Introduction
Traditional use of insects as food is widespread in
tropical and subtropical countries because it provides nutritional
and economic benefits in rural communities (1). Certain insects, including silkworms,
have also been used as therapeutics in Korean traditional medicine
(2). Because of its high
nutritional value, the silkworm has also been considered as a
potential solution to the exhaustion of food sources (3). Silkworm pupa (SWP) is a traditional
food that is commonly consumed following boiling with soybean
sauce. In China, oil-fried, water-boiled or ground pupa is often
consumed. It has been reported that >1,000 patients per year
suffer anaphylactic reactions following consumption of SWP in China
(4). A foreign tourist was
reported to enter anaphylactic shock after consuming SWP (4). However, a high prevalence of skin
test reactivity (9.4%) to total extract of SWP among patients with
allergies unrelated to SWP was reported, suggesting that
non-specific or clinically irrelevant immunoglobulin (Ig) E binding
is a substantial problem in the diagnosis of SWP allergy (5).
A retrospective analysis of serum IgE to silkworm
moth and 9 common inhalant allergens among patients with
respiratory allergy in Guangzhou, China, demonstrated a positive
correlation between allergies to silkworm moths and to cockroaches
or house dust mites. Cross-allergenicity between moth and midge was
also described in Japanese asthmatic patients (6). Baldo and Panzani (7) described IgE-binding components of ~37
kDa, potentially tropomyosin, from various arthropods, including
house fly, blowfly, common clothes moth, warehouse moth, Bogong
moth, grain borer, locust, silverfish, cockroach, carpet beetle and
house dust mite (7). However, the
IgE reactivity of silkworm tropomyosin has not been
investigated.
It is well established that the pan-allergen
tropomyosin, especially from invertebrates including shellfish and
mollusks, is an important cause of food allergy (8). Tropomyosin from dust mites,
cockroaches, and helminthes elicits allergic symptoms and it is
conceivable that tropomyosin is important in SWP allergy,
especially considering its heat stable nature. In the present
study, recombinant SWP tropomyosin was produced and its IgE
reactivity was evaluated for diagnosis of SWP allergy.
Materials and methods
SWP protein extract preparation
A frozen SWP was provided by Anysilk Co., Ltd.
(Cheongju, Korea). Following pulverization in liquid nitrogen, the
pupa was defatted with five volumes of a 1:1 mixture of ethyl ether
and ethyl acetate. Proteins were extracted with phosphate-buffered
saline (PBS; pH 7.4) containing 6 mM 2-mercaptoethanol, protease
inhibitor cocktail set III (1:1,000; Calbiochem; Merck KGaA,
Darmstadt, Germany), and 1 mg/ml 1-phenyl-3-(2-thiazyl)-2-thiourea
(Sigma-Aldrich; Merck KGaA) at 4°C. The supernatant was collected
after centrifugation at 10,000 × g for 30 min at 4°C,
filtered through a syringe (0.22 µm; Merck KGaA), and stored at
−70°C until use. The protein concentration was determined by
Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Subjects and serum samples
Serum samples were obtained from 15 patients (age
range, 17–43 years; mean, 34) who visited the Allergy-Asthma Center
at Severance Hospital (Seoul, Korea) between 2014 and 2015. The
patients were diagnosed with SWP allergy based on the temporal
relationship between SWP intake and onset of cutaneous allergic
symptoms and a positive skin prick test to total extract of SWP
(Table I). These symptoms included
urticaria and anaphylaxis, and occurred within 30 min following
intake. ImmunoCAP (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) assay was performed for common allergens, including shrimp
(f24) and crab (f23), in order to estimate potential
cross-reactivity. ImmunoCAP was not performed for SWP because it is
not commercially available. Serum samples from 9 individuals (age
range, 3–47 years; mean, 21) who were not allergic to SWP and
exhibited no positive reaction to the allergen by skin prick test
and ImmunoCAP were also included (healthy controls). The present
study was approved by the relevant institutional review board at
the Yonsei University College of Medicine (4–2013-0397).
 | Table I.Clinical features of enrolled silkworm
allergy patients. |
Table I.
Clinical features of enrolled silkworm
allergy patients.
