Introduction
Deep vein thrombosis (DVTs) is the third most common
cardiovascular disease (CVD) after ischemic heart disease and
stroke (1). The determinant
factors for DVTs also include environmental factors such as
exposure to atmospheric pollution (2–5),
genetic factors (6) and unbalanced
lifestyle habits, such as poor diet (7–9),
physical inactivity, smoking and alcohol consumption (10). The risk for human health derived
from an unbalanced diet, can lead to a deficiency of essential
metals. Essential trace elements are those compounds that need to
be present in the human diet to maintain normal physiological
functions. Copper (Cu), manganese (Mn) and zinc (Zn) have been
recognized as essential elements due to their presence in important
proteins and enzymes such as pyruvate carboxylase and SOD (11).
Conversely, others metals such as lead (Pb), iron
(Fe), chromium (Cr) and cobalt (Co) undergo cycling reactions and
have the possibility to produce reactive oxygen species (ROS) in
biological systems. Therefore, disruption of metal ion homeostasis
may lead to oxidative stress that induces DNA damage, lipid
peroxidation and protein modification. This leads to numerous
diseases, including cancer, cardiovascular disease, diabetes,
atherosclerosis, venous thromboembolism, neurological disorders,
chronic inflammation and others. The mechanism of action for all
these metals involves formation of the ROS, finally producing
malondialdehyde (MDA), 4-hydroxynonenal (4-HNE) and metabolic
consumption of antioxidant species such as SOD (12).
Our study compared the essential elements Cu, Zn and
Mn, crucial for normal physiology maintenance, and Pb levels as
environmental pollutant, in hair of subjects suffering of deep vein
thrombosis of lower limbs (DVTs) vs. healthy subjects. Furthermore,
we evaluated oxidative stress parameters, the thiobarbituric acid
reactive substances (TBARS) as the sum of malondialdehyde or
1,1,3,3-tetraethoxypropane (MDA) and 4-hydroxynonenal (4-HNE) and
cytosolic superoxide dismutase (Cu/Zn-SOD) plasma concentration in
both groups.
Materials and methods
Study design
The study was based on a case-control design. The
participants were recruited by the Department of Clinic and
Experimental Medicine, University of Catania. The study was
approved by the Ethics Committee ‘Catania 1’ and a written informed
consent was obtained from each participant.
The socio-demographic, habits and lifestyle data
were collected via questionnaire. Cases included in the study were
selected by the following criteria: subjects with clinically
diagnosed DVTs with increased volume limb, erythematosus skin tone
(sometimes cyanotic), hot and glossy skin, sign of the fovea, pain
after muscle strain, DVTs confirmed instrumentally; subjects with
asymptomatic DVTs instrumentally diagnosed.
Some cases were excluded from the study with the
following criteria: subjects aged >75 years and suffering of
active chronic diseases; subjects with recently surgical
procedures; subjects with a history of congenital thrombophilia;
subjects with tattoos, metal joint implants and metal dentures;
subjects with treated hair (permanent and color).
Sampling of hair and blood
Samples of hair were collected for analysis of
transition metals Cu, Mn and Zn and toxic metal Pb, because easily
accessible samples, available in sufficient quantities,
non-invasive sampling and stable material that does not require
special treatments. In addition, minerals do not deteriorate or
disappear after collection.
Hair was cut with a stainless steel pair of
scissors, which were cleaned and visually inspected between samples
to prevent cross-contamination. Hair was collected in three strands
from the left, central and right area of the nape. The first 3 cm
next to the hairline were selected for metal determination,
accurately washed into 50 ml of falcon tube (Thermo Fischer
Scientific, Pittsburgh, PA, USA) with bi-distilled water until
water did not appear clean. Samples were then stored at −20°C until
processing.
Blood sample for TBARS and SOD analysis was
collected by venipuncture, immediately centrifuged at 1,500 × g for
10 min at 4°C to separate the plasma. Plasma was then preserved in
amber vials (Eppendorf AG, Hamburg, Germany) and stored at −80°C
until analysis.
Analysis of the metals
For metal extraction and quantification, aliquots of
0.5 g of each hair sample was weighed. An acid digestion was
conducted with an Ethos TC microwave system (Milestone, Sorisole,
Italy) with 6 ml of 65% nitric acid (HNO3) (Carlo Erba,
Milan, Italy) and 2 ml of 30% peroxide hydrogen
(H2O2) (Carlo Erba) for 50 min operation
cycle at 200°C. After the cycle, at a temperature <25°C the
Teflon vessels (Milestone, Sorisole, Italy) were opened and Milli-Q
water (Q-Gard® 1; Merck Millipore, Darmstadt, Germany)
was added to the samples up to 50 ml; an ICP-MS (ELAN®
DRC-e; PerkinElmer, Inc., Waltham, MA, USA) was used for metal
quantification. Standards for the instrument calibration were
prepared with mono element certified reference solution ICP
standard (Merck Millipore, Darmstadt, Germany). To validate
analysis we spiked real samples in duplicate with 5 µg/l of each
element, and the percentage of recovery ranged between 97.8 and
113.8%. The method detection limits (MDL) estimated with 3 σ of the
procedure blanks were (mg/kg w.w.): Cu 0.02, Mn 0.25, Zn 0.10 and
Pb 0.011.
TBARS evaluation
TBARS were evaluated as the sum of MDA and 4-HNE
concentrations according to the methodology previously described
(13,14).
Briefly, 125 µl of thiobarbituric acid (TBA) (0.25 g
in 50 ml H2O; Sigma-Aldrich St. Louis, MO, USA), 150 µl
HPLC-grade H2O, and 325 µl phosphoric acid
(H3PO4, 0.15 M; Sigma-Aldrich) were added to
100 µl plasma (in EDTA blood vials). The sample was incubated at
45°C for 1 h for 4-HNE TBA derivatization and 90°C for 1 h for MDA,
then placed in ice for 4 min and centrifuged at 1,500 × g for 10
min at 4°C and syringe-filtered (0.45 mm; Superchrom S.r.l., Milan,
Italy). Twenty microliters of the sample was then successively
injected in HPLC (Series 200; Perkin-Elmer, Inc., Waltham, MA, USA)
equipped with a UV detector, and a fluorescence detector using a
Lichrospher® 100 RP-18 (250×4 mm) column. The HPLC
setting was: mobile phase consisting of 200 ml methanol and 300 ml
phosphate-buffered saline (50 mM, pH 7.4) with a flow of 1.0 ml/min
and detection was carried out using an excitation of 532 nm and an
emission of 553 nm. The calibration curve was carried out using
commercial MDA (purity >96%; Merck Millipore) and 4-HNE (purity
>99%, Cayman Chemical Co., Ann Arbor MI, USA) external
standards, the calibration (five points) was performed using a
pre-column derivatization with TBA. Mean recoveries of spiked
matrices were 92 and 103% for MDA e 4-HNE, respectively. The MDL
were 0.02 µM-L for MDA and 0.01 µM-L for 4-HNE.
SOD analysis
SOD was dosed in plasma (EDTA) samples using a
certificate assay kit of Cayman Chemical Co. (Ann Arbor kits, MI
48108). SOD activity was assessed by measuring the dismutation of
superoxide radicals generated by xanthine oxidase and hypoxanthine
in a 96-well plate. A SOD unit (U) is defined as the amount of
enzyme required to the 50% of dismutation. In our study, cytosolic
Cu-Zn-SOD was detected. Briefly, the plasma was centrifuged at
1,500 × g for 10 min at 4°C. We collected only the top yellow
plasma layer without disturbing the white buffy layer. The samples
were diluted 1:5 with the equipped sample buffer. Sample processing
and plate development were carried out according to the
manufacturer's instructions. The plates were read at 440 nm through
a Multiskan™ Thermo Fisher spectrophotometer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 96-well plates. MDL was
0.005 U/ml.
Statistical analysis
Data were analyzed using SPSS, version 20.0 (SPSS,
Inc., Chicago, IL, USA). The differences in the proportions of
cases and controls responding to each question were tested by the
Chi-square test. The Mann-Whitney U test was used to compare all
quantitative variables between cases and controls. Results are
presented as percentages and the corresponding p-value and as
medians and interquartile range (IQR).
Results
Seventy volunteers (24 cases and 46 controls) were
included in the study. Volunteers were males and females aged
between 43 and 71 years, residents in the province of Catania. The
small number of participants was due to the limited prevalence of
the DVTs (2–4% of the population) and it is frequently
asymptomatic. In Tables I and
II are summarized the results
obtained from the administered questionnaires. Among the subjects
recruited, there is a prevalence of women in the control group.
Among the other socio-demographic parameters, habits and
lifestyles, there is no association between case and control
groups. According to the diet, it was revealed that the cases
consumed fewer vegetables than the controls (p<0.001).
 | Table I.Frequencies of some qualitative
variables stratified by cases and controls. |
Table I.
Frequencies of some qualitative
variables stratified by cases and controls.
| Variables | Cases n (%) | Controls n (%) |
P-valuea |
|---|
| Gender |
|
|
<0.05 |
|
Male | 17 (70.8) | 20 (43.5) |
|
Female | 7 (29.2) | 26 (56.5) |
| Sport |
|
| 0.764 |
|
Yes | 8 (33.3) | 17 (37) |
| No | 16 (66.7) | 29 (63) |
| Smoking habits |
|
| 0.252 |
| Current
smokers | 6 (25) | 13 (28.3) |
| Never
smoked | 9 (37.5) | 24 (52.2) |
|
Ex-smokers | 9 (37.5) | 9 (19.6) |
| Drinking water
type |
|
| 0.277 |
|
Mineral | 14 (58.3) | 32 (69.6) |
| Tap
water | 10 (41.7) | 12 (26.1) |
|
Both | 0 | 2 (4.3) |
| Fruit
consumption |
|
| 0.400 |
|
Yes | 16 (66.7) | 35 (76.1) |
| No | 8 (33.3) | 11 (23.9) |
| Vegetables
consumption |
|
|
<0.001 |
|
Yes | 9 (37.5) | 39 (84.8) |
| No | 15 (62.5) | 7 (15.2) |
| Fish
consumption |
|
| 0.314 |
|
Yes | 10 (41.7) | 25 (54.3) |
| No | 14 (58.3) | 21 (45.7) |
| Alcohol
consumption |
|
| 0.100 |
|
Yes | 7 (29.2) | 6 (13) |
| No | 17 (70.8) | 40 (87.0) |
| Mineral supplements
use |
|
| 0.874 |
|
Yes | 4 (16.7) | 7 (15.2) |
| No | 20 (83.3) | 39 (84.8) |
| Herbal products
use |
|
| 0.304 |
|
Yes | 2 (8.3) | 8 (17.4) |
| No | 22 (91.7) | 38 (82.6) |
| Hair products
use |
|
| 0.229 |
|
Yes | 11 (45.8) | 28 (60.9) |
| No | 13 (54.2) | 18 (39.1) |
| Cosmetics use |
|
| 0.126 |
|
Yes | 7 (29.2) | 21 (45.7) |
| No | 17 (70.8) | 22 (47.8) |
 | Table II.Distribution of some quantitative
variables stratified by cases and controls. |
Table II.
Distribution of some quantitative
variables stratified by cases and controls.
| Variables | Cases median
(IQR) | Controls median
(IQR) |
P-valuea |
|---|
| Age | 64 (52–71) | 54 (43–66.8) | 0.201 |
| Weight (kg) | 74 (59.5–89.3) | 71.5
(61.8–77.3) | 0.310 |
| Height (m) | 1.65
(1.57–1.75) | 1.65
(1.60–1.71) | 0.598 |
| Liters of
drinking | 1 (1–2) | 1 (1–2) | 0.702 |
| water per day |
|
|
|
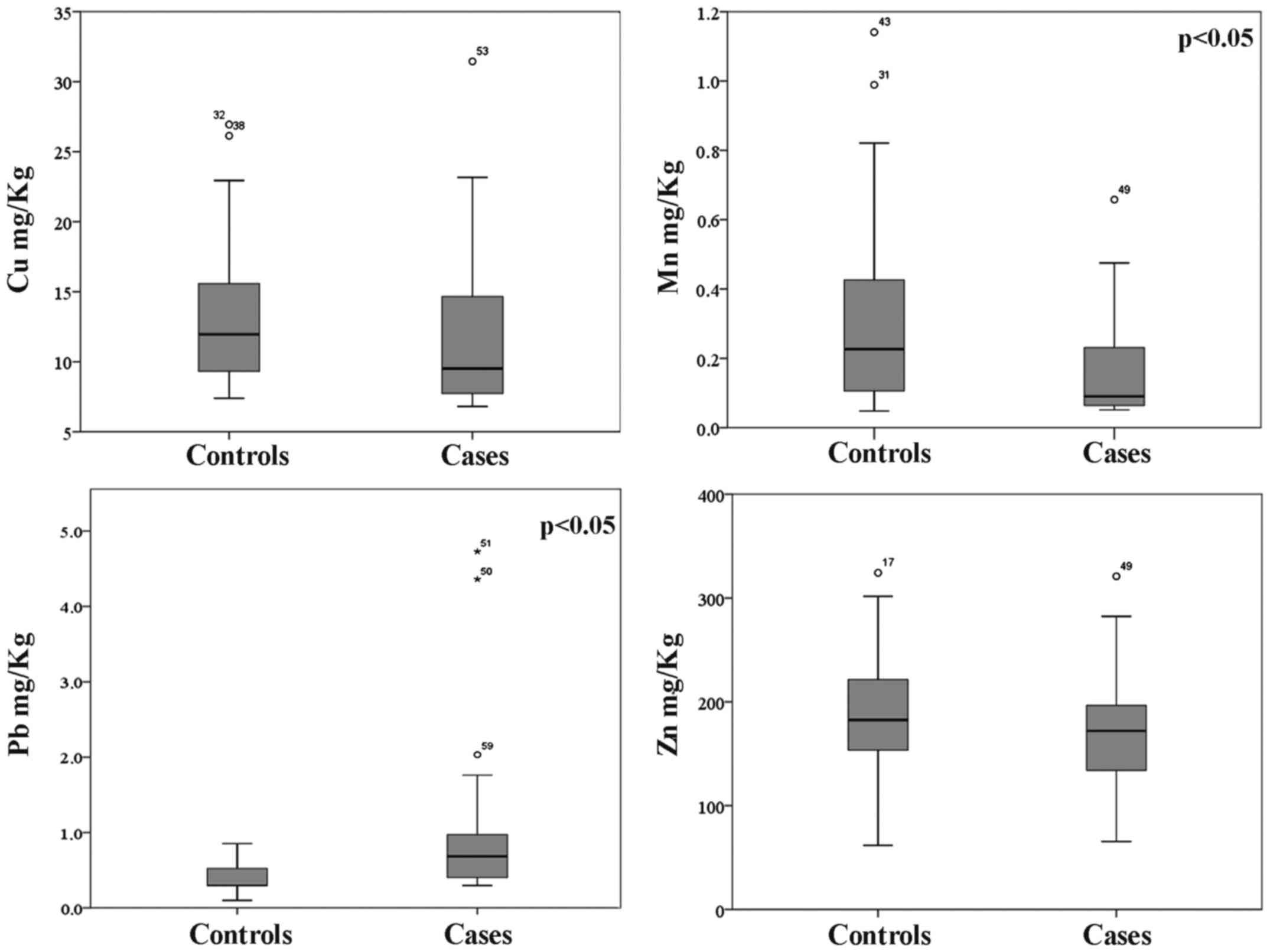
With regard to metal concentrations detected in hair
sample, as shown in Fig. 1, the
overall trend is a lower concentration of essential metals Cu, Mn
and Zn in the hair of subjects suffering of DVTs with respect to
the control one. We found the median concentrations 9.52, 0.090 and
172 mg/kg, respectively, in the cases and 11.9, 0.227 and 182.5
mg/kg in the control group.
Conversely, the highest levels of the toxic metal Pb
were found in the cases group (0.684 vs. 0.297 mg/kg). Although
results indicate a clear pattern of accumulation between cases and
control groups, statistically significant differences are related
only to Mn (p=0.011) and Pb (p=0.042) concentrations.
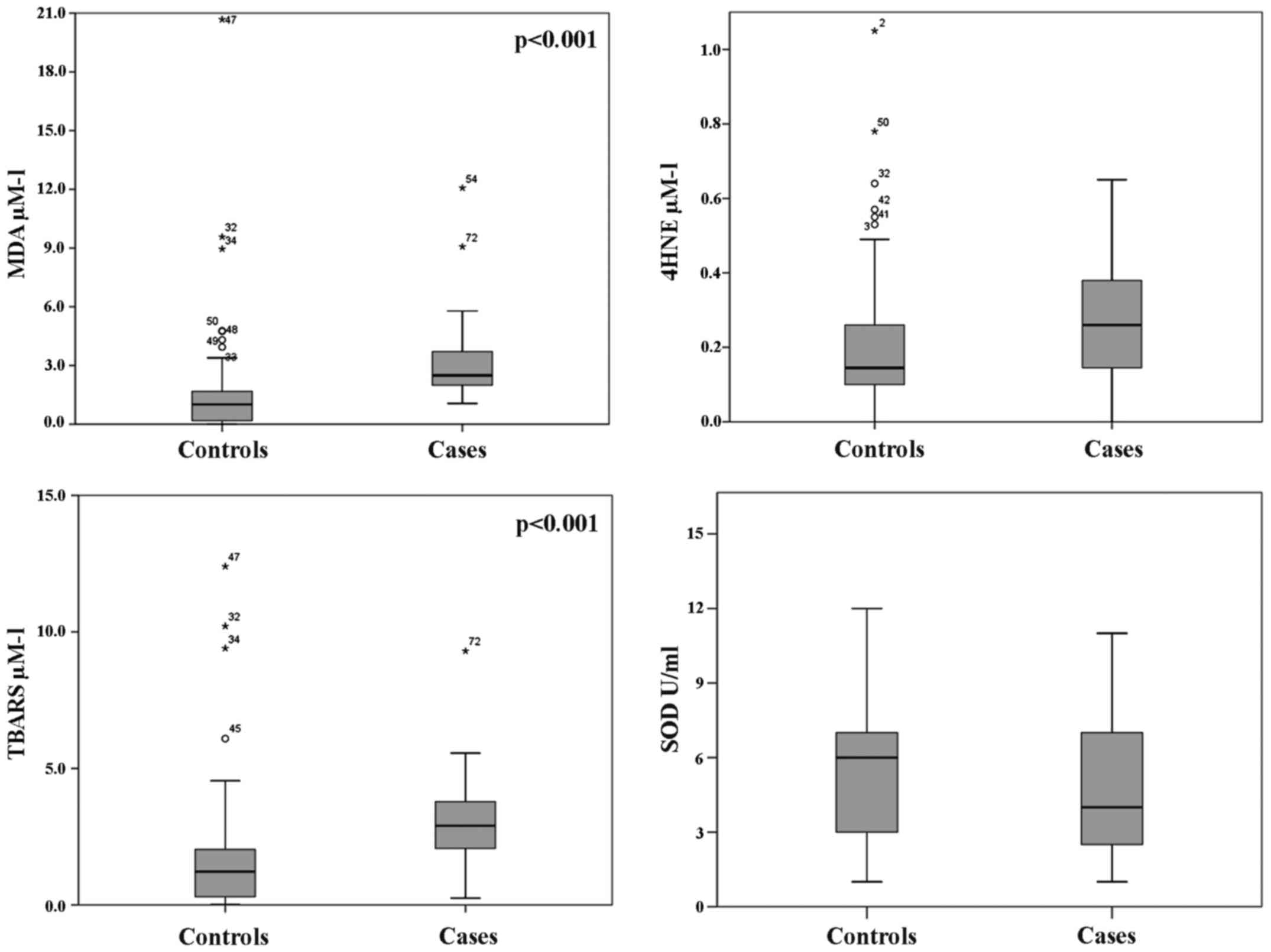
With regard to oxidative stress parameters (Fig. 2), we found significantly higher
median values in the cases than the control group for MDA (2.49 vs.
1.01 µM/l; p<0.001) and TBARS (5.39 vs. 1.16 µM/l; p<0.001),
as well as for 4-HNE (0.26 vs. 0.15 µM/l); however, only 4-HNE was
without a significant p-value (p=0.330) (Fig. 2). Conversely, the antioxidant
enzyme SOD was found higher in the control group than in the cases
(5.92 vs. 4.37 U/ml), but without significance (p=0.117). These
results, even without strong statistical significance, indicate a
status of oxidative stress in DVT subjects as well as deficiency in
the oxide reductive system.
Discussion
Subjects with DVTs were recruited to evaluate
bioaccumulation of essential metals (Cu, Mn and Zn). The toxic
metal Pb can be a determinant factor in DVTs, and at which level
the antioxidant defense system can be altered. In particular, the
MDA, TBARS and 4-HNE levels, which are products of lipid
peroxidation, were used as surrogate oxidation markers. A parameter
reflecting antioxidant defenses measured in our study was
Cu/Zn-SOD.
The status of oxidative stress occurs when the
balance between the production of ROS and the antioxidant defense
system is lacking. This imbalance can cause various diseases,
including CVDs, thus DVTs. The endothelial integrity may therefore
be less in case of excess of ROS or deficiency of antioxidant
defense enzymes. The enzymes reduce the activity of ROS, and are
able to maintain at vessel level the vasodilatory effects of nitric
oxide (NO), conversely, ROS species can decrease the biological
activity of NO (15), produced in
the respiratory tract as part of the inflammatory process (16).
In several systemic diseases with an inflammatory
component (cardiovascular, pulmonary, hepatic, retinal, and
neurodegenerative), the products of lipid peroxidation are
detected. The oxidation markers MDA and 4-HNE are among the
breakdown products most commonly used to validate index of
oxidative stress (17). These
products are relatively long-lived compared to ROS, forming
covalent adducts with target molecules such as proteins, with
important pathophysiological consequences (18).
Our findings show higher oxidative stress parameters
in DVTs compared to controls, indicating a physiological condition
of oxidative stress, but highlighting significant differences only
for MDA and TBARS. Low concentration of 4-HNE was detected in all
subjects, while in the cases, it was found slightly higher. These
results could be explain by the normal 4-HNE generation only
amounting to 10% of that of MDA, as demonstrated in several in
vitro oxidation studies (19).
The first line of enzymatic defense against
oxidative stress is represented by the antioxidant enzyme SOD, and
to a lesser extent by other enzymes such as ubiquinone
oxidoreductase and catalase (NADH) (20). In particular, SOD catalyzes the
dismutation of superoxide radicals into hydrogen peroxides
(H2O2) and prevents the NO2 and
peroxinitrites formation (21,22).
Deficit of some essential metals (Cu, Zn and Mn) may be the
determinant of the reduction of SOD activity. It is known that
Cu/Zn-SOD and not Mn-SOD is inactivated, at a temperature of 37°C
and a 7.4 pH, in the presence of H2O2 and
superoxide ion (23,24). In a healthy status, platelets
possess high levels of the SOD, in particular, 77% of Cu/Zn-SOD and
the remaining part of Mn-SOD (25), thus, SOD plays and important role
in the platelet membrane fluidity and in the prevention of
thrombosis. Our results highlight lower concentrations of the
essential metals in DVTs, especially Mn that is significantly lower
in this group. As said before, these metals are above all,
essential to activate the SOD antioxidant defense, and we found a
decreased activity of this enzyme in the DVT groups; SOD adds to
the increased production of surrogate oxidative markers.
As indicated by the analysis of the questionnaries,
there are no significant differences among socio-demographics,
habits and lifestyle parameters, except for vegetable consumption,
lower in the controls group, justifying, even if only in part, the
increase of the oxidative stress in DVTs. In fact, vegetables as
well as fruits are known to provide several important health
benefits (26). The dietary intake
of antioxidant nutrients, as well as fruit and vegetable
consumption, may reduce oxidative stress, once they are able to
eliminate free radicals in a direct way, as is the case of
vitamins, or in an indirect way, through minerals, which act as
co-factors of antioxidant enzymes (27). Nevertheless, several pollutants can
be absorbed by diet (28–31), and even if it is balanced, to date,
the balance between benefits and risks due to the ingestion of
contaminated food has been poorly characterized (32).
With regard to the toxic metal investigation, the
highest levels of Pb were found in DVTs. In human blood, ~99% of
the lead is found in the erythrocytes, leaving ~1% in the plasma
and serum (33), indicating that
erythrocytes could be an important target of lead toxicity in the
cardiovascular system. Pb can increase thrombin generation and
accelerating the coagulation process by tissue factor in plasma. Pb
interferes with normal red blood cell formation by inhibiting
important enzymes, inhibits SOD and reduces glutathione levels,
increasing erythrocyte vulnerability to oxidative stress (34). In association with DVTs, Pb
decreases the plasminogen activator antigen (t-PA:Ag), increasing
the production of this inhibitor (PAI-1). Plasminogen degrades many
blood proteins, and in particular the fibrin of thrombus. Its
absence reduces fibrinolysis and therefore favors DVTs (35). In our patients Pb could have a role
in the pathogenesis of DVTs, determining alterations in the
erythrocyte membrane and their adhesiveness to the endothelium. It
indirectly reduces the antioxidant defense mechanisms, exposing the
endothelium to radicals and leading to endothelial dysfunction.
The Pb exposure can be caused by atmospheric
pollution (36,37), which is associated with alterations
of physiological balance related to cardiovascular health such as
inflammation, hypercoagulability, thrombosis, vascular dysfunction
of the autonomic nervous system and atherosclerosis. The
experimental results of the studies carried out by other authors
indicate that long-term exposure (years or decades) to air
pollutants contributes to pulmonary (38) and systemic oxidative stress,
inflammation, atherosclerosis and increased risk of ischemic heart
disease and death (5). The
short-term exposure (days or weeks) favors the risk of acute
coronary syndrome due to atherosclerotic plaque rupture and
thrombosis (39,40). Furthermore, air pollution has been
associated with CVDs (heart failure, stroke, arrhythmias and
cardiac dead) (41) and a few
recent studies indicate that air pollutants can play a role in the
DVT risk (2).
Arterial and venous thrombosis are commonly
considered two distinct diseases, to date a growing body of
evidence indicate several pathophysiological links. There are many
common risk factors such as age, obesity, metabolic syndrome and
most recently, the negative effect of short or prolonged exposure
to air contaminants (42–44).
It is known that air pollution plays a role on
hypercoagulative condition. Consequently, there is a link between
pollutants and venous thrombosis.
However, we possess robust knowledge concerning the
role of air pollution on arterial thrombosis, while its role on
venous thrombosis is still unclear.
Since our study showed an important significance on
public health due to the high risk of venous thromboembolic disease
(45), it has a limit concerning
the number of recruited subjects.
A larger amount of high-quality scientific
experimental data are required to confirm our preliminary results
and to correlate the atmospheric pollution with the residential
areas of the recruited subjects. The results of this research are
an important step in the understanding of the relationship between
lifestyles, environmental pollution and venous thromboembolism,
deepening the knowledge on their bond through the study of
oxidative stress.
Acknowledgements
This study was supported by the Department of
Clinical and Experimental Medicine, University of Catania and the
Department of Medical, Surgical Sciences and Advanced Technologies,
‘G.F. Ingrassia’ Hygiene and Public Health, University of
Catania.
References
|
1
|
Goldhaber SZ: Pulmonary embolism
thrombolysis: A clarion call for international collaboration. J Am
Coll Cardiol. 19:246–247. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baccarelli A, Martinelli I, Zanobetti A,
Grillo P, Hou LF, Bertazzi PA, Mannucci PM and Schwartz J: Exposure
to particulate air pollution and risk of deep vein thrombosis. Arch
Intern Med. 168:920–927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Franchini M, Guida A, Tufano A and Coppola
A: Air pollution, vascular disease and thrombosis: Linking clinical
data and pathogenic mechanisms. J Thromb Haemost. 10:2438–2451.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franchini M and Mannucci PM: Particulate
air pollution and cardiovascular risk: Short-term and long-term
effects. Semin Thromb Hemost. 35:665–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franchini M and Mannucci PM:
Thrombogenicity and cardiovascular effects of ambient air
pollution. Blood. 118:2405–2412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Signorelli SS, Fiore V, Puccia G,
Mastrosimone G and Anzaldi M: Thrombophilia in patients with lower
limb deep veins thrombosis (LDVT). Results of a monocentric survey
on 103 consecutive outpatients. Clin Appl Thromb Hemost.
20:589–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fiore M, Ledda C, Rapisarda V, Sentina E,
Mauceri C, DAgati P, Conti Oliveri G, Serra-Majem L and Ferrante M:
Medical school fails to improve Mediterranean diet adherence among
medical students. Eur J Public Health. 25:1019–1023. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agodi A, Barchitta M, Valenti G,
Quattrocchi A, Marchese AE, Conti Oliveri G, Fallico R, Sciacca S
and Ferrante M: Dietary folate intake and blood biomarkers reveal
high-risk groups in a Mediterranean population of healthy women of
childbearing potential. Ann Nutr Metab. 63:179–185. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sciacca S and Conti Oliveri G: Mutagens
and carcinogens in drinking water. Med J Nutrition Metab.
2:157–162. 2009. View Article : Google Scholar
|
|
10
|
World Health Organisation: Global status
report on noncommunicable diseases 2010. World Health Organisation;
Geneva: 2010
|
|
11
|
Goldhaber SB: Trace element risk
assessment: Essentiality vs. toxicity. Regul Toxicol Pharmacol.
38:232–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santo SS, Sergio N, Luigi DP, Giuseppe M,
Margherita F, Gea OC, Roberto F, Gabriella C, Giuseppe P and
Massimiliano A: Effect of PLC on functional parameters and
oxidative profile in type 2 diabetes-associated PAD. Diabetes Res
Clin Pract. 72:231–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Signorelli SS, Fatuzzo P, Rapisarda F,
Neri S, Ferrante M, Conti Oliveri G, Fallico R, Di Pino L, Pennisi
G, Celotta G, et al: A randomised, controlled clinical trial
evaluating changes in therapeutic efficacy and oxidative parameters
after treatment with propionyl L-carnitine in patients with
peripheral arterial disease requiring haemodialysis. Drugs Aging.
23:263–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reguig S, Bouanane S, Merzouk H, Soufi N
and Merzouk S: Oxidative stress and thrombotic disorders: Study in
patients with venous thromboembolism. Int J Health Sci Res.
6:185–194. 2016.
|
|
16
|
Zhang X, Staimer N, Gillen DL, Tjoa T,
Schauer JJ, Shafer MM, Hasheminassab S, Pakbin P, Vaziri ND,
Sioutas C, et al: Associations of oxidative stress and inflammatory
biomarkers with chemically-characterized air pollutant exposures in
an elderly cohort. Environ Res. 150:306–319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Busch CJ and Binder CJ: Malondialdehyde
epitopes as mediators of sterile inflammation. Biochim Biophys
Acta. Jun 26–2016.(Epub ahead of print).
|
|
18
|
Papac-Milicevic N, Busch CJ and Binder CJ:
Malondialdehyde epitopes as targets of immunity and the
implications for atherosclerosis. Adv Immunol. 131:1–59. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esterbauer H and Zollner H: Methods for
determination of aldehydic lipid peroxidation products. Free Radic
Biol Med. 7:197–203. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uriu-Adams JY and Keen CL: Copper,
oxidative stress, and human health. Mol Aspects Med. 26:268–298.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chambers JC, Haskard DO and Kooner JS:
Vascular endothelial function and oxidative stress mechanisms in
patients with Behçets syndrome. J Am Coll Cardiol. 37:517–520.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brawn K and Fridovich I: Superoxide
radical and superoxide dismutases: Threat and defense. Acta Physiol
Scand (Suppl). 492:9–18. 1980.PubMed/NCBI
|
|
23
|
Sinet PM and Garber P: Inactivation of the
human CuZn superoxide dismutase during exposure to O2
and H2O2. Arch Biochem Biophys. 212:411–416.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harzallah O, Kerkeni A, Baati T and
Mahjoub S: Oxidative stress: Correlation with Behçets disease
duration, activity and severity. Eur J Intern Med. 19:541–547.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng YY, Trachtenburg J, Ryan US and
Abendschein DR: Potentiation of endogenous nitric oxide with
superoxide dismutase inhibits platelet-mediated thrombosis in
injured and stenotic arteries. J Am Coll Cardiol. 25:269–275. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ames BN, Shigenaga MK and Hagen TM:
Oxidants, antioxidants, and the degenerative diseases of aging.
Proc Natl Acad Sci USA. 90:7915–7922. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alissa E and Ferns G: Functional foods and
nutraceuticals in the primary prevention of cardiovascular
diseases. J Nutr Metab. 2012:5694862012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conte F, Copat C, Longo S, Conti GO,
Grasso A, Arena G, Brundo MV and Ferrante M: First data on trace
elements in Haliotis tuberculata (Linnaeus, 1758) from
southern Italy: Safety issues. Food Chem Toxicol. 81:143–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Conti GO, Copat C, Ledda C, Fiore M,
Fallico R, Sciacca S and Ferrante M: Evaluation of heavy metals and
polycyclic aromatic hydrocarbons (PAHs) in Mullus barbatus
from Sicily Channel and risk-based consumption limits. Bull Environ
Contam Toxicol. 88:946–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Copat C, Bella F, Castaing M, Fallico R,
Sciacca S and Ferrante M: Heavy metals concentrations in fish from
Sicily (Mediterranean Sea) and evaluation of possible health risks
to consumers. Bull Environ Contam Toxicol. 88:78–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Copat C, Vinceti M, DAgati MG, Arena G,
Mauceri V, Grasso A, Fallico R, Sciacca S and Ferrante M: Mercury
and selenium intake by seafood from the Ionian Sea: A risk
evaluation. Ecotoxicol Environ Saf. 100:87–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Copat C, Arena G, Fiore M, Ledda C,
Fallico R, Sciacca S and Ferrante M: Heavy metals concentrations in
fish and shellfish from eastern Mediterranean Sea: Consumption
advisories. Food Chem Toxicol. 53:33–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rabinowitz MB: Toxicokinetics of bone
lead. Environ Health Perspect. 91:33–37. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hossain S, Bhowmick S, Islam S, Rozario L,
Jahan S, Hassan M, Sarkar M, Choudhury BK, Ahmed S and Shahjalal H:
Oral administration of Ganoderma lucidum to lead-exposed
rats protects erythrocytes against hemolysis: Implicates to
anti-anemia. Evid Based Complement Alternat Med. 2015:4637032015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaji T, Yamamoto C, Sakamoto M and Kozuka
H: Inhibitory effect of lead on the release of tissue plasminogen
activator from human vascular endothelial cells in culture.
Toxicology. 73:219–227. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Y, Zhuang G, Zhang Z, Wang Y and
Zhuang Y: Characteristics and sources of lead pollution after
phasing out leaded gasoline in Beijing. Atmos Environ.
40:2973–2985. 2016. View Article : Google Scholar
|
|
37
|
Sangani RG, Soukup JM and Ghio AJ: Metals
in air pollution particles decrease whole-blood coagulation time.
Inhal Toxicol. 22:621–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ghozikali Ghanbari M, Heibati B, Naddafi
K, Kloog I, Conti Oliveri G, Polosa R and Ferrante M: Evaluation of
chronic obstructive pulmonary disease (COPD) attributed to
atmospheric O3, NO2, and SO2 using
Air Q Model (2011–2012 year). Environ Res. 144:(Pt A). 99–105.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bind MA, Baccarelli A, Zanobetti A,
Tarantini L, Suh H, Vokonas P and Schwartz J: Air pollution and
markers of coagulation, inflammation, and endothelial function:
Associations and epigene-environment interactions in an elderly
cohort. Epidemiology. 23:332–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Madrigano J, Baccarelli A, Mittleman MA,
Wright RO, Sparrow D, Vokonas PS, Tarantini L and Schwartz J:
Prolonged exposure to particulate pollution, genes associated with
glutathione pathways, and DNA methylation in a cohort of older men.
Environ Health Perspect. 119:977–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mills NL, Donaldson K, Hadoke PW, Boon NA,
MacNee W, Cassee FR, Sandström T, Blomberg A and Newby DE: Adverse
cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc
Med. 6:36–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Piazza G and Goldhaber SZ: Venous
thromboembolism and atherothrombosis: An integrated approach.
Circulation. 121:2146–2150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ageno W, Becattini C, Brighton T, Selby R
and Kamphuisen PW: Cardiovascular risk factors and venous
thromboembolism: A meta-analysis. Circulation. 117:93–102. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prandoni P: Venous and arterial
thrombosis: Two aspects of the same disease? Clin Epidemiol. 1:1–6.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Signorelli SS, Fiore V, Ruggieri M and
Basile A: Acute deep vein thrombosis (DVT) of the lower limbs in a
32-year-old man with chronic hypoplasia of the inferior vena cava
(HIVC) without risk factors. Intern Emerg Med. 11:273–275. 2016.
View Article : Google Scholar : PubMed/NCBI
|