Introduction
Polycyclic aromatic hydrocarbons (PAHs) are
considered a dangerous pollutant for human health due to their
carcinogenic and mutagenic activity. PAHs are generated by the
incomplete combustion of fossil fuels, tobacco smoke and grilled or
broiled foods (1–3). Benzo(a)pyrene (BaP) is the most
widely investigated PAH as a marker for the carcinogenic risk of
PAHs in ambient air. BaP has been classified as carcinogenic to
humans (4) and it can generate
reactive oxygen species that may result in cell apoptosis (5,6)
and/or generalized inflammations in several tissues (7–10).
Both BaP and its metabolites are known for their capacity of
producing BP diol-epoxide-DNA adducts (BPDE). It is generally
agreed that (±)-anti-BaPDE is the metabolite that forms the major
adducts with DNA in vivo (11). BPDE adducts can interfere or alter
DNA replication (12). Biomarkers
are required to develop novel therapeutics and risk-assessment
methodologies. In fact, BPDE adducts reflect individual variation
of exposure, absorption, metabolic activation and DNA repair, also,
there is no dose of exposure below which BPDE adducts are not
produced. Therefore, they represent valuable environmental risk
biomarkers (13). In particular,
several authors have measured the anti-BPDE hydrolyzed DNA
adducts or Tetrol I-1
(r-7,c-10t-8,t-9-tetrahydroxy-7,8,9,10-tetrahydrobenzo(a)pyrene)
and syn-BPDE hydrolyzed DNA adducts or Tetrol II-2
(8r-7,t-9,t-10,t-8-tetrahydroxy-7,8,9,10-tetrahydrobenzo(a)pyrene)
(11). Several studies report a
correlation between BPDE adducts and various diseases, such as
cancer (14) and behavioral
outcomes in children (15,16), whereas very few studies report
altered fertility in male adults (17,18).
At present, male fertility represents a relevant worldwide issue.
There are no exhaustive data for global prevalence of male
infertility but, according to World Health Organization (WHO),
nearly 60–80 million couples worldwide currently suffer from
infertility (19). It varies
across regions of the world and it is estimated to affect 8–12% of
couples on average. According to a WHO's multicentric study, in 20%
of cases the couple's infertility was attributed to male
infertility, 38% to female infertility, 27% due to infertility of
both partners, and 15% could not be attributed to either partner
(20). Moreover, often no
detectable cause can be associated to a male infertility by
measuring the conventional parameters on sperm analysis. This is
defined as unexplained or idiopathic infertility (20).
Few studies have investigated the association
between BPDE adducts in DNA of human spermatozoa and male fertility
(12,17,18).
Only two articles are available to date about the association
between BPDE adduct numbers measured by immunofluorescence
technique in sperm-extracted DNA and fertility, but these studies
were carried out only in infertile men (17,18).
The immunofluorescence, immunostaining, COMET assay and
23P-postlabelling techniques are all used for BPDE
adducts tests, but these techniques are not able to measure a
single Tetrol or only BaP-adducts, thus providing a total generic
PAH-DNA adduct concentration.
Therefore, the aim of the present study was to
verify this hypothesis measuring the Tetrol I-1 and Tetrol II-2 in
population's semen of eastern Sicily (Italy) by high-performance
liquid chromatography-fluorescence (HPLC-FL) method. The present
study is the first carried out on general population and that
reported both Tetrol I-1 and Tetrol II-2 individually in human
sperm.
Materials and methods
Sampling
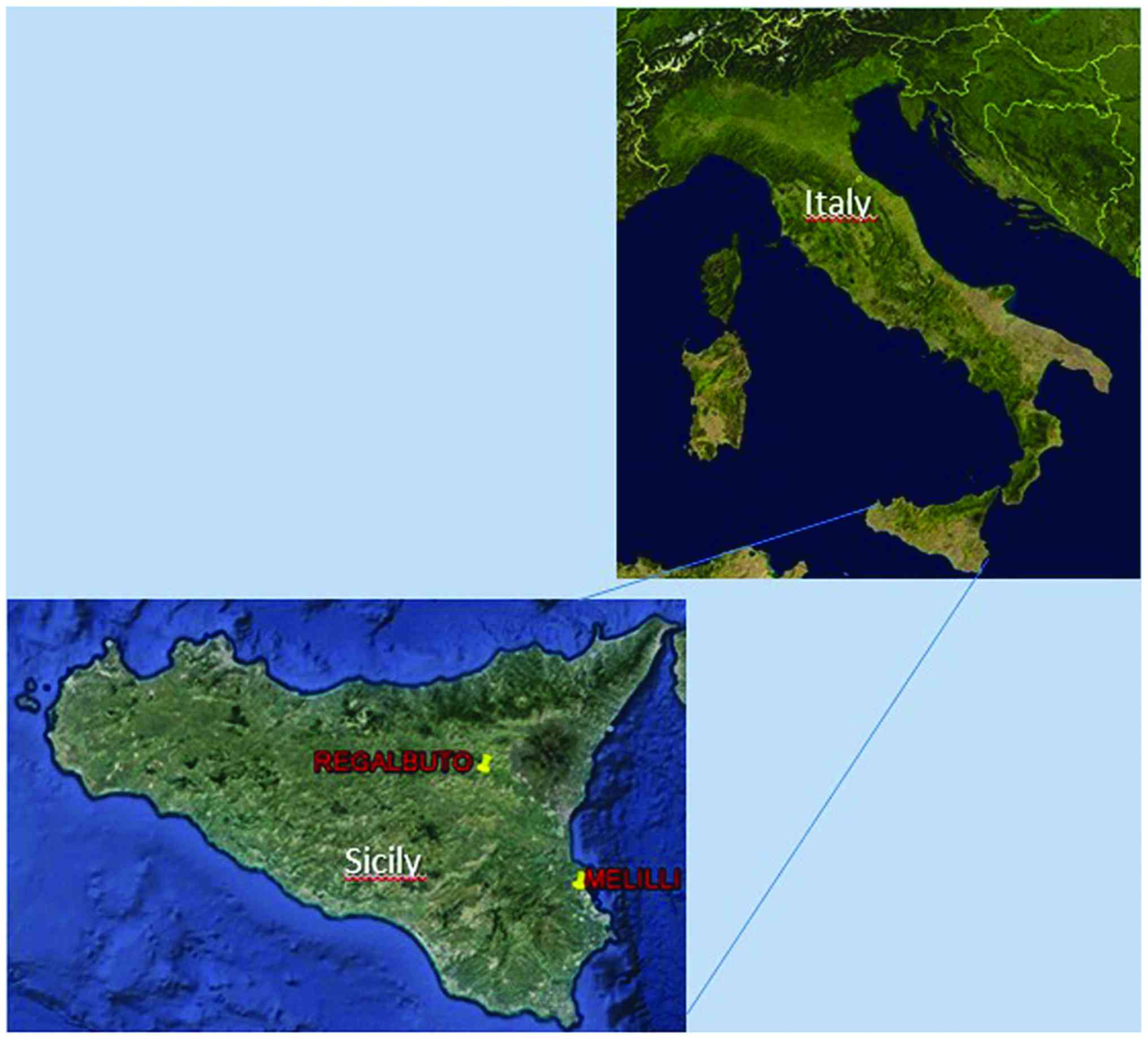
Semen of 86 volunteers from Eastern Sicilian cities
(from Regalbuto, a small town in a rural agricultural and Melilli a
small town in an industrial district) (Fig. 1) was collected at Division of