| Age | Gender | SPT wheal to silkworm
pupa (cm) | Symptoms | ImmunoCAP
(kUA/l) | SPT positive
allergens (degree of skin test response) |
|---|
| 39 | F | ND |
Anaphylaxis | f23 (1.28), f24
(1.48) | ND |
| 35 | F | 3.5 | Urticaria | f23 (0.68), f24
(0.78) | ND |
| 36 | F | ND | Urticaria | f23 (0.06), f24
(0.04) | ND |
| 37 | F | 4.5 | Urticaria | f23 (6.55), f24
(6.84) | f4 (2+) |
| 40 | M | ND | Urticaria | f23 (0.00), f24
(0.05) | ND |
| 25 | M | ND | Urticaria | f23 (0.02), f24
(0.05) | ND |
| 43 | M | ND | Anaphylaxis | f23 (11.0), f24
(8.41) | ND |
| 28 | F | 8.5 | Urticaria | f23 (3.18), f24
(3.64), f4 (0.02) | Silkworm pupa (3+),
f23 (1+), f24 (1+) |
| 27 | F | 4 | Urticaria | d2 (1.35), i6 (0.64),
f23 (0.87), f24 (1.11) | d1 (2+), i206 (2+),
silkworm pupa (1+) |
| 39 | M | 5.5 | Urticaria | f23 (3.27), f24
(3.27) | Silkworm pupa
(−) |
| 18 | M | 8.5 | Urticaria | f23 (11.3), f24
(10.8) | Silkworm pupa
(3+), f290 (2+), saury (2+), f206 (1+) |
| 30 | M | 2.5 | Anaphylaxis | f23 (14.2), f24,
(11.6), c1 (0.15), c5 (0.35), c7 (0.28) | Silkworm pupa
(1+) |
| 26 | M | ND | Urticaria | d2 (6.34), i6
(3.37), f23 (5.51), f24 (5.46) | d1 (3+), d2
(3+), i206 (2+) |
| 43 | F | ND | Urticaria | f23 (0.07), f24
(0.12) | ND |
| 17 | M | 2 |
Urticaria | d2 (23.0), f23
(8.59), f24 (8.97) | f24 (2.5),
silkworm pupa (1+) |
Multiple sequence alignment of
silkworm tropomyosin with allergenic species
Analysis of silkworm tropomyosin was performed using
the Basic Local Alignment Search Tool (BLAST) software from the
National Center for Biotechnology Information (NCBI; Bethesda, MD,
USA) (9). A homology search in
GenBank was performed using the NCBI BlastX program. Sequence
alignment with the retrieved allergen sequences was performed using
the CLUSTAL X software (10).
Production of recombinant
tropomyosin
Total RNA was prepared from frozen pupae, which were
provided by Anysilk Co., Ltd., with TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). First strand cDNA was synthesized
from 5 µg of total RNA using AMV reverse transcriptase (Promega
Corporation, Madison, WI, USA). The reaction mixture contained 0.5
µM oligo-dT (T18) primer (Promega Corporation), 1.9 U of
RNase inhibitor (RNasin®; Promega Corporation), 1x
reaction buffer (Promega Corporation), 0.5 mM dNTPs (Promega
Corporation), 4 mM sodium pyrophosphate (Sigma-Aldrich; Merck KGaA)
and 5 U of reverse transcriptase, reverse transcription was
performed at 42°C for 1 h. Polymerase chain reaction (RT-PCR) was
performed using oligonucleotide primers designed based on the
tropomyosin GenBank sequence (accession no. NM_001110312), as
follows: Forward, 5′-ATGGACGCGATCAAGAAGAA-3′; and reverse,
5′-TTATTCCTTGAGGATGAGCT-3′. After 5 min of initial denaturation at
95°C, 35 cycles of PCR reaction (5 sec at 94°C, 5 sec at 50°C and
10 sec at 72°C) were performed with HiPi Super DNA polymerase (HiPi
Super 5x PCR Master Mix; ELPIS-Biotech, Inc., Daejeon, Korea). The
PCR-amplified DNA fragment, without purification, was directly
ligated into pEXP-5NT/TOPO vector (Invitrogen). Following
transformation of Escherichia coli BL21 (DE3), transformants
were cultured at 37°C in 1 L of Luria Bertani broth and expression
of recombinant protein was induced by the addition of 0.8 mM
isopropyl-1-thio-β-D-galactopyranoside when absorbance at 600 nm
was 0.5. The culture was harvested 4 h after induction. The
cellular pellets were resuspended in buffer (10 mM imidazole, 300
mM NaCl, 50 mM NaH2PO4, pH 8.0), and lysed by
sonication. The lysates were centrifuged at 10,000 × g at
4°C for 30 min, and the recombinant protein was purified from the
supernatant (soluble fraction) using nickel-nitrilotriacetic acid
agarose (Qiagen GmbH, Hilden, Germany) and analyzed by 10% SDS-PAGE
under reducing conditions. Protein concentration was determined by
Bradford assay.
IgE reactivity of recombinant
tropomyosin
IgE reactivity of the recombinant protein was
examined by ELISA. A microplate was coated with recombinant protein
(2 µg/ml) overnight in 0.05 M sodium bicarbonate buffer (pH 9.6).
Following blocking with 3% skim milk in PBS containing 0.05%
Tween-20 (PBST) overnight at 4°C, serum samples (diluted 1:4) were
added and the plate was incubated for 1 h at room temperature. IgE
antibodies were detected by incubation with biotinylated goat
anti-human IgE (1:1,000; cat. no. BA 3040; Vector Laboratories,
Inc., Burlingame, CA, USA) for 1 h, followed by incubation with
streptavidin-peroxidase conjugate (1:1,000; Sigma-Aldrich; Merck
KGaA) for 30 min. The plate was washed at least three times with
PBST between incubation steps. Color development was initiated by
addition of the substrate 3,3′5,5′-tetramethyl-benzidine
(Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD, USA).
Absorbance at 450 nm was measured following addition of 0.5 M
H2SO4 to stop the reaction. The mean
absorbance plus 2 standard deviations of the sera from healthy
controls was used as a cutoff value for significance.
For ELISA inhibition, microtiter plates were coated
with SWP total extract (10 µg/ml) and incubated at 4°C overnight.
