Introduction
The adrenal cortex comprises three layers: Zona
glomerulosa, zona fasciculata and zona reticularis. The adrenal
cortex mediates the stress response through the production of
cortisol. Following bilateral adrenalectomy for the treatment of
Cushing's disease, adrenocorticotropic hormone (ACTH)-independent
macronodular adrenal hyperplasia and pheochromocytoma, cortisol
replacement is necessary for the rest of patients' lives (1–4).
However, patients experience side effects from long-term steroid
treatment and are at risk of adrenal insufficiency.
Autotransplantation of the adrenal cortex may be an alternative to
steroid replacement therapy following bilateral pheochromocytoma,
which is a form of catecholamine-producing neuroendocrine tumor
(4). To avoid the side effects of
cortisol replacement, autotransplantation following bilateral
adrenalectomy is required. Successful, autotransplantation may
lower the risk of adrenal insufficiency and improve the quality of
life for patients.
Upregulation of glucocorticoids and ACTH levels in
blood following autotransplantation has been reported in patients
with pheochromocytomas following bilateral adrenalectomy (2,5).
Notably, there have not been any reports detailing the function of
the hypothalamus and pituitary gland following adrenal
autotransplantation. Because the autotransplanted adrenal gland
does not have the full function of the original adrenal gland
(6), dysfunction of the
hypothalamus-pituitary axis may occur in patients following
autotransplantation. However, the functional alterations in the
hypothalamus and pituitary gland following autotransplantation are
poorly understood. In the current study, the gene expression in the
hypothalamus and pituitary were examined following adrenal
autotransplantation using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis as a pilot study.
Materials and methods
Ethical approval
All experiments were conducted in accordance with
the Guidelines (7) and were
approved by the Ethics Committee on Animal Experiments of Kansai
Medical University (Hirakata, Japan; approval ID: 15-002).
Animal preparation
A total of nine male Wistar rats (age, 8- to
9-weeks-old; weight, 180–240 g) were housed in sound-attenuated
light-controlled cages (light on 8:00 a.m. and off 8:00 p.m.; 12 h
light-dark cycle; constant environment at 25±1°C and 50±10%
relative humidity). Food and water were available ad
libitum. Bilateral adrenalectomy was performed on 4 rats
following laparotomy under general anesthesia by inhalation of 2%
isoflurane (Pfizer Japan Inc., Tokyo, Japan) and 3 l/min oxygen.
Resection of the adrenal medulla and midline and horizontal
incision was conducted under a stereomicroscope. The 4
chopped-bilateral adrenal capsules and cortex with zona glomerulosa
and undifferentiated cell zone (8)
were autotransplanted in 4 rats in two abdominal muscle pockets
that were formed by a pair of fine scissors (9). Tissue collections were performed at 4
weeks after autotransplantation between 9:00 a.m. and 11:00 a.m.
(zeitgeber time (ZT) 1 to ZT3) in all animals. As a control,
sham-operations without adrenalectomy were performed in 5 rats, and
their tissues were also collected at 4 weeks after surgery. All
animals received saline instead of water during the 10 days after
surgery, because adrenal-autotransplanted rats cannot survive
without saline for 10 days after surgery (10). The animals did not receive any
steroid replacement, as rats can survive without steroid
replacement following adrenalectomy (11). Rat hypothalamus was dissected
coronally from the optic chiasma to the mammillary bodies (−6 mm
from the chiasma) using a brain slicer (Zivic Instruments,
Pittsburgh, PA, USA). The dorsal limit of the hypothalamus was the
roof of the third ventricle, and the lateral limit was the amygdala
(12). A total of 32 male Wistar
rats (age, 11-12-weeks-old) were housed in same aforementioned
conditions and decapitated to identify their diurnal variation in
housekeeping genes at 4 h intervals over 24 h from ZT0 to ZT20 of
the hypothalamus (n=4 for each ZT) or at ZT2 and ZT14 of the
pituitary (n=4 for each ZT).