Andrology, University of Catania. Semen samples were collected
after 3–5 days of abstinence and analyzed according to the WHO
criteria (21). The volunteers
joined the study by signing the informed consent and by filling out
a semi-structured questionnaire. The questionnaire elicited
anthropometric information, demographic, lifetime residential
history (location of birth and duration of residence), history of
active and passive smoking (including number of household members
who smoke), date of smoking cessation, occupational exposure,
medication information, and consumption of PAH-containing meat
(frequency of eating fried, broiled, or barbecued meat, mineral or
drink water during the last 2 weeks). Socioeconomic information
related to income, education, hobbies and sports preferred was also
collected.
Semen quality assessment
Sperm parameters (volume, sperm concentration, total
sperm counts, progressive motility, and normal forms) were
evaluated in all samples according to the WHO criteria (21).
DNA adducts analysis
We analyzed hydrolyzed BPDE adducts or Tetrol I-1
and Tetrol II-2 in extracted sperm cell DNA using the modified
HPLC-FL method according to Alexandrov et al (22) modified. Briefly, the semen samples
were collected at the Laboratory of the Division of Andrology and
aliquoted, for semen quality parameter assessment and for molecular
analysis. DNA was extracted using the DNA IQ™ system
(Promega Corp., Madison, WI, USA) according to the manufacturer's
instructions. Then, the DNA was subjected to a procedure of
hydrolysis and purification.
Reagent
HPLC-grade water was produced in-house using a
Milli-Q® system (Merck KGaA, Darmstadt, Germany).
Methanol, ethyl acetate, ethanol, and acetonitrile, all
LiChrosolv® hypergrade, were purchased from
Sigma-Aldrich (Saint Louis, MO, USA).
Hydrochloric acid (HCl) hypergrade and NaOH were
purchased from Merck KGaA (Darmstadt, Germany).
Purification of acid
The HCl, also if hypergrade, contains fluorescent
impurities. One of which in particular, having the same retention
time, interferes with Tetrol I-1, hence influencing the
concentration of this hydrolyzed BaP-adduct. To eliminate this
preparative bias all fluorescent impurities were deleted from HCl
(0.4 N) heating this for 4 h at 90°C and successively extracted
three times with ethyl acetate.
Analytical procedures
A specialized laboratory was dedicated to the
biochemical analysis as the detection of Tetrols is difficult. To
avoid the fluorescent impurities contaminating samples, the vials,
tips, tubes, syringes and capillaries for HPLC mobile phases and
any other equipment used were washed with methanol hypergrade.
The extracted and purified DNA was dissolved in 1 ml
of water and analyzed in a Perkin Elmer Series 200 HPLC
(Perkin-Elmer, Milano, Italy).
Preparation of samples was carried out through the
following steps: i) hydrolysis of purified DNA diluted with
purified HCl (0.4 N) and then neutralized with NaOH (1 N) to pH
7-7.5; ii) the sample was then diluted (20%) with methanol; iii)
subsequently it was purified using Sep-Pak C18 (Waters Corp.,
Milford, MA, USA) cartridges SPE-equilibrated with 20% methanol
drop by drop with a flow rate of 2.0 ml/min for 10 min; iv) Tetrols
I-1 and II-2 were eluted with 1 ml of methanol (55%). v) Samples
were evaporated to dryness with a gentle stream of nitrogen and
reconstituted with 100 µl of methanol and of these, 30 µl were
injected into HPLC column.
Chromatographic settings and
materials
Automated analysis was performed using an integrated
system of Perkin Elmer coupled to Series 200 quaternary pump,
peltier column, autosampler, UV detector, fluorescence detector and
a degasser apparatus, all Series 200 (Perkin-Elmer).