Following blocking with 3% skim milk in PBST overnight at 4°C, the
wells were incubated for 1 h at room temperature with patient serum
(1:4 dilution, pooled from two patients who showed a positive IgE
reaction to the recombinant protein), which had been preincubated
for 2 h at room temperature and overnight at 4°C with solutions
containing various concentrations of the extract or recombinant
proteins. Subsequently, IgE antibodies were detected as described
above. The recombinant 27-kDa hemolymph glycoprotein, a silkworm
allergen, was used as an inhibitor for positive control. Production
of recombinant 27-kDa glycoprotein was previously described
elsewhere (11).
For inhibition of immunoblotting, 10 µg of the
extract was run on a 12% SDS-PAGE gel under reducing conditions.
The separated proteins were transferred onto polyvinylidene
difluoride (PVDF) membrane (0.45 µm; Merck KGaA). Following
blocking with 3% skim milk in PBST, the membrane was cut into 4-mm
wide strips and incubated overnight with serum sample (1:4
dilution, pooled from two patients who exhibited a positive
reaction to recombinant protein), which had been preincubated for 2
h at room temperature and overnight at 4°C with solutions
containing 20 µg recombinant protein. Bound IgE antibodies were
detected by incubation with alkaline phosphatase-conjugated goat
anti-human IgE (1:1,000; cat. no. A3525; Sigma-Aldrich; Merck KGaA)
for 1 h at room temperature. Color development was initiated by
addition of nitro blue tetrazolium (NBT) and
5-bromo-4-chloro-3-indolyl-phosphate (BCIP; Promega
Corporation).
Western blot analysis
In order to confirm the presence of tropomyosin in
the extract, western blot analysis was performed using the
monoclonal antibody, 2G32, raised against German cockroach
tropomyosin, Bla g 7 (12).
Monoclonal antibody 2G32 has also been demonstrated to recognize
tropomyosin from house dust mite and dusky brown cockroach
(13). SWP extract (10 µg) was
separated on 12% SDS-PAGE under reducing conditions and Proteins
were transferred onto a PVDF membrane. Following blocking overnight
with 3% skim milk in PBST at 4°C, the membrane was incubated for 1
h with hybridoma culture supernatant (undiluted, collected after
2–3 days of culture). The blots were then incubated with goat
anti-mouse IgG conjugated with alkaline phosphatase (1:1,000; cat.
no. A1293; Sigma-Aldrich; Merck KGaA) for 1 h at room temperature
and developed in a substrate solution of NBT/BCIP.
Statistical analysis
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla,
CA, USA) was used for evaluating the statistical parameters.
Difference in IgE levels between healthy control and allergic
subjects was analyzed with unpaired t-test. The correlation between
IgE to the allergens was evaluated by calculating the Spearman rank
correlation coefficient (ρ).
Results
Homology to allergenic
tropomyosins
An alignment analysis was performed on the amino
acid sequence of SWP tropomyosin. SWP tropomyosin exhibited up to
92.3% sequence identity to Chi k 10, a chironomid tropomyosin,
followed by 90.1% to Per a 7 and 89.4% to Bla g 7, two cockroach
tropomyosins (Fig. 1). SWP
tropomyosin also shared 78.5–81.0% identity with mite tropomyosins
(Der p 10, Der f 10, Tyr p 10, and Lep d 10) and 73.5% identity
with shrimp (Pen a 1) and crab (Hom a 1) tropomyosins.
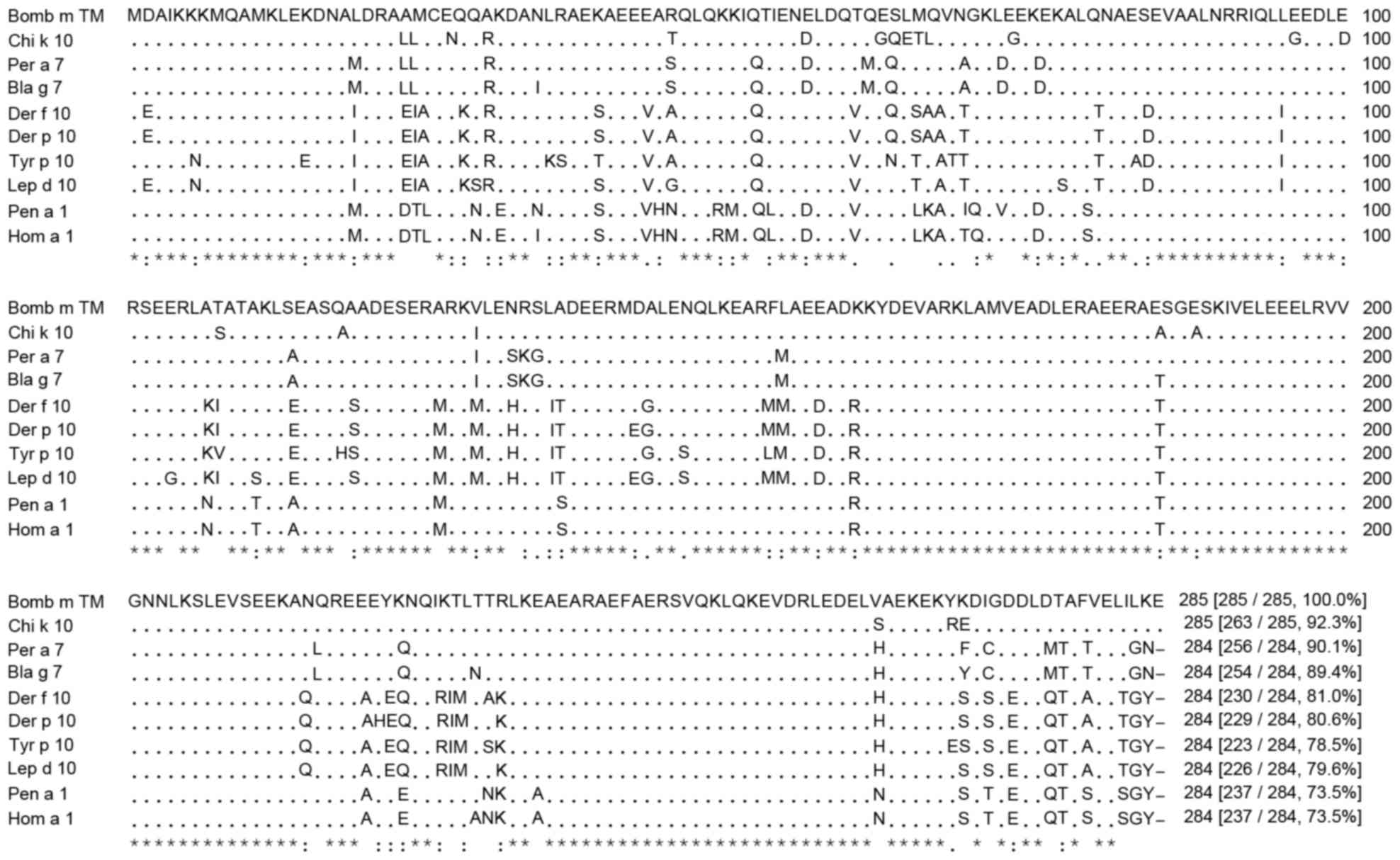 | Figure 1.Amino acid sequence alignment of
silkworm tropomyosin with other allergenic tropomyosins. The
percentage of sequence identity is presented in square brackets.
Bomb m TM, Bombyx mori accession no. NM_001110312; Chi k 10,
Chironomus kiiensis accession no. O96764; Per a 7,
Periplaneta americana accession no. AF106961; Bla g 7,
Blattella germanica accession no. Q9NG56; Der f 10,
Dermatophagoides farinae accession no. D17682; Der p 10,
Dermatophagoides pteronyssinus accession no. AF016278; Tyr p
10, Tyrophagus putrescenatiae accession no. AY623832; Lep d
10, Lepidoglyphus destructor accession no. AJ250096; Pen a
1, Farfantepenaseus aztecus accession no. DQ151457; Hom a 1,
Homarus americanus accession no. AAC48288; *, identical;:,
highly conserved;., less conserved. |
Expression and IgE reactivity of
recombinant SWP tropomyosin
The open reading frame of native SWP tropomyosin
encodes a 285-amino acid protein with a calculated molecular mass
of 32.8 kDa and an isoelectric point of 4.5. The coding sequence of
SWP tropomyosin was cloned by RT-PCR using specific primers and
expressed in E. coli. The resulting purified recombinant
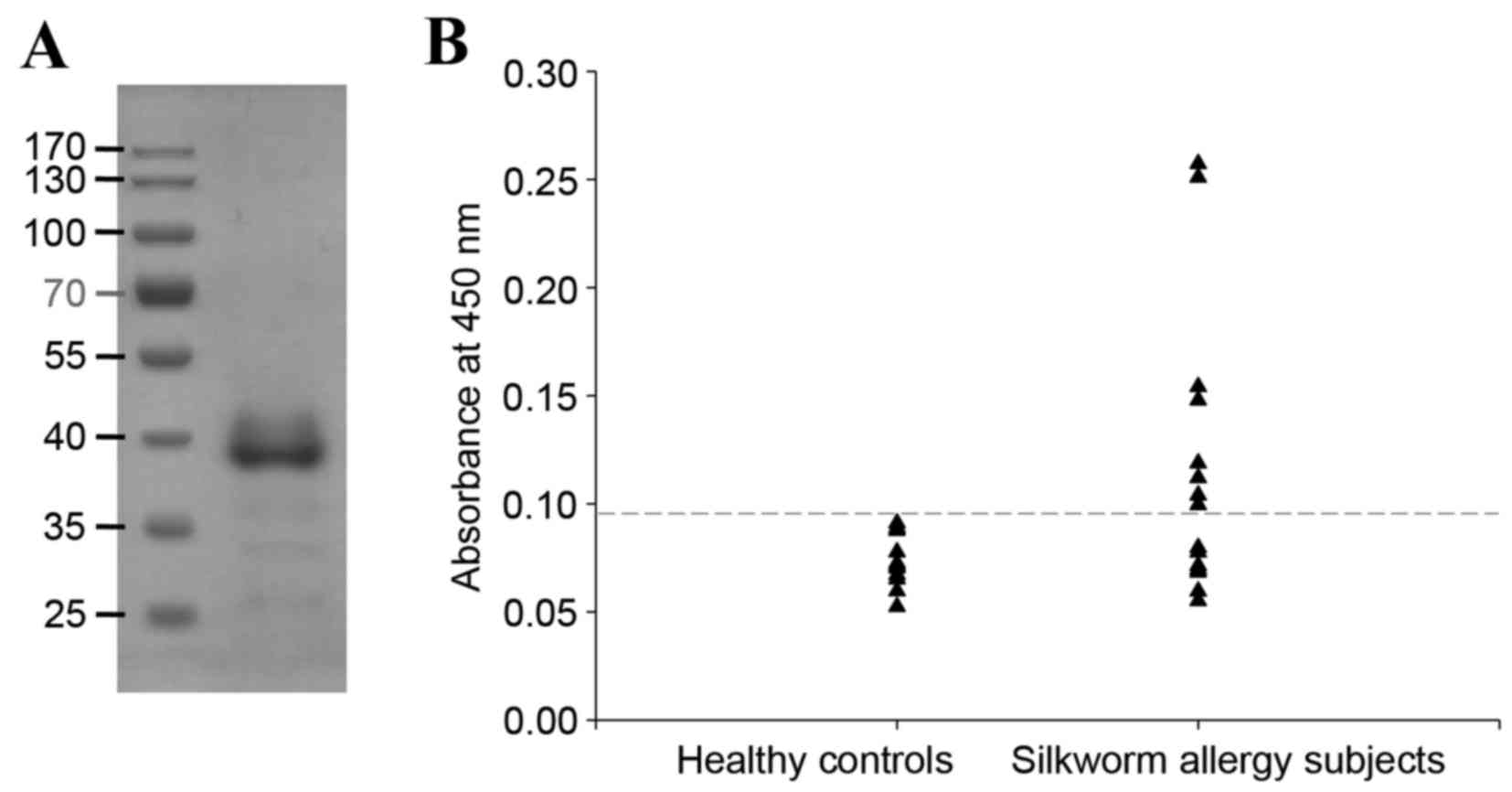
tropomyosin migrated as an ~38 kDa protein on SDS-PAGE, even though
the molecular mass of recombinant tropomyosin containing the
N-terminus 22 amino acid poly-histidine tag (MSG SHH HHH HGS SGE
NLY FQS L) was calculated at 35.3 kDa (Fig. 2A). The yield of the purified
recombinant tropomyosin was 6.618 mg/l of bacteria culture. The
frequency of IgE binding to recombinant silkworm tropomyosin was
53.3% (eight of 15 samples), as examined by ELISA of sera from
patients with SWP allergy compared with healthy controls (Fig. 2B).
Inhibition of specific IgE binding to
total extract
Specific IgE inhibition analysis against total
extract of SWP was performed using pooled sera from two patients
who exhibited the strongest IgE reactivity to the recombinant SWP
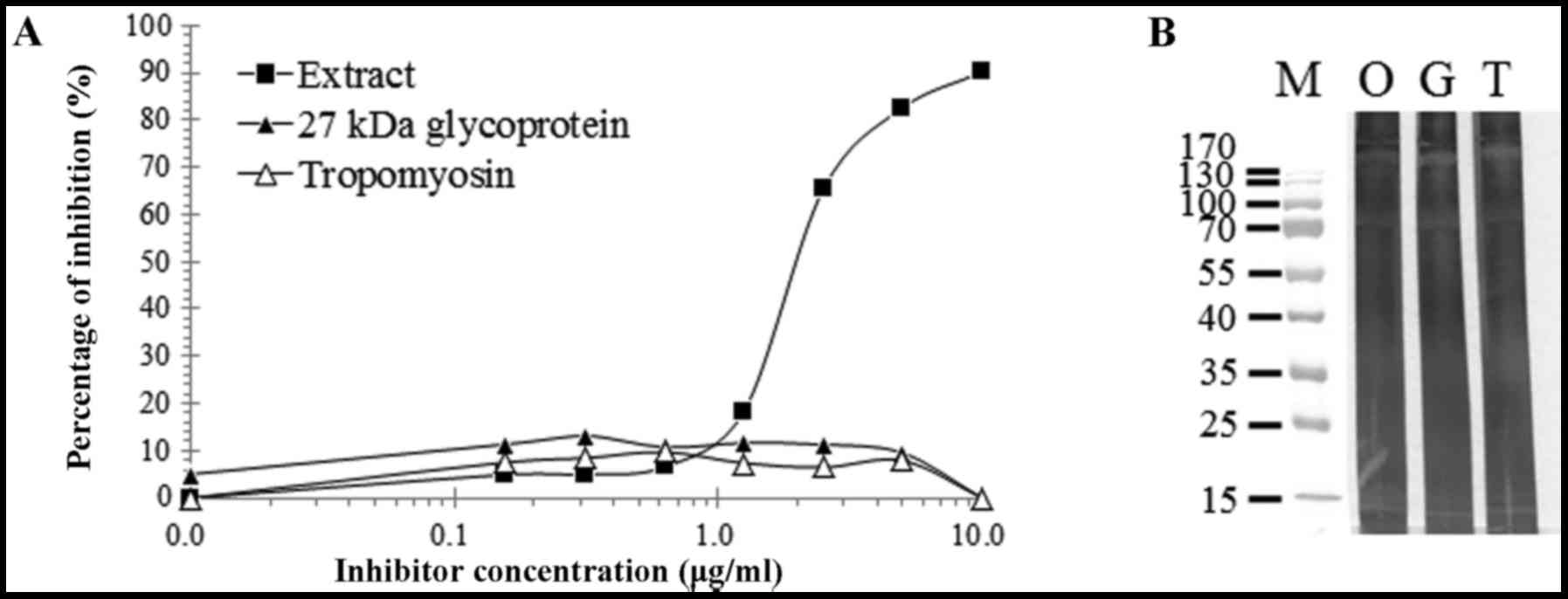
tropomyosin. Total extract of SWP inhibited up to 90.2% of IgE
reactivity to total extract of SWP, whereas the recombinant
tropomyosin exhibited a maximum 9.7% inhibition (Fig. 3A). Recombinant 27-kDa hemolymph
glycoprotein inhibited IgE reactivity to the extract by up to
11.7%. Inhibition immunoblotting was also performed. However, no
specific inhibition of response to the silkworm extract by the
recombinant tropomyosin and 27 kDa glycoprotein was observed
(Fig. 3B).
Identification of tropomyosin in the
SWP extract
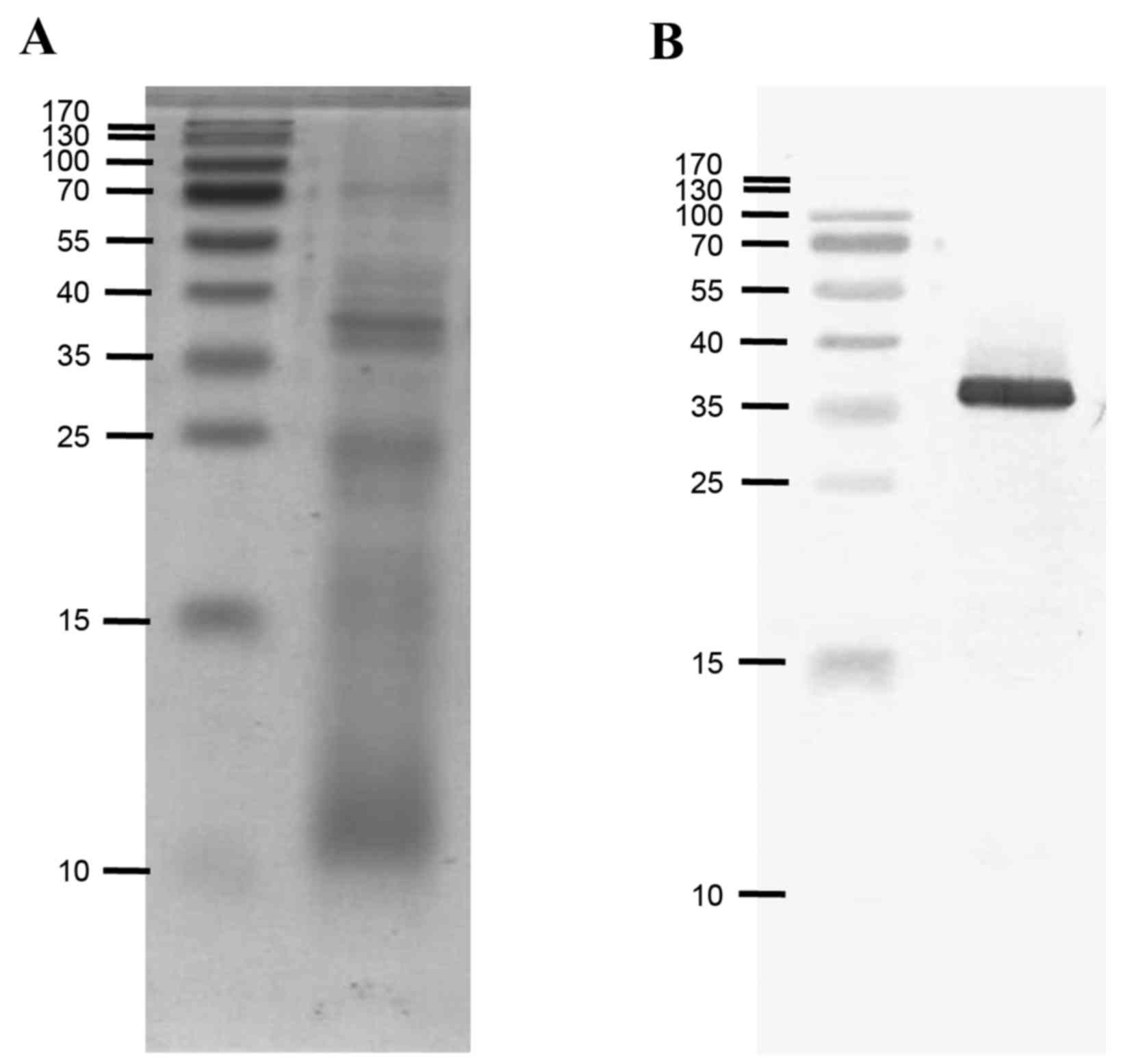
Western blotting was performed to identify
tropomyosin in the total SWP extract (Fig. 3), since IgE reactivity to total SWP
extract was not inhibited by recombinant tropomyosin. Native
silkworm tropomyosin, at ~36 kDa, was strongly recognized by a
monoclonal antibody raised against recombinant German cockroach
tropomyosin, indicating that the minimum inhibition of IgE
reactivity by recombinant tropomyosin was not due to a low
concentration of native tropomyosin in the extract (Fig. 4).
Correlation of IgE reactivity to
silkworm and shrimp/crab tropomyosins
Since shellfish tropomyosins account for >80% of
IgE reactivity to shellfish in patients, IgE reactivity to
shellfish (shrimp and crab) was measured by ImmunoCAP in order to
examine its possible cross-reactivity with SWP (Table I). Of the 15 sera tested from
patients with silkworm allergy, 11 exhibited IgE reactivity to both
shrimp and crab (ImmunoCAP>0.35 kUA/l; Table I). Sensitization to SWP tropomyosin
and shrimp or crab extracts did not exhibit a correlation [ρ=−0.066
(shrimp, P=0.815) and −0.125 (crab, P=0.658], whereas specific IgE
reactivity to shrimp and crab were strongly correlated (ρ=0.987,
P=1.304e-011). However, six of eight sera that exhibited positive
reactivity to SWP tropomyosin also displayed positive IgE
reactivity to shrimp and crab, implying potential cross-reactivity
between SWP tropomyosin and shellfish (shrimp/crab) tropomyosin
(Table I).
Discussion
IgE reactivity of recombinant silkworm
tropomyosin
Epidemics of food allergy reflect the regional
culture, including eating habits. For example, buckwheat, chestnut,
chickpea, bird's nest and royal jelly are frequent causes of food
allergy in Asian populations (14). In particular, parvalbumin and
tropomyosin proteins are known to be responsible for fish and
shellfish allergies (15). SWP is
an important cause of food allergy in East Asia. In Korea, SWP is
commonly consumed after boiling with soybean sauce. Silkworm
belongs to the phylum Arthropoda, similar to crustaceans, and the
well-established, heat-stable, allergenic nature of tropomyosin in
crustaceans was the motivation behind investigating silkworm
tropomyosin in the present study. The current study demonstrated
that silkworm tropomyosin shared 73.5 to 92.3% sequence identity
with other allergenic tropomyosins, implying possible
cross-reactivity. Additionally, recombinant SWP tropomyosin was
recognized by serum IgE from 53.3% of the patients tested with SWP
allergy. However, the IgE titer to tropomyosin was not high and its
inhibition of IgE reactivity to the total extract of SWP was
minimal. Minimum inhibition of IgE reactivity by recombinant
tropomyosin may reflect its low IgE reactivity, as an abundant
amount of native tropomyosin was detected in the total SWP extract
by western blotting. Non-specific binding or the presence of
clinically irrelevant specific IgE binding are common problems in
the diagnosis of SWP allergy (5,16–18),
as inhibition of the total extract may not reflect clinical
significance. In fact, a high prevalence of IgE reaction to SWP is
detected in Koreans without clinical symptoms. Therefore, there is
an urgent need for component-resolved diagnosis of SWP allergy
based on molecular studies.
Potential cross-reactivity with
shellfish tropomyosins
The IgE reactivity to shellfish tropomyosin (shrimp
and crab), which is known to be responsible for most cases of
shellfish allergy, was measured in sera from patients with silkworm
allergy by ImmunoCAP, (Table I).
Of the 15 sera tested, 11 exhibited IgE reactivity to shrimp and
crab (>0.35 kUA/l), suggesting possible
cross-reactivity between SWP and shellfish tropomyosins. However,
none of the patients enrolled were allergic to shrimp and crab. The
small amount of tropomyosin in the SWP extract may in part explain
the low allergenicity of SWP tropomyosin. For example,
cross-reactivity between house dust mite and storage mite
tropomyosins (Der f 10 and Tyr p 10) is limited because of its low
concentration (19). However,
chironomid tropomyosin, Chi k 10, has been demonstrated to be a
major allergen, implying a high concentration of tropomyosin in
dried dead debris (20). In a
recent study, tropomyosin was demonstrated to have a minor role in
cross-reactivity among edible insects (21). However, low degree of IgE
reactivity may explain a minor role of tropomyosin in the case of
SWP, because it was strongly recognized by monoclonal antibody in
the extract used in the current study.
Arginine kinase (Bomb m 1) was identified as a major
allergen from SWP (22). However,
this result was not reproduced in the present study. IgE reactivity
to a 27 kDa-hemolymph glycoprotein and high molecular weight
proteins have been demonstrated to be increased following heat
treatment (11). Recently,
proteins homologous to paramyosin and chitinase have been
identified to be allergenic by proteomic analysis (23). In addition, six IgE reactive
components [vitellogenin, chitinase, 30 K protein homologous to
microvitellogenin (Bom m 9), triosephosphate isomerase, heat shock
protein, and chymotrypsin inhibitor] from SWP were demonstrated to
be recognized by sera from asthma subjects (24). However, IgE reactivity of the
purified proteins has not been examined in detail. Therefore, more
investigations are needed to characterize allergens and develop
improved diagnostics for SWP allergy.
In conclusion, recombinant SWP tropomyosin, sharing
strong homology with other allergenic tropomyosins, was produced
and demonstrated to exhibit an IgE binding frequency of 53.3% among
patients with SWP allergy. Tropomyosin may contribute significantly
to SWP allergy and its importance should not be underestimated
because of potential strong cross-reactivity. Large-scale
investigations of SWP tropomyosin and its potential allergenic
cross-reactivity will be required in the future for further
characterization in order to develop more efficient diagnostic
tests for SWP allergy.
Acknowledgements
This research was supported by a grant of the Korea
Healthcare Technology R&D Project through the Korean Health
Industry Development Institute, Funded by the Ministry of Health,
Welfare & Family Affairs, Republic of Korea (grant no.
HI14C1324).
References
|
1
|
DeFoliart GR: Insects as food: Why the
Western attitude is important. Annu Rev Entomol. 44:21–50. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pemberton RW: Insect and other arthropods
used as drugs in Korean traditional medicine. J Ethnopharmacol.
65:207–216. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramos-Elorduy J: Insects: A sustainable
source of food? Ecol Food Nutr. 36:247–276. 1997. View Article : Google Scholar
|
|
4
|
Ji KM, Zhan ZK, Chen JJ and Liu ZG:
Anaphylactic shock caused by silkworm pupa consumption in China.
Allergy. 63:1407–1408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SH, Kang HR, Kim KM, Kim TB, Kim SS,
Chang YS, Kim CW, Bahn JW, Kim YK, Cho SH, et al: The sensitization
rates of food allergens in a Korean population: A multi-center
study. J Asthma Allergy Clin Immunol. 23:502–514. 2003.
|
|
6
|
Komaze Y, Sakata M, Azuma T, Tanaka A and
Nakagawa T: IgE antibodies against midge and moth found in Japanese
asthmatic subjects and comparison of allergenicity between these
insects. Allergy. 52:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baldo BA and Panzani RC: Detection of IgE
antibodies to a wide range of insect species in subjects with
suspected inhalant allergies to insects. Int Arch Allergy Appl
Immunol. 85:278–287. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong KY, Hong CS and Yong TS: Allergenic
tropomyosins and their cross-reactivities. Protein Pept Lett.
13:835–845. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altschul SF, Gish W, Miller W, Myers EW
and Lipman DJ: Basic local alignment search tool. J Mol Bio.
215:403–410. 1990. View Article : Google Scholar
|
|
10
|
Larkin MA, Blackshields G, Brown NP,
Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm
A, Lopez R, et al: Clustal W and Clustal X version 2.0.
Bioinformatics. 23:2947–2948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong KY, Son M, Lee JY, Park KH, Lee JH
and Park JW: Allergenic characterization of 27-kDa glycoprotein, a
novel heat stable allergen, from the pupa of silkworm, Bombyx
mori. J Korean Med Sci. 31:18–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong KY, Lee J, Lee IY, Ree HI, Hong CS
and Yong TS: Allergenicity of recombinant Bla g 7, German cockroach
tropomyosin. Allergy. 58:1059–1063. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeong KY: Characterization of allergenic
properties of German cockroach tropomyosin using recombinant
proteins, Yonsei University College of Medicine. 2004.
|
|
14
|
Lee BW, Shek LP, Gerez IF, Soh SE and Van
Bever HP: Food allergy-lessons from Asia. World Allergy Organ J.
1:129–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leung NY, Wai CY, Chu S, Wang J, Kenny TP,
Chu KH and Leung PS: Current immunological and molecular biological
perspectives on seafood allergy: A comprehensive review. Clin Rev
Allergy Immunol. 46:180–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee MH, Kim YY and Kang SY: Two cases of
pupa allergy. Allergy. 2:23–25. 1982.
|
|
17
|
Kim S, Paik SW and Kang SY: A case of
anaphylaxis after ingestion of pupae. Allergy. 3:175–178. 1983.
|
|
18
|
Koh YI, Choi IS, Chung SU and Cho S:
Clinical features of adult patients with anaphylaxis associated
with food in Gwangju and Chonnam area. J Asthma Allergy Clin
Immunol. 24:217–223. 2004.
|
|
19
|
Jeong KY, Lee H, Lee JS, Lee J, Lee IY,
Ree HI, Hong CS and Yong TS: Molecular cloning and the allergenic
characterization of tropomyosin from Tyrophagus
putrescentiae. Protein Pept Lett. 14:431–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeong KY, Yum HY, Lee IY, Ree HI, Hong CS,
Kim DS and Yong TS: Molecular cloning and characterization of
tropomyosin, a major allergen of Chironomus kiiensis, a
dominant species of nonbiting midges in Korea. Clin Diagn Lab
Immunol. 11:320–324. 2004.PubMed/NCBI
|
|
21
|
Srinroch C, Srisomsap C,
Chokchaichamnankit D, Punyarit P and Phiriyangkul P: Identification
of novel allergen in edible insect, Gryllus bimaculatus and
its cross-reactivity with Macrobrachium spp. Allergens. Food
Chem. 184:160–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Xia L, Wu Y, Xia Q, Chen J and Roux
KH: Identification and characterization of an arginine kinase as a
major allergen from silkworm (Bombyx mori) larvae. Int Arch
Allergy Immunol. 150:8–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Li L, Kuang Z, Luo G and Li B:
Proteomic and immunological identification of two new allergens
from silkworm (Bombyx mori L.) pupae. Cent Eur J Immunol.
40:30–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zuo J, Lei M, Yang R and Liu Z: Bom m 9
from Bombyx mori is a novel protein related to asthma.
Microbiol Immunol. 59:410–418. 2015. View Article : Google Scholar : PubMed/NCBI
|