RT-qPCR
Total RNA was isolated from each individual
hypothalamus and whole pituitary gland using Sepasol-RNA I Super G
reagent (Nacalai Tesque, Inc., Kyoto, Japan). Single-stranded cDNA
was synthesized using the PrimeScript RT reagent kit with gDNA
Eraser (Takara Bio, Inc., Otsu, Japan). The expression level of
each mRNA was determined by RT-qPCR with an ABI 7300 system
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) using the THUNDERBIRD qPCR mix (Toyobo Co., Ltd., Osaka,
Japan) and gene-specific primers (Table I). PCR products were amplified
using the following thermocycling conditions: 1 cycle, 1 min, 95°C;
40 cycles, 10 sec, 95°C, 60 sec, 60°C.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene symbol | Accession
number | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| Gh1 | NM_001034848.2 |
tgtttgccaatgctgtgctc |
tgaatggaatagcgctgtcc |
| Prl | NM_012629.1 |
tttggtgcactcctggaatg |
agccgcttgttttgttcctc |
| Tshb | NM_013116.2 |
ttccgtgcttttcgctcttg |
agatggtggtgttgatggtcag |
| Fshb | NM_001007597.1 |
tgaagtcgatccagctttgc |
atgcagaaacggcactcttc |
| Lhb | NM_012858.2 |
ttctgatgcccacccactaac |
aagcctttattgggagggatgg |
| Cga | NM_053918.2 |
atcagtgtatgggctgttgc |
atgatttggccacacagcac |
| Pomc | NM_139326.2 |
ttcatgacctccgagaagagc |
tgtgcgcgttcttgatgatg |
| Crh | NM_031019.1 |
gaatacttcctccgcctggg |
ggaaaaagttagccgcagcc |
| Crhr1 | NM_030999.3 |
ttctgaacagtgaggtccgc |
aggtggggatggacatagct |
| Crhr2 | NM_022714.1 |
ccgaatcgccctcatcatca |
ttcgtggtcgatgagttgca |
| Nr3c1 (GR) | NM_012576.2 |
aaatgggcaaaggcgatacc |
agcaaagcagagcaggtttc |
| Trh | NM_013046.3 |
aaaagggcattgggtcatcc |
acttgtgggctttgcttcac |
| Trhr | NM_013047.3 |
ccatcaacccggtgatttac |
aaagcggtctgactccttga |
| Ucn2 | NM_133385 |
atgtttctgaacccctcacg |
gacacagctaggcacgacaa |
| Hprt1 | NM_012583.2 |
cctgttgatgtggccagtaaag |
atcaaaagggacgcagcaac |
| Rplp2 | NM_001030021.1 |
attgaggatgtcatcgctcagg |
tctttcttctcctctgctgcag |
| Ywhaz | NM_013011.3 |
ttcgcagccagaaagcaaag |
ttgtcatcaccagcagcaac |
The housekeeping gene with minimum diurnal variation
was identified using a Rat Housekeeping Gene Primer set (Takara
Bio, Inc.) in the hypothalamus at 4 h intervals over a 24 h period.
Hypoxanthine phosphoribosyltransferase-1 (Hprt1), ribosomal
protein large P2 (Rplp2) and tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein-z
(Ywhaz) primers were newly synthesized for the relative
quantification of the gene expression in the hypothalamus and
pituitary (Table I). Subsequently,
the relative level of target gene expression was evaluated using
the 2−ΔΔCq method (13)
with Hprt1 as an internal control.
Statistical analysis
Distributions [mean ± standard deviation (SD)] of
relative gene expressions were compared using unpaired Student's
t-test in Microsoft Excel software. P<0.05 was considered to
indicate a statistically significant difference.
Results
Pituitary hormones and hypothalamic releasing
hormones possess diurnal variation. In addition, certain
housekeeping genes may also have this variation. Therefore, to
avoid false-positive results caused by the sampling time and to
increase the stringency of relative hormone mRNA measurements, the
housekeeping genes with minimum diurnal variation were examined.
The relative quantity of housekeeping gene expression was evaluated
using the 2−ΔΔCq method with ATP synthase H+
transporting mitochondrial Fo complex subunit B1 used as
the reference gene. The Hprt1 gene had minimal variation
over a 24 h period in the rat hypothalamus (mean ± SD; 1.08±0.05,
coefficient of variation; 4.55%; Fig.
1; Table II). There was no
significant difference in the relative expression of Hprt1
in pituitary tissue between ZT2 (1.16±0.15) and ZT14 (1.18±0.20;
P=0.865). Hprt1 had minimal variation, when compared with
Rplp2 and Ywhaz, which were the housekeeping genes
with the second and third lowest diurnal variation in the
hypothalamus. In addition, there was no significant difference of
Rplp2 in the pituitary gland between ZT2 (1.37±0.26) and
ZT14 (1.17±0.13; P=0.106) but, notably, there was a significant
difference in the pituitary gland of Ywhaz between ZT2
(1.61±0.13) and ZT14 (1.09±0.09; P<0.001).
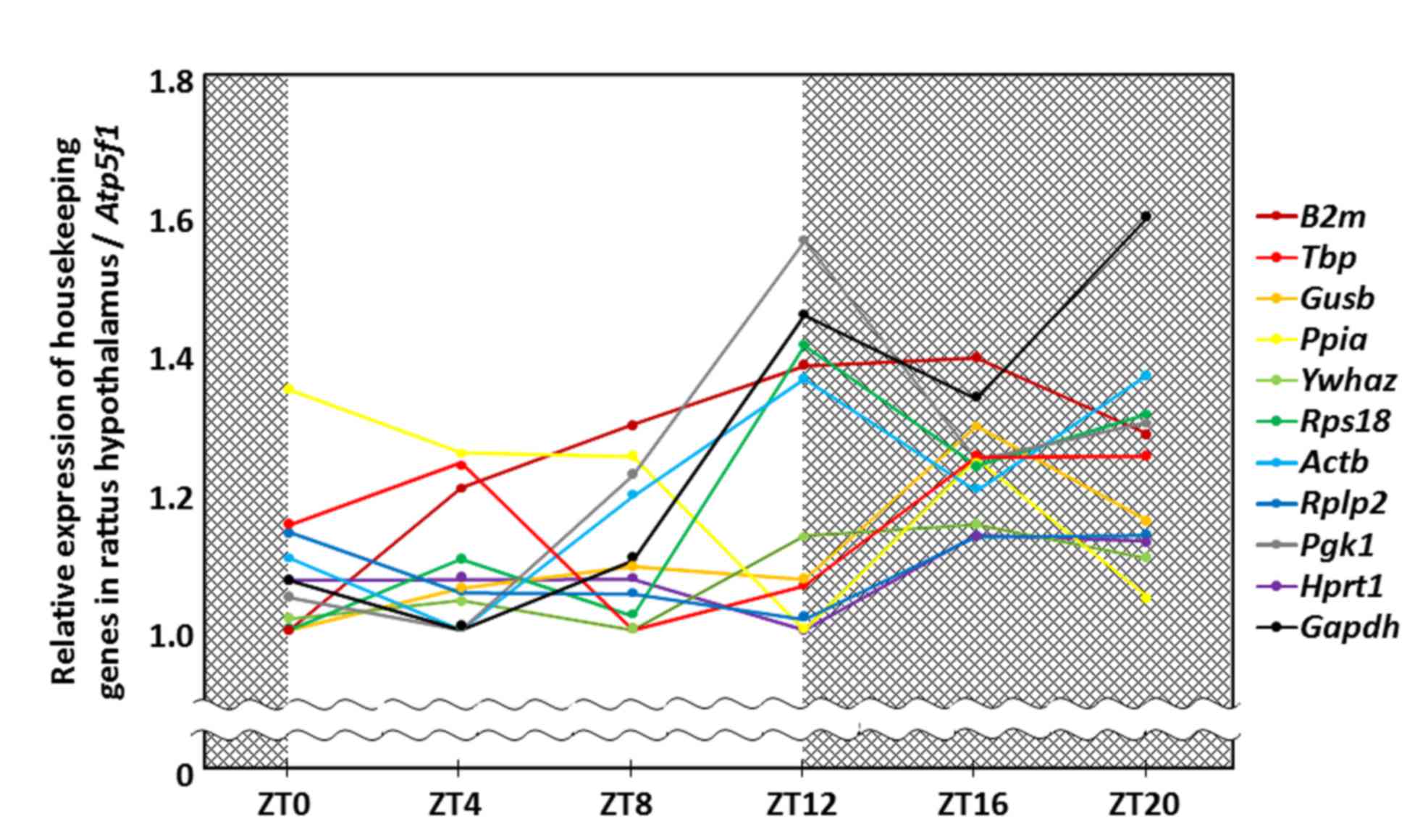 | Figure 1.Diurnal rhythm of housekeeping gene
expression in the rat hypothalamus. Each dot represents the mean
value of relative expression of housekeeping genes at different
ZTs. Atp5f1, ATP synthase, H+ transporting,
mitochondrial Fo complex subunit B1; B2m, β-2
microglobulin; Tbp, TATA box binding protein; Gusb,
glucuronidase-β; Ppia, peptidylprolyl isomerase A; Ywhaz, tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein z;
Rps18, ribosomal protein S18; Actb, β-actin; Rplp2, ribosomal
protein large P2; Pgk1, phosphoglycerate kinase 1; Hprt1,
hypoxanthine phosphoribosyltransferase-1; Gapdh,
glyceraldehyde-3-phosphate dehydrogenase; ZT, zeitgeber time. |
 | Table II.Diurnal variations of housekeeping
genes in the rat hypothalamus. |
Table II.
Diurnal variations of housekeeping
genes in the rat hypothalamus.
| Gene symbol | Mean
expressiona | Standard
deviation | Coefficient of
variation (%) |
|---|
| Hprt1 | 1.080 | 0.049 | 4.545 |
| Rplp2 | 1.089 | 0.055 | 5.045 |
| Ywhaz | 1.075 | 0.064 | 5.940 |
| Gusb | 1.112 | 0.102 | 9.154 |
| Tbp | 1.159 | 0.107 | 9.250 |
| Ppia | 1.191 | 0.135 | 11.356 |
| Actb | 1.205 | 0.144 | 11.965 |
| B2m | 1.259 | 0.144 | 11.459 |
| Rps18 | 1.180 | 0.165 | 14.007 |
| Pgk1 | 1.229 | 0.201 | 16.313 |
| Gapdh | 1.259 | 0.239 | 18.953 |
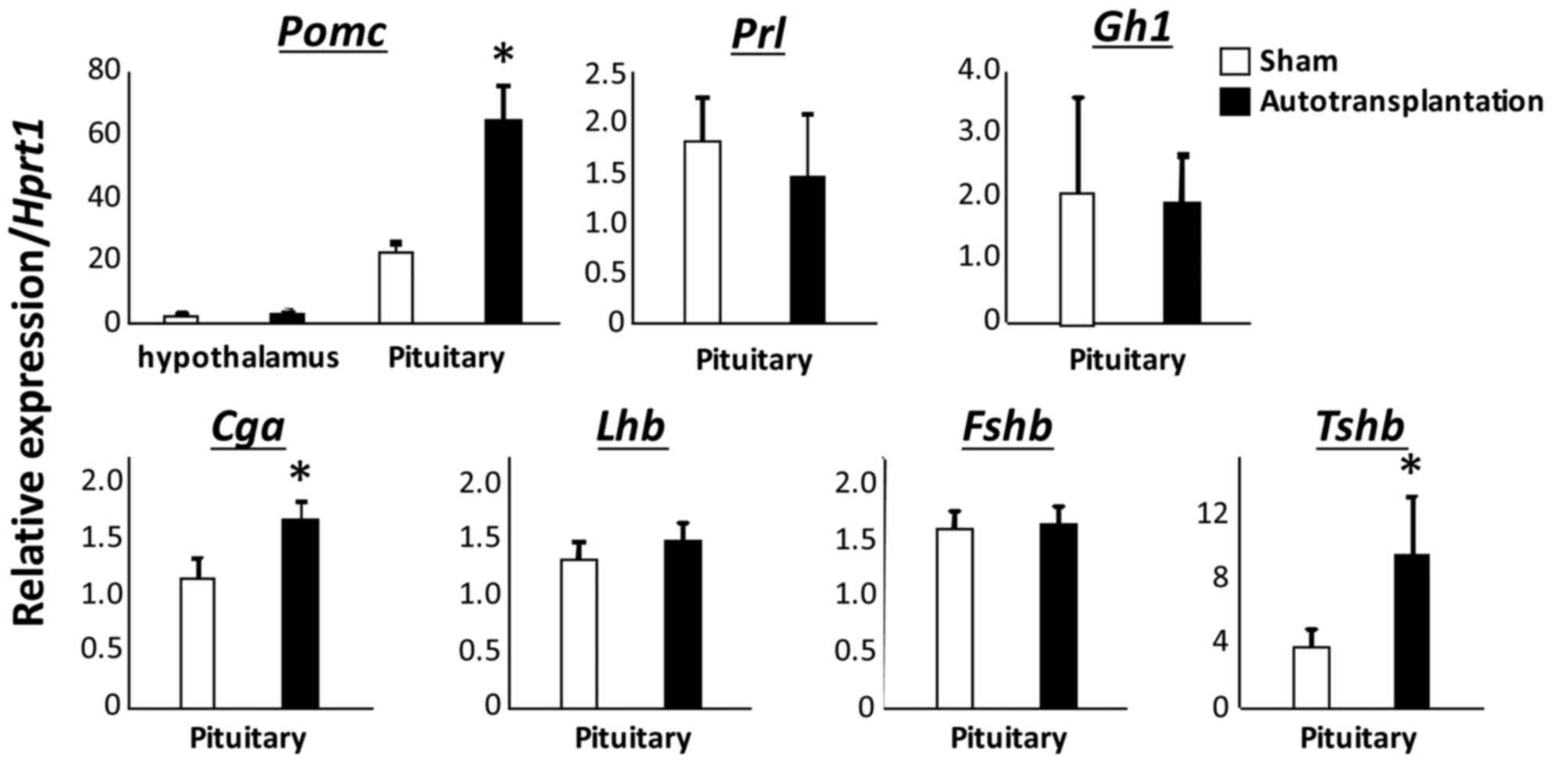
Subsequently, Hprt1 was used as the internal
control. Proopiomelanocortin (Pomc; 64.28±11.39 vs.
22.63±3.39; P<0.005), glycoprotein hormones α polypeptide
(Cga; 1.69±0.14 vs. 1.16±0.17; P<0.01) and thyroid
stimulating hormone β (Tshb; 9.60±3.61 vs. 3.90±1.02;
P<0.05) were demonstrated to be significantly elevated in the
pituitary gland of autotransplanted rats, when compared with
sham-operated rats (Fig. 2). There
was no significant difference in prolactin (Prl; 1.83±0.46
vs. 1.47±0.64), growth hormone-1 (Gh1; 2.06±1.53 vs.
1.89±0.77), luteinizing hormone β polypeptide (1.32±0.22 vs.
1.51±0.39), follicle stimulating hormone β polypeptide (1.59±0.51
vs. 1.66±0.45) and urocortin-2 (Ucn2; 14.97±11.99 vs.
11.80±5.74) in the pituitary gland between sham-operated rats and
autotransplanted rats (Figs. 2 and
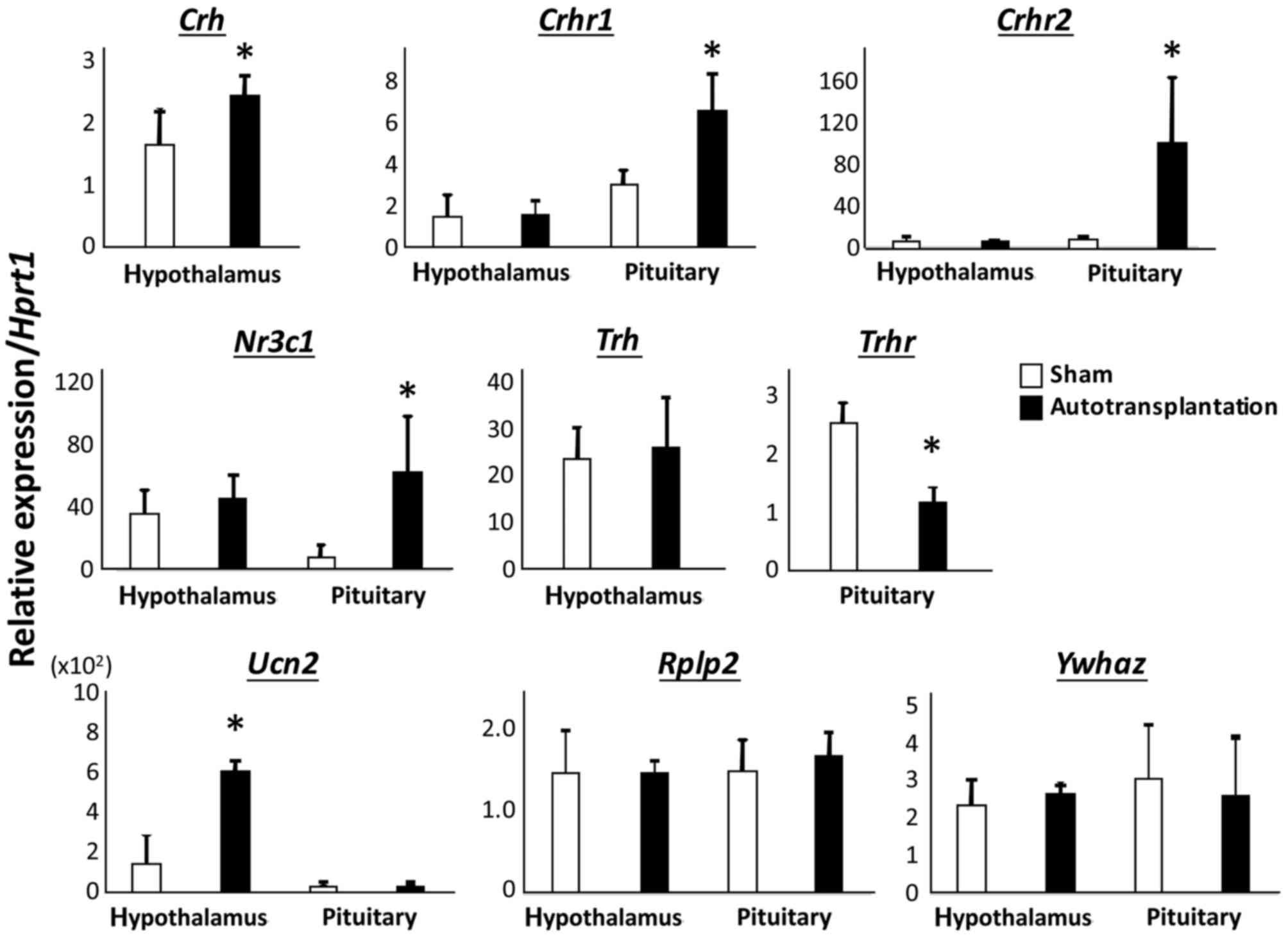
3). There were significant
differences in expression of corticotropin releasing hormone
receptor 1 (Crhr1; 3.03±0.68 vs. 6.61±1.78; P<0.01),
Crhr2 (9.55±1.90 vs. 102.96±61.14; P<0.05), nuclear
receptor subfamily 3 group C member 1 (Nr3c1; 7.86±7.81 vs.
63.35±34.86; P<0.05) and thyrotropin releasing hormone receptor
(Trhr; 2.55±0.24 vs. 1.17±0.24; P<0.005) in the pituitary
gland between sham-operated rats and autotransplanted rats
(Fig. 3). In the hypothalamus,
corticotropin releasing hormone (Crh; 1.65±0.55 vs.
2.45±0.31; P<0.05) and Ucn2 (150.03±127.97 vs.
611.46±252.98; P<0.01) were significantly upregulated in
autotransplanted rats compared with sham-operated rats (Fig. 3). There were no significant
differences in levels of Pomc (1.46±0.32 vs. 1.82±0.53),
Trh (23.67±6.78 vs. 26.29±10.55), Crhr1 (1.53±0.99
vs. 1.60±0.65), Crhr2 (7.27±4.31 vs. 4.51±3.31) and
Nr3c1 (35.53±15.35 vs. 46.11±14.90) in the hypothalamus
between sham-operated rats and autotransplanted rats (Fig. 3). In both the pituitary gland and
the hypothalamus, there was no difference in Rplp2
(1.48±0.52 vs. 1.46±0.15 in the hypothalamus; and 1.51±0.38 vs.
1.70±0.28 in the pituitary gland) and Ywhaz (2.34±0.67 vs.
2.70±0.21 in the hypothalamus; and 3.06±1.43 vs. 2.60±1.58 in the
pituitary gland) between sham-operated rats and autotransplanted
rats (Fig. 3).
Discussion
Autotransplantation following bilateral
adrenalectomy helps to avoid steroid replacement therapy in
postoperative pheochromocytoma patients. To the best of our
knowledge, there are no studies regarding the
hypothalamo-hypophysial system without ACTH in subjects following
autotransplantation. To clarify the precise effect of adrenal
autotransplantation on the pituitary and hypothalamic function, the
authors examined whether there were significant differences in the
hypothalamus-pituitary-adrenal axis, and other hormonal systems
following adrenal autotransplantation. In the current study, there
were increased levels of Pomc, Cga, Tshb,
Crhr1, Crhr2 and Nr3c1 transcripts in the
pituitary gland and Crh and Ucn2 transcripts in the
hypothalamus of autotransplanted rats compared with sham rats. In
addition, the results demonstrated decreased levels of Trhr
in the pituitary gland of autotransplanted rats compared with sham
rats.
The hypothalamus neuropeptide, CRH is secreted from
the paraventricular nucleus during stress responses. CRH activates
the hypothalamic-pituitary-adrenal axis, modulating stress-induced
ACTH secretion from the pars distalis. ACTH is proteolytically
synthesized from the large precursor protein, POMC, by the anterior
pituitary corticotrophs. The increase in blood ACTH level results
in the adrenocortical release of cortisol and aldosterone (14,15).
CRH itself is inhibited by glucocorticoids, which acts as a
classical negative feedback loop. Therefore, the elevations of
Pomc, Crhr1, Crhr2, Nr3c1 and
Crh transcripts in the present study are in line with the
decrease in the negative feedback of glucocorticoids, due to the
hypofunctioning autotransplanted adrenal cortex (6). The CRHR1 mediates the effects of CRH
on the hypothalamus-pituitary-adrenal axis (16,17).
The stress-inducible ACTH secretion from the anterior pituitary
corticotrophs is impaired in Crhr1−/− mice
(18,19). Hypersensitivity of the
hypothalamus-pituitary-adrenal axis against stress conditions has
been demonstrated in Crhr2 null mice (20,21).
One member of the CRH family, the UCN2 protein, selectively binds
to CRHR2 (22) and an elevation of
Ucn2 mRNA was identified in the hypothalamus of the
autotransplanted rats in the current study. Therefore, Pomc
transcription in autotransplanted rats may be regulated by
hypothalamic CRH and UCN2 in a coordinated manner.
In addition, Cga and Tshb expression
are upregulated in the pituitary gland of autotransplanted rats.
Glucocorticoids inhibit Cga expression mediated by the
glucocorticoid responsive element (GRE) to the 5′-flanking region
containing the cAMP-response element with a tissue-specific element
of the Cga gene (23–25).
Basal and thyrotropin-releasing hormone (TRH)-stimulated total TSH,
CGA and TSHB secretion were decreased following dexamethasone
administration in patients with hypothyroiditis (26). As chronic insufficiency of
adrenocortical function due to autotransplantation induces low
blood levels of glucocorticoids, it is speculated that subclinical
hyperthyroiditis was induced in adrenal autotransplanted
animals.
Tshb expression was upregulated in the
pituitary gland of autotransplanted rats, however there is no
report on the direct effect of glucocorticoid on Tshb
transcription. The GRE in the upstream region of the Tshb
gene has not yet been identified (27). By contrast, there is a GRE in the
5′-flanking region of the Trh gene. Trh transcription
is directly regulated by glucocorticoids (28,29),
therefore it was suggested that the increase in Trh
expression and subsequent Tshb elevation had occurred in the
autotransplanted rats by the mechanism reported by Walter et
al (30). Unexpectedly, there
was no significant change in Trh expression in the current
study. Prepro-TRH is synthesized in the neuronal cell bodies of
various brain regions (31).
Although several hypothalamic nuclei synthesize TRH, the TRH
neurons regulating pituitary TSH release are localized exclusively
to the paraventricular nucleus (32,33).
In the present study, the whole hypothalamus was used to determine
the expression level of Trh, therefore changes in Trh
expression caused by autotransplantation in the paraventricular
nucleus could not be detected in the present samples. Subsequently,
the downregulation of Trhr expression was demonstrated to
occur in the pituitary gland of the autotransplanted rats. The
direct transcriptional enhancement of Trhr induced by
glucocorticoids via GRE has been well described (34–36).
These results suggested that Tshb expression in the
pituitary gland of autotransplanted rats was regulated by a
different pathway from the TRH-TRHR system or direct glucocorticoid
effect.
In conclusion, the results identified an elevation
in gene expression of the hypothalamus-pituitary-adrenal axis and
adenohypophysis thyrotrophs in autotransplanted rats, suggesting
that a small amount of cortisol replacement is required even
following autotransplantion. Future studies will examine gene
expression in other tissues following adrenal
autotransplantation.
Acknowledgements
The present study was supported by the Japan Society
for the Promotion of Science KAKENHI fund (grant nos. 25280052 and
15K08224 to Dr Susumu Tanaka), the research grant from Kansai
Medical University to Dr Nae Takizawa, and MEXT-Supported Program
for the Strategic Research Foundation at Private Universities
(grant nos. S1101034 and S1201038 to Dr Hisao Yamada). The authors
would like to thank Dr Kiyoshi Kurokawa (Osaka International
University, Hirakata, Japan) and Dr Yukie Hirahara-Wada (Kansai
Medical University, Hirakata, Japan) for their helpful
comments.
Glossary
Abbreviations
Abbreviations:
|
ACTH
|
adrenocorticotropic hormone
|
|
Atp5f1
|
ATP synthase H+
transporting mitochondrial Fo complex subunit B1
|
|
Cga
|
glycoprotein hormones α
polypeptide
|
|
Crh
|
corticotropin releasing hormone
|
|
Crhr1
|
corticotropin releasing hormone
receptor 1
|
|
Crhr2
|
corticotropin releasing hormone
receptor 2
|
|
Fshb
|
follicle stimulating hormone β
polypeptide
|
|
Gh1
|
growth hormone 1
|
|
GRE
|
glucocorticoid responsive element
|
|
Hprt1
|
hypoxanthine phosphoribosyltransferase
1
|
|
Lhb
|
luteinizing hormone β polypeptide
|
|
Nr3c1
|
nuclear receptor subfamily 3 group C
member 1
|
|
Prl
|
prolactin
|
|
Rplp2
|
ribosomal protein large P2
|
|
Trh
|
thyrotropin releasing hormone
|
|
Trhr
|
thyrotropin releasing hormone
receptor
|
|
Tshb
|
thyroid stimulating hormone β
|
|
Ucn2
|
urocortin 2
|
|
Ywhaz
|
tyrosine 3-monooxygenase/tryptophan
5-monooxygenase activation protein z
|
|
Pomc
|
proopiomelanocortin
|
References
|
1
|
Erdogan G, Kologlu S, Kamel N, Baskal N,
Cesur V and Eraslan S: Adrenal autotransplantation after total
adrenalectomy: Delayed determined function. Endocr J. 41:45–48.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lucon AM, Mendonça BB, Domenice S, Chambô
JL, Wajchemberg BL and Arap S: Adrenal autografts following
bilateral adrenalectomy. J Urol. 149:977–979. 1993.PubMed/NCBI
|
|
3
|
Kubo N, Onoda N, Ishikawa T, Ogawa Y,
Takashima T, Yamashita Y, Tahara H, Inaba M and Hirakawa K:
Simultaneous bilateral laparoscopic adrenalectomy for
adrenocorticotropic hormone-independent macronodular adrenal
hyerplasia: Report of a case. Surg Today. 36:642–646. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inabnet WB, Caragliano P and Pertsemlidis
D: Pheochromocytoma: Inherited associations, bilaterality and
cortex preservation. Surgery. 128:1007–1012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okamoto T, Obara T, Ito Y, Yamashita T,
Kanbe M, Iihara M, Hirose K and Yamazaki K: Bilateral adrenalectomy
with autotransplantation of adrenocortical tissue or unilateral
adrenalectomy: Treatment options for pheochromocytomas in multiple
endocrine neoplasia type 2A. Endocr J. 43:169–175. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taniguchi A, Tajima T, Nonomura K,
Shinohara N, Mikami A and Koyanagi T: Expression of vascular
endothelial growth factor and its receptors Flk-1 and Flt-1 during
the regeneration of autotransplanted adrenal cortex in the
adrenalectomized rat. J Urol. 171:2445–2449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Research Council of The National
Academies: Guide for the care and use of laboratory animals. 8th.
The National Academies Press (US); Washington, DC: 2011
|
|
8
|
Mitani F: Functional zonation of the rat
adrenal cortex: The development and maintenance. Proc Jpn Acad Ser
B Phys Biol Sci. 90:163–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Belloni AS, Neri G, Musajo FG, Andreis PG,
Boscaro M, D'Agostino D, Rebuffat P, Boshier DP, Gottardo G,
Mazzocchi G, et al: Investigations on the morphology and function
of adrenocortical tissue regenerated from gland capsular fragments
autotransplanted in the musculus gracilis of the rat.
Endocrinology. 126:3251–3262. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirose J, Masuda H, Ushiyama T, Ohtawara
Y, Ohta N, Suzuki K, Tajima A and Aso Y: Histological and
encrinological observations on the regeneration of the
autotransplanted adrenal gland in the rat. Nihon Hinyokika Gakkai
Zasshi. 79:666–672. 1988.(In Japanese). PubMed/NCBI
|
|
11
|
Srougi M and Gittes RF: Adrenal
autotransplantation. Urol Surv. 28:41–48. 1978.PubMed/NCBI
|
|
12
|
Terao A, Wisor JP, Peyron C,
Apte-Deshpande A, Wurts SW, Edgar DM and Kilduff TS: Gene
expression in the rat brain during sleep deprivation and recovery
sleep: An affymetrix genechip study. Neuroscience. 137:593–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Owens MJ and Nemeroff CB: Physiology and
pharmacology of corticotropin-releasing factor. Pharmacol Rev.
43:425–473. 1991.PubMed/NCBI
|
|
15
|
Steckler T and Holsboer F:
Corticotropin-releasing hormone receptor subtypes and emotion. Biol
Psychiatry. 46:1480–1508. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skutella T, Criswell H, Moy S, Probst JC,
Breese GR, Jirikowski GF and Holsboer F: Corticotropin-releasing
hormone (CRH) antisense oligodeoxynucleotide induces anxiolytic
effects in rat. Neuroreport. 5:2181–2185. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skutella T, Montkowski A, Stöhr T, Probst
JC, Landgraf R, Holsboer F and Jirikowski GF:
Corticotropin-releasing hormone (CRH) antisense
oligodeoxynucleotide treatment attenuates social defeat-induced
anxiety in rats. Cell Mol Neu. 14:579–588. 1994. View Article : Google Scholar
|
|
18
|
Timpl P, Spanagel R, Sillaber I, Kresse A,
Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F and Wurst W:
Impaired stress response and reduced anxiety in mice lacking a
functional corticotropin-releasing hormone receptor 1. Nat Genet.
19:162–166. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith GW, Aubry JM, Dellu F, Contarino A,
Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA,
et al: Corticotropin releasing factor receptor 1-deficient mice
display decreased anxiety, impaired stress response and, aberrant
neuroendocrine development. Neuron. 20:1093–1102. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bale TL, Contarino A, Smith GW, Chan R,
Gold LH, Sawchenko PE, Koob GF, Vale WW and Lee KF: Mice deficient
for corticotropin-releasing hormone receptor-2 display anxiety-like
behaviour and are hypersensitive to stress. Nat Genet. 24:410–414.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coste SC, Kesterson RA, Heldwein KA,
Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA,
Hohimer AR, et al: Abnormal adaptations to stress and impaired
cardiovascular function in mice lacking corticotropin-releasing
hormone receptor-2. Nat Genet. 24:403–419. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reyes TM, Lewis K, Perrin MH, Kunitake KS,
Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW and
Sawchenko PE: Urocortin II: A member of the corticotropin-releasing
factor (CRF) neuropeptide family that is selectively bound by type
2 CRF receptors. Proc Natl Acad Sci USA. 98:2843–2848. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akerblom IE, Slater EP, Beato M, Baxter JD
and Mellon PL: Negative regulation by glucocorticoids through
interference with a cAMP responsive enhancer. Science. 241:350–353.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gurr JA and Kourides IA: Regulation of the
transfected human glycoprotein hormone alpha-subunit gene by
dexamethasone and thyroid hormone. DNA. 8:473–480. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chatterjee VK, Madison LD, Mayo S and
Jameson JL: Repression of the human glycoprotein hormone
alpha-subunit gene by glucocorticoids: Evidence for receptor
interactions with limiting transcriptional activators. Mol
Endocrinol. 5:100–110. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kourides IA, Weintraub BD, Re RN, Ridgway
EC and Maloof F: Thyroid hormone, oestrogen and glucocorticoid
effects on two different pituitary glycoprotein hormone alpha
subunit pools. Clin Endocrinol (Oxf). 9:535–542. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reddy TE, Pauli F, Sprouse RO, Neff NF,
Newberry KM, Garabedian MJ and Myers RM: Genomic determination of
the glucocorticoid response reveals unexpected mechanisms of gene
regulation. Genome Res. 19:2163–2171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cote-Vélez A, Pérez-Martínez L, Charli JL
and Joseph-Bravo P: The PKC and ERK/MAPK pathways regulate
glucocorticoid action on TRH transcription. Neurochem Res.
33:1582–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Díaz-Gallardo MY, Cote-Vélez A, Charli JL
and Joseph-Bravo P: A rapid interference between glucocorticoids
and cAMP-activated signalling in hypothalamic neurones prevents
binding of phosphorylated cAMP response element binding protein and
glucocorticoid receptor at the CRE-Like and composite GRE sites of
thyrotrophin-releasing hormone gene promoter. J Neuroendocrinol.
22:282–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walter KN, Corwin EJ, Ulbrecht J, Demers
LM, Bennett JM, Whetzel CA and Klein LC: Elevated thyroid
stimulating hormone is associated with elevated cortisol in healthy
young men and women. Thyroid Res. 5:132012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Joseph-Bravo P, Jaimes-Hoy L and Charli
JL: Regulation of TRH neurons and energy homeostasis-related
signals under stress. J Endocrinol. 224:R139–R159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fekete C and Lechan RM: Central regulation
of hypothalamic-pituitary-thyroid axis under physiological and
pathophysiological conditions. Endocr Rev. 35:159–194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fliers E, Kalsbeek A and Boelen A: Beyond
the fixed setpoint of the hypothalamus-pituitary-thyroid axis. Eur
J Endocrinol. 171:R197–R208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Høvring PI, Matre V, Fjeldheim AK, Loseth
OP and Gautvik KM: Transcription of the human thyrotropin-releasing
hormone receptor gene-analysis of basal promoter elements and
glucocorticoid response elements. Biochem Biophys Res Commun.
257:829–834. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J and Tashjian AH Jr: Regulation of
endogenous thyrotropin-releasing hormone receptor messenger RNA in
GH4C1 cells: Roles of protein and RNA synthesis. Mol Endocrinol.
7:1144–1150. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang J and Tashjian AH Jr: Transcriptional
regulation by dexamethasone of endogenous thyrotropin-releasing
hormone receptor messenger ribonucleic acid in rat pituitary GH4C1
cells. Endocrinology. 133:487–490. 1993. View Article : Google Scholar : PubMed/NCBI
|