoptosis (11).
In conclusion, the present study suggested that
Tan-IIA may exert tumor-suppressing effects via inducing ER stress
in cancer cells, and may have potential as a novel therapeutic
strategy for the treatment of patients with pancreatic cancer.
Acknowledgements
The present study was supported by the Research
Section of the Changhua Christian Hospital, Changhua, Taiwan (grant
no. 103-CCH-IRP-023).
References
|
1
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2016. Ca Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Che AJ, Zhang JY, Li CH, Chen XF, Hu ZD
and Chen XG: Separation and determination of active components in
Radix Salviae miltiorrhizae and its medicinal preparations by
nonaqueous capillary electrophoresis. J Sep Sci. 27:569–575. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li C, Han X, Zhang H, Wu J and Li B: The
interplay between autophagy and apoptosis induced by tanshinone IIA
in prostate cancer cells. Tumour Biol. 37:7667–7674. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie J, Liu J, Liu H, Liang S, Lin M, Gu Y,
Liu T, Wang D, Ge H and Mo SL: The antitumor effect of tanshinone
IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on
the human non-small cell lung cancer A549 cell line. Acta Pharm Sin
B. 5:554–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang L, Guo H, Dong L, Wang L, Liu C and
Wang X: Tanshinone IIA inhibits the growth, attenuates the stemness
and induces the apoptosis of human glioma stem cells. Oncol Rep.
32:1303–1311. 2014.PubMed/NCBI
|
|
8
|
Munagala R, Aqil F, Jeyabalan J and Gupta
RC: Tanshinone IIA inhibits viral oncogene expression leading to
apoptosis and inhibition of cervical cancer. Cancer Lett.
356:536–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fronza M, Murillo R, Ślusarczyk S, Adams
M, Hamburger M, Heinzmann B, Laufer S and Merfort I: In vitro
cytotoxic activity of abietane diterpenes from Peltodon longipes as
well as Salvia miltiorrhiza and Salvia sahendica. Bioorg Med Chem.
19:4876–4881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang CY, Chiu TL, Kuo SJ, Chien SY, Chen
DR and Su CC: Tanshinone IIA inhibits the growth of pancreatic
cancer BxPC-3 cells by decreasing protein expression of TCTP, MCL-1
and Bcl-xL. Mol Med Rep. 7:1045–1049. 2013.PubMed/NCBI
|
|
11
|
Su CC: Tanshinone IIA could inhibit
pancreatic cancer BxPC-3 cells through increasing PERK, ATF6,
caspase-12 and CHOP expression to induce apoptosis. J Biomedical
Sci Engineering. 8:149–159. 2015. View Article : Google Scholar
|
|
12
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Y and Hendershot LM: The role of the
unfolded protein response in tumour development: Friend or foe? Nat
Rev Cancer. 4:966–977. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim R, Emi M, Tanabe K and Murakami S:
Role of the unfolded protein response in cell death. Apoptosis.
11:5–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rasheva VI and Domingos PM: Cellular
responses to endoplasmic reticulum stress and apoptosis. Apoptosis.
14:996–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan TL, Wang PW, Hung YC, Huang CH and Rau
KM: Proteomic analysis reveals tanshinone IIA enhances apoptosis of
advanced cervix carcinoma CaSki cells through mitochondria
intrinsic and endoplasmic reticulum stress pathways. Proteomics.
13:3411–3423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiu SC, Huang SY, Chen SP, Su CC, Chiu TL
and Pang CY: Tanshinone IIA inhibits human prostate cancer cells
growth by induction of endoplasmic reticulum stress in vitro and in
vivo. Prostate Cancer Prostatic Dis. 2013:315–322. 2013. View Article : Google Scholar
|