Chromatographic analysis was carried out in a Tosoh (C18 RP 25×0.46
cm, 5 µm) eluted with a elution program of 15 min with 20%
water/acetonitrile of equilibrium phase, followed by 5 min with 20%
water/acetonitrile and 60 min to acetonitrile (100%) with a curve
of 1 and finally other 10 min to 100% acetonitrile a flow rate of
0.85 ml/min. The excitation wavelength was set at 344 nm and the
fluorescence emission wavelength at 388 nm. The sensitivity of the
detector was fixed to high modality. The wavelength of UV detector
was set at 238 nm for the double detection of both Tetrols. The
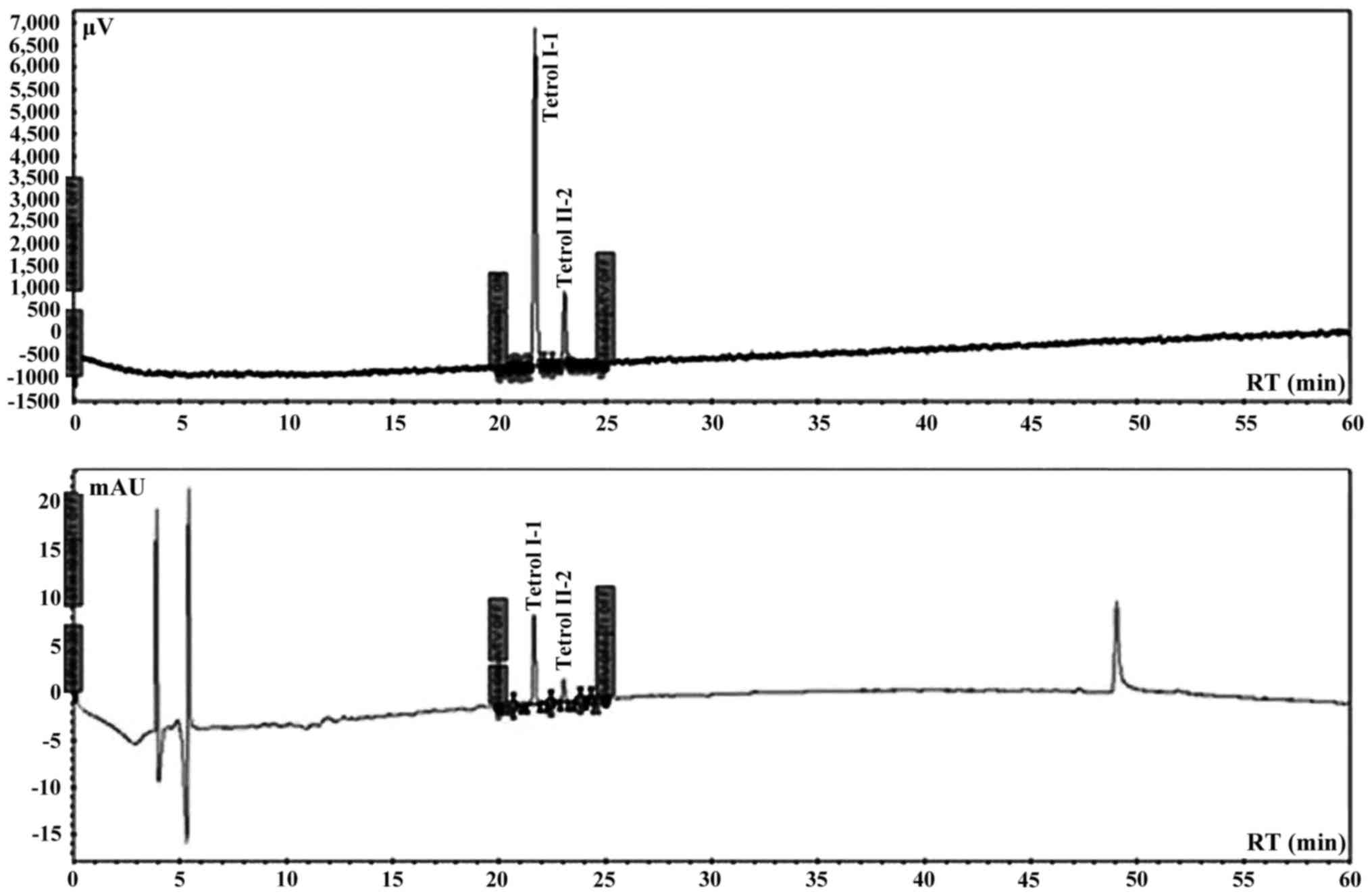
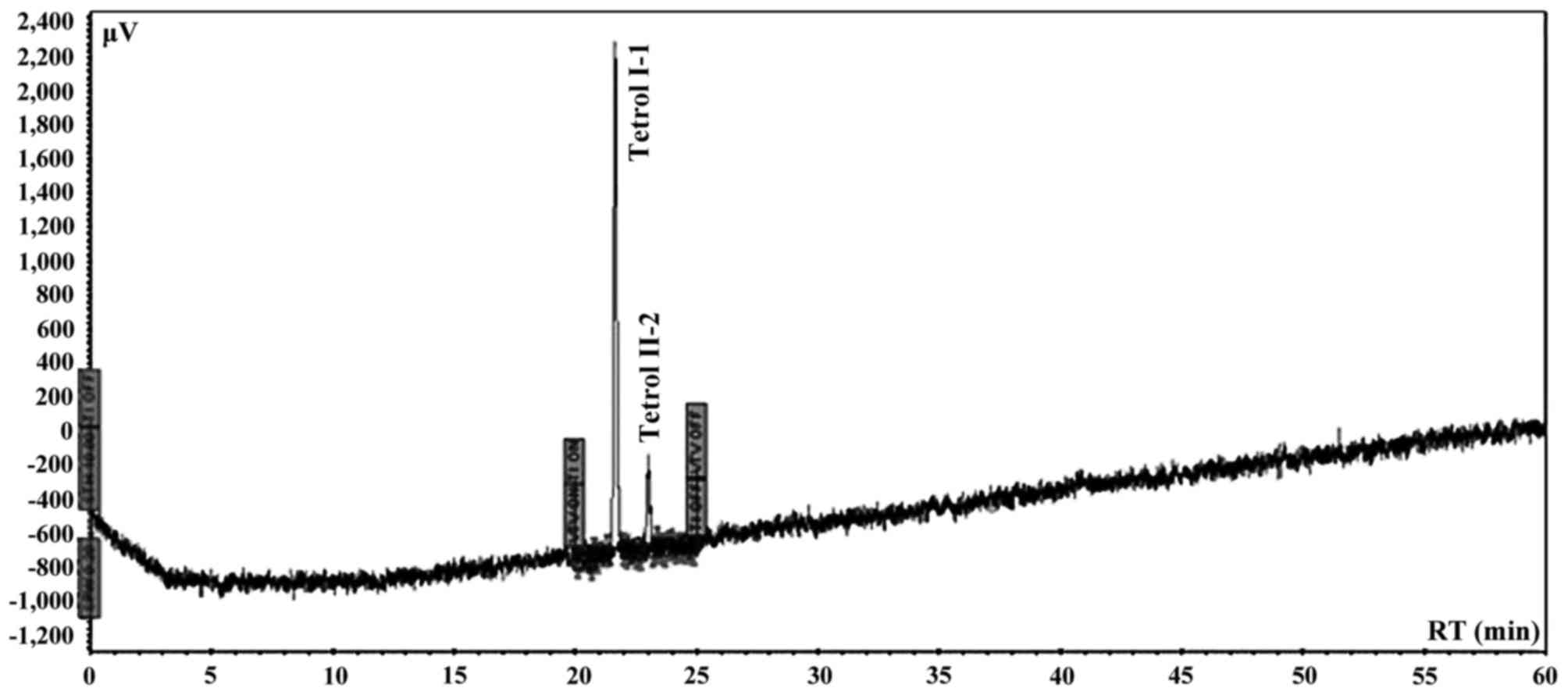
chromatographic system was calibrated using Tetrol I-1 and Tetrol
II-2 certified pure standards of NCl (Chemical Carcinogen Reference
Standard Repository, Kansas City, MO, USA). Tetrol I-1 and Tetrol
II-2 (purity 99.0%; both from Sigma Aldrich) were used as external
standards (Fig. 2). Recovery was
93% and 81% for Tetrol I-1 and Tetrol II-2, respectively (Fig. 3). Processing of reagent blank
disclosed no trace of Tetrol I-1 and Tetrol II-2. Linearities
(R2) of calibrated chromatographic system were 0.9980
and 0.9854 for fluorescence and UV detectors, respectively for
Tetrol I-1. For Tetrol II-2, the linearities were 0.9950 and 0.9943
for fluorescence and UV detectors, respectively. MDL were 2.0 and
3.1 pg/ml for Tetrol I-1 and Tetrol II-2, respectively. The HPLC
software used for area integrations and linearity computing was
TotalChrome (version 2010; Agilent Technologies, Santa Clara, CA,
USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 21.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered
to be statistically significant.
Descriptive analyses were performed using
interquartile interval, min, max and 95% confidence intervals (95%
CI) for quantitative variables.
The differences between Tetrol levels were assessed
by the Wilcoxon signed-rank test and the Mann-Whitney U test, as
appropriate. Correlation analysis between sperm quality parameters
and Tetrol levels was performed by Spearman's correlation
coefficient (ρ).
Results
The numbers of Tetrol adducts (n.
adducts/108 nucleotides) detected in sperm samples are
reported in Tables I and II.
 | Table I.Distribution of conventional sperm
parameters. |
Table I.
Distribution of conventional sperm
parameters.
| Variables | Min | 25th | Median | 75th | Max |
|---|
| Sperm concentration
(millions/ml) | 0.0 | 32.5 | 76.0 | 113.5 | 250.0 |
| Total sperm count
(millions/ejaculate) | 0.0 | 95.6 | 204.0 | 350.0 | 825.0 |
| Progressive motility
(%) | 1.0 | 24.0 | 33.0 | 42.0 | 62.0 |
| Normal forms (%) | 8.0 | 13.0 | 16.0 | 20.0 | 33.0 |
 | Table II.Distribution of Tetrol I-1 and Tetrol
II-2 (n. Adduct/108 nucleotides). |
Table II.
Distribution of Tetrol I-1 and Tetrol
II-2 (n. Adduct/108 nucleotides).
| Variables | Min | 25th | Median | 75th | Max |
|---|
| Tetrol I-1 | 0,00100 | 0,00240 | 0,00540 | 0,05226 | 103,58500 |
| Tetrol II-2 | 0,00200 | 0,00731 | 0,01712 | 0,17862 |
52,67400 |
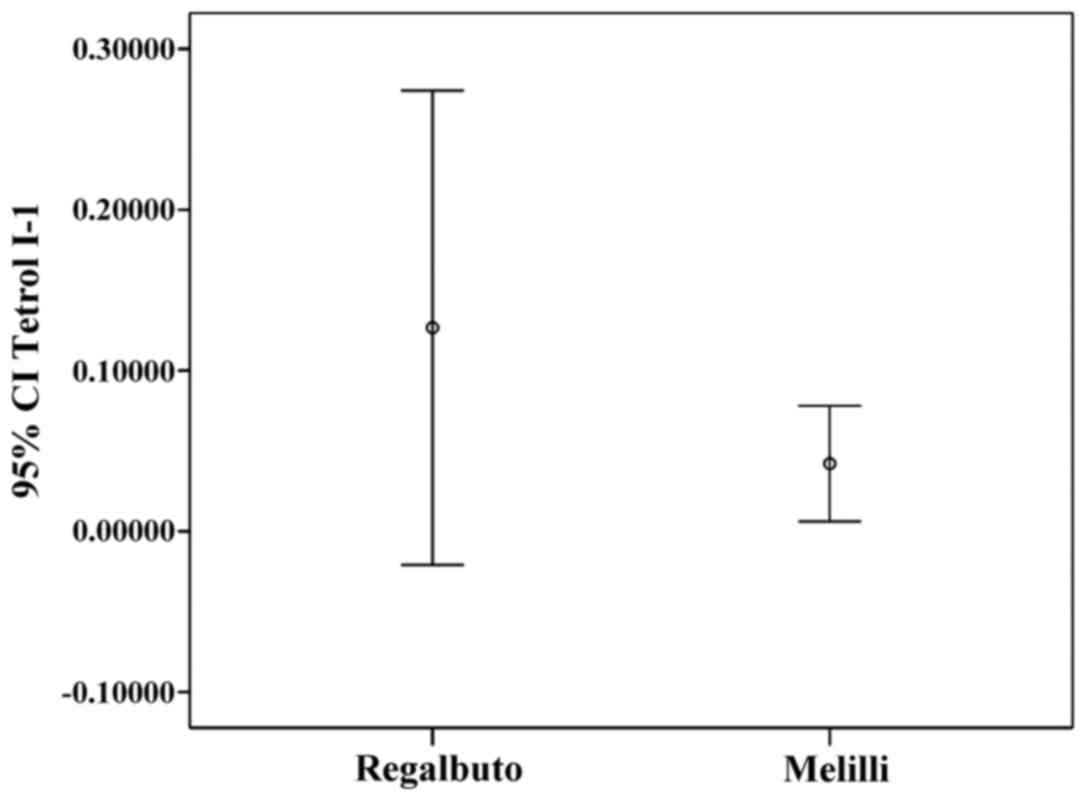
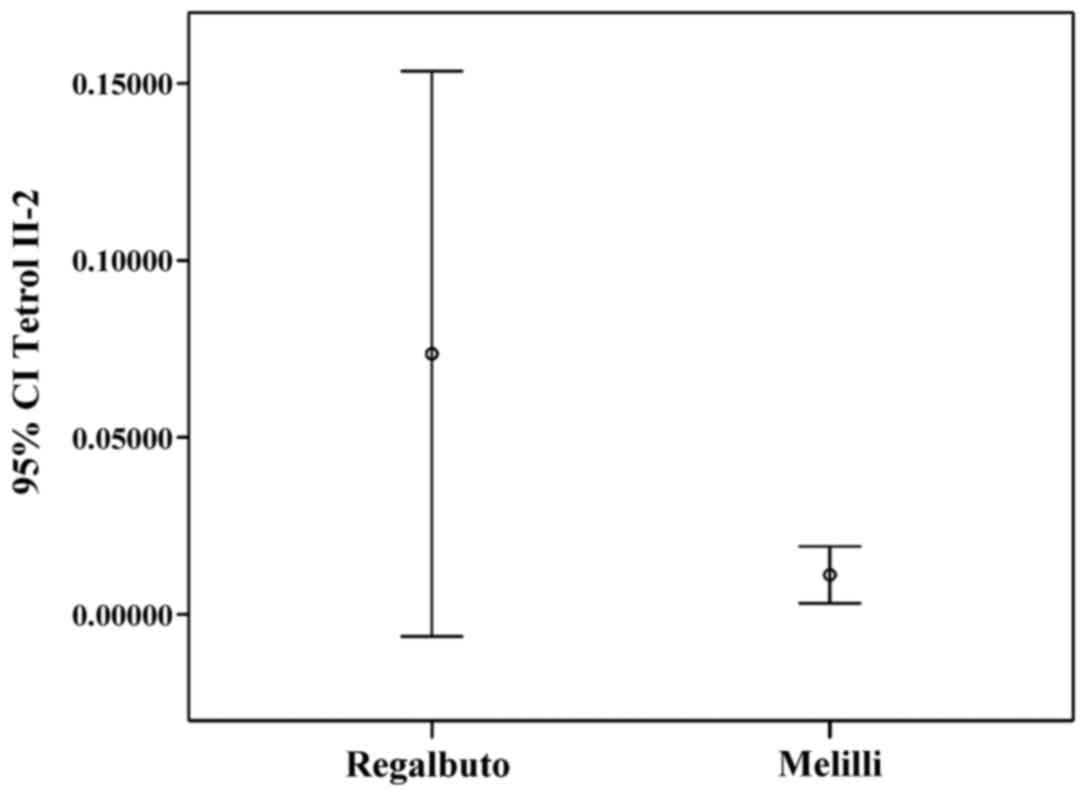
Tetrol I-1 and Tetrol II-2 stratifications by
residence (IC 95%) are shown in Figs.
4 and 5. Table III shows the correlations between
sperm parameters, age, cigarette/day and adducts Tetrol I-1 and
Tetrol II-2. A significant inverse correlation between sperm
progressive motility and both TI-1 and TII-2 was observed (Table III).
 | Table III.Correlation between conventional
parameters and Tetrols. |
Table III.
Correlation between conventional
parameters and Tetrols.
|
|
| Tetrol I-1 | Tetrol II-2 | Sperm
concentration | Progressive
motility | MNP c | Normal forms | Total count | Age | Cigarette/day |
|---|
| Tetrol I-1 | R | 1.000 |
|
|
|
|
|
|
|
|
|
| P | . |
|
|
|
|
|
|
|
|
|
| N | 86 |
|
|
|
|
|
|
|
|
| Tetrol II-2 | R | 0.869b | 1.000 |
|
|
|
|
|
|
|
|
| P | 0.000 | . |
|
|
|
|
|
|
|
|
| N | 86 | 86 |
|
|
|
|
|
|
|
| Sperm
concentration | R | 0.077 | 0.022 | 1.000 |
|
|
|
|
|
|
|
| P | 0.480 | 0.840 | . |
|
|
|
|
|
|
|
| N | 86 | 86 | 86 |
|
|
|
|
|
|
| Progressive
motility | R | −0.264a | −0.222a | 0.052 | 1.000 |
|
|
|
|
|
|
| P | 0.014 | 0.040 | 0.502 | . |
|
|
|
|
|
|
| N | 86 | 86 | 86 | 86 |
|
|
|
|
|
| MNP c | R | 0.234a | 0.279b | 0.025 | −0.606b | 1.000 |
|
|
|
|
|
| P | 0.030 | 0.009 | 0.750 | 0.000 | . |
|
|
|
|
|
| N | 86 | 86 | 86 | 86 | 86 |
|
|
|
|
| Normal forms | R | 0.137 | 0.201 | 0.123 |
0.161a | −0.015 | 1.000 |
|
|
|
|
| P | 0.209 | 0.063 | 0.109 | 0.035 | 0.848 | . |
|
|
|
|
| N | 86 | 86 | 86 | 86 | 86 | 86 |
|
|
|
| Total count | R | 0.061 | 0.042 |
0.820b | 0.143 | −0.013 | 0.228b | 1.000 |
|
|
|
| P | 0.579 | 0.702 | 0.000 | 0.063 | 0.866 | 0.003 | . |
|
|
|
| N | 86 | 86 | 86 | 86 | 86 | 86 | 86 |
|
|
| Age | R | −0.152 | −0.225a | −0.006 | 0.041 | −0.060 | 0.021 | 0.056 | 1.000 |
|
|
| P | 0.165 | 0.038 | 0.941 | 0.595 | 0.440 | 0.786 | 0.465 | . |
|
|
| N | 85 | 85 | 86 | 86 | 86 | 86 | 86 | 86 |
|
| Cigarette/day | R | 0.101 | 0.191 | 0.015 | 0.028 | 0.126 | 0.253a | 0.143 | 0.358b | 1.000 |
|
| P | 0.575 | 0.286 | 0.903 | 0.823 | 0.312 | 0.040 | 0.248 | 0.002 | . |
|
| N | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
Discussion
Male infertility is an alarming global health issue
that has not been studied to truly understand its magnitude and
prevalence. PAHs are a class of organic compounds produced by
incomplete combustion or high-pressure processes (23,24)
that can affect human health. The present study investigated the
hypothesis of an association between BPDE-DNA adducts in human
sperms and fertility. The results of the present study showed that
Σ(Tetrol I-1+Tetrol II-2) were significantly higher in
the volunteers' semen from Regalbuto (a rural area) than in those
of men living in an industrial area. Moreover, we found that Tetrol
I-1 was higher than Tetrol II-2 in samples from Regalbuto, whereas
Tetrol II-2 was higher than Tetrol I-1 in Melilli samples.
Furthermore, a greater dispersion of adduct levels was observed in
these specimens (Figs. 4 and
5). A significant inverse
correlation was found between sperm progressive motility and the
two Tetrols (Table III).
Furthermore, no correlation was found between smoking and BPDE-DNA
adducts. In fact, the non-smokers (46 subjects) showed median value
of Tetrols higher than smokers (40 subjects) (Tetrol I-1: 0.00540
vs. 0.00375; Tetrol II-2: 0.01618 vs. 0.01605).
The present study showed that BaP negatively affects
sperm parameters by BPDE-DNA adducts production. Few studies
(12,17,18)
have found that increased levels of DNA adducts in humans are
associated with sperm motility and impaired male fertility, but up
to now there are inconsistencies in the evidence for a link with
smoking (17,25–27)
except for the lung tissue. In fact, the relationship between
smoking-related DNA adducts must be considered on a
tissue-by-tissue basis (25). In a
similar Italian study, Gaspari et al (17) showed that PAH-DNA adducts, measured
as sum by immunofluorescence analysis, were negatively correlated
with the percentage of spermatozoa with normal morphology and neck
morphological abnormalities, whereas a positive correlation was
found with head abnormalities. Furthermore, these authors reported
a significantly association between occupational exposure to PAH
and PAH-DNA adducts in spermatozoa and none significantly
association between smoking and sperm PAH-DNA adducts. Lastly, a
higher number of PAH-DNA adducts was found in infertile patients
vs. fertile men. A weak association between tobacco consumption and
BPDE-DNA adducts (measured with immunofluorescence technique) in
spermatozoa of professional exposed infertile men was reported
(18). However, several authors
reported that BaP-DNA adducts were associated significantly with
environmental BaP exposure and in particular to air BaP pollution
(1,28).
The presence of a major adducts dispersion and
concentration in men from Regalbuto is a very original and
interesting finding. No data are available about male population
fertility or environment pollution of Regalbuto until today. Our
hypothesis is that the residents of this town are exposed to air
pollution due to archaic agricultural practices such as the
controlled fires of fields (fires are high natural BaP source of
emission in atmosphere). This is a usual practice in this
geographical area and no control or monitoring is applied to verify
the pollution produced. This hypothesis is supported by some
available data on the topic (29).
In contrast, the residents of Melilli are also
exposed to air pollution but, being a city with an industrial pole
devoted to oil refining and energy and chemical production, this is
a very controlled area through a network of fixed stations for the
control of atmospheric pollution. This continued monitoring allows
an appropriate management of the production cycle to prevent the
increasing air pollution.
No data are available today on the relationship
between smoked foods consume and BaP-DNA adducts in spermatozoa
but, for other tissue, Tang et al (13) reported that dietary PAHs do not
significantly contribute to adducts number (13). No data are available on the
different number of Tetrol I-1 and Tetrol II-2, for this reason the
present study added very original data to the scientific
literature. Indeed, the higher number of Tetrol II-2 compared to
Tetrol I-1 can be explained through some molecular mechanisms, such
as a higher susceptibility of one chain of DNA vs. genotoxic
effects of BaP or, inefficiency of some enzymes for DNA repair
(30–32). In particular, there is available
evidence about the XRCC1 polymorphisms that may modify sperm
PAH-DNA adduct levels (32). Thus,
this highly sensitive and specific procedure is suitable for
measuring BPDE-DNA adducts in human spermatozoa from
environmentally exposed subjects and it could be adapted to measure
PAH effects in genital organs.
Despite the considerable interest raised regarding
the air pollution for the multiple adverse effects reported on
human health from inflammation to cancer, its impact on fertility
remains still unclear (33,34).
A significant impact of air pollution on miscarriage and clinical
pregnancy rates in the general population was reported.
Furthermore, some air contaminants seem to negatively impact on
fertility outcomes, including miscarriage and live birth rates, in
subfertile patients (33,34).
In conclusion, the present study, for first time,
investigated the hypothesis of an association between BaP-DNA
adducts and human male infertility. The results seem to indicate
that this hypothesis may be real and very interesting data were
found including a new molecular strategy to study male infertility.
Because spermatozoa are not the target cells for BaP exposure, the
relationship between PAH-DNA adducts and these germinal cells
remain to be determined.
References
|
1
|
Guerreiro CBB, Horálek J, de Leeuw F and
Couvidat F: Benzo(a)pyrene in Europe: Ambient air concentrations,
population exposure and health effects. Environ Pollut.
214:657–667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sciacca S and Conti Oliveri G: Mutagens
and carcinogens in drinking water. Med J Nutrition Metab.
2:157–162. 2009. View Article : Google Scholar
|
|
3
|
Conte F, Copat C, Longo S, Conti G, Grasso
A, Arena G, Dimartino A, Brundo MV and Ferrante M: Polycyclic
aromatic hydrocarbons in Haliotis tuberculata (Linnaeus,
1758) (Mollusca, Gastropoda): Considerations on food safety and
source investigation. Food Chem Toxicol. 94:57–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
International Agency for Research on
Cancer (IARC): Chemical Agents and Related OccupationsIARC
Monographs. 100F. International Agency for Research on Cancer;
Lyon: 2012
|
|
5
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferecatu I, Borot MC, Bossard C, Leroux M,
Boggetto N, Marano F, Baeza-Squiban A and Andreau K: Polycyclic
aromatic hydrocarbon components contribute to the
mitochondria-antiapoptotic effect of fine particulate matter on
human bronchial epithelial cells via the aryl hydrocarbon receptor.
Part Fibre Toxicol. 21:(7). 182010. View Article : Google Scholar
|
|
7
|
Signorelli SS, Anzaldi M, Libra M,
Navolanic PM, Malaponte G, Mangano K, Quattrocchi C, Di Marco R,
Fiore V and Neri S: Plasma levels of inflammatory biomarkers in
peripheral arterial disease: Results of a cohort study. Angiology.
67:870–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Liu G, Lin Z, Wang Y, He H, Liu T
and Kamp DW: Pro-inflammatory response and oxidative stress induced
by specific components in ambient particulate matter in human
bronchial epithelial cells. Environ Toxicol. 31:923–936. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Signorelli SS, Fatuzzo P, Rapisarda F,
Neri S, Ferrante M, Conti Oliveri G, Fallico R, Di Pino L, Pennisi
G, Celotta G, et al: A randomised, controlled clinical trial
evaluating changes in therapeutic efficacy and oxidative parameters
after treatment with propionyl L-carnitine in patients with
peripheral arterial disease requiring haemodialysis. Drugs Aging.
23:263–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Signorelli SS, Fatuzzo P, Rapisarda F,
Neri S, Ferrante M, Conti Oliveri G, Fallico R, Di Pino L, Pennisi
G, Celotta G, et al: Propionyl-L-carnitine therapy: Effects on
endothelin-1 and homocysteine levels in patients with peripheral
arterial disease and end-stage renal disease. Kidney Blood Press
Res. 29:100–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alexandrov K, Sala M and Rojas M:
Differences in the DNA adducts formed in cultured rabbit and rat
dermal fibroblasts by benzo(a)pyrene and
(−)benzo(a)pyrene-7,8-diol. Cancer Res. 48:7132–7139.
1988.PubMed/NCBI
|
|
12
|
U.S. Environmental Protection Agency
(EPA): Toxicity and Exposure Assessment for Children's Health
(TEACH). https://archive.epa.gov/region5/teach/web/html/index.htmlAccessed.
January 10–2017.
|
|
13
|
Tang D, Li T, Liu JJ, Chen J, Qu L and
Perera F: PAH-DNA adducts in cord blood and fetal and child
development in a Chinese cohort. Environ Health Perspect.
114:1297–1300. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
United States Environmental Protection
Agency (U.S. EPA): IRIS Toxicological Review of Benzo[a]pyrene
(Final Report)U.S. Environmental Protection Agency. Washington, DC:
EPA/635/R-17/003F. 2017, https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=329750
|
|
15
|
Perera FP, Chang HW, Tang D, Roen EL,
Herbstman J, Margolis A, Huang TJ, Miller RL, Wang S and Rauh V:
Early-life exposure to polycyclic aromatic hydrocarbons and ADHD
behavior problems. PLoS One. 5:e1116702014. View Article : Google Scholar
|
|
16
|
Perera FP, Tang D, Wang S, Vishnevetsky J,
Zhang B, Diaz D, Camann D and Rauh V: Prenatal polycyclic aromatic
hydrocarbon (PAH) exposure and child behavior at age 6–7 years.
Environ Health Perspect. 120:921–926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gaspari L, Chang SS, Santella RM, Garte S,
Pedotti P and Taioli E: Polycyclic aromatic hydrocarbon-DNA adducts
in human sperm as a marker of DNA damage and infertility. Mutat
Res. 535:155–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zenzes MT, Bielecki R and Reed TE:
Detection of benzo(a)pyrene diol epoxide-DNA adducts in sperm of
men exposed to cigarette smoke. Fertil Steril. 72:330–335. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rutstein SO and Shah IH: Infecundity,
infertility, and childlessness in developing countries. DHS
Comparative Reports No 9ORC Macro and the World Health
Organization. Calverton, MD: 2004
|
|
20
|
Kumar N and Singh AK: Trends of male
factor infertility, an important cause of infertility: A review of
literature. J Hum Reprod Sci. 8:191–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
World Health Organization (WHO): WHO
Laboratory Manual for the Examination and Processing of Human
Semen. 5th. WHO Press; Geneva: pp. 1–271. 2010
|
|
22
|
Alexandrov K, Rojas M, Geneste O,
Castegnaro M, Camus AM, Petruzzelli S, Giuntini C and Bartsch H: An
improved fluorometric assay for dosimetry of benzo(a)pyrene
diol-epoxide-DNA adducts in smokers' lung: Comparisons with total
bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer
Res. 52:6248–6253. 1992.PubMed/NCBI
|
|
23
|
Hayakawa K: Environmental behaviors and
toxicities of polycyclic aromatic hydrocarbons and nitropolycyclic
aromatic hydrocarbons. Chem Pharm Bull (Tokyo). 64:83–94. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conti GO, Copat C, Ledda C, Fiore M,
Fallico R, Sciacca S and Ferrante M: Evaluation of heavy metals and
polycyclic aromatic hydrocarbons (PAHs) in Mullus barbatus
from Sicily channel and risk-based consumption limits. Bull Environ
Contam Toxicol. 88:946–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Phillips DH and Venitt S: DNA and protein
adducts in human tissues resulting from exposure to tobacco smoke.
Int J Cancer. 131:2733–2753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gilberson T, Peluso ME, Munia A,
Luján-Barroso L, Sánchez MJ, Navarro C, Amiano P, Barricarte A,
Quirós JR, Molina-Montes E, et al: Aromatic adducts and lung cancer
risk in the European Prospective Investigation into Cancer and
Nutrition (EPIC) Spanish cohort. Carcinogenesis. 35:2047–2054.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palli D, Saieva C, Munnia A, Peluso M,
Grechi D, Zanna I, Caini S, Decarli A, Sera F and Masala G: DNA
adducts and PM(10) exposure in traffic-exposed workers and urban
residents from the EPIC-Florence City study. Sci Total Environ.
403:105–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jayachandran S: Air quality and early-life
mortality: Evidence from Indonesia's wildfires. J Hum Resour.
44:916–954. 2009. View Article : Google Scholar
|
|
29
|
Conti Oliveri G, Heibati B, Kloog I, Fiore
M and Ferrante M: A review of AirQ Models and their applications
for forecasting the air pollution health outcomes. Environ Sci
Pollut Res Int. Jan 4–2017.(Epub ahead of print). PubMed/NCBI
|
|
30
|
Miri M, Derakhshan Z, Allahabadi A, Ahmadi
E, Conti Oliveri G, Ferrante M and Aval HE: Mortality and morbidity
due to exposure to outdoor air pollution in Mashhad metropolis,
Iran. The AirQ model approach. Environ Res. 151:451–457. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghozikali Ghanbari M, Heibati B, Naddafi
K, Kloog I, Conti Oliveri G, Polosa R and Ferrante M: Evaluation of
Chronic Obstructive Pulmonary Disease (COPD) attributed to
atmospheric O3, NO2, and SO2 using
Air Q Model (2011–2012 year). Environ Res. 144:99–105. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Kloog I, Coull BA, Kosheleva A,
Zanobetti A and Schwartz JD: Estimating causal effects of long-term
PM2.5 exposure on mortality in New Jersey. Environ Health Perspect.
124:1182–1188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nieuwenhuijsen MJ, Basagaña X, Dadvand P,
Martinez D, Cirach M, Beelen R and Jacquemin B: Air pollution and
human fertility rates. Environ Int. 70:9–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frutos V, González-Comadrán M, Solà I,
Jacquemin B, Carreras R and Checa Vizcaíno MA: Impact of air
pollution on fertility: A systematic review. Gynecol Endocrinol.
31:7–13. 2015. View Article : Google Scholar : PubMed/NCBI
|