Introduction
Basalt and volcanic ash are natural constituents of
the ground surrounding volcanic areas such as Mount Etna. The dust
may be daily inhaled by the general population as well as by
several types of workers, such as tourist guides, forest guards,
road maintenance workers, dump and construction workers.
In some of these occupational areas following the
manufacturing process, these materials can be mechanically broken
down to small particles, easy to inhale. One of these occupational
areas is the construction one, where basalt and volcanic ash are
often excavated in the early construction working phases. Besides,
basalt rock, basalt crushed rock (locally called azolo) and
volcanic ash are generally exploited as construction material,
usually mixed with cement. Volcanic ash is composed of fragments of
pulverized rock, minerals and volcanic glass, produced during
volcanic eruptions and measuring no more than 2 mm in diameter
(1).
With the word azolo we refer to a specific
type of grey-black, sand-like, sharp, less than 2 mm in diameter
stone dust of Mount Etna's area, which derives from the crushing of
basaltic rock due either to weathering factors or man's
intervention (2). Cement is a
binder, a substance exploited in construction that sticks and
hardens and can bind materials together. It is used as a component
in the production of mortar in masonry and of concrete, which is a
mixture of cement and an aggregate (i.e. azolo) to form a
strong construction material.
The grain size of construction materials is of
critical importance and is conventionally defined in terms of
aerodynamic diameter. Thoracic and respirable fractions of
particulate matter (PM) are defined as the fragment of inhaled
particles capable of going through the larynx and ciliated airways,
respectively, during inhalation (3). The PM less than 10 µm
(PM10) in size has a high penetration rate in the
airways (4,5).
Potential toxicity of PM depends on its chemical
and/or physical composition in association with the size that
classifies it as fine (<1 µm) and ultrafine (<0.01 µm) and
makes it breathable and inhalable, reaching the alveolar region
(3).
So far, few studies on the toxicity of volcanic dust
exist. Studies of exposure to ash related to volcanic activity
among the inhabitants of the Etna area (Sicily, Italy) have
revealed an increase in the rate of acute respiratory and
cardiovascular diseases (6–8) and
accumulation of heavy metals in the airways (9). Censi and co-workers (10) theorized that inhalation of air
borne particulate from Mt. Etna eruptions may account for the
occurrence of fibrotic lung disease. In this study, we analyzed the
potential mutagenic and cytotoxic effects of the materials used in
construction industry, derived from Mt. Etna.
Materials and methods
Sampling
Ground basalt (A), volcanic ash (B), mixed basalt
and cement (C) and cement (D) were sampled from the construction
site of a subway line in Catania, Sicily. The samples are
representative of the volcanic and volcaniclastic lithologies of
the place; to make the sample more representative the actual dust
produced by excavation works was collected (C and D). Samples were
then taken to the laboratory in polyethylene bags. They were dried
in an oven at 40°C and preserved in polyethylene containers and
underwent characterizations by scanning electron microscope (SEM)
(Cambridge Stereoscan 360) equipped with energy dispersive X-ray
(EDX) system (Oxford Instruments INCA Energy), as previously
described (11).
Afterwards, respirable fractions were isolated.
Approximately 5 g samples was aerosolised with HEPA-filtered
compressed air using a Naneum AERO PA100 Powder Aerosolizer Model
(Particle Measuring Systems, Boulder, CO, USA). The aerosol was
passed through a gravitational separator where large particles and
agglomerates sedimented after the aerosol. Particles below a
maximum theoretical aerodynamic diameter of 6 µm (i.e., within the
respirable range) (3), were
processed for a specific airflow and particle density in the
system, then entered a sampling collection chamber.
Mutagenic assessment: Ames test
In order to assess the mutagenic effect, a leaching
procedure was carried out, using deionized water (Merck KGaA,
Darmstadt, Germany) as a leaching agent. The solid/liquid ratio was
1:20 according to leaching procedure with a speed of 30 rpm for 18
h. The leachates were then filtered through a 0.45-µm filter. The
Ames mutagenicity test (kindly provided by B.N. Ames; Berkeley, CA,
USA) was performed with a standard plate incorporation assay with
the TA98, TA100, and TA1535 Salmonella typhimurium strains
with and without metabolic activation (12). In short, a preliminary toxic dose
range testing was conducted first to select a proper dose range for
the mutagenicity test. TA98, TA100 or TA1535 were grown in nutrient
broth (NB) at 37°C for 18 h, and then diluted for 106-
and 107-fold by PBS. Leachates of 5, 50, 100, 200, 250
or 500 µl were mixed with diluted bacteria (100 µl) and soft agar
(2 ml). Furthermore, mixed solutions were put onto plates and
incubated for 24 h. Mutagenicity effects of leachates were assessed
by comparing the bacterial counts between leachates and blank
control (deionized water) samples. The results revealed that 500
µl/plate of different leachates can be used for Ames assay.
Therefore, five volume levels (100, 200, 300, 400 and 500 µl/plate)
of leachates were selected in line with a previous report and
underwent a mutagenicity test (13,14).
The Ames test was validated by counting the number
of revertants by means of negative and positive controls, with the
spontaneous revertants being within the normal values for the three
strains. Moreover, a 2-fold criterion was used to dissect the
mutagenic activity, as shown below (12).
Cytotoxic assessment:
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Primary lines of normal, non immortalized, human
adult lung fibroblasts were detected by means of outgrowth from
explant in line with Jordana et al (15,16).
Human lung fibroblasts were made to proliferate in 10% fetal calf
serum RPMI, 100 U/ml penicillin, 100 µg/ml streptomycin, and 25
µg/ml fungizone (Life Technologies, Paisley, UK) and incubated at
37°C and 5% CO2. The medium was substituted every 3 days
and subcultures were isolated every 10–12 days. All in all, cell
lines were withdrawn just before the tenth passage. A549, which are
human bronchoalveolar carcinoma-derived cells with some features
peculiar of alveolar epithelial type II cells, were isolated from
American Type Culture Collection (ATCC, Rockville, MD, USA) and
routinely maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented as before. Cells were trypsinized, counted in a
haemocytometer, plated onto 96- or 6-well plates (Costar,
Cambridge, MA, USA), and grown in incubator at 37°C with 5% of
CO2.
J774 cells, a murine monocyte-macrophage
immortalized cell line, were acquired from ATCC. The cells were
grown in 4.5 g/l glucose DMEM with 1 mM sodium pyruvate. Samples
were suspended in medium and, after sonication, combined with the
experimental cultures at concentrations of 5, 50, and 100 µg/ml
(1.06, 10.6, and 21.2 µg/cm2) for 24 h before cell
collection.
The MTT proliferation assay is based on the
conversion by mitochondrial dehydrogenases of the substrate
containing a tetrazolium loop into blue, spectrophotometrically
quantifiable formazan (17). The
level of blue formazan is then used as indirect index of cell
density. The optical density of each sample was assessed by a
microplate spectrophotometer reader (Multiskan™ GO Microplate
Spectrophotometer, Thermo Scientific, Waltham, MA, USA) at λ
570 nm. Four replicates were carried out for every sample.
Statistical analysis
Ames test results are reported as mean of replicate
frequencies and standard deviation (SD). MMT outcomes are shown as
percentage of cell viability. Data analysis was performed by
GraphPad Prism ver. 7 (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
The Ames test revealed that cement (sample D),
showed a higher and more significant mutagenicity than the samples
A, B and C. Table I reports the
outcome. The revertants of samples A (ground basalt) and B
(volcanic ash) were comparable and below the 2-fold criterion of
the control sets, either with or without S9 activation for the
strains TA98, TA100 and TA1535. These data showed that sample A and
B revealed no mutagenic activity. However, mutagenicity was
observed in samples C (mixed basalt and cement) and D in a
positive, dose-dependent manner. The levels of revertants in
samples C and D increased roughly from 2- to 8-fold by 100, 200,
300, 400 and 500 µl/plate doses of samples in the TA98 and TA100
strain without S9 activation. Furthermore, no dose-response effects
of revertants were detected for TA1535, base-pair substitution
mutations, in sample C and D (Table
I).
 | Table I.Ames test results (mean ± SD). |
Table I.
Ames test results (mean ± SD).
|
| Sample A | Sample B | Sample C | Sample D |
|---|
|
|
|
|
|
|
|---|
| Strains | -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | -S9 | +S9 |
|---|
| TA98 |
|
|
|
|
|
|
|
|
| 100
µl/plate | 17±9 | 14±10 | 15±6 | 17±7 | 27±9 | 31±8 | 32±2 | 41±3 |
| 200
µl/plate | 15±8 | 16±7 | 13±8 | 16±6 | 28±8 | 32±9 | 31±3 | 42±4 |
| 300
µl/plate | 16±9 | 17±8 | 17±4 | 18±5 | 26±12 | 31±9 | 34±2 | 39±6 |
| 400
µl/plate | 11±7 | 13±8 | 16±6 | 20±6 | 24±7 | 27±6 | 34±5 | 43±4 |
|
Blank | 14±9 | 18±6 | 15±6 | 14±8 | 15±8 | 16±5 | 16±4 | 17±3 |
|
Positive | 154±30 | 161±25 | 149±24 | 168±26 | 164±27 | 171±40 | 159±23 | 167±36 |
| TA100 |
|
|
|
|
|
|
|
|
| 100
µl/plate | 19±9 | 18±12 | 24±9 | 23±4 | 131±21 | 134±24 | 145±50 | 142±51 |
| 200
µl/plate | 20±10 | 21±7 | 25±7 | 26±9 | 135±20 | 139±19 | 144±60 | 145±48 |
| 300
µl/plate | 24±8 | 26±10 | 24±8 | 30±11 | 137±19 | 142±23 | 146±49 | 139±50 |
| 400
µl/plate | 19±9 | 24±12 | 27±13 | 28±9 | 141±29 | 145±32 | 147±61 | 145±52 |
|
Blank | 106±36 | 121±37 | 102±30 | 116±38 | 109±34 | 118±41 | 117±66 | 120±68 |
|
Positive | 698±140 | 1409±212 | 653±127 | 1398±197 | 687±131 | 1425±209 | 654±128 | 1387±214 |
| TA1535 |
|
|
|
|
|
|
|
|
| 100
µl/plate | 13±5 | 16±7 | 16±8 | 17±6 | 23±9 | 25±10 | 36±12 | 38±19 |
| 200
µl/plate | 16±7 | 19±11 | 18±4 | 21±5 | 24±11 | 26±8 | 34±13 | 40±13 |
| 300
µl/plate | 15±8 | 16±12 | 17±6 | 18±10 | 23±7 | 28±9 | 30±15 | 32±16 |
| 400
µl/plate | 19±13 | 17±9 | 18±7 | 21±12 | 26±12 | 25±14 | 26±17 | 29±16 |
|
Blank | 14±6 | 15±6 | 12±4 | 16±4 | 11±8 | 13±7 | 12±8 | 14±10 |
|
Positive | 649±78 | 697±81 | 661±59 | 689±76 | 653±80 | 689±89 | 668±75 | 703±101 |
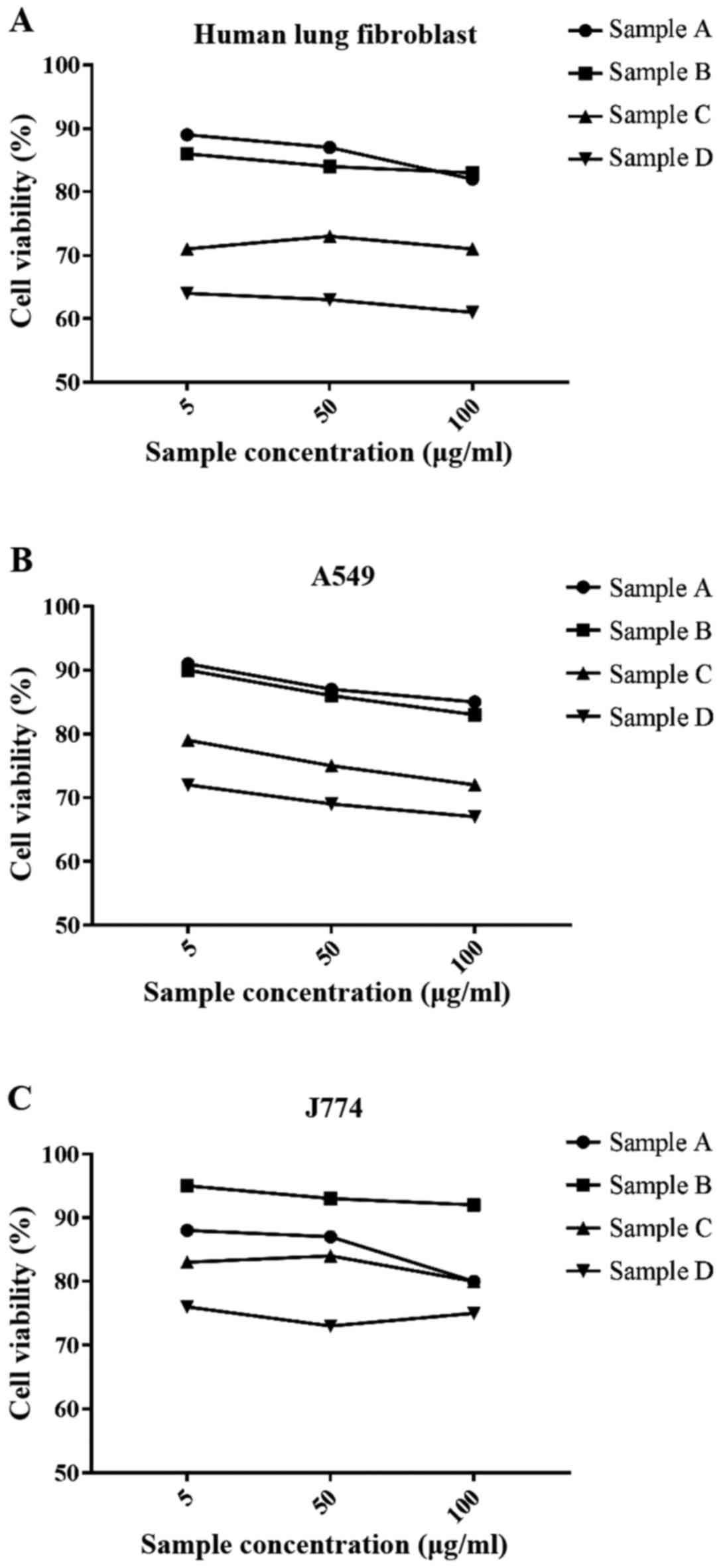
MTT assay showed that samples C and D, at lesser
concentration (5 µg/ml) for 24 h, had a slightly negative impact on
cell viability. Instead, samples A and B at lower concentration had
none. At superior concentrations (50 and 100 µg/ml) for 24 h all of
the samples (A-D), showed a slightly negative impact on cell
ability to metabolize tetrazolium salts (Fig. 1A-C).
Discussion
Basaltic rocks coming from Mount Etna are an
outstanding source of construction materials. These materials have
always been exploited without taking into due account the possible
risks for the workers' health as well as for the people actually
living in these houses (3,9,11).
Recently, in the South-west area of Mt. Etna a
natural asbestiform fibre has been detected, called Fluoro-edenite
(FE) (18). FE contaminated lava
rocks were used for approximately 50 years to build houses and
roads. FE was recognized as carcinogenic to humans and there are
several experimental and epidemiological studies showing its
toxicity for workers and the population in general (19–31).
In this study, we analyzed the potential mutagenic and/or cytotoxic
effects of basaltic materials used in the construction field.
To date, there are no studies that have assessed the
toxicity of lava stones deriving from Mt. Etna (6–9). We
have only data relative to the acute effects on men of volcanic
dust and ash after a volcanic eruption. Previously, the potential
health consequences undergone by construction workers handling
basaltic rock have been investigated in an ecotoxicological study
that showed minimal toxicity of the volcanic ash (11).
The results of our study suggested that samples C
(mixed basalt and cement) and D (cement) might generate derivative
metabolites after reactions in the living beings, thereby inducing
frame-shift mutations (TA98 and TA1535) and base-pair substitution
(TA100) (12).
Little is known about the long-term exposure to
volcanic ash and their effects on respiratory tract, even less the
mainstay of risk assessment in occupational studies. Eruption
activity is a natural source of breathable nanoparticles (1,10).
The long-term exposure to breathable volcanic ash (<4 µm Ø),
either because of long-lasting exposure to ash fallout or
re-mobilization of ash owing to human activity and wind, has been
hypothesized as a potential cause of chronic and/or
potentially-fatal lung diseases (silicosis, which occurs through
inhaling of crystalline silica), chronic obstructive pulmonary
disease, non-specific pneumoconiosis and lung cancer (32).
Our study points out poor mutagenic and cytotoxic
effects of ground basalt (A) and volcanic ash (B); whereas cement
reveals higher mutagenic and cytotoxic activity, both by itself
(D), and in combination with basalt (C). These results are in
accordance with an earlier study, conducted with a Vibrio
fischeri light inhibition test that highlighted the biotoxicity
of the same sample. The results indicated that sample C and D were
toxic to V. fischeri (11).
Recently, Cervini-Silva et al (32) demonstrated that degeneration of
free radicals by ash particles is due to the presence of iron
(Fe3+). These observations can account for the high
reactivity of samples C and D. As a matter of fact, in these
samples the content of Fe is significantly greater than that of A
and B. The results were reported by Ledda et al (11).
Some categories of workers may be exposed to stone
dusts both at their workplace and at home. The dust can determine
pathological responses in the respiratory tract (33,34).
Frequently, illnesses deriving from inhalation of mineral dusts and
fibres are usually progressive and cumulative, with 20 to 30-year
latency. Interstitial fibrosis is the most common disease
originating from inhaled mineral dusts, that can develop other
chronic diseases and, eventually cancer (3).
The study of long-term exposure risks to
christobalite-bearing ash, after the eruption of Mt. St. Helens and
Soufrière Hills, showed several cases of silicosis (35), although the toxicity of volcanic
cristobalite had not yet been established (36). In-vitro and in-vivo
toxicological tests on volcanic ash produced different results
depending on the concentration of silica (Si) (37,38).
Yet, no outstanding intra-study differences in reactivity among
samples with variable cristobalite contents were detected (39–41).
In our study, the content of Si is significantly
greater in samples C than in the others (11). Many work categories, particularly
those employed in the construction, are exposed to basaltic and
volcanic dusts, as well as cement. Furthermore, cement and basalt
dusts are often mixed to make mortar. Exposure to cement dust may
result in a reduced pulmonary function and chronic respiratory
symptoms (42,43). Moreover, in construction workers,
there is a significantly higher risk of lung cancer than in the
general population (24).
As a conclusion, we can say that no particular risks
seem to exist for construction industry workers, while the
exploitation of cement and cement mixed with basalt seems to be a
risk for workers, given the high percentage of Si and Fe,
especially in the latter sample C. Thus, our findings could
contribute to identifying further risk factors adding to the
increased rate of lung cancer in this working category (44–46).
The results of our study stimulate further tests in order to better
clarify the health effects of basaltic dust, especially when used
in combination with other substances due to their various
manufacturing scopes.
References
|
1
|
Rose WI and Durant AJ: Fine ash content of
explosive eruptions. J Volcanol Geotherm Res. 186:32–39. 2009.
View Article : Google Scholar
|
|
2
|
Venerando R, Amati M, Coloccini S,
Bolognini L, Gobbi L and Duscio D: The in vitro release of hydroxyl
radicals from dust containing fluoro-edenite fibers identified in
the volcanic rocks of Biancavilla (eastern Sicily). Med Lav.
94:200–206. 2003.(In Italian). PubMed/NCBI
|
|
3
|
Brown JS, Gordon T, Price O and Asgharian
B: Thoracic and respirable particle definitions for human health
risk assessment. Part Fibre Toxicol. 10:122013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Workplace atmospheres-size fraction
definitions for measurement of airborne particles. (Report No. BS
EN 481: 1993). 1993.
|
|
5
|
American Conference of Governmental
Industrial Hygienists (ACGIH): TLVs and BEIs: Based on the
Documentation of the Threshold Limit Values for Chemical Substances
and Physical Agents and Biological Exposure IndicesAmerican
Conference of Governmental Industrial Hygienists. Cincinnati, OH:
2005
|
|
6
|
Lombardo D, Ciancio N, Campisi R, Di Maria
A, Bivona L, Poletti V, Mistretta A, Biggeri A and Di Maria G: A
retrospective study on acute health effects due to volcanic ash
exposure during the eruption of Mount Etna (Sicily) in 2002.
Multidiscip Resp Med. 8:512013. View Article : Google Scholar
|
|
7
|
Hansell AL, Horwell CJ and Oppenheimer C:
The health hazards of volcanoes and geothermal areas. Occup Environ
Med. 63:149–156, 125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fano V, Cernigliaro A, Scondotto S, Cuccia
M, Forastiere F, Nicolosi A, Oliveri C, Scillieri R, Distefano P
and Perucci CA: Health effects of environmental contamination due
to volcanic ash of Mount Etna in autumn 2002. Epidemiol Prev.
29:180–187. 2005.(In Italian). PubMed/NCBI
|
|
9
|
Censi P, Zuddas P, Randazzo LA, Tamburo E,
Speziale S, Cuttitta A, Punturo R, Aricò P and Santagata R: Source
and nature of inhaled atmospheric dust from trace element analyses
of human bronchial fluids. Environ Sci Technol. 45:6262–6267. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Censi P, Tamburo E, Speziale S, Zuddas P,
Randazzo LA, Punturo R, Cuttitta A and Aricò P: Yttrium and
lanthanides in human lung fluids, probing the exposure to
atmospheric fallout. J Hazard Mater. 186:1103–1110. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ledda C, Rapisarda V, Bracci M, Proietti
L, Zuccarello M, Fallico R, Fiore M and Ferrante M: Professional
exposure to basaltic rock dust: Assessment by the Vibrio
fischeri ecotoxicological test. J Occup Med Toxicol. 8:232013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mortelmans K and Zeiger E: The Ames
Salmonella/microsome mutagenicity assay. Mutat Res.
455:29–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radetski CM, Ferrari B, Cotelle S,
Masfaraud JF and Ferard JF: Evaluation of the genotoxic, mutagenic
and oxidant stress potentials of municipal solid waste incinerator
bottom ash leachates. Sci Total Environ. 333:209–216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen PW, Liu ZS, Wun MJ and Ran CL:
Evaluating the mutagenicity of leachates obtained from the bottom
ash of a municipal solid waste incinerator by using a
Salmonella reverse mutation assay. Chemosphere. 124:70–76.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jordana M, Schulman J, McSharry C, Irving
LB, Newhouse MT, Jordana G and Gauldie J: Heterogeneous
proliferative characteristics of human adult lung fibroblast lines
and clonally derived fibroblasts from control and fibrotic tissue.
Am Rev Respir Dis. 137:579–584. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cardile V, Renis M, Scifo C, Lombardo L,
Gulino R, Mancari B and Panico A: Behaviour of the new asbestos
amphibole fluor-edenite in different lung cell systems. Int J
Biochem Cell Biol. 36:849–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miozzi E, Rapisarda V, Marconi A, Costa C,
Polito I, Spandidos DA, Libra M and Fenga C: Fluoro-edenite and
carbon nanotubes: The health impact of ‘asbestos-like’ fibres. Exp
Ther Med. 11:21–27. 2016.PubMed/NCBI
|
|
19
|
Rapisarda V, Rapisarda G, Vico GD, Gobbi
L, Loreto C and Valentino M: Monitoring of fluoro-edenite fibre
pollution through the study of sheep lymph nodes as a model of a
biological indicator. Occup Environ Med. 62:6562005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinez G, Loreto C, Rapisarda V,
Masumeci G, Valentino M and Carnazza ML: Effects of exposure to
fluoro-edenite fibre pollution on the respiratory system: An in
vivo model. Histol Histopathol. 21:595–601. 2006.PubMed/NCBI
|
|
21
|
Loreto C, Rapisarda V, Carnazza ML,
Musumeci G, Valentino M, Fenga C and Martinez G: Fluoro-edenite
fibres induce lung cell apoptosis: An in vivo study. Histol
Histopathol. 23:319–326. 2008.PubMed/NCBI
|
|
22
|
Szychlinska MA, Parenti R, Loreto C,
Salvatorelli L, Spadola S, Trovato FM, Pirri C, Rapisarda V, Pace
MM, et al: Fluoro edenite-associated pathogenesis in pleural
malignant mesothelioma. Acta Med Mediter. 30:981–989. 2014.
|
|
23
|
Musumeci G, Loreto C, Szychlinska MA,
Imbesi R, Rapisarda V, Aiello FC, Castorina S and Castrogiovanni P:
N-Cadherin, ADAM-10 and Aquaporin 1 expression in lung tissue
exposed to fluoro-edenite fibers: An immunohistochemical study.
Histol Histopathol. 30:987–999. 2015.PubMed/NCBI
|
|
24
|
Rapisarda V, Ledda C, Ricceri V, Arena F,
Musumeci A, Marconi A, Fago L, Bracci M, Santarelli L and Ferrante
M: Detection of pleural plaques in workers exposed to inhalation of
natural fluoro-edenite fibres. Oncol Lett. 9:2046–2052.
2015.PubMed/NCBI
|
|
25
|
Rapisarda V, Ledda C, Migliore M, Salemi
R, Musumeci A, Bracci M, Marconi A, Loreto C and Libra M: FBLN-3 as
a biomarker of pleural plaques in workers occupationally exposed to
carcinogenic fibers: A pilot study. Future Oncol. 11:(Suppl).
35–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ledda C, Loreto C, Pomara C, Rapisarda G,
Fiore M, Ferrante M, Bracci M, Santarelli L, Fenga C and Rapisarda
V: Sheep lymph-nodes as a biological indicator of environmental
exposure to fluoro-edenite. Environ Res. 147:97–101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Musumeci G, Loreto C, Giunta S, Rapisarda
V, Szychlinska MA, Imbesi R, Castorina A, Annese T, Castorina S,
Castrogiovanni P, et al: Angiogenesis correlates with macrophage
and mast cell infiltration in lung tissue of animals exposed to
fluoro-edenite fibers. Exp Cell Res. 346:91–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ledda C, Pomara C, Bracci M, Mangano D,
Ricceri V, Musumeci A, Ferrante M, Musumeci G, Loreto C, Fenga C,
et al: Natural carcinogenic fiber and pleural plaques assessment in
a general population: A cross-sectional study. Environ Res.
150:23–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rapisarda V, Salemi R, Marconi A, Loreto
C, Graziano AC, Cardile V, Basile MS, Candido S, Falzone L,
Spandidos DA, et al: Fluoro-edenite induces fibulin-3
overexpression in non-malignant human mesothelial cells. Oncol
Lett. 12:3363–3367. 2016.PubMed/NCBI
|
|
30
|
Ledda C, Loreto C, Matera S, Massimino N,
Cannizzaro E, Musumeci A, Migliore M, Fenga C, Pomara C and
Rapisarda V: Early effects of fluoro-edenite: Correlation between
IL-18 serum levels and pleural and parenchymal abnormalities.
Future Oncol. 12:59–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ledda C, Loreto C, Bracci M, Mangano D,
Migliore M, Ricceri V, Musumeci A, Costa C, Pomara C and Rapisarda
V: High risk of pleural plaques and parenchymal abnormalities in
women living in Biancavilla (Italy). Future Oncol. 12:63–65. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cervini-Silva J, Antonio-Nieto-Camacho
Gomez-Vidales V, Ramirez-Apan MT, Palacios E, Montoya A, Kaufhold
S, Abidin Z and Theng BK: Lipid peroxidation and cytotoxicity
induced by respirable volcanic ash. J Hazard Mater. 274:237–246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fubini B and Otero Areán C: Chemical
aspects of the toxicity of inhaled mineral dusts. Chem Soc Rev.
28:373–381. 1999. View
Article : Google Scholar
|
|
34
|
Shukla A, Ramos-Nino M and Mossman B: Cell
signaling and transcription factor activation by asbestos in lung
injury and disease. Int J Biochem Cell Biol. 35:1198–1209. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Horwell CJ and Baxter PJ: The respiratory
health hazards of volcanic ash: A review for volcanic risk
mitigation. Bull Volcanol. 69:1–24. 2006. View Article : Google Scholar
|
|
36
|
Damby DE, Murphy FA, Horwell CJ, Raftis J
and Donaldson K: The in vitro respiratory toxicity of
cristobalite-bearing volcanic ash. Environ Res. 145:74–84. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sanders CL, Rhoads K and Mahaffey JA:
Long-term reactivity of lung and mediastinal lymph nodes following
intratracheal instillation of sandy loam soil or Mount St. Helens
volcanic ash. Environ Res. 32:188–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wehner AP, Dagle GE, Clark ML and Buschbom
RL: Lung changes in rats following inhalation exposure to volcanic
ash for two years. Environ Res. 40:499–517. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Housley DG, Bérubé KA, Jones TP, Anderson
S, Pooley FD and Richards RJ: Pulmonary epithelial response in the
rat lung to instilled Montserrat respirable dusts and their major
mineral components. Occup Environ Med. 59:466–472. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vallyathan V, Robinson V, Reasor M,
Stettler L and Bernstein R: Comparative in vitro cytotoxicity of
volcanic ashes from Mount St. Helens, El Chichon, and Galunggung. J
Toxicol Environ Health. 14:641–654. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wilson MR, Stone V, Cullen RT, Searl A,
Maynard RL and Donaldson K: In vitro toxicology of respirable
Montserrat volcanic ash. Occup Environ Med. 57:727–733. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alvear-Galindo MG, Mendez-Ramirez I,
Villegas-Rodriguez JA, Chapela-Mendoza R, Eslava-Campos CA and
Laurell AC: Risk indicator of dust exposure and health effects in
cement plant workers. J Occup Environ Med. 41:654–661. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
AbuDhaise BA, Rabi AZ, al Zwairy MA, el
Hader AF and el Qaderi S: Pulmonary manifestations in cement
workers in Jordan. Int J Occup Med Environ Health. 10:417–428.
1997.PubMed/NCBI
|
|
44
|
Ledda C and Rapisarda V: Malignant pleural
mesothelioma: The need to move from research to clinical practice.
Arch Med Res. 47:4072016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rapisarda V, Loreto C, Ledda C, Musumeci
G, Bracci M, Santarelli L, Renis M, Ferrante M and Cardile V:
Cytotoxicity, oxidative stress and genotoxicity induced by glass
fibers on human alveolar epithelial cell line A549. Toxicol In
Vitro. 29:551–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Palmucci S, Torrisi SE, Caltabiano DC,
Puglisi S, Lentini V, Grassedonio E, Vindigni V, Reggio E, Giuliano
R, Micali G, et al: Clinical and radiological features of
extra-pulmonary sarcoidosis: A pictorial essay. Insights Imaging.
7:571–587. 2016. View Article : Google Scholar : PubMed/NCBI